Chapter 31 Pacing Technology and Its Indications
Advances in Threshold Management, Automatic Mode Switching, and Sensors
Since the first endocardial pacing lead implantation in 1958, pacemaker therapy has undergone remarkable technologic advances. For example, the number of circuitry components has increased from a mere two to three transistors in early pacemakers to nearly one million components with RAM (random access memory) sizes up to 124,000 bytes.1 This increased sophistication has led to pacemaker features that the average pacemaker implanter may not have the time to understand or to program appropriately. In addition, threshold and sensor assessment may take up to 40% of time in an average follow-up (Figure 31-1).2 Thus, automatic optimization of many pacing parameters has become a pressing need. This chapter reviews the current state of the art in three important modern pacemaker functions: (1) capture management, (2) automatic mode switching (AMS), and (3) implantable sensors. Particular attention is given to their indications and automaticity in programming.
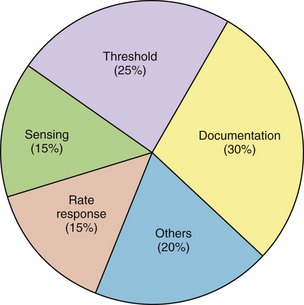
FIGURE 31-1 Time used for different activities during a routine pacemaker follow-up.
(From Marshall M, Butts L, Flaim G, et al: Predictors of time requirements for pacemaker clinic evaluation [abstract], Pacing Clin Electrophysiol 18:952, 1995.)
Capture Management
The primary function of a pacemaker is to pace effectively at an efficient energy output. This depends on the pacing threshold, which varies significantly from individual to individual and within an individual over time. The latter may occur because of the spontaneous threshold rise after implantation, the occurrence of gross dislodgment or microdislodgment, diurnal changes, and the changes introduced by drugs and myocardial ischemia.3,4 Thus, the ability to track threshold automatically will maximize patient safety, minimize battery drain for pacing, and, importantly, simplify programming. Box 31-1 presents a list of reasons for the need for automatic capture management.
Box 31-1 Needs and Potential Benefits of Capture Management
St. Jude/Pacesetter Autocapture
The Autocapture pacing system was first introduced in the single-chamber Microny pacemaker from St. Jude Medical in 1995. (See Box 31-2 for information on the manufacturers referred to in this chapter.) It is designed to verify a response to each pacemaker stimulation that represents capture or myocardial depolarization and to automatically adjust the pacing output accordingly on a beat-to-beat basis. After a VP stimulus, the Autocapture algorithm opens an evoked response (ER) detection window for 46 ms after a 15-ms blanking period. Detection of an ER is used to diagnose capture. If an ER is not detected (loss of capture), a high-energy backup pulse of 4.5 V is discharged at 100 ms after the VP stimulus. If two consecutive backup pulses are delivered, the algorithm starts a stimulation threshold search by increasing the output to effect two consecutive captures (Figure 31-2). In single-chamber devices (Microny and Regency SR), a margin of 0.3 V is added. In addition, to avoid pacing at high output caused by diurnal fluctuation in threshold, the device automatically performs a threshold search once every 8 hours. Again a safety margin of 0.3 V is added to the detected threshold. In dual-chamber devices, the A-V interval is shortened to 50 ms (Ap) or 25 ms (atrial sensing [As]) to ensure overdrive of intrinsic ventricular rhythm. In the Affinity DR, automatic decrements and increments of output during threshold search are 0.25 V and 0.125 V, respectively (see Figure 31-2). In addition, beat-to-beat capture verification has only recently been extended to atrial stimulation in the Zephyr pacemaker by St. Jude Medical (ACP confirm), as the small atrial electrical signal represents a major challenge in discrimination between the ER signal and the pace-induced after-polarization (see below).
Box 31-2 Pacemaker Manufacturers and Models
Efficacy
Factors that affect the Autocapture’s detection of an ER are listed in Box 31-3. One major challenge to this approach is the difficulty in distinguishing the ER signal and the pace-induced after-potential. A large electrode polarization artifact relative to the size of the ER can affect ER detection. This can be reduced with the use of low-polarization electrodes (made possible by increasing the microscopic electrode-tissue interface area).5 An alternative is to use a biphasic waveform that comprises a fast precharge followed by a negative postcharge to minimize the polarization effect.6 In one study, the effect of a modified fast prepulse on the Autocapture was tested in 45 patients with leads from two manufacturers (Medtronic 4024 Cap Sure, and Pacesetter 1450 K/T and 1470 T leads).7 Whereas the ER was independent of the type of pacing pulse, the polarization artifact was significantly less during the modified pulse compared with the conventional pacing pulse, resulting in an improved efficacy of the Autocapture algorithm (94% vs. 71% success rate in ER detection). An adequate ER amplitude of greater than 2.5 mV is recommended before activation of the Autocapture algorithm; this was present in 93% of 60 patients in one study.8 Neither the clinical data nor the conventional electrical parameters were effective in predicting the size of the ER signal. Body posture and exercise had relatively little effect on the ER.9 Recently, a new ER algorithm measuring the depolarization integral (area) instead of ER signal amplitudes (voltage) to determine ER has enhanced the accuracy of capture verification; in fact, the algorithm allows ER determination even with old high-polarization bipolar leads. Because of enhanced sensitivity, it has become possible to distinguish a small atrial ER from a pace-induced after-potential.
In a multicenter study, 113 patients received the Pacesetter Microny SR+ and were followed up for 1 year. ER was satisfactory for Autocapture in 102 of 113 patients.10 Even though ER was stable over time it correlated poorly with the R wave at the time of implantation. Acute and chronic pacing thresholds measured at the clinic using a standard surface ECG VARIO threshold test significantly correlated with that derived from the Autocapture, although the Autocapture threshold was higher (0.11 ± 0.22 V) because of the way in which threshold was derived. During Holter recordings, no failure of ventricular capture occurred, and backup pulses were used in 1.1% of all paced beats. Most of these were caused by fusion or pseudo-fusion beats (87%), undersensing of either the R wave or the ER (4.6%), and was the true cause of loss of capture in only 7%. Although these did not affect pacing performance, the need for backup pulses may negate the energy saving by the Autocapture itself. Similar positive results from the Autocapture algorithm in the medium term for safety and efficacy have been published.11,12
Projected increases in battery longevity afforded by the Autocapture have been reported.13,14 Compared with the factory-set pacemaker setting of 5 V, the Autocapture reduced the energy drain in the Microny SR+ (with 0.35 Ah) and increased device longevity by 53%. For the Regency SR+ with a larger battery (0.79 Ah), the increase in device longevity was even more significant (245%). However, when the conventional output was reduced to 2.5 V, the benefit of the Autocapture on battery life was much less impressive.13,14
Clinical Implications
The main benefits of any automatic capture management algorithm are patient safety and effective capture during threshold changes. Programming of threshold can be simplified as the Autocapture threshold has been significantly correlated with bedside threshold assessment. The energy saving would be more important in patients with chronic high thresholds. Conversely, fusion and pseudo-fusion beats appear to be the main limitation, not only because they reduce battery energy but also because they may lead to erroneous threshold determination.15
Biotronik Capture Control
The Logos pacemakers measure the ER signals from several successful capture beats to generate a reference curve, against which failure of capture is compared.16,17 No backup pacing pulses are present, but persistent loss of capture results in the increase of pulse output in 2-V steps. After a programmable period, the output is reduced to the programmed value. This algorithm ensures patient safety through beat-by-beat capture verification.
Medtronic Ventricular Capture Management
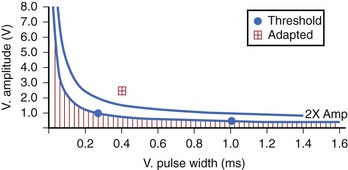
The Kappa 700 pacemakers incorporate a threshold assessment based on ER: The Pacing Threshold Search (ambulatory) and Capture Management Threshold Test (bedside). During the procedure, the threshold at the Rheobase is determined at 1 ms by amplitude decrement until loss of capture and then by amplitude increment until capture is confirmed. The chronaxie is then determined by doubling the programmed amplitude and decreasing the pulse width (and subsequently increasing the amplitude to capture). A recommended pacing setting is then determined (Figure 31-3). The physician can use the ambulatory threshold data to automatically adjust threshold (adaptive), use the data only for monitoring, or turn off the algorithm. A minimal adapted output needs to be programmed. The ventricular capture management can be activated once every 15 minutes for 42 days and is not a beat-by-beat threshold tracking algorithm.
Automatic Mode Switching
Because the ventricular response of a DDD pacemaker is dependent on the atrial rate, rapid VP can occur in a DDD pacemaker during episodes of atrial tachycardias (AT), especially during AF (Figure 31-4). This is managed in contemporary pacemakers by using an algorithm known as automatic mode switching. Patients with dual chamber pacemakers will develop AT for several reasons: (1) Nearly one half of patients receiving pacemakers have sinus node disease, and a substantial proportion of these patients have bradycardia-tachycardia syndrome. (2) As many as 30% of patients with complete AV block either have coexistent bradycardia-tachycardia syndrome or will develop this problem with time.18 (3) A dual chamber pacemaker is often used in patients after AV nodal ablation for refractory AT. (4) The incidence of AF increases markedly with age.19
Several registries and controlled trials have generated data on the incidence of AF. From 1988 to 1990, 12.9% of Medicare pacemaker recipients who received dual-chamber pacemakers had underlying paroxysmal AF.20 After implantation of a dual-chamber pacemaker, patients have an overall 2% to 3% per year risk of developing AF. In patients with sinus node disease, the risk of developing paroxysmal or persistent AF is increased to 8% per year.21–24
Conventional or specially designed pacemakers can convert automatically to another pacing mode under a variety of circumstances.25–32 The term automatic mode switching is now used to define an automatic function whereby a device is designed to switch temporarily to a nonatrial tracking destination mode during an AT and to switch spontaneously back to the original mode on resumption of sinus rhythm.
Components of an Automatic Mode Switching Algorithm
Atrial Tachycardia Detection
A device can detect AT in four main ways (Table 31-1): (1) Most devices use a rate cutoff criterion, in which a sensed atrial rate exceeding a programmable value will result in AMS. Some systems are designed to avoid mode switch during atrial ectopic beats or short runs of AT. For example, four short cycles of seven consecutive beats are required before AMS occurs in the Medtronic Kappa 700. Interval number summation is used in the incremental and decremental counter of the Meta DDDR for short cycles and long cycles, respectively. (2) Some devices use a mean atrial rate, or matched atrial rate, based on a moving value related to the duration of the prevailing sensed atrial cycle as a criterion to move toward AMS. AMS will occur when the matched atrial interval has shortened to a predetermined duration. This algorithm is used in the Medtronic Thera DR, the Kappa 400 (GEM DR ICD), and the St. Jude Trilogy DR+/Affinity family. Because the process is gradual, the rapidity of AMS will depend not only on the AT detection rate or interval but also on the pre-existing sinus rate. It is easier for the matched atrial interval to reach the tachycardia detection interval when AT occurs in the setting of a higher resting sinus rate than from a sinus bradycardia. This is because the matching atrial interval starts from a shorter baseline duration on its gradual way to reach the tachycardia detection interval. (3) Sensors can be used to determine the physiological rate (e.g., Diamond and SmarTracking in Marathon). To take into account the fluctuation in sinus rate, a physiological heart rate range based on the sensor-indicated rate is used to define sinus rhythm, and rates beyond the upper end of the physiological range will activate AMS. (4) Complex algorithms are a combination of algorithms, or they use additional criteria (often from implantable cardioverter-defibrillators [ICDs]) to distinguish AF and other rhythms. For example, a PR logic and a rate criterion are instrumented in the AT500 (Medtronic, Inc.) to detect AF and AT.33
Table 31-1 Classification of Different Methods of AT Detection in Current AMS Algorithms
| CRITERION | EXAMPLES | INDICATIONS FOR MODE SWITCHING |
|---|---|---|
| Rate cutoff | Pulsar/Vigor/Meridian/Discovery | Incremental/decremental counter |
| Inos/Logos | Ratio of short/total cycles (e.g., 4 of 7 consecutive cycles) | |
| Kappa 400/700 | Ratio of short/total cycles (e.g., 4 of 7 consecutive cycles) | |
| Marathon DR | Consecutive short cycles | |
| Meta DDDR (model 1254/1256) | Incremental/decremental counter | |
| Running average rate | Thera DR | Matched atrial interval computed from prevailing atrial rate |
| Trilogy DR Affinity | Filtered atrial interval | |
| Sensor-based physiological rate | Clarity/Diamond | Single beat outside a physiological rate band (15 or 30 beats/min) |
| Marathon DR | SmarTracking rate range (accelerometer sensor) | |
| Meta DR (model 1250) | Sensor-controlled PVARP | |
| Living 1/Living 1 Plus | Sensor-indicated rate to define tachycardia detection | |
| Complex | Marathon DR | SmarTracking and rate cutoff |
| AT 500 | Rate cutoff and PR relationship |
AT, Atrial tachycardia; AMS, automatic mode switching; PVARP, postventricular atrial refractory period.
The Ideal Automatic Mode Switching Algorithm
An ideal AMS algorithm (Table 31-2) should have an appropriate onset speed after AT begins. Prolonged rapid VP caused by a slow algorithm or oscillation between mode switching and tracking during short-lasting AT in a fast algorithm will result in undesirable ventricular rate fluctuation, AV dissociation, or both. It is clear that speed of response and rate stability are two competing parameters. Atrial and ventricular responses during AMS should result in a pacing rate appropriate to the pathophysiological state of the patient. In general, this rate is sensor determined. At the termination of AT, and to avoid AV dissociation during the process, the algorithm should resynchronize to sinus rhythm at the earliest opportunity. Many algorithms incorporate a rate fallback mechanism to ensure smooth rate control during mode transitions. With ideal sensing and programming, these characteristics are dependent entirely on the AMS algorithm.
| CHARACTERISTICS | REMARKS |
|---|---|
| Onset | Is rapid and avoids high-rate ventricular pacing without causing frequent mode oscillations during unsustained AT |
| Response | Avoids excessive rate fluctuation |
| Avoids inappropriate atrial pacing | |
| Resynchronization | Restores AV synchrony to sinus rhythm at the earliest opportunity |
| Sensitivity | Has the ability to sense AT of varying rates and signal sizes |
| Has the ability to sense atrial flutter | |
| Specificity | Avoids switching during VA cross-talk, sinus tachycardia, and extraneous electrical noises |
AMS, Automatic mode switching; AV, atrioventricular; AT, atrial tachycardia; VA, ventriculoatrial.
In clinical practice, however, arrhythmia-related and sensing-related issues affect the sensitivity and specificity of the AMS response significantly. Sensitivity of an AMS algorithm refers to its ability to detect AT (i.e., avoid false-negative response), whereas specificity refers to the absence of AMS during sinus rhythm (i.e., avoid false-positive response) (Table 31-3). With AMS algorithms, the greater the sensitivity, the lower is the specificity, and vice versa.
Table 31-3 Factors Affecting AT Detection in Dual-Chamber Pacemakers with AMS Algorithms
| UNDERDETECTION (SENSITIVITY) | OVERDETECTION (SPECIFICITY) | |
|---|---|---|
| Arrhythmia related | Atrial flutter | Sinus tachycardia |
| AF with small or widely varying signal amplitudes | — | |
| Slow atrial tachycardia (actual or drug-induced) | — | |
| Pacemaker related | ||
| Lead configurations | — | Unipolar sensing (far-field sensing and myopotentials) |
| Low atrial lead positions | ||
| Lead in the coronary sinus | ||
| Dual-site atrial or bi-atrial sensing | ||
| Atrial sensitivity | Insufficient atrial sensitivity to sense AF | |
| Atrial blanking | Reduced sensed AT rate | Inadequate blanking in A–V interval and after ventricular pacing |
| A–V interval | Reduced sensed AT rate (some devices) | — |
| PVARP | Reduced sensed AT rate (some devices) | PVARP-mediated AMS: AMS can occur during sinus tachycardia or ectopy |
| VA cross-talk | — | Increased sensed atrial rate |
AF, Atrial fibrillation; AMS, automatic mode switching; AT, atrial tachycardia; AV, atrioventricular; PVARP, postventricular atrial refractory period.
Automatic Mode Switching Sensitivity
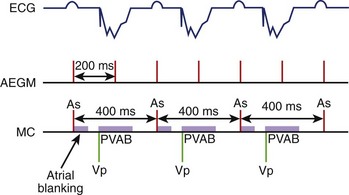
As most AMS algorithms detect AT by a rate cutoff criterion, a slow atrial rate (e.g., atrial rate slowing after antiarrhythmic medications) may fall below the tachycardia detection rate, and AMS will not occur. Conversion of AF to atrial flutter is a special situation in which alternate flutter waves coincide with the postvertricular atrial blanking period (PVAB), and the effectively detected atrial rate falls below the tachycardia detection rate and prevents AMS (Figure 31-5).
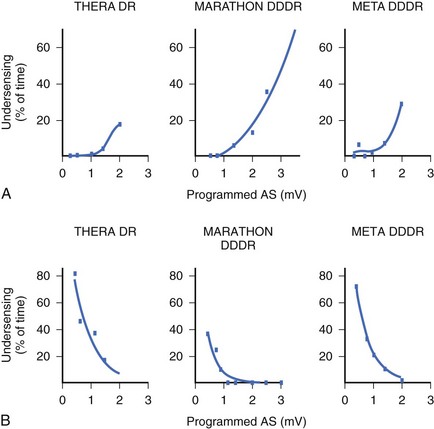
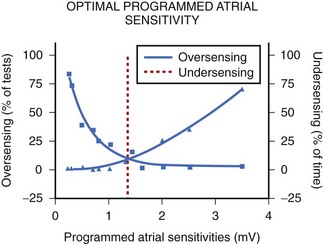
In the currently available devices, the postventricular atrial refractory period (PVARP) is opened (completely or on a conditional high rate) to enhance AMS. In other words, sensing occurs in the second part of the PVARP beyond the PVAB. The latter is designed to prevent far-field R-wave sensing. Atrial undersensing may occur because of an inappropriately long PVAB. Undersensing can often be avoided by appropriate adjustment of the PVAB, provided the atrial channel exhibits no far-field sensing. The widely varying amplitude of atrial ECG in AF, both temporally in a patient and between patients, can result in AMS failure when the atrial sensitivity is programmed incorrectly. During electrophysiological study, acutely recorded atrial ECGs (ECGs) during AF and sinus rhythm show similar mean amplitude, but the variability in amplitude is substantially wider and the minimum amplitude considerably smaller in AF compared with sinus rhythm (minimum atrial ECG: 1.4 ± 1.1 and 2.0 ± 0.8 mV, respectively).34 A high-programmed atrial sensitivity may cause As of far-field signals or noise, whereas a low atrial sensitivity can lead to undersensing during AF (Figure 31-6).35 Optimal programming of atrial sensitivity for AMS requires three times the safety margin compared with two times for sinus P-wave sensing (Figure 31-7).35
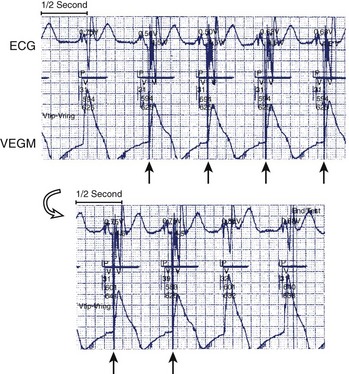
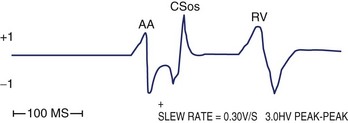
Far-field sensing of the tail end of the QRS complex by the atrial channel is the most common cause of a false-positive AMS response. Such far-field sensing of the QRS complex (almost always from a paced beat) causes VA cross-talk in opposite direction to the well-known form of AV cross-talk. Several investigators have studied the incidence of far-field R wave as recorded by an atrial-lead VDD system or a single-lead VDD system.36–38 In general, unipolar As, paced QRS complex, longer dipole lengths (30 vs. 17.8 mm), and septal and low right atrial implants may predispose to far-field R-wave sensing. In one study, at an atrial sensitivity of 0.1 mV, all 30 bipolar leads had a far-field R wave sensed.38 The median far-field QRS complex sensing threshold was 0.3 mV, and it occurred at 67 to 202 ms following the VP stimuli. These have implications on the highest atrial sensitivity and the duration of blanking period needed. In addition to atrial sensitivity, VA cross-talk occurs when the PVAB is too short or when the QRS is too long. Anything that prolongs the QRS complex (e.g., flecainide, amiodarone, or hyperkalemia) favors such VA cross-talk. Without PVAB programmability, false AMS from such far-field R-wave sensing can often be corrected by decreasing the atrial sensitivity. Less commonly, oversensing of atrial signals can occur within the A-V interval. In such a case, the atrial blanking period connected to the initial part of the A-V interval (post-atrial blanking period) must terminate before the A-V interval has timed out. Thus, double sensing of the P wave (near-field), especially caused by a large after-potential following Ap or sensing of the early part of the spontaneous QRS complex (far field), can occur within the A-V interval. Far-field sensing of the spontaneous QRS complex during the A-V interval is probably less common than sensing the terminal part of the QRS complex beyond the PVAB. The atrial channel can sense the early part of the spontaneous QRS only if it is detected before the ventricular channel senses it as a near-field signal.
A low-lying atrial lead or one in the coronary sinus may pick up both atrial and ventricular signals. The recent use of dual-site—right-atrial (with a posteriorly situated lead near the coronary sinus) and bi-atrial—pacing necessitates special algorithms or optimal lead positioning to avoid far-field R-wave sensing or even double sensing of the P wave. The latter may occur when the atrial conduction time between the two atrial sites is longer than the atrial blanking period (Figure 31-8). The sensitivity and specificity of AMS can now be validated by ECG and data storage of current implanted devices.
Automatic Mode Switching Diagnostics
AMS diagnostics provide an assessment of the frequency and pattern of AT episodes. These data may be useful for consideration of pacemaker mode reprogramming, such as from a dual-chamber mode to the VVIR mode when a patient develops permanent AF, and to assess the need for adjunctive antiarrhythmic and anticoagulation drug therapies in patients with a large number of AF episodes, long duration of AF episodes, or both. Indeed, recent studies suggest that asymptomatic episodes of AF occur at least 12 times more frequently than do symptomatic episodes in patients with implantable rhythm control devices.39,40 Although these episodes may not be symptomatically relevant, their impact on thromboembolism and heart failure is probably similar to that of symptomatic episodes and may require similarly aggressive treatment. Indeed, AMS was used 66% of the time in patients with a known history of AT or AF and in 55% of the time in patients without this history.39 However, the critical issue is the specificity of the recorded episodes characterized as AT or AF, which is now better defined through recording of atrial ECGs.
Event Counters
In general, event counters record the number of intrinsic and pacemaker-mediated events that occur during an event recording period. These counters are either triggered by the onset of a high atrial rate or AMS (Figure 31-9). Some current devices also allow patients to trigger the event counter, using an external magnet, to record data for a preset number of beats. This feature is useful for documentation and assessment of the pattern of symptomatic AF episodes.41 Either the AMS counter or the atrial high rate episode monitor can be used for the detection and assessment of AT or AF episodes.
The AMS counter records the actual number of mode switches that occur. Previous studies on the Medtronic Thera DR pacemaker have demonstrated that in 12% to 40% of patients, mode-switching episodes were not attributed to AT. As described above, the majority of inappropriate mode switching was due to far-field R wave, or near-field A-wave sensing of atrial paced beats. Furthermore, the mode switch count recorded is affected by the speed of response, the sensitivity of the algorithm, and the speed of resynchronization to sinus rhythm or atrial paced rhythm as well.42–50
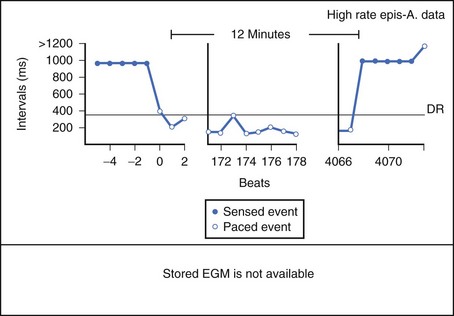
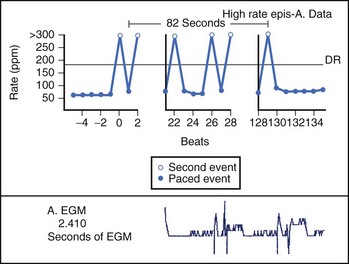
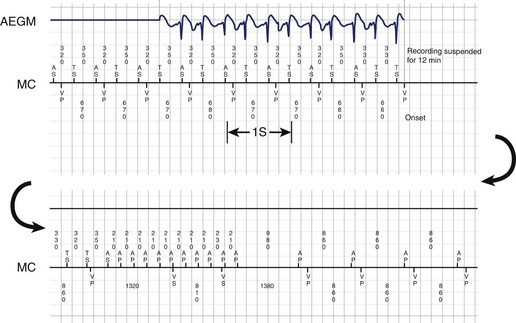
Theoretically, the atrial high-rate episode monitor should be independent of the mode switch algorithm and should be more accurate than the AMS counter. For example, intermittent atrial undersensing can mimic frequent short episodes of paroxysmal AF by registering repeated AMS (Figure 31-10). Seidl and colleagues suggested that optimal programming of the atrial high rate episode monitor in the Medtronic Thera DR pacemaker could reliably detect AT with high sensitivity and specificity.44 However, false-negative detection during short episodes of AF and false-positive detection due to far-field R-wave oversensing were still observed. A specific pattern of oscillations in the atrial rate profile consistent with atrial oversensing has been described (Figure 31-11). With additional criteria to exclude oversensing, off-line analysis of the recorded signals can significantly reduce false-positive detection to 2.9%.45
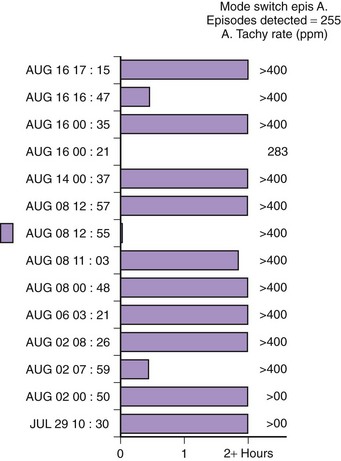
FIGURE 31-10 Mode switch episode log in the same patient as in Figure 31-9 who developed persistent atrial fibrillation (AF) 4 years later. Note the very close spacing between each recorded episode, which gave an impression of very frequent paroxysms of AF. However, this was probably due to mode switching in and out of persistent AF due to AF undersensing.
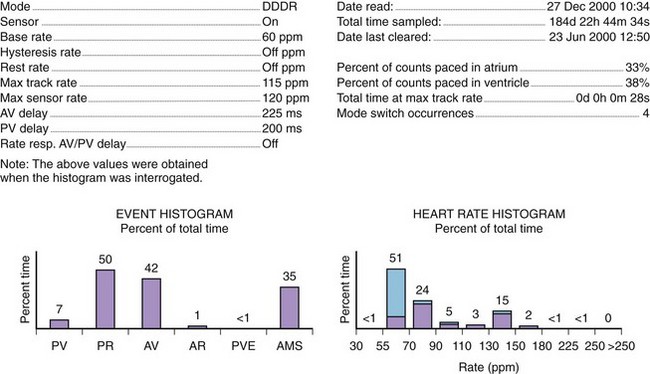
Histograms
Histograms provide details of the pacing operation of the device (e.g., As-Vs, As-Vp, Ap-Vs, Ap-Vp, and ventricular ectopy) and of the AMS operations and also provide the number of AF episodes (Figure 31-12). The rate histogram during the mode switch episodes may be useful for identification of inappropriate mode switching. When mode switching episodes occur in the atrial rate of 175 to 250 beats/min, it may represent double counting because of oversensing either far-field or near-field events. Episodes where the atrial rate is greater than 400 beats/min are likely to represent true AF, if lead fracture and myopotential sensing can be excluded.46
Stored Atrial Electrocardiogram
A number of devices have telemetered atrial ECGs that allow online assessment of pacemaker operations. These are very useful to assess atrial sensing issues when adjusting the parameters of AMS. The incorporation of the stored atrial ECG data is very useful in confirming the etiology of the recorded arrhythmia (see Figures 31-11 and 31-13). Atrial stored ECGs can increase the accuracy of the event counters and identify the type of atrial arrhythmias.47–50 They may provide important insight on the onset and termination of the arrhythmias (Figure 31-13). In a recent study, when atrial ECGs were available for confirmation, it was found that as many as 62.7% of mode switching episodes were erroneously executed.44 In patients with an implanted single-lead VDD system, only 35% of 235 episodes of suspected AF were confirmed as AF, whereas the other episodes were diagnosed to be atrial undersensing.50 At present, most devices provide only limited duration of stored atrial ECGs due to the limitation of pacemaker memory capability.
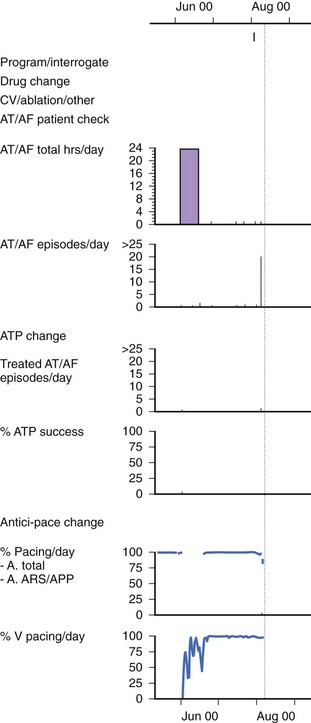
Atrial Fibrillation Burden
Several clinical studies have attempted to use AMS diagnostics to evaluate the effects of Ap on the total burden of AF (both symptomatic and asymptomatic episodes).51,52 This provides a powerful tool to assess the effectiveness of a therapy to treat AF (Figure 31-14). However, the accuracy of the measurement of AF burden by using the AMS diagnostics is limited by the following factors: (1) the specificity and sensitivity of the AMS diagnostics as described, (2) the availability of the atrial stored ECG to confirm AF, and (3) the storage capacity of the pacemaker data logs because the event counters in most pacemakers can easily be saturated.
Clinical Benefits
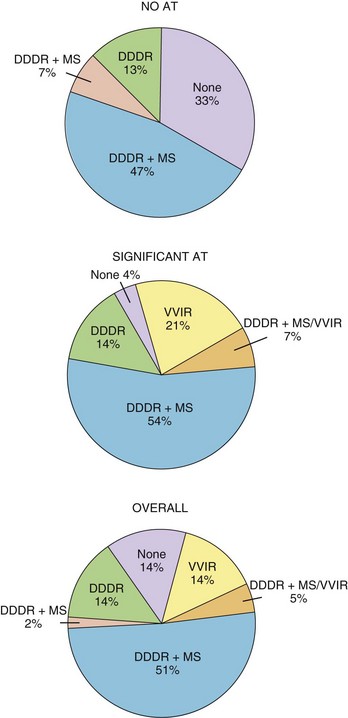
The hemodynamic benefits of maintaining a regular ventricular rate have been reported.53–57 Anecdotal reports of the symptomatic benefit of AMS and improvement of tachycardia-related symptoms by avoiding a rapid-paced ventricular rate at the onset of AT are also available. A randomized, crossover, prospective study has reported on the addition of AMS in patients after AV nodal ablation and DDDR pacing.58 In this study, 48 patients were randomly assigned to DDDR pacing with and without activation of AMS. It was found that the VVIR mode alone was the least well tolerated, and the DDDR with mode switching was the most acceptable (Figure 31-15). Patient-perceived well-being was superior with AMS activated than with AMS inactivated, and early crossover was observed in 3% and 19% of patients, respectively. This study documented a short-term symptomatic benefit of AMS over conventional DDDR mode in a population with high AT incidence.
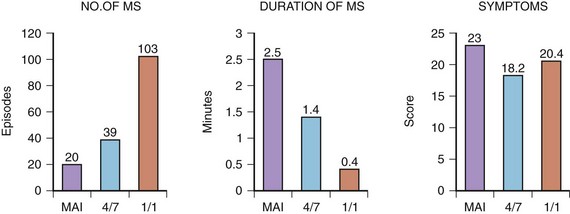
Whether different AMS algorithms may have an impact on symptoms was the subject of another study.59 Three different AMS onset criteria (mean atrial rate, “4 of 7” and “1 of 1”) of an AMS algorithm were injected into an implanted DDDR pacemaker (Thera DR models 7142, 7952, and 7960i; Medtronic, Inc.) in a group of patients with frequent AT episodes. The faster AMS criteria, 4 of 7 and 1 of 1, were better tolerated than were the slow-onset criteria using mean atrial rate (Figure 31-16). Conversely, the fast-responding algorithms led to shorter but more frequent episodes of AF that were sensed, suggesting that the device may be switching in and out of AMS because of lack of stability. This study demonstrated that different types of AMS algorithms might also have an impact on patients’ symptoms. An inappropriate AMS algorithm can have a deleterious effect on patients’ symptoms. For example, in the first version of the DDDR Meta, frequent mode switching can result in AV dissociation, which may cause more symptoms than AF itself.60 In patients with a high sinus rate, undesirable mode switching will occur during sinus rhythm. The extended ventricular escape interval caused by mode switching will prevent VP, as the device will function in a DDI/VVI mode.61 In the presence of a prolonged P-R interval, this may lead to a very long A-V interval and pacemaker syndrome.62
Two recent studies on the stability of the sinus node after AV nodal ablation questioned the long-term clinical benefit of AMS.63,64 In one study, after AV nodal ablation and implantation of a DDDR device, patients were symptomatically better and achieved better quality of life and NYHA class, compared with a control group of patients with continuation of medical therapy.63 The long-term maintenance of sinus rhythm was poor in the ablated and paced group. As many as 24% developed chronic AF by 12 months, necessitating reprogramming DDDR pacemakers to the VVIR mode. In another study, 12 of 37 patients developed chronic AF at 6 weeks after AV nodal ablation versus 0 of 19 in those who continued medical treatment.64 The cause was most likely the withdrawal of antiarrhythmic medications after AV nodal ablation but also possibly an atrial proarrhythmic effect of the ablation and pacing procedure. However, should a dual chamber device be implanted after AV nodal ablation, AMS remains an integral part of device therapy to avoid the occurrence of rapid VP during AF.
Follow-up and Troubleshooting
Because of the highly variable AF amplitudes, a routine setting of nominal atrial sensitivity margin or two times the atrial sensitivity margin may not be adequate for optimal AT or AF detection. If an episode of AF becomes available, sensitivity adjustment can be guided by the measured amplitude of the atrial ECG. Leung and associates have suggested that the optimal atrial sensitivity setting for AMS appears to be at three times versus two times the detected amplitude of the atrial ECG in sinus rhythm.35
AMS failure caused by atrial events occurring within the atrial blanking periods was reported in a series of 7 patients with Meta DDDR (Model 1254).60 All patients experienced palpitations. In 6 of 7 patients, AMS failure was due to atrial flutter, with alternate flutter waves falling within the atrial blanking periods (values: 120 ms after atrial sensed/paced event and 150 ms for PVAB). In these patients, relatively slow atrial flutter was related to drugs used to control AF. By either shortening the A-V interval or prolonging the A-V interval and PVARP so that flutter waves could be sensed outside the atrial blanking interval, normal AMS function was restored. In 1 of 6 patients, the cause of AMS failure was due to a low atrial ECG amplitude during AF, and it was corrected by reprogramming atrial sensitivity.
Palma and colleagues systematically evaluated the effect of varying atrial sensitivity, A-V interval, and detection criteria for AMS in 18 patients.65 Pacemaker models studied included the Meta DDDR (Model 1254, Medtronic, Inc.), the Thera DR (Model 7940, Medtronic, Inc.) and the Relay (Model 293-03 Intermedics, Inc.). Although an atrial sensitivity of 1 mV allowed correct sensing of sinus rhythm in 13 of 14 patients (93%), only 43% of the patients had effective AMS during AF. A-V intervals between 120 and 200 ms were found to be most effective to detect AF, whereas AF sensing was reduced when a longer A-V interval was used. The use of stringent criteria for AF detection might interfere with AMS onset. This study points out the importance of adjusting the conventional pacing parameters to optimize AMS function.60
Implantable Sensors
Sensor Classification
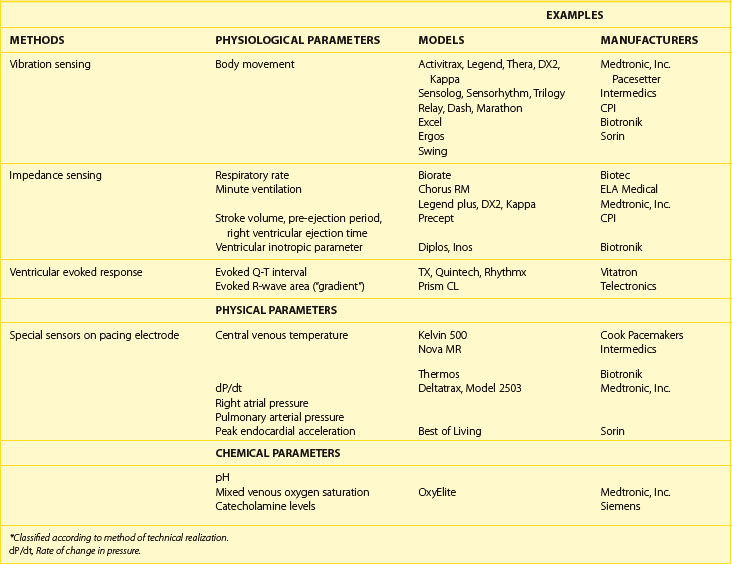
Sensors derived from a common technical principle share similar hardware requirements and sensor stability as well as similar drawbacks and limitations.66 Thus, it is more practical to classify sensors according to the technical methods that are used to measure the sensed parameter (Table 31-4). During isotonic exercise, body movements (especially those produced by heel strike during walking) result in changes in acceleration forces that act on the pacemaker. Sensors that are capable of measuring the acceleration or vibration forces in the pulse generator are broadly referred to as activity sensors. The sensing of body vibrations is, therefore, a simple way to indicate the onset of exercise. Technically, detection of body movement can be achieved using a piezoelectric crystal, an accelerometer, a tilt switch, or an inductive sensor. Each of these devices transduces the motion of the sensor either directly into voltage or indirectly into measurable changes in the electrical resistance of the crystal. Although it is a tertiary sensor, activity is the most widely used control parameter in rate-adaptive pacing because of its ease of implementation and its compatibility with standard unipolar and bipolar pacing leads. Minimal or no energy expenditure is required for such a system, and because the sensor does not need to have contact with body fluid, it is usually stable over time. Other advantages are that all the hardware is within the pacemaker case, it does not depend on the electrode arrangement, and it can be used with standard unipolar and bipolar pacing electrodes. This makes it ideal for combining it with other sensors and for pacemaker upgrading. It is used often as a backup sensor when a new sensor is being investigated. However, as a group, because body movement has only a loose relationship to workload, the sensor-indicated rate has low proportionality, and physical activities that do not involve body movement will not be detected by these sensors.
Impedance is a measure of all factors that oppose the flow of electric current and is derived by measuring resistance to an injected subthreshold electric current across a tissue. This principle has been used extensively for measuring respiratory parameters and relative stroke volume in situations involving invasive monitoring.67,68 The elegant simplicity of impedance has enabled it to be used with implantable pacing leads, including both standard pacing leads and specialized multi-electrode catheters. The pulse generator casing has been used as one electrode for the measurement of impedance in most of these pacing systems. Impedance can be used to detect relative changes in ventilatory mechanics, right ventricular mechanical function, or their combination. Relative motions between electrodes for impedance sensing also lead to changes in impedance measured, and this is inversely related to the number of electrodes used to measure impedance. In rate-adaptive pacemakers, motion artifacts are usually the result of arm movements that cause the pulse generator to move within the prepectoral pocket, thereby changing the relative electrode separation between the pacemaker and the intracardiac electrodes.69 Because arm movement accompanies normal walking, these artifacts in the impedance signal occur with both walking and upper limb exercises. Similarly, exogenous electrical interference such as diathermy may lead to erroneous sensing.
The intracardiac ventricular ECG resulting from a suprathreshold pacing stimulus has been used to provide several parameters that can guide rate modulation. The area under the curve inscribed by the depolarization phase of the paced ventricular ECG (the intracardiac R wave) has been termed the ventricular depolarization gradient or paced depolarization integral (PDI).70 In addition to depolarization, the total duration of depolarization and repolarization can be estimated by the interval from the pacing stimulus to the intracardiac T wave (the Q-T or stimulus-T interval). Both these parameters are sensitive to changes in heart rate and circulating catecholamines and can be derived from the paced intracardiac ECG with conventional pacing electrodes. Because a large polarization effect occurs after a pacing stimulus, a modified waveform of the output pulse that compensates for after-potentials is needed to eliminate this effect so that these parameters can be accurately measured.
The last group of sensors comprises those that are incorporated into the pacing lead. These dedicated sensors allow the chemical compositions of the bloodstream or intracardiac hemodynamics to be measured and may result in a more physiological sensor system. However, the long-term stability of these sensors is questionable. They are also energy expensive. Examples of these specialized leads include thermistors (used to measure blood temperature), piezoelectric crystals (used to measure right ventricular pressure), optical sensors (used to measure oxygen [SvO2]), and accelerometers at the tip of pacing leads. Some of these sensors measure highly physiological parameters. These include the measurement of pH, catecholamines, SvO2, and right ventricular pressure.71,72
These sensors have only a limited application in the field of rate-adaptive pacing because their long-term stability in the blood environment is questionable, but some important developments have occurred in this field, and these sensors may open the possibility for ambulatory monitoring of intracardiac environment using an implanted device;. Included in this group are sensors that detect right ventricular dP/dt as an index of contractility and an accelerator sensor at the tip of a pacing lead to monitor the peak endocardial acceleration, which has the possibility for optimizing the AV interval.73–81
Characteristics of an Ideal Rate-Adaptive Pacing System
The normal human sinus node increases the rate of its spontaneous depolarization during exercise in a manner that is linearly related to oxygen consumption (VO2). Because this response undoubtedly has evolutionary advantages, the goal of rate-adaptive pacemakers that modulate pacing rate by artificial sensors has been to simulate the chronotropic characteristics of the sinus node (Table 31-5). However, it is uncertain whether the sinus node provides the ideal rate response in patients who require permanent pacemakers. Nevertheless, until evidence indicating otherwise becomes available, rate-adaptive pacemakers will strive to reproduce this physiological standard. Keeping these uncertainties in mind, the ideal rate-adaptive pacing system should provide pacing rates that are proportional to the level of metabolic demand. One of the best indicators of sensor proportionality is the correlation between the sensor-indicated pacing rate and the level of oxygen consumption during exercise (Figure 31-17). In general, parameters such as minute ventilation and the paced Q-T interval are proportional sensors. Some sensors using specialized pacing leads are also highly proportional. For example, a properly functioning SvO2 sensor will result in a rate closely related to oxygen consumption during exercise.
Table 31-5 Characteristics of an Ideal Sensor for Rate-Responsive Pacing
| CONSIDERATIONS | EXAMPLES AND REMARKS |
|---|---|
| SENSOR CONSIDERATIONS | |
| Proportionality | Oxygen saturation sensing has good proportionality. |
| Speed of response | Activity sensing has the best speed of response. |
| Sensitivity | QT sensing can detect non–exercise-related changes such as anxiety reaction. |
| Specificity | Activity sensing is affected by environmental vibration. |
| Respiratory sensing is affected by voluntary hyperventilation. | |
| TECHNICAL CONSIDERATIONS | |
| Stability | Stability of early pH sensor was a problem. |
| Size | Large size or requirement for additional electrodes may be a problem. |
| Energy consumption must not unduly harm pacemaker longevity. | |
| Energy consumption must not unduly harm pacemaker longevity. | |
| Biocompatibility | It is important for the sensor to be in direct contact with the bloodstream. |
| Ease of programming | Programming was difficult in early QT-sensing pacemakers. |
In addition, an appropriate speed of response of the pacing rate to the onset of and recovery from exercise is an essential feature of a rate-adaptive pacing system. The change in pacing rate should occur with the kinetics (or speed of response) of a sensor that is similar to that of the sinus node. An anticipatory response of the heart rate occurs in many individuals before exercise. With both supine and upright isotonic exercises, heart rate and cardiac output increase within 10 seconds of the onset of exercise.82,83 Both cardiac output and sinus rate increase exponentially, with a half-time that ranges from 10 to 45 seconds, the rate of rise being proportional to the intensity of work.82 At the termination of upright exercise, a delay of approximately 5 to 10 seconds occurs before cardiac output starts to decrease, followed by an exponential fall with a half-time of 25 to 60 seconds. If the rate decay is faster than is physiologically appropriate, adverse hemodynamic consequences may occur in the presence of a substantial drop in heart rate. In one study, pacing rate was reduced either abruptly or gradually after identical exercise, and it was shown that an appropriately modulated rate recovery was associated with a higher cardiac output, lower sinus rate, and faster lactate clearance than with an unphysiological rate recovery pattern. Appropriate adjustment of the rate recovery curve is important to enhance recovery from exercise.
The exercise responses of six different types of rate-adaptive pacemakers (with sensors for activity, Q-T interval, respiratory rate, minute ventilation, and right ventricular dP/dt) were compared with normal sinus rate in one study (Figure 31-18).84 The results of this study demonstrated that the activity-sensing pacemakers best simulated the normal speed of rate response at the start of exercise. The rate response of activity sensors is usually immediate (no delay time), and the time needed to attain half the maximal change in rate occurred within 45 seconds from the onset of exercise. The maximal change in pacing rate is reached within 2 minutes of beginning an ordinary activity such as walking. The respiratory rate and the right ventricular dP/dt sensors had a longer delay time. The slowest sensor to respond to exercise was an early version of the QT-sensing pacemaker, which required up to 1 minute to initiate a rate response, and the maximal change in pacing rate was attained only in the recovery period following a short duration of exercise.
Exercise is but one of the many physiological requirements for variation in heart rate. Emotions such as anxiety may trigger a substantial change in heart rate. The sinus rate is higher when an individual moves from the supine posture to the upright posture, with a fall in the cardiac output. Isometric exercise also results in an increase in cardiac output and heart rate in most individuals. The changes in heart rate that occur during various physiological maneuvers (e.g., Valsalva maneuver), baroreceptor reflexes, and anxiety reactions may also be potentially important. An appropriate compensatory heart rate response is especially important in pathophysiological conditions such as anemia, acute blood loss, or other causes of hypovolemia. The artificial sensor should be sensitive enough to detect exercise needs and nonexercise needs for changes in heart rate and yet be specific enough not to be affected by unrelated signals arising from both internal and external environments. Although the ideal sensor should provide these functional characteristics, it must also be technically feasible to implement, with a reliability that is acceptable with modern implantable pacemakers (see Table 31-5).
Automaticity
See material at www.expertconsult.com/.
Clinical Outcome
The ultimate goal of pacemaker therapy is to improve symptoms and quality of life, and these criteria have been used to compare pacing modes. In terms of symptomatology, VVIR pacing is superior to VVI pacing. However, the overall contribution of improved control of symptoms to enhanced quality of life is probably small in the usual pacemaker recipient in whom quality of life is already close to that of age-matched normal individuals.85 No comparative study of sensors and their effects on symptomatology or quality of life has yet been completed. A patient-randomized, double-blinded crossover study was done in 10 patients using the combined activity and minute ventilation dual-sensor VVIR pacemaker for-high grade AV block and chronic or persistent paroxysmal AF. The patients in this study performed 2 weeks of out-of-hospital activity in the activity only, minute ventilation only, and dual-sensor VVIR and VVI modes. Patients were assessed on their perceived general well-being by using visual analog scale scores, Specific Activities Scale functional status questionnaire, and objective improvement by standardized daily activity protocols and graded treadmill testing. Subjective perception of exercise capacity and functional status was significantly reduced in the VVI mode compared with the VVIR modes. However, no clear advantage of dual-sensor VVIR pacing over activity sensor pacing was demonstrated. Four of the 10 patients preferred the activity VVIR mode, 3 preferred the dual-sensor mode, and 3 had no preference. Three patients found dual-sensor VVIR the least acceptable, 3 patients found minute ventilation least acceptable, and 1 patient found both dual-sensor and minute ventilation sensor pacing unacceptable. There was no significant difference in the objective performance among the 3 VVIR modes. These are not unexpected results, which suggests that no major differences exist among sensors and their combinations in gross clinical terms. However, the overall numbers of patients studied were small and hence the data do not have sufficient statistical power to unveil less-than-major differences, which may be important for assessing the long-term effects of a pacing mode. The difficulty of multiple comparisons and the order of pacing modes studied are further limitations. Lukl and colleagues also assessed quality of life with regard to cardiovascular symptoms, physical activity, psychosocial and emotional functioning, and self-perceived health during DDD and dual-sensor VVIR pacing.86 Significant improvement during DDD pacing was demonstrated in all subgroups of patients (sick sinus syndrome, patients with chronotropic competence or incompetence, and patients with high-degree AV block). The overall result shows that DDD pacing offers better quality of life than does dual-sensor VVIR pacing. Thus, dual-sensor VVIR pacing cannot compensate for the lack of AV synchrony. Because most rate-adaptive pacemakers have an activity sensor, it is of interest if the addition of a minute ventilation sensor contributes to better clinical performance. A preliminary study suggests that, although the maximal exercise capacity may not be affected by the combined sensor, better rate-adaptation profiles may enhance quality of life.87
Key References
Barold SS. Automatic mode switching during antibradycardia pacing in patients without supraventricular tachycardia. In Barold SS, Mugica J, editors: New perspectives in cardiac pacing, ed 3, Mt. Kisco, NY: Futura, 1993.
Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: A randomized, controlled study. Circulation. 1998;98:953-960.
Callaghan F, Vollmann W, Livingston A, et al. The ventricular depolarization gradient: Effects of exercise, pacing rate, epinephrine, and intrinsic heart rate control on the right ventricular evoked response. Pacing Clin Electrophysiol. 1989;12:1115-1130.
Clarke M, Liu B, Schuller H, et al. Automatic adjustment of pacemaker stimulation output correlated with continuously monitored capture thresholds: A multicenter study. European Microny Study Group. Pacing Clin Electrophysiol. 1998;21:1567-1575.
Clementy J. Dual chamber rate responsive pacing system driven by contractility: Final assessment after 1-year follow-up. The European PEA Clinical Investigation Group. Pacing Clin Electrophysiol. 1998;21:2192-2197.
Connolly SJ, Kerr C, Gent M, Yusuf S. Dual-chamber versus ventricular pacing. Critical appraisal of current data. Circulation. 1996;94:578-583.
Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385-1391.
Ellenbogen KA, Wood MA, Mond HG, et al. Clinical applications of mode-switching for dual-chamber pacemakers. In: Singer I, Barold SS, Camm AJ, editors. Nonpharmacological therapy of arrhythmias for the 21st century. The state of the art. Armonk, NY: Futura, 1998.
Kamalvand K, Tan K, Kotsakis A, et al. Is mode switching beneficial? A randomized study in patients with paroxysmal atrial tachyarrhythmias. J Am Coll Cardiol. 1997;30:496-504.
Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998;338:1097-1104.
Lau CP, Butrous GS, Ward DE, Camm AJ. Comparison of exercise performance of six rate-adaptive right ventricular cardiac pacemakers. Am J Cardiol. 1989;63:833-838.
Lau C, Cameron DA, Nishimura SC, et al. A cardiac evoked response algorithm providing threshold tracking: A North American multicenter study. Clinical Investigators of the Microny-Regency Clinical Evaluation Study. Pacing Clin Electrophysiol. 2000;23:953-959.
Lau CP, Leung SK, Tse HF, Barold SS. Automatic mode switching of implantable pacemakers: II. Clinical performance of current algorithms and their programming. Pacing Clin Electrophysiol. 2002;25:1094-1113.
Marshall HJ, Harris ZI, Griffith MJ, et al. Prospective randomized study of ablation and pacing versus medical therapy for paroxysmal atrial fibrillation: Effects of pacing mode and mode-switch algorithm. Circulation. 1999;99:1587-1592.
Schuchert A, Ventura R, Meinertz T. Automatic threshold tracking activation without the intraoperative evaluation of the evoked response amplitude. AUTOCAP Investigators. Pacing Clin Electrophysiol. 2000;23:321-324.
1 Ohm OJ, Danilovic D. Improvements in pacemaker energy consumption and functional capability: Four decades of progress. Pacing Clin Electrophysiol. 1997;20:2-9.
2 Marshall M, Butts L, Flaim G, et al. Predictors of time requirements for pacemaker clinic evaluation [abstract]. Pacing Clin Electrophysiol. 1995;18:952.
3 Preston TA, Fletcher RD, Lucchesi BR, Judge RD. Changes in myocardial threshold. Physiologic and pharmacologic factors in patients with implanted pacemakers. Am Heart J. 1967;74:235-242.
4 Dohrmann ML, Goldschlager NF. Myocardial stimulation threshold in patients with cardiac pacemakers: Effect of physiologic variables, pharmacologic agents, and lead electrodes. Cardiol Clin. 1985;3:527-537.
5 Schaldach M, Hubmann M, Weikl A, Hardt R. Sputter-deposited TiN electrode coatings for superior sensing and pacing performance. Pacing Clin Electrophysiol. 1990;13:1891-1895.
6 Curtis AB, Vance F, Miller K. Automatic reduction of stimulus polarization artifact for accurate evaluation of ventricular evoked responses. Pacing Clin Electrophysiol. 1991;14:529-537.
7 Provenier F, Germonpre E, De Wagter X. Improved differentiation of the ventricular evoked response from polarization by modification of the pacemaker impulse. Pacing Clin Electrophysiol. 2000;23:2073-2077.
8 Schuchert A, Ventura R, Meinertz T. Automatic threshold tracking activation without the intraoperative evaluation of the evoked response amplitude. AUTOCAP Investigators. Pacing Clin Electrophysiol. 2000;23:321-324.
9 Schuchert A, Ventura R, Meinertz T. Effects of body position and exercise on evoked response signal for automatic threshold activation. Pacing Clin Electrophysiol. 1999;22:1476-1480.
10 Clarke M, Liu B, Schuller H, et al. Automatic adjustment of pacemaker stimulation output correlated with continuously monitored capture thresholds: A multicenter study. European Microny Study Group. Pacing Clin Electrophysiol. 1998;21:1567-1575.
11 Sermasi S, Marconi M, Libero L, et al. Italian experience with AutoCapture in conjunction with a membrane lead. Pacesetter Automatic Control of Energy and Membrane Automatic Threshold Evaluation (Pacemate) Study Group. Pacing Clin Electrophysiol. 1996;19:1799-1804.
12 Lau C, Cameron DA, Nishimura SC, et al. A cardiac evoked response algorithm providing threshold tracking: A North American multicenter study. Clinical Investigators of the Microny-Regency Clinical Evaluation Study. Pacing Clin Electrophysiol. 2000;23:953-959.
13 Boriani G, Biffi M, Branzi A, et al. Benefits in projected pacemaker longevity and in pacing related costs conferred by automatic threshold tracking. Pacing Clin Electrophysiol. 2000;23:1783-1787.
14 Simeon L, Duru F, Fluri M, et al. The impact of automatic threshold tracking on pulse generator longevity in patients with different chronic stimulation thresholds. Pacing Clin Electrophysiol. 2000;23:1788-1791.
15 Duru F, Bauersfeld U, Schuller H, Candinas R. Threshold tracking pacing based on beat by beat evoked response detection: Clinical benefits and potential problems. J Interv Card Electrophysiol. 2000;4:511-522.
16 Guilleman D, Bussillet H, Scanu P, et al. Output adjustment with the DDD pacemaker with automatic capture-control algorithm. Prog Biomed Res. 1999;4:291-294.
17 Novak M, Kamaryt P, Haeuser T, Mach P. Simplifying pacemaker follow-up using automatic threshold determination in ventricle. Prog Biomed Res. 1999;4:287-290.
18 Gross JN, Moser S, Benedek ZM, et al. DDD pacing mode survival in patients with a dual-chamber pacemaker. J Am Coll Cardiol. 1992;19:1536-1541.
19 Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-844.
20 Lamas GA, Pashos CL, Normand SL, McNeil B. Permanent pacemaker selection and subsequent survival in elderly Medicare pacemaker recipients. Circulation. 1995;91:1063-1069.
21 Connolly SJ, Kerr C, Gent M, Yusuf S. Dual-chamber versus ventricular pacing. Critical appraisal of current data. Circulation. 1996;94:578-583.
22 Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
23 Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998;338:1097-1104.
24 Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385-1391.
25 Van Wyhe G, Sra J, Rovang K, et al. Maintenance of atrioventricular sequence after His-bundle ablation for paroxysmal supraventricular rhythm disorders: A unique use of the fallback mode in dual chamber pacemakers. Pacing Clin Electrophysiol. 1991;14:410-414.
26 Mayumi H, Uchida T, Shinozaki K, Matsui K. Use of a dual chamber pacemaker with a novel fallback algorithm as an effective treatment for sick sinus syndrome associated with transient supraventricular tachyarrhythmia. Pacing Clin Electrophysiol. 1993;16:992-1000.
27 Barold SS: Automatic mode switching during antibradycardia pacing in patients without supraventricular tachycardia. In Barold SS, Mugica J, editors: New perspectives in cardiac pacing, ed 3, Mt Kisco, NY, 1993, Futura.
28 Barold SS, Mond HG. Optimal antibradycardia pacing in patients with paroxysmal supraventricular tachyarrhythmias: Role of fallback and automatic mode switching mechanisms. In Barold SS, Mugica J, editors: New perspectives in cardiac pacing, ed 3, Mt Kisco, NY: Futura, 1993.
29 Mond HG, Barold SS. Dual chamber, rate adaptive pacing in patients with paroxysmal supraventricular tachyarrhythmias: Protective measures for rate control. Pacing Clin Electrophysiol. 1993;16:2168-2185.
30 Lau CP, Leung SK, Tse HF, Barold SS. Automatic mode switching of implantable pacemakers: II. Clinical performance of current algorithms and their programming. Pacing Clin Electrophysiol. 2002;25:1094-1113.
31 Lau CP, Leung SK, Tse HF, Barold SS. Automatic mode switching of implantable pacemakers: I. Principles of instrumentation, clinical, and hemodynamic considerations. Pacing Clin Electrophysiol. 2002;25:967-983.
32 Lau CP, Tai YT, Fong PC, et al. Atrial arrhythmia management with sensor controlled atrial refractory period and automatic mode switching in patients with minute ventilation sensing dual chamber rate adaptive pacemakers. Pacing Clin Electrophysiol. 1992;15:1504-1514.
33 Medtronic, Inc. Medtronic AT500 Technical Manual. Minneapolis, MN: Medtronic; 2003.
34 Kerr CR, Mason MA. Amplitude of atrial electrical activity during sinus rhythm and during atrial flutter-fibrillation. Pacing Clin Electrophysiol. 1985;8:348-355.
35 Leung SK, Lau CP, Lam CT, et al. Programmed atrial sensitivity: A critical determinant in atrial fibrillation detection and optimal automatic mode switching. Pacing Clin Electrophysiol. 1998;21:2214-2219.
36 Frohlig G, Helwani Z, Kusch O, et al. Bipolar ventricular far-field signals in the atrium. Pacing Clin Electrophysiol. 1999;22:1604-1613.
37 Theres H, Sun W, Combs W, et al. P wave and far-field R wave detection in pacemaker patient atrial electrograms. Pacing Clin Electrophysiol. 2000;23:434-440.
38 Brandt J, Worzewski W. Far-field QRS complex sensing: Prevalence and timing with bipolar atrial leads. Pacing Clin Electrophysiol. 2000;23:315-320.
39 Love CJ, Wilkoff BL, Hoggs KS, et al. Incidence of mode switch in a general pacemaker population [abstract]. Pacing Clin Electrophysiol. 1997;20:1137.
40 Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: Prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369-382.
41 Machado C, Sullivan C, Johnson D, et al. Pacemaker patient triggered event recording: Accuracy and utility for pacemaker follow-up clinic [abstract]. Pacing Clin Electrophysiol. 1996;19:720.
42 Ricci R, Puglisi A, Azzolini P, et al. Reliability of a new algorithm for automatic mode switching from DDDR to DDIR pacing mode in sinus node disease patients with chronotropic incompetence and recurrent paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1996;19:1719-1723.
43 Ovsyshcher IE, Katz A, Bondy C. Initial experience with a new algorithm for automatic mode switching from DDDR to DDIR mode. Pacing Clin Electrophysiol. 1994;17:1908-1912.
44 Seidl K, Meisel E, VanAgt E, et al. Is the atrial high rate episode diagnostic feature reliable in detecting paroxysmal episodes of atrial tachyarrhythmias? Pacing Clin Electrophysiol. 1998;21:694-700.
45 Fitts SM, Hill MR, Mehra R, Gillis AM. High rate atrial tachyarrhythmia detections in implantable pulse generators: Low incidence of false-positive detections. The PA Clinical Trial Investigators. Pacing Clin Electrophysiol. 2000;23:1080-1086.
46 Ellenbogen KA, Wood MA, Mond HG, et al. Clinical applications of mode-switching for dual-chamber pacemakers. In: Singer I, Barold SS, Camm AJ, editors. Nonpharmacological therapy of arrhythmias for the 21st century. The state of the art. Armonk, NY: Futura, 1998.
47 Mabo P, Daubert C, Limousin M, et al. Atrial electrogram storage: A new tool for atrial arrhythmia diagnosis in pacemaker patients [abstract]. Pacing Clin Electrophysiol. 1996;19:721.
48 Petersen B, Huikuri H, Benzer W, et al. Specificity of pacemaker diagnostics verified with stored E-grams [abstract]. Europace. 2000;1:D214.
49 Huikuri H. Effect of stored electrograms on management in the paced patient. Am J Cardiol. 2000;86:101K-103K.
50 Israel CW, Gascon D, Nowak B, et al. Diagnostic value of stored electrograms in single-lead VDD systems. Pacing Clin Electrophysiol. 2000;23:1801-1803.
51 Gillis AM, Wyse DG, Connolly SJ, et al. Atrial pacing periablation for prevention of paroxysmal atrial fibrillation. Circulation. 1999;99:2553-2558.
52 Lau CP, Tse HF, Yu CM, et al. Dual-site right atrial pacing in paroxysmal atrial fibrillation without bradycardia [NIPP-AF study]. Am J Cardiol. 2001;88:371-375.
53 Lau CP, Leung WH, Wong CK, Cheng CH. Haemodynamics of induced atrial fibrillation: A comparative assessment with sinus rhythm, atrial and ventricular pacing. Eur Heart J. 1990;11:219-224.
54 Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039-1045.
55 Wittkampf FH, de Jongste MJ, Lie HI, Meijler FL. Effect of right ventricular pacing on ventricular rhythm during atrial fibrillation. J Am Coll Cardiol. 1988;11:539-545.
56 Lau CP, Jiang ZY, Tang MO. Efficacy of ventricular rate stabilization by right ventricular pacing during atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:542-548.
57 Brunner-La Rocca HP, Rickli H, Weilenmann D, et al. Importance of ventricular rate after mode switching during low intensity exercise as assessed by clinical symptoms and ventilatory gas exchange. Pacing Clin Electrophysiol. 2000;23:32-39.
58 Kamalvand K, Tan K, Kotsakis A, et al. Is mode switching beneficial? A randomized study in patients with paroxysmal atrial tachyarrhythmias. J Am Coll Cardiol. 1997;30:496-504.
59 Marshall HJ, Kay GN, Hess M, et al. Mode switching in dual chamber pacemakers: Effect of onset criteria on arrhythmia-related symptoms. Europace. 1999;1:49-54.
60 Pitney MR, May CD, Davis MJ. Undesirable mode switching with a dual chamber rate responsive pacemaker. Pacing Clin Electrophysiol. 1993;16:729-737.
61 Leung SK, Lau CP, Leung WH, et al. Apparent extension of the atrioventricular interval due to sensor-based algorithm against supraventricular tachyarrhythmias. Pacing Clin Electrophysiol. 1994;17:321-330.
62 Jais P, Barold S, Shah DC, et al. Pacemaker syndrome induced by the mode switching algorithm of a DDDR pacemaker. Pacing Clin Electrophysiol. 1999;22:682-685.
63 Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: A randomized, controlled study. Circulation. 1998;98:953-960.
64 Marshall HJ, Harris ZI, Griffith MJ, et al. Prospective randomized study of ablation and pacing versus medical therapy for paroxysmal atrial fibrillation: Effects of pacing mode and mode-switch algorithm. Circulation. 1999;99:1587-1592.
65 Palma EC, Kedarnath V, Vankawalla V, et al. Effect of varying atrial sensitivity, AV interval, and detection algorithm on automatic mode switching. Pacing Clin Electrophysiol. 1996;19:1735-1739.
66 Lau CP. The range of sensors and algorithms used in rate adaptive cardiac pacing. Pacing Clin Electrophysiol. 1992;15:1177-1211.
67 Pacela AF. Impedance pneumography—a survey of instrumentation techniques. Med Biol Eng. 1966;4:1-15.
68 Rushmer RF, Crystal DK, Wagner C, Ellis RM. Intracardiac impedance plethysmography. Am J Physiol. 1953;174:171-174.
69 Lau CP, Ritche D, Butrous GS, et al. Rate modulation by arm movements of the respiratory dependent rate responsive pacemaker. Pacing Clin Electrophysiol. 1988;11:744-752.
70 Callaghan F, Vollmann W, Livingston A, et al. The ventricular depolarization gradient: Effects of exercise, pacing rate, epinephrine, and intrinsic heart rate control on the right ventricular evoked response. Pacing Clin Electrophysiol. 1989;12:1115-1130.
71 Stangl K, Wirtzfeld A, Heinze R, Laule M. First clinical experience with an oxygen saturation controlled pacemaker in man. Pacing Clin Electrophysiol. 1988;11:1882-1887.
72 Lau CP, Tai YT, Leung WH, et al. Rate adaptive cardiac pacing using right ventricular venous oxygen saturation: Quantification of chronotropic behavior during daily activities and maximal exercise. Pacing Clin Electrophysiol. 1994;17:2236-2246.
73 Bennett T, Sharma A, Sutton R, et al. Development of a rate adaptive pacemaker based on the maximum rate-of-rise of right ventricular pressure (RV dP/dtmax). Pacing Clin Electrophysiol. 1992;15:219-234.
74 Ovsyshcher I, Guetta V, Bondy C, Porath A. First derivative of right ventricular pressure, dP/dt, as a sensor for a rate adaptive VVI pacemaker: Initial experience. Pacing Clin Electrophysiol. 1992;15:211-218.
75 Kay GN, Philippon F, Bubien RS, Plumb VJ. Rate modulated pacing based on right ventricular dP/dt: Quantitative analysis of chronotropic response. Pacing Clin Electrophysiol. 1994;17:1344-1354.
76 Rickards AF, Bombardini T, Corbucci G, Plicchi G. An implantable intracardiac accelerometer for monitoring myocardial contractility. The Multicenter PEA Study Group. Pacing Clin Electrophysiol. 1996;19:2066-2071.
77 Leung SK, Lau CP, Lam CT, et al. Automatic optimization of resting and exercise atrioventricular interval using a peak endocardial acceleration sensor: Validation with Doppler echocardiography and direct cardiac output measurements. Pacing Clin Electrophysiol. 2000;23:1762-1766.
78 Bongiorni MG, Soldati E, Arena G, et al. Is local myocardial contractility related to endocardial acceleration signals detected by a transvenous pacing lead? Pacing Clin Electrophysiol. 1996;19:1682-1688.
79 Clementy J. Dual chamber rate responsive pacing system driven by contractility: Final assessment after 1-year follow-up. The European PEA Clinical Investigation Group. Pacing Clin Electrophysiol. 1998;21:2192-2197.
80 Langenfeld H, Krein A, Kirstein M, Binner L. Peak endocardial acceleration-based clinical testing of the “BEST” DDDR pacemaker. European PEA Clinical Investigation Group. Pacing Clin Electrophysiol. 1998;21:2187-2191.
81 Binner L. One year follow-up of a new DDDR pacemaker based on contractility: A multicenter European study on Peak Endocardial Acceleration (PEA) [abstract]. Pacing Clin Electrophysiol. 2000;23:1762-1766.
82 Loeppky JA, Greene ER, Hoekenga DE, et al. Beat-by-beat stroke volume assessment by pulsed Doppler in upright and supine exercise. J Appl Physiol. 1981;50:1173-1182.
83 Miyamoto Y, Tamura T, Takahashi T, Mikami T. Transient changes in ventilation and cardiac output at the start and end of exercise. Jpn J Physiol. 1981;31:153-168.
84 Lau CP, Butrous GS, Ward DE, Camm AJ. Comparison of exercise performance of six rate-adaptive right ventricular cardiac pacemakers. Am J Cardiol. 1989;63:833-838.
85 Lau CP, Rushby J, Leigh-Jones M, et al. Symptomatology and quality of life in patients with rate-responsive pacemakers: A double-blind, randomized, crossover study. Clin Cardiol. 1989;12:505-512.
86 Lukl J, Doupal V, Heinc P. Quality-of-life during DDD and dual sensor VVIR pacing. Pacing Clin Electrophysiol. 1994;17:1844-1848.
87 Leung SK, Lau CP, Lam C, et al. Does dual sensor rate adaptive pacing improve quality of life? [abstract]. Europace. 2000;1:D195.

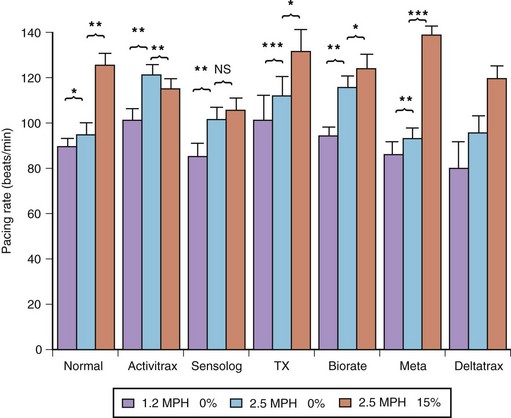
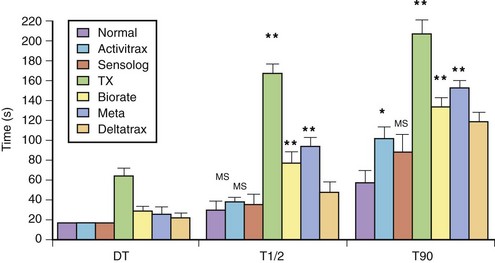
 and T90) with those of the normal sinus response. Ninety percent of the maximal rate for this exercise was reached within the exercise period in all patients, except in those with the QT-sensing pacemaker (TX), and these patients achieved this pacing rate only in the recovery phase. DT, Delay time; T
and T90) with those of the normal sinus response. Ninety percent of the maximal rate for this exercise was reached within the exercise period in all patients, except in those with the QT-sensing pacemaker (TX), and these patients achieved this pacing rate only in the recovery phase. DT, Delay time; T and T90, times needed to reach 50% and 90% of maximal heart rate.
and T90, times needed to reach 50% and 90% of maximal heart rate.