Packaging
Sudaxshina Murdan
Chapter contents
Key points
• The closure, such as a stopper, lid, top or cap, is an integral part of the pack.
• A pack can be single-unit, e.g. a sachet, or multiple-unit, e.g. a bottle containing many tablets.
Introduction
Packaging is the means of economically providing containment, protection, presentation, identification, information, convenience and compliance, for a product during storage, distribution, display and use.
Pharmaceutical packaging enables the packaged medicine’s requirements of efficacy, safety, uniformity, reproducibility, integrity, purity and stability to be maintained throughout the product’s shelf-life. Furthermore, some pharmaceutical packaging is essential for the product’s use and the drug’s administration, i.e. the pack is the delivery device. Examples of this are pressurized metered dose inhalers, nasal sprays, transdermal patches and pre-filled syringes.
The role of packaging seems to be constantly expanding, for example, in anti-counterfeiting, branding and providing distinguishing features to the drug product to avoid errors as well as in the monitoring of patient adherence.
The pharmaceutical pack
A pharmaceutical pack contains, protects and delivers a safe, efficacious drug product. At the same time it provides identification and information, enabling patient compliance and convenience. The primary pack, which is in direct contact with the product during storage and delivery (e.g. a glass bottle and cap), contains the product, while the secondary pack (e.g. a carton box for a glass bottle) contains the primary pack, as well as ancillary components, such as dispensing spoons and information leaflets.
Primary packs
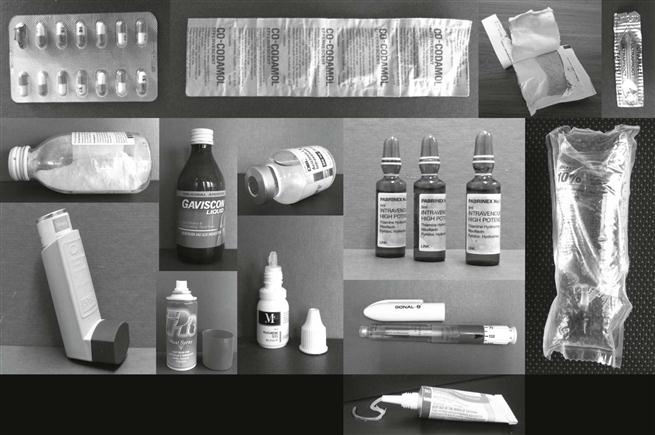
The wide range of pharmaceutical products, such as solid powders, granules, tablets, capsules, semi-solids (e.g. creams, ointments, gels), liquids (such as solutions, suspensions, emulsions), some of which are sterile, obviously require a great diversity, both in primary pack design and in packaging materials. The latter include paper, glass, plastics, rubber, metal or combination materials such as laminates. Examples of primary packs include blister packs, strip packs, sachets, bottles, ampoules, vials, bags, tubes and syringes. The primary pack may contain many doses (i.e. be a multiple-unit pack, e.g. a bottle containing many tablets) or a single dose (i.e. be a single-unit pack, e.g. blister pack, sachet). Examples of the range of pharmaceutical primary packs are shown in Figure 47.1.
The primary pack must offer child-resistance to restrict children’s access to the product. Child-resistant packs have been successful in reducing accidental poisoning of children. At the same time, the pack must allow access to the user, who may be elderly or frail and may have difficulty opening packs. Difficult-to-open packs are not always fully reclosed between administration events – and this may compromise the product. In addition, the primary pack must be tamper-resistant and tamper-evident to improve the product’s security from pilferage and deliberate contamination and safeguard the product’s legitimate user. Ideally, packs would be completely tamper-proof, though this is probably impossible to achieve against determined malice.
The primary pack must be compatible with the product, and take nothing out of the product and add nothing in. Drug absorption or adsorption into the pack would reduce product potency, while chemicals leaching out of the pack and into the product could induce drug degradation. In addition, the primary pack must protect the product against atmospheric factors, such as extremes of temperature, light, moisture, oxygen, carbon dioxide, particulates (e.g. dust, dirt), as well as biological hazards, such as microorganisms, insects and rodents, and enable product stability.
Drug molecules can undergo chemical reactions triggered by light, heat, moisture, or atmospheric gases, such as oxygen (see Chapters 48 for more details). For example, light can provide the energy necessary for a drug isomer to change its configuration. Protection from light is usually achieved by using an opaque or amber-coloured container. Oxygen can cause drug degradation via oxidation. Carbon dioxide can dissolve in the water in unbuffered aqueous products, and lower their pH by forming carbonic acid. Water can cause drug degradation via hydrolysis. Moisture gain into a product can also cause dilution of liquid products, wetting of solid products and an aqueous environment can support microbial growth. Solvent loss from a product can also occur if the container is permeable. Secondary packs also contribute to protection against atmospheric factors to some extent, although their major role is to provide protection against mechanical hazards, such as shock (e.g. when dropped), compression, vibration, abrasion, puncture, etc., during handling, storage and transport.
Closures
A closure is a device – e.g. stopper, lid, top or cap – which is used to close a container, and is an integral part of the pack. The word ‘pack’ therefore covers both the container and the closure. Without the latter, the functions of a pack, such as containment, presentation, protection and convenience, cannot be fulfilled, and like the container, the closure must be inert, compatible with the contents, and protect the latter against environmental hazards, such as oxygen, light, moisture, etc.
Certain closures must maintain sterility, e.g. in multi-use parenteral vials. A good seal between the container and the closure prevents anything from leaking out or gaining access into the pack, and is obtained by a snug fit between the inner face of the closure and the external face of the container finish. Resilient liners inside the closure are sometimes used to achieve a snug fit, although many plastic closures are internally moulded to achieve a good seal and are liner-free.
The closure has to be user-friendly, allow easy opening to legitimate consumers and be easy to reclose (for multi-unit packs), as well as child-resistant, tamper-resistant and tamper-evident. Closures may also include dispensing devices, e.g. a pump on bottles containing creams. The outer surface of the closure may also be ribbed to allow good grip when opening by twisting. Pharmaceutical closures are mostly made of plastic (thermosets and thermoplastics), although metal is also used, e.g. on parenteral vials.
The word ‘closure’ does not always refer to a stopper-type device. Metal tubes have two closures – a cap at one end while the other end of the tube is sealed by folding and crimping. Flexible packaging, such as pouches, sachets and blister packs do not contain a closure as defined above. They are instead sealed by heat and/or pressure, or with adhesives, and are non-reclosable packs.
Packaging materials
Once the function(s) of the desired packaging has been defined, selection of the primary packaging material is the first step in the packaging process, and takes into account the dosage form, the route of administration, drug/product stability, need for terminal sterilization and for visual inspection of the packaged medicine, patient compliance and convenience, aesthetics, cost, environmental-friendliness, etc. Liquids, which are in constant intimate contact with the primary pack, as opposed to solids such as tablets and capsules, require greater quality from a pack in order to ‘take nothing out of the product or add nothing in’.
Injectable liquids require even greater quality from the pack compared to oral liquids, to maintain sterility and freedom from other possible contaminants, such as extraneous particulates. Medicines which are terminally sterilized in their final packs need to be made from materials that can withstand the sterilization procedure.
Semi-solids need to be able to be dispensed from the container, under slight pressure, e.g. squeezing of a tube. Drugs which are sensitive to atmospheric conditions such as sunlight need a packaging material which stops light transmission into the pack. Patient convenience and compliance can be aided by the use of blister packaging, which allows transport of the required doses (rather than the whole bottle) and which can also be printed with information to aid memory, for example, for contraceptive pills.
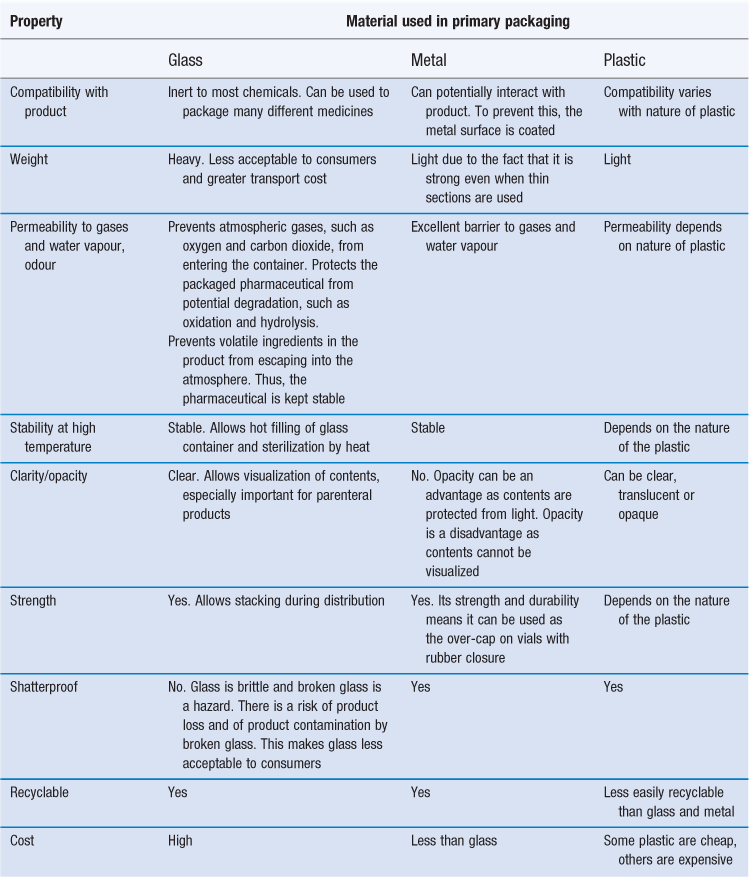
The packaging material is obviously very important and the different materials are described next. Glass, metal and plastic are the materials most commonly used in primary packs; their properties with respect to their use in packaging are compared in Table 47.1.
Glass
Glass – believed to have been first discovered around 3000 BC in the East Mediterranean and which has been used for thousands of years – is widely used for packaging pharmaceuticals due to its excellent barrier properties, inertness and compatibility with pharmaceuticals. Its many advantages are shown in Table 47.1, and it has traditionally been the gold standard in pharmaceutical packaging.
Glass is produced by heating together various inorganic substances to form a molten mass, and then rapidly cooling the latter which solidifies in a non-crystalline state. Sand (or more properly silicon dioxide; silica) which is the main constituent of sand), limestone (calcium carbonate) and soda ash (sodium carbonate) are heated to very high temperatures (e.g. 1500 °C) in a furnace. The ingredients melt and gradually react and form a homogeneous molten mass. The latter is then converted into glass containers by one of two basic methods: blow moulding or tubular glass fabrication. Blow moulding, the older method is when a ‘gob’ (a small piece of highly viscous molten glass) of glass is moulded into a container. The second method is used to manufacture ampoules and vials. Once a homogeneous molten glass mass is formed at high temperature, the molten mass is converted into glass tubing as it moves out of the furnace. The tubing is cut to proscribed lengths and the individual glass tubes are then converted into ampoules or vials.
In addition to silica, soda ash and limestone, other compounds are added in trace amounts to achieve certain properties in the glass formed. For example:
• alumina (Al2O3) increases the hardness, durability and clarity of the glass
• selenium or cobalt oxides improve clarity
• lead oxide gives clarity and sparkle (but makes glass soft)
• boron compounds give low thermal expansion and high heat-shock resistance
• arsenic trioxide and sodium sulphate are added to reduce blisters in the glass.
Opacity and different colours are achieved by the inclusion of a range of compounds, as shown in Table 47.2. The different colours convey specific properties, for example:
• amber glass is widely used to package pharmaceuticals susceptible to degradation by sunlight
• green glass is mostly used for packaging beverages
• blue glass makes white products appear whiter
• opaque white (opal) conveys prestige to upmarket toiletries and cosmetics.
Table 47.2
Additives that are generally used to achieve different colours of glass
| Colour | Possible additives |
| Amber | Iron oxides, manganese oxides, carbon oxides, sulphur compounds |
| Browns | Iron oxides, carbon oxides, sulphur compounds |
| Greens | Iron oxides |
| Yellow greens | Uranium oxides |
| Yellow | Lead with antimony |
| Deep blue | Cobalt oxide |
| Light blue | Copper compounds |
| Reds | Gold chloride, selenium compounds, copper compounds |
| Amethyst | Manganese oxides |
| Black | Mix of manganese, cobalt, iron |
| White | Tin compounds, antimony oxides |
Glass is not totally inert
Although glass is fairly inert, it is not totally so. Basic colourless glass used for most packaging applications contains silica at 59–75%, calcium oxide at 5–12%, sodium oxide at 12–17%, alumina at 0.5–3% and possibly trace amounts of other compounds, such as ferric oxide, titanium dioxide, potassium and magnesium oxide. Some of the glass components can leach out of the glass and into the contents. For example, sodium which is loosely combined with silicon can leach out from the glass surface into water contained within the glass, thus increasing the alkalinity of the contents. Another problem occurs when glass is stored at high temperature and high humidity or when ambient temperature and humidity conditions fluctuate greatly. Salts in the glass migrate from the body of the glass and accumulate at its surface. This physical change is called blooming. Such problems are obviously unacceptable. To reduce leaching, the glass can be soaked in heated water or a dilute acid solution, which removes most of the surface-leachable salts. The glass surface can also be treated, for example, with a sulphur compound, to make it more resistant to water or acidic (but not alkaline) solutions. Thus, different types of glass exist and the least reactive are used to package pharmaceuticals.
Pharmaceutical types of glass
Four types of glass – defined precisely in the United States Pharmacopoeia (USP) as types I, II, III and NP glass – are used to package pharmaceuticals.
Type I glass.
This is the best pharmaceutical grade and is produced by adding boron oxide to glass, hence this glass is also called borosilicate glass. It is the most inert glass, shows the least amount of leaching of glass components, and is used in ampoules and vials for liquid parenteral products. Due to its lowest coefficient of thermal expansion, this glass is highly resistant to temperature changes, e.g. during severe sterilization procedures. Type I glass is used to package slightly acidic solutions (as mentioned above, standard glass can increase the alkalinity of its contents). This glass is also the most expensive to produce.
Type II glass.
This is the next (lower) grade and is made from the same materials as standard glass (soda lime glass) but its surface is treated with sulphur dioxide. The latter reacts with the oxides found on the glass surface, for example, sodium oxide is converted to sodium sulphate, which can then be removed by washing the glass. Such surface treatment, thus, reduces the amount of ions that can leach out of the glass. Hence, type II glass is also referred to as treated soda lime glass or dealkalized soda lime glass. Type II glass is suitable for solutions that can be buffered to remain below pH 7. At higher pH, the oxides in the glass (which are free to move) are more easily leached out.
Type III glass.
This is the next (lower) grade and is standard soda lime glass and is analogous with glass used in food packaging. It is used to package large volumes (normally >100 mL). The large volume to surface area of product in contact with the glass surface limits the amount of glass components that can leach into the product.
NP glass.
This is the lowest grade and is called nonparenteral glass, and, as the name suggests, is not used for packaging parenteral products, as small volumes of the latter would be easily contaminated by sodium and other materials leaching out of the glass. NP glass is however suitable for packaging large volumes (normally >100 mL) of topical products like creams and of oral products, such as mouthwashes.
Plastics
Use of plastics in packaging
The versatility of plastics has led to their use in almost all parts of our lives, and they are used to package a wide variety of domestic products. They are widely used as containers (e.g. bottles, trays), closures (e.g. screw-tops), cling films, carrier bags, sacks, overwraps, etc. Plastics are also widely used to package medicines in a variety of containers, such as:
Packaging plastics in use
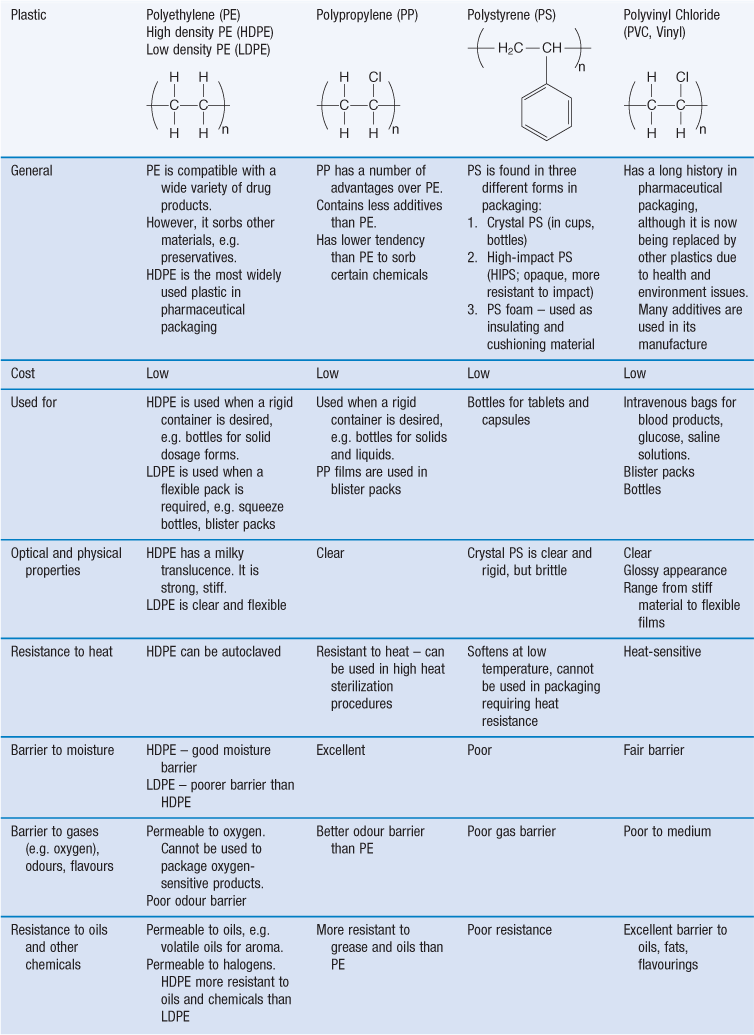
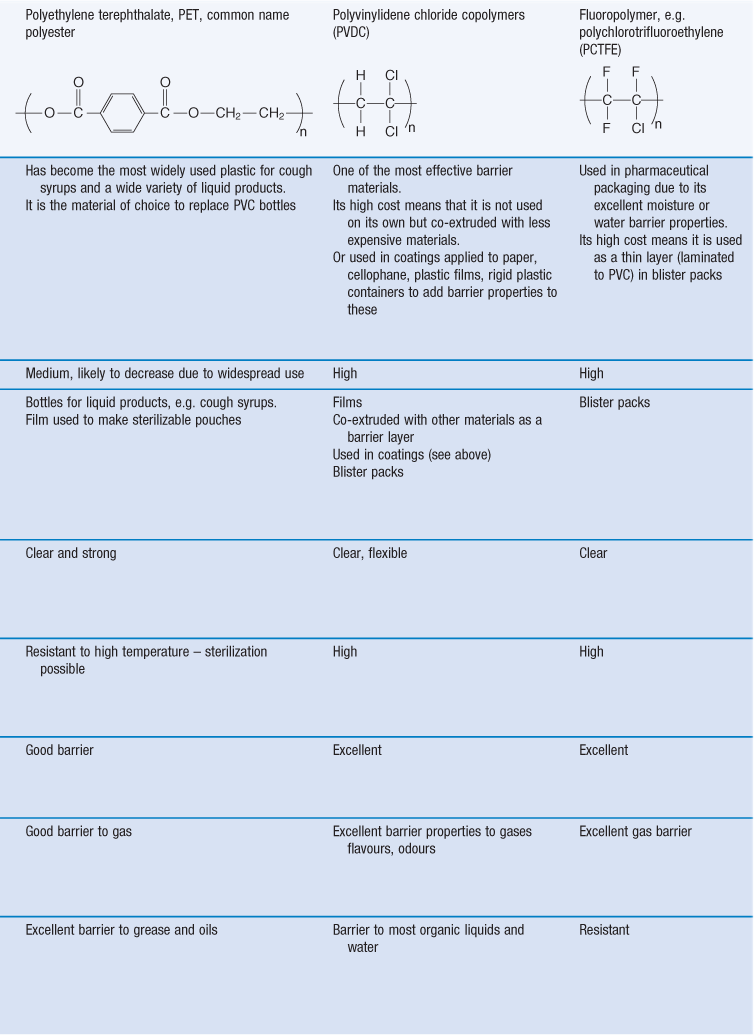
There are many different types of polymers, or polymer mixes, used as packaging plastics. In addition, the different plastics are available in many different grades. Commonly used packaging plastics include polyethylene, polystyrene, polypropylene, polyethylene terephthalate, polyvinyl chloride, polyvinylidene chloride, polyamides (nylons), polycarbonates. The most commonly used plastics in pharmaceutical packaging and their properties are shown in Table 47.3.
General properties of plastics
Their use as a pharmaceutical packaging material is growing due to the significant advantages and consumer preference for plastic. Plastics are light, shatterproof and can be clear or opaque (clarity may be desired for product inspection; opacity to protect the contained medicine). Plastics are easily shaped and sealed, which gives great versatility in the design of the pack, and allows the inclusion of administration aids, such as a squeezable dropper.
Plastics do however suffer from certain disadvantages compared to traditional packaging materials, such as glass and metal, which limit their use. No plastic can yet match the chemical inertness and impermeability (to environmental gases, such as oxygen) of type I glass. Plastics are less resistant to heat and long-term light exposure than glass and metal. Plastics are also liable to undergo stress cracking, where the presence of solvents, such as alcohols, acids or oils, cause a plastic pack to become brittle, crack and eventually fail over time. Certain components of the plastic packaging material can also leach out of the plastic and into the contained product. The wide range of plastic materials results in a range of physical, chemical, optical and performance properties.
Molecular structure of plastics
Plastics are polymeric materials. Polymers – macromolecules of repeating units called monomers – are produced by addition or condensation reactions, where one chemical species reacts with another (or itself) to form a new and larger compound. Thus, polymers may be copolymers (which consist of more than one type of monomer) or homopolymers (contain only one type of monomer). For example, ethylene can be polymerized into the homopolymer polyethylene, or be made to react with a different species such as vinyl acetate which would produce the copolymer ethylene vinyl acetate. Polymers may be linear, branched, cross-linked, and contain amorphous and/or crystalline regions where the polymer chains are arranged in a random or highly ordered manner, respectively (Fig. 47.2).
A linear uncrosslinked polymeric material can be visualized as a bowl of spaghetti (Fig. 47.2a), where the individual spaghetti strands represent polymer chains. The number of monomer parts in a polymer chain adds up to the molecular weight of the polymer. The polymer chains in a polymeric material are of different lengths, i.e. contain different numbers of monomer units, thus, the polymeric material’s molecular weight is not exact, but can be considered as the average of all the strands contained within a sample. Common plastic polymers used in pharmaceutical packaging have molecular weights ranging from approximately 10 000 to 1 000 000 Da. In contrast to the ‘bowl of spaghetti’, a cross-linked polymer can be considered as one very large molecule, where all the monomer parts and polymer chains are irreversibly linked (Fig. 47.2c).
Thermoplastic and thermosetting polymers
Plastics can be divided into two classes: thermoplastics and thermosetting plastics. In general, thermoplastics have linear and branched polymer chains, while thermoset polymers are cross-linked.
At high temperature, thermoplastic polymers melt and become liquid, the polymer chains flow and the material can be moulded into a variety of shapes, such as bottles, tubes and films. Softening and re-shaping by the application of heat and mechanical force can be performed multiple times. Examples of thermoplastics include: polyvinyl chloride, polyethylene, polystyrene, polypropylene, nylon, polyester, polycarbonate. The properties and uses of the most commonly used plastics in pharmaceutical packaging are shown in Table 47.3. Domestic ‘plastic containers’ are made from thermoplastics.
Thermoplastics are employed in blow-fill-seal technology, which is used to produce liquid filled plastic containers, with container formation, filling and sealing occurring in one continuous operation, which occurs over a period of less than a few seconds. In the container blowing step, pharmaceutical grade plastic granules, most usually polypropylene or polyethylene, are melted at high temperature and extruded into a hollow tube, termed the ‘parison’. The parison is cut to the desired length, then two halves of the container mould close around it, the lower end is sealed and then air is blown into the parison, causing it to expand to the shape of the container. This container is partially cooled, filled with liquid via a needle and sealed. The completed, filled containers are removed from the filling equipment, and excess plastic is removed (see Figure 36.6). The ability to operate this process in a controlled environment without the intervention of operators makes this process particularly suitable for aseptically producing sterile pharmaceutical products (see Chapter 36).
In contrast to thermoplastics, thermoset polymers can only be shaped once following the polymer formation, due to the fact that the cross-linked polymer chains cannot flow. Further heating would lead to breakage of the bonds in the polymer and polymer degradation. Examples of thermoset polymers are: urea formaldehyde, epoxides, urethanes, unsaturated polyesters and rubbers. These are mainly used to produce closures, as metal coatings, and adhesives in the pharmaceutical packaging industry.
Process residues and additives in plastics
During polymer synthesis a variety of chemicals, such as solvents, catalysts, initiators and accelerators, are needed to assist the polymerization process. These chemicals are therefore present to some extent in the final product; they are then known as process residues. Unreacted monomers may also be present. When the polymer is subsequently made into a finished product, e.g. a bottle, further chemicals – called additives and processing aids – are added in order to control or enhance the properties of the polymer/finished product, or to aid the manufacturing process. These chemicals include plasticizers, fillers, toughening agents, stabilizers, antioxidants, opacifiers, colourants, UV absorbers, lubricants, slip and anti-blocking agents and internal release agents. The function of these additives and processing aids is shown in Table 47.4; their inclusion (or not) will depend on the plastic and the finished product.
Table 47.4
| Additives and Processing Aids | Role | Examples |
| Plasticizer | Improve flow properties. Increase softness and flexibility. | Phthalate ester |
| Filler, extender | Inert solid substance. May reduce plastic degradation. May be used to reduce cost. | Talc |
| Toughening agent/impact modifier | Improve strength of brittle plastics. | Rubber added to polystyrene |
| Stabilizer | Increase stability of plastic, to combat effects of heat and light. | Calcium-zinc salts added to PVC. |
| Antioxidant | Prevent or retard oxidative degradation of plastic. | Cresols |
| UV absorber | To protect plastic or packaged product from UV degradation. | Substituted phenols |
| Opacifier | Make plastic opaque. | Titanium dioxide |
| Whitener | Give a ‘whiter than white’ appearance. | Ultramarine |
| Colourant | Colour the plastic. | Pigments and dyes |
| Lubricant | Prevent adhesion of plastic to metal parts during fabrication. | Waxes, liquid paraffin. |
| Internal release agent | Provide release from moulds. | Metal stearates, silicone fluids. |
| Anti-block or slip agent | Used for films made or used on high-speed equipment, where non-slip or film sticking together would interfere with processing. | Amides, finely divided silica. |
| Anti-static agent | Reduce static accumulation on plastic. | Surfactants |
Monomers, process residues, additives and processing aids present in a plastic pack may leach out of the plastic material and into the packaged product. For this reason, pharmaceutical grade polymers should not contain certain additives and have strict limits on the amount of chemicals that can leach out of the plastic material.
Rubbers and Elastomers
These are extensively used as stoppers (closures on parenteral containers). When used as a closure they allow a hypodermic needle to enter the container, and reseal when the needle is removed. They are soft enough to mould and conform to the opening of the container and allow a tight seal. The materials used must therefore be resilient, resistant to coring (must not fragment when penetrated by a needle) and compatible with the pack contents.
The terms rubbers and elastomers are sometimes used interchangeably although some authors do differentiate between the two words as follows:
Elastomers may be natural (extracted from rubber trees) or synthetic (derived from petrochemicals). Common pharmaceutical examples include butyl, chlorobutyl, natural and silicone elastomers. Butyl and chlorobutyl elastomers are least permeable to oxygen and water vapour. These materials are not used alone, however. For example, natural rubber is mixed with chlorobutyl to help resist coring in closures which must withstand multiple penetrations by a needle. Neoprene (polychloroprene) is included in rubber formulations when a pharmaceutical product contains mineral oil. Like other plastics, rubbers are not totally inert. They are permeable to some extent; they may also sorb components of the packaged product and may leach residues and low-molecular weight components into the packaged contents.
To produce rubber formulations, the elastomer and other required materials are placed in a mixer which breaks the materials into small fragments and produces a uniform dispersion. The latter – a viscous liquid – is placed in a heated mould, where heat and pressure promote polymer cross-linking and ‘cure’ the formulation, such that a strong, tough and elastic rubber is produced. The rubber is then trimmed and washed to remove residual materials that may have migrated to the surface during moulding. Residual materials may also be extracted out of the rubber by different techniques such as autoclaving. The rubber surface may be treated with chlorine to produce a shiny glaze or coated with materials, such as silicon oils, to reduce their coefficient of friction.
Metal
Metal is widely used to package food, beverages, aggressive products, etc. Aluminium and tinplate (a sheet of steel that is coated with a thin deposit of tin) are the metals used in the packaging of pharmaceuticals. They are used in the form of cans (e.g. pressurized metered dose inhaler (pMDI) containers), tubes (for creams, ointments, gels), pouches (for powders, granules, liquids, suppositories), blister packs and in closures.
Metal has many advantages as a packaging material, it is mechanically strong and can withstand the high internal pressure in pMDI containers, it is shatterproof, lightweight (due to the fact that it is strong even when thin layers are used), impermeable to gases and light and is malleable; it can be tailored in hardness and flexibility with respect to the desired container. Both soft and hard forms of aluminium and tinplate are used in pharmaceutical packaging; hard material is used for its strength and durability in containers such as aerosol cans, while soft and malleable metal is used to produce collapsible tubes, flexible pouches and as the collar on parenteral vials with rubber stoppers. Malleability allows the metal collar to be crimped in place and flexible pouches and metal tubes to be crimped closed. The metal’s malleability also means that when metal tubes are squeezed to expel the product, e.g. a cream, the tube does not spring back into its original shape and air is not sucked back into the container, which could otherwise react with the product or cause it to dry out.
Metal can however interact with the pharmaceutical product. To isolate the metal from the product, the metal surface is coated with vinyl-, acrylic- and epoxy-based resins. The outside of the metal container is also coated to protect the metal and to enable printing.
Paper
Paper is one of the oldest pharmaceutical packaging materials. It has diverse applications including labels and leaflets, collapsible and rigid cartons, bags and sacks, and sachets. Unlike the other packaging materials, glass, metal and plastic, paper is not usually used in the manufacture of the primary pack, i.e. the container holding and in direct contact with the pharmaceutical product. An exception is the sachet, which is the primary pack, where the paper is separated from the product by a layer of another material. A traditional OTC cold remedy (Beecham’s Powders™) is still sold in powdered form wrapped in paper only as the primary pack.
Paper is defined as a matted or felted sheet usually composed of natural plant fibre. When the paper material weighs 250 g/m2 or more or is 300 µm or more in thickness it is known as paperboard. Softwood from spruce, fir, pine and eucalyptus trees is now the most common source of fibre in papermaking although bagasse (from sugar cane), cotton, straw, flax, bamboo, jute, hemp, grass, esparto, rags and sisal have also been used.
To produce paper, cellulose fibre is extracted from the wood by pulping the latter (mechanically and/or chemically). The pulp is then mechanically treated to break down any fibre bundles, to hydrate and break up the surface of the fibres and then bleached if desired. A variety of non-fibrous additives are then added to the treated pulp to control water and ink permeation (rosins), to increase strength (starches, gums, resins), and improve the optical brightness and printing qualities (clay, talc, titanium dioxide). The mixture (consisting of water (99%), fibre and additives, known as ‘furnish’) is then fed to the paper-making machine, where most of the water is removed, and the solid material is turned into sheets of paper. The latter is pressed between multiple stacks of heavy rollers which smooth out the surface of the paper and make it more suitable for printing. A number of coatings may then be applied to the paper to further improve its surface properties, such as to reduce its porosity and liquid penetration rate (using gelatin, starch, modified rosins or waxes), or increase its opacity, gloss, brightness and printability (using clay, calcium carbonate or titanium dioxide).
Advantages and disadvantages of paper as a packaging material
Paper has many advantages as a packaging material:
• relatively low cost and readily available
• generally of non-toxic origin
• readily torn or cut open, an advantage when paper is used in sachets
Paper does have certain disadvantages and these result in it not generally being used on its own in the primary pack. These disadvantages include:
• no barrier properties against moisture, gases and odours
• no heat- or cold-seal properties and hence cannot be sealed without adhesives or special coatings
• poor transparency and gloss compared to certain plastic films.
To overcome such disadvantages, paper can be combined with other materials. For example, paper can be further coated by polymers or laminated to plastic or to aluminium foil to improve its barrier properties to gases and moisture, and to create heat-sealing ability when used in the primary pack.
Laminates
A laminate is made by bonding together two or more plies (layers) of different materials, such as paper, plastic and metal. The aim is to combine the desirable properties of the different plies into a single packaging structure. A minimum amount of material is used and the laminate is cost-effective.
Laminates are used to produce pharmaceutical packs such as sachets, blister packs, tubes, pouches, etc. An example is a structure consisting of paper/ metal foil/polythene plies, used for sachet packaging. The paper provides strength, printability and the ability to easily tear the package, the foil provides an excellent barrier to light, moisture and gases and the polythene enables heat sealability.
Packaging and regulatory bodies
Like the medicine, the pharmaceutical packaging is subject to regulation and approval by government agencies, such as the United States Food and Drug Administration (FDA), the European Medicines Agency (EMA) and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA). The packaging is considered part of the product, and manufacturers must submit large amounts of data to show that the packaging is safe, efficacious and performs as claimed. The regulatory bodies produce guidance documents to assist manufacturers, such as on labelling, patient information leaflets, closures, and the testing of containers. The United States Pharmacopeia include requirements for containers, and many of the drug product monographs include the requirements for packaging, such as ‘preserve in single-dose containers, preferably in Type I glass, protected from light’. Labelling and patient information leaflets are part of the pack and are also subject to regulation, although they have not been covered in this chapter.
Repackaging
An original pack is one which is intended to be dispensed directly to the patient without modification except for the addition of appropriate labelling. In many countries, many medicines are packaged by the manufacturer into such packs which can be dispensed to the patient without the need for repackaging in the pharmacy. Repackaging – transfer of medicines from their original pack into different packs – is performed to a small extent in community and hospital pharmacy, for distribution to hospital wards, clinics, nursing homes or upon patient request, for example, by an arthritic patient who might struggle to open the original pack, or into dose administration aids.
Repackaging is mostly carried out for tablets and capsules. The new primary container should be chosen with care, ensuring good containment and protection of the medicine, and compatibility between product and pack. The repackager must be aware of the issues regarding the repackaging of medicines, such as potential for errors, the physical and chemical stability of drugs and medicines, cleanliness, cross-contamination, shelf-life of repackaged products, legal aspects, clear and full labelling, etc.
Bibliography
1. Bauer EJ. Pharmaceutical Packaging Handbook. New York: Informa Healthcare; 2009.
2. Dean DA, Evans ER, Hall IH. Pharmaceutical Packaging Technology. London: Taylor and Francis; 2000.
3. FDA Guidance for Industry, Container Closures Systems for Packaging Human Drugs and Biologics, Chemistry, Manufacturing and Controls Documentation. at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070551.pdf; accessed 25 August 2011.
4. Jenkins WA, Osborn KR. Packaging Drugs and Pharmaceuticals. Technomic Publishing Co. Inc, Lancaster 1993.
5. MHRA. http://www.mhra.gov.uk/Howweregulate/Medicines/Labelspatientinformationleafletsandpackaging/index.htm; accessed 30 August 2011.
6. Soroka W, Emblem A, Emblem H. Fundamentals of Packaging Technology. USA: The Institute of Packaging; 1996.
7. Winfield AJ, Rees JA, Smith I. Pharmaceutical Practice. 4th edn Churchill Livingstone, Edinburgh: Elsevier; 2009.