Chapter 37 Pacemaker Follow-up
Introduction
Since the implantation of the first human pacemaker in 1958, electrical myocardial stimulation against various types of rhythm disorders of the heart has become available. Pacemakers were initially introduced for the treatment of heart block and later on for sinus node disease. Currently, they are used for a variety of conditions, including tri-fascicular block and syncope related to a variety of pathologic reflexes. This implies that pacemakers now have an important role as lifesaving devices.1 They are important tools for improving quality of life in several of the cited conditions; thus pacemaker therapy has become one of the most effective medical treatments. The recent innovation of cardiac resynchronization added another dimension to pacing because combined left and right ventricular stimulation is a useful tool in improving heart function by restoring synchronous ventricular activation and contraction.
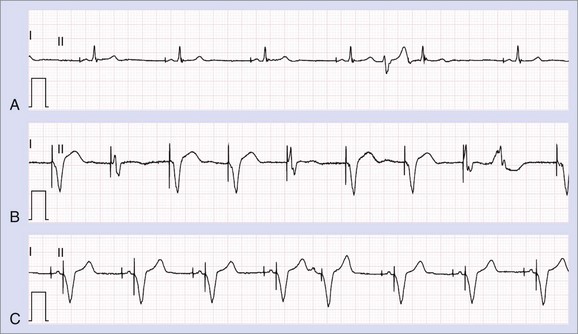
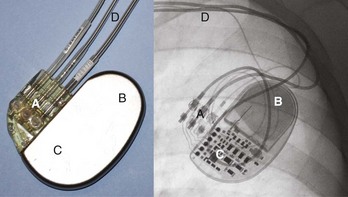
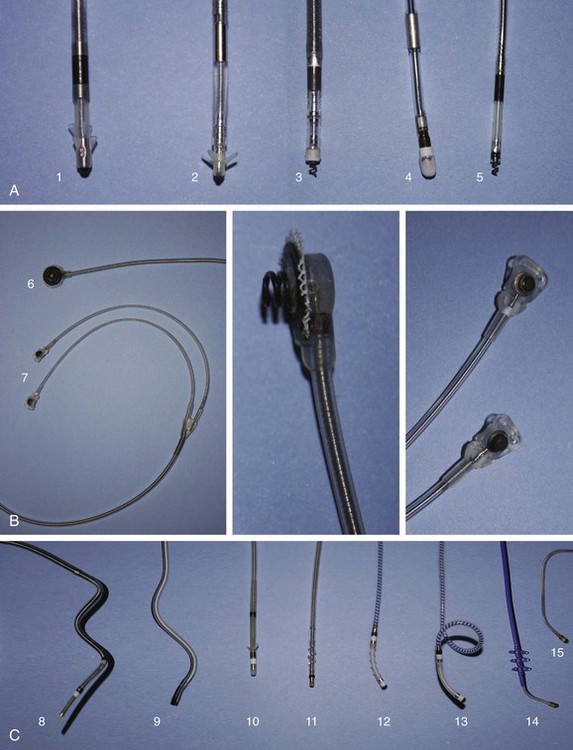
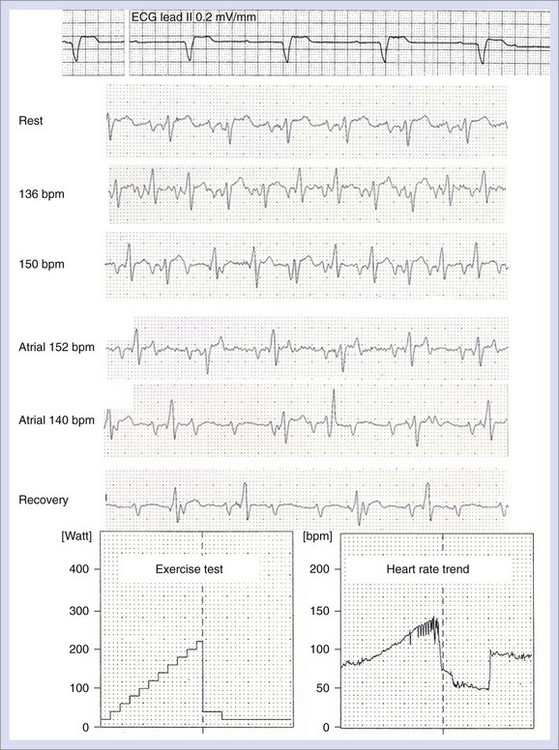
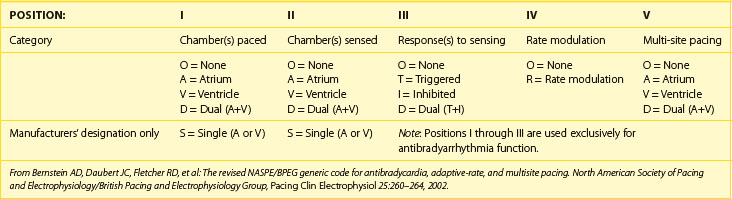
The international revised North American Society of Pacing and Electrophysiology and the British Pacing and Electrophysiology Group (NASPE/BPEG) generic (NBG) pacemaker code (Table 37-1) is generally used to address the pacing chamber, the chambers sensed, and the response to sensing as well as to determine if rate modulation and multi-site pacing have been activated.2 Figure 37-1 shows electrocardiograms (ECGs) with the three most often used methods of cardiac stimulation. In Figure 37-2, a photograph and the x-ray image of a pacemaker with the most important components are shown. These are the connectors for the electrodes leading to the heart; the pulse generator itself comprises the power source and the electronic circuit that controls the timing, the impulses, and a variety of programmable features. The pacemaker is connected to one or more pacemaker electrodes (epicardiac or endocardiac), which allow conduction of the stimulus from the pulse generator to the myocardium (Figure 37-3). Pacemaker electrodes can be implanted in the right atrium, the right ventricle, and the coronary sinus for left-sided stimulation by venous access or inserted surgically for epicardial access. Electrodes can be unipolar or bipolar.
Table 37-1 The Revised NASPE/BPEG Generic (NBG) Code for Antibradycardia, Adaptive-Rate, and Multi-site Pacing
The third part of the pacemaker system (beside the pacemaker itself and the electrodes) is the pacemaker programmer (Figure 37-4), which usually is not considered as critical for pacemaker operation. However, this tool is used to check the integrity of the pacemaker system and control the functioning of the pacemaker. Nowadays, these programmers have evolved into dedicated computers, which have made possible extensive programming of pacemakers and advanced interrogation of pacemaker diagnostics. This chapter focuses on the essential elements and developments of modern pacemaker follow-up.
Organization of the Pacemaker Clinic
In the early pacemaker era, follow-up largely was intended to monitor the proper technical operation of the electronic device itself. Over time, pacemakers have evolved into a stable and trustable technology, and they are now equipped with multiple diagnostic tools. Although the proper technical functioning of the pacemaker still is the most critical part of therapy, a shift from pure technical device checkup to clinical rhythm control is often necessary. Therefore modern pacemaker follow-up takes place in a highly specialized, multi-disciplinary environment. There is general consensus about the various aspects of monitoring cardiovascular implantable electronic devices (CIEDs).3 The essential elements of a well-organized pacemaker clinic are summarized below.
Staff and Assisting Personnel
A well-organized pacemaker clinic can depend on personnel from multiple disciplines. The pacemaker follow-up clinic is under the supervision of the cardiologist and an allied professional with a technical background such as a medical engineer or a skilled nurse or dedicated nurse practitioner. Both physicians and allied professionals should have internationally accepted competencies.4,5 The pacemaker clinic is typically integrated into a general cardiology facility, which facilitates easy access to administrative and other supporting personnel. Involvement of a dedicated psychologist and pediatric cardiologists may be required.
Equipment
Routine pacemaker follow-up is often performed in a dedicated pacemaker control room (Figure 37-5), which allows efficient, high-quality device follow-up. This room should be equipped with an examination table, an electrocardiogram (ECG) recorder, pacemaker programmers with appropriate and updated device software, advanced life support material such as external defibrillator with trans-cutaneous pacing capabilities and resuscitation materials, technical documentation, and specifications of pacemakers in use. Computers connected to the hospital network and the Internet, with online access to electronic patient records, the pacemaker database, and remote follow-up web pages, should be available.
Normal Routine Pacemaker Follow-up
Postimplantation and Predischarge Follow-up
Proper pacemaker functioning and connection of the electrodes must be confirmed in the peri-operative and immediate postoperative periods. After the implantation of a pacemaker system, pacemaker follow-up should be performed on a regular basis to confirm the efficacy of the applied therapy.3,6,7 The first follow-up should be performed before hospital discharge.
Long-Term Device Follow-up
A technical control was normally required 3 months after implantation to verify the output of the pacemaker and to fine-tune the stimulation as the threshold (which varies the most in the first few months because of the endocardiac healing process) returns to the baseline. The output could be optimized at this point in time. If an automatic capture control function is available, checked at the time of discharge, and activated, the first control can be delayed.8
Contents of Routine Ambulatory Pacemaker Follow-up
To achieve the goals of the pacemaker clinic, routine pacemaker follow-up should be performed in a structured way. Box 37-1 summarizes all the elements of a methodical, routine, more advanced control (as needed).
Box 37-1 Follow-up Procedures at the Pacemaker Clinic
History
Technical Examination
Patient History and Physical Examination
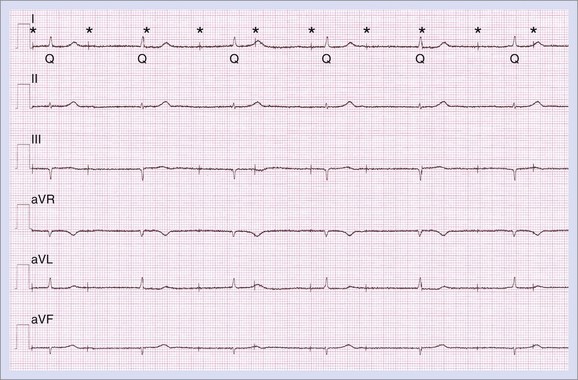
A routine ECG will show whether the patient has pacing or no pacing in his or her daily life and is often useful to understand data from the programmer.9 Also, the ECG will reveal many other features at the atrial and ventricular levels. This is highly important for cardiac resynchronization therapy (CRT) because QRS width and axis can be proof of biventricular pacing versus right or left ventricular stimulation alone and because the axis, in general, tells something about the pacing site (Figure 37-6).10
Technical Pacemaker Control
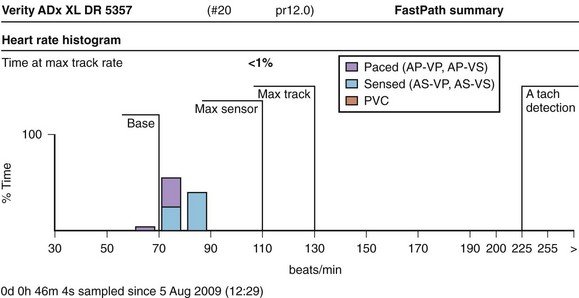
Checkup of the technical integrity is another routine that may be dealt with simply by interrogating the device and printing out or reading a completely automated report. This typically shows the actual battery voltage, the impedance over the system and the leads, and data on pacing and sensing, which are often expressed as histograms or tables (Figure 37-7). At the impending end of (battery) life (EOL, end-of-life, or EOS, end-of-service) an early warning is reported, called the elective replacement index (ERI). At this time, the appointment for elective replacement should be scheduled within a reasonable time, according to the specifications of the device. When the battery is almost completely depleted, the device often reverts to a backup mode, simply delivering single-chamber synchronous pacing and later reverting to asynchronous pacing until no more output is possible (Figure 37-8). The behavior of current lithium iodine batteries is adequately predictable, so when a normal (remote) follow-up scheme is followed, premature battery depletion or asynchronous EOL behavior should no longer appear on pacemaker follow-up. To prolong the generator life, several precautions can be taken during control. One is to use automatic capture; if this is not available, the output should be programmed at a level that ensures safety (usually two to three times the measured threshold), without depleting the energy of the battery.8,11 Several systems now have an automatic threshold function, a system to verify automatically the sensing the various levels of the pacemaker and automatic sensor optimization capabilities. This may lead to reprogramming, if possible. Otherwise, automatic adjustments may have occurred all the time, and a printout of this function will be generated. It is generally supposed that activation of the automatic features simplifies follow-up without problems for selected patients.12 These automatic features can always be checked manually, if preferred, during routine follow-up.
The integrity of the conductors should also be examined by observing the intracardiac electrogram (IEGM). When diagnostics are available, more appropriate programming of the device can be performed to avoid the unnecessary features. Alternatively, new features can be included to provide more comfort for the patient. In general, it is wise to start the pacemaker therapy by simply providing basic functions, depending on the patient’s indications for a pacemaker. Features such as rest rate or night drop and flywheel or rate smoothing (Figure 37-9) can be activated later, on the basis of the collected pacemaker diagnostics and Holter or histogram information.13 These features should be activated only when adequate lower and upper rates are programmed and after correct steepness of the sensor response has been studied, such as by exercise testing or a 6-minute walk test.14 Furthermore, as it is known, right ventricular apical pacing should be avoided.15 This can be ensured with programming the pacemaker modus into AAI, a low backup rate in VVI, DDI with a low rate if an atrial lead is necessary, or ventricular lead implantation on alternative sites such as the right ventricular septum or the outflow tract.16 The alternatives are automatic mode switching algorithms from AAI to DDD or automatic adjustment of the AV delay if the pacemaker system allows it.17 However, if ventricular pacing is needed, correct programming in DDD mode with adapted AV delay is necessary.18,19
More Advanced Control and Troubleshooting
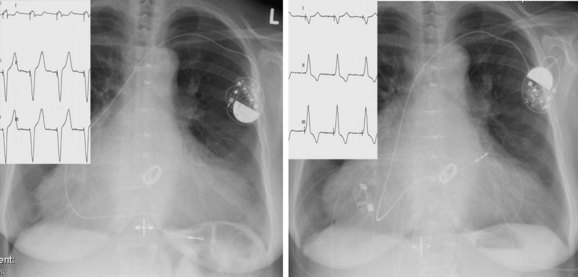
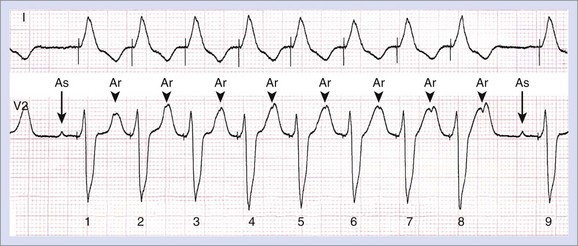
More advanced fine-tuning of the pacemaker may be necessary; it is often done unexpectedly or may occur when a patient is admitted to the hospital. The reason for such an event might be syncope or a surgical or medical event such as myocardial infarction, which requires reprogramming or renewed programming. It is almost impossible to describe general rules to solve the diverse problems related to pacemakers; each patient deserves an independent, individualized, and tailored approach. For example, pacemaker fine-tuning is required for persistent atrial fibrillation and atrioventricular block in an older adult who is wheelchair dependent rather than for paroxysmal atrial fibrillation and rate-dependent AV block (Figure 37-10) in a young cyclist, even when a comparable pacemaker system could have been implanted.20
Troubleshooting for the pacemaker system is not always easy. A routine ECG or the reading of the programmer may reveal the reasons for the (potential) dysfunction of the pacemaker system. Box 37-2 lists several potential complications and problems that should be solved with more expert knowledge and systematic analysis of the electrograms and surface ECG.
Various IEGM recordings that display noise, high frequency, or other noncardiac signals can help discover interference that may be caused by cellular phones or magnetic fields. Because of improved signal filtering and detection algorithms, pacemaker malfunction caused by such phenomena seems to have become relatively rare.21,22
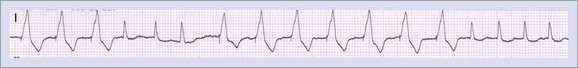
Specific problems such as pacemaker-mediated tachycardia (Figure 37-11) require some understanding of the normal conduction behavior of the heart and of the particular pacemaker algorithms designed to avoid them.23 Some maneuvers may be necessary to provoke or reproduce complaints associated with pacemaker-mediated tachycardia. Pacemaker syndrome is another problem typically associated with (but not limited to) ventricular pacing and retrograde conduction.
Complaints associated with far-field R-wave sensing or undersensing of supraventricular activity can be resolved by individual adjustment of blanking and refractory periods.24 Marker annotations combined with IEGMs help interpret these problems.
Additional Tests
Hemodynamic fine-tuning can be done using echocardiography to adjust the A-V interval, the V-V interval (for resynchronization devices), and the intraventricular interval.15,25 Optimization of cardiac resynchronization devices requires more attention than described here and is discussed in other chapters in this text.
Device Advisories and Safety Alerts
Good databases also allow taking fast action when a device or lead advisory is issued. The affected devices or leads can be easily queried from the database. Alert notifications as well as specific device advisory information from the manufacturer should be added to the concerned patient’s files. The patient should be given clear information from both the manufacturer and the physician. Finally, all the essential technical and clinical information from the device or the lead and information about patients involved in safety advisories should be reported to, or shared with, the relevant government authorities, manufacturers, and other physicians.26,27 With these efforts, remote monitoring will be of significant assistance.28,29
Key References
Andersen RA, Nielsen JC, Bloch Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
Aranda JMJr, Woo GW, Schofield RS, et al. Management of heart failure after cardiac resynchronization therapy: Integrating advanced heart failure treatment with optimal device function. J Am Coll Cardiol. 2005;46:2193-2198.
Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993-3001.
Carlson MD, Wilkoff BL, Maisel WH, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm. 2006;3(10):1250-1273.
de Cock CC, Meyer A, Kamp O, Visser CA. Hemodynamic benefits of right ventricular outflow tract pacing: Comparison with right ventricular apex pacing. Pacing Clin Electrophysiol. 1998;21:536-541.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:e350-e408.
Faust M, Fraser J, Schurig L, et al. Educational guidelines for the clinically associated professional in cardiac pacing and electrophysiology. Pacing Clin Electrophysiol. 1990;13:1448-1455.
Hayes DL, Naccarelli GV, Furman S, et al. North American Society of Pacing and Electrophysiology: NASPE training requirements for cardiac implantable electronic devices: Selection, implantation, and follow-up. Pacing Clin Electrophysiol. 2003;26:1556-1562.
Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing, for the Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998;338:1097-1104.
Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines. Heart Rhythm. 2009;6(6):869-885.
Mayumi H, Kohno H, Yasui H, et al. Use of automatic mode change between DDD and AAI to facilitate native atrioventricular conduction in patients with sick sinus syndrome or transient atrioventricular block. Pacing Clin Electrophysiol. 1996;19:1740-1747.
Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: Calendar year 2005, an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202-1212.
Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: Impact on medical management and health-care resource utilization. Europace. 2008;10:164-170.
Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Europace. 2007;9:959-998.
Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5(6):907-925.
1 Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: Calendar year 2005, an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202-1212.
2 Bernstein AD, Daubert JC, Fletcher RD, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol. 2002;25:260-264.
3 Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5(6):907-925.
4 Hayes DL, Naccarelli GV, Furman S, et al. North American Society of Pacing and Electrophysiology. NASPE training requirements for cardiac implantable electronic devices: Selection, implantation, and follow-up. Pacing Clin Electrophysiol. 2003;26:1556-1562.
5 Faust M, Fraser J, Schurig L, et al. Educational guidelines for the clinically associated professional in cardiac pacing and electrophysiology. Pacing Clin Electrophysiol. 1990;13:1448-1455.
6 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:e350-e408.
7 Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Europace. 2007;9:959-998.
8 Ribeiro AL, Rincón LG, Oliveira BG, et al. Automatic adjustment of pacing output in the clinical setting. Am Heart J. 2004;147:127-131.
9 Byrd CB, Hallberg BS, Byrd CL. Computerized pacemaker patient analysis. Pacing Clin Electrophysiol. 1990;13:1779-1781.
10 Aranda JMJr, Woo GW, Schofield RS, et al. Management of heart failure after cardiac resynchronization therapy: Integrating advanced heart failure treatment with optimal device function. J Am Coll Cardiol. 2005;46:2193-2198.
11 Schuchert A, Meinertz T, Low Output Programming (LOP) Investigators. A randomized study on the effects of pacemaker programming to a lower output on projected pulse generator longevity. Pacing Clin Electrophysiol. 2001;24:1234-1239.
12 Alings M, Vorstenbosch JM, Reeve H. Automaticity: Design of a registry to assess long-term acceptance and clinical impact of automatic algorithms in Insignia pacemakers. Europace. 2009;11:370-373.
13 Duckers HJ, van Hemel NM, Kelder JC, et al. Effective use of a novel rate-smoothing algorithm in atrial fibrillation by ventricular pacing. Eur Heart J. 1997;18:1951-1955.
14 Provenier F, Jordaens L. Evaluation of six minute walking test in patients with single chamber rate responsive pacemakers. Br Heart J. 1994;72:192-196.
15 Andersen RA, Nielsen JC, Bloch Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
16 de Cock CC, Meyer A, Kamp O, Visser CA. Hemodynamic benefits of right ventricular outflow tract pacing: Comparison with right ventricular apex pacing. Pacing Clin Electrophysiol. 1998;21:536-541.
17 Mayumi H, Kohno H, Yasui H, et al. Use of automatic mode change between DDD and AAI to facilitate native atrioventricular conduction in patients with sick sinus syndrome or transient atrioventricular block. Pacing Clin Electrophysiol. 1996;19:1740-1747.
18 Lamas GA, Orav EJ, Stambler BS, et al. for the Pacemaker Selection in the Elderly Investigators: Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. N Engl J Med. 1998;338:1097-1104.
19 Provenier F, Boudrez H, Deharo JC, et al. Quality of life in patients with complete heart block and paroxysmal atrial tachyarrhythmias: A comparison of permanent DDIR versus DDDR pacing with mode switch to DDIR. Pacing Clin Electrophysiol. 1999;22:462-468.
20 Bailey SM, Wilkoff BL. Complications of pacemakers and defibrillators in the elderly. Am J Geriatr Cardiol. 2006;15:102-107.
21 Hekmat K, Salemink B, Lauterbach G, et al. Interference by cellular phones with permanent implanted pacemakers: An update. Europace. 2004;6:363-369.
22 Trigano A, Blandeau O, Sougues M, et al. Clinical study of interference with cardiac pacemakers by a magnetic field at power line frequencies. J Am Coll Cardiol. 2005;45:896-900.
23 Den Dulk K, Lindemans FW, Bär FW, et al. Pacemaker related tachycardias. Pacing Clin Electrophysiol. 1982;5:476-485.
24 Kolb C, Wille B, Maurer D, et al. Management of far-field R wave sensing for the avoidance of inappropriate mode switch in dual chamber pacemakers: Results of the FFS-test study, on behalf of the FFS-Test Study Group. J Cardiovasc Electrophysiol. 2006;17:992-997.
25 Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993-3001.
26 Carlson MD, Wilkoff BL, Maisel WH, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm. 2006;3(10):1250-1273.
27 Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: Developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009;6(6):869-885.
28 Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: Impact on medical management and health-care resource utilization. Europace. 2008;10:164-170.
29 Scholten MF, Thornton AS, Theuns DA, et al. Twiddler’s syndrome detected by home monitoring device. Pacing Clin Electrophysiol. 2004;27:1151-1152.