Chapter 77 Pacemaker and Implantable Cardioverter-Defibrillator Therapy in Pediatric Patients with and Without Congenital Heart Disease
Overview of Pacemaker and Implantable Cardioverter-Defibrillator Therapy
No large clinical trials or prospective studies in children are available to offer guidelines for pacemaker and device implantation in children. For this reason, the adult guidelines have served as a basis for pediatric implantations. The most recent indications for device implantation are shown in Box 77-1 (pacing) and Box 77-2 (ICD or cardiac resynchronization therapy [CRT]).1 This chapter discusses the unique circumstances and special indications in children and adolescents and in those with congenital heart disease that are not addressed by the adult studies and guidelines.
Box 77-1 Indications for Pacing in Children and Adolescents
Class I
Class IIa
Pacemaker Therapy for Children and Patients with Congenital Heart Disease
Several important considerations in young patients separate them from adults. Children, especially neonates, require higher heart rates to maintain an adequate cardiac output. An increasing population of survivors of palliative procedures for various forms of congenital heart disease has suboptimal cardiovascular physiology, compromised myocardial function, or both. They are vulnerable to the effects of bradycardia, which may leave them with inadequate cardiac output and increased vulnerability to both atrial and ventricular arrhythmias. Moreover, loss of AV synchrony associated with complete heart block or junctional bradycardia may result in symptoms at heart rates that would be tolerated in individuals with normal cardiovascular physiology.2,3 In most patients, pacemaker implantation is indicated when symptoms associated with bradycardia are present, with subsequent improvement of those symptoms with chronotropic support, restoration of AV synchrony, or both. Because of the lack of large pediatric randomized double-blind clinical trials, the level of evidence for most recommendations is based on the consensus of experts.
Specific Pacing Issues
Sinus Node Dysfunction
Isolated sinus node dysfunction is extremely rare in children with structurally normal hearts. Pediatric sinus node disease is most commonly encountered after repair of congenital heart disease, with device implantation indicated only for symptomatic patients.4 It can be challenging to correlate bradycardia with symptoms of fatigue, dizziness, or syncope. Before implanting a pacemaker, it is important to exclude reversible causes of bradycardia such as seizure, increased intracranial pressure, hypothyroidism, hypothermia, apnea, sepsis, medications, or other systemic conditions. Since average heart rates decrease as children grow, bradycardia is a relative term, and no specific heart rate “cut-off” that defines an indication for pacing exists.
In a carefully selected subgroup of children with profound bradycardia and asystole associated with pallid breath-holding spells, pacing has been successful in alleviating symptoms.5
The combination of sinus bradycardia with atrial tachyarrhythmias (tachycardia-bradycardia syndrome) is becoming more common as patients survive complex repairs for congenital heart disease. The loss of sinus rhythm is considered an independent risk factor for the development of these intra-atrial re-entrant arrhythmias.6 Although antiarrhythymic drugs may be useful, their use may be limited by pre-existing bradycardia.7 Long-term atrial pacing and atrial anti-tachycardia pacing (ATP) have both been used with equivocal results.7,8
Postoperative Atrioventricular Block
Natural history studies have established a very poor prognosis with up to 50% mortality in patients with permanent heart block after surgical repair of congenital heart disease.9 Advanced second-degree or third-degree postoperative heart block that has persisted for at least 7 days and is not expected to resolve is considered a class I indication for pacemaker placement.1 Patients with transient AV block after surgery have a small but definite risk of late progression to complete AV block decades after surgery and need close monitoring and follow-up.10,11 Patients with residual bi-fascicular block and progressive P-R interval prolongation may be particularly at risk for development of late-onset AV block.12 Because of this possibility, unexplained syncope is a class IIa indication for pacing in these patients once other cardiac and noncardiac etiologies of syncope have been ruled out.1
Congenital Complete Atrioventricular Block
In children with structurally normal hearts, congenital complete AV block (CCAVB) is the most common indication for permanent pacing, and accounts for 20% to 30% of the entire pediatric paced population in large single-center reports.13,14 CCAVB is often caused by fetal exposure to maternal lupus antibodies but may be idiopathic as well. The subgroup of patients with maternal lupus associated CCAVB tend to present with the need for pacing at an earlier age and are more likely to develop dilated cardiomyopathy compared with those with idiopathic CCAVB.15
Class I indications for permanent pacing include a wide QRS escape rhythm, complex ventricular ectopy, or ventricular dysfunction.1 In neonates with a narrow QRS escape rhythm, the class I indication is met when the average heart rate is below 55 beats/min or below 70 beats/min for infants with significant congenital heart disease. Beyond the first year of life, the class IIa indication states that pacing is appropriate for average heart rates below 50 beats/min, or when abrupt pauses of two to three times the basic cycle length occur, or when symptoms of chronotropic incompetence are present.1
Long QT Syndrome
In long QT syndrome (LQTS), pacing prevents bradycardia and pauses and may even shorten the QT interval. Pacing alone has been shown to be efficacious for certain high-risk patients with LQTS, and although most high-risk patients in the modern era are treated with ICD therapy, a simple pacemaker may be a good option for small patients in whom ICD implantation is not feasible.16
Lyme Carditis
Lyme disease is a common cause of acquired heart block in children and should be considered in any child presenting with heart block. In one recent large study of 207 children with Lyme disease, 33 (16%) developed carditis.17 Nine patients had complete heart block, and three of those received temporary pacing, but no patient required permanent pacing. With very rare exceptions, heart block in the setting of Lyme disease is transient and responds to antibiotic therapy. In cases of advanced second-degree or third-degree AV block, some have recommended corticosteroid therapy in addition to antibiotics. The opinion on using steroids is mixed, and evidence is limited to isolated case reports and series.18
Technical Considerations and Challenges of Implantation
General Principles
Pediatric pacemakers should be implanted by specialized teams with expertise in the unique aspects of pacing in children and familiarity with the anatomic variations associated with congenital heart disease.19 Procedures are usually performed with the patient under general anesthesia and monitored by a pediatric cardiac anesthesiologist.
The psychosocial adjustments of the patient and family must also be considered, and trained nurses, play/child-life therapists, and other psychosocial professionals should be made an essential part of the implantation team. The techniques of epicardial and transvenous pacemaker implantation are discussed in detail in other chapters of this book and elsewhere.19
Transvenous Versus Epicardial Systems
Transvenous systems are more reliable and have greater longevity compared with epicardial systems. In one large pediatric cohort, transvenous leads had lower thresholds and significantly more longevity compared with steroid-eluting epicardial leads.20 Epicardial pacing is generally preferred for smaller patients and for patients with congenital heart disease who have intracardiac shunts.21 For the infant who requires lifelong pacing, an epicardial pacing system preserves the venous anatomy and ideally defers transvenous implantation until the child has grown closer to adult size. Although transvenous pacing is technically feasible in infants, lead survival is significantly decreased, and the risk of venous occlusion may present an obstacle to placement of additional leads in the future.22,23 As small patients grow and the distance between the generator and the heart increases, tension can develop on the lead. This problem is sometimes addressed by placing a right atrial loop to introduce more “slack” in the lead, although these loops can promote adhesions of the lead body to the vessel walls, making extraction difficult, and often may not straighten out as planned with growth.24–26
It is clear that infants should receive epicardial pacing systems when indicated, but the choice between the epicardial and transvenous approaches is more difficult in toddlers and young children. The transvenous approach is attractive because it avoids thoracotomy and is associated with increased lead survival time. However, venous thrombosis may be a problem when adult-sized pacing leads are placed into small vessels. One study evaluated 63 children with transvenous pacing leads with echocardiography and venography to determine the incidence of venous thrombosis and found that 18% of them had moderate or severe thrombosis at follow-up.27 Children who had thrombosis were younger at implant (4.5 vs. 8.2 years) and had a higher ratio of lead diameter to body surface area.27 This suggests that children in the age range of 3 to 6 years may have better long-term outcomes with epicardial pacing because venous anatomy is preserved. It also suggests that a two-lead system may have a higher thrombosis risk than a single-lead system and should be avoided unless absolutely necessary.
In the setting of congenital heart disease with intracardiac shunting, a risk of thromboembolic events exists, and epicardial pacing is preferred at any age.21 Epicardial pacing may be used and should be strongly considered in small children, even when trans-venous pacing is technically feasible in the interests of maintaining venous access for future use. Steroid-eluting epicardial leads are preferred, and the lead should be secured in a scar-free area to optimize pacing and sensing thresholds. A limited left thoracotomy approach may be successful in older children or in children with scars from prior thoracotomies.
Single-Chamber Versus Dual-Chamber Pacing
In the setting of heart block, dual-chamber DDD pacing provides AV synchrony and is obviously more physiological than single-chamber ventricular pacing. However, children with normal hearts or repaired congenital heart disease do surprisingly well with rate-responsive VVIR pacing and can be expected to lead active lives.25 The merits of AV synchrony may be outweighed by the challenges of implanting a two-lead transvenous system, including increased surgical risk and operative time and the late risk of venous thrombosis and lead malfunction. When the child reaches adolescence or young adulthood, an upgrade to a dual-chamber system may be indicated. For infants, single-lead epicardial implantation can be accomplished with a smaller incision and with less intrathoracic dissection than in the case of a dual-chamber system. This shortens the recovery time and preserves the chest, heart, and thoracic cavity for future operations. For a school-aged child undergoing transvenous implantation, a single ventricular lead is less likely to cause venous occlusion compared with a two-lead system.
Pectoral Versus Abdominal Implantation
In general, epicardial leads are attached to abdominal pulse generators and transvenous leads are attached to pectoral devices. Although a transvenous lead can be tunneled to an abdominal device, this approach is generally avoided because it exposes the lead to trauma. Submuscular pockets are preferred for pediatric patients and are thought to minimize trauma and improve cosmetic results.28
Single-Lead VDD Pacing Systems
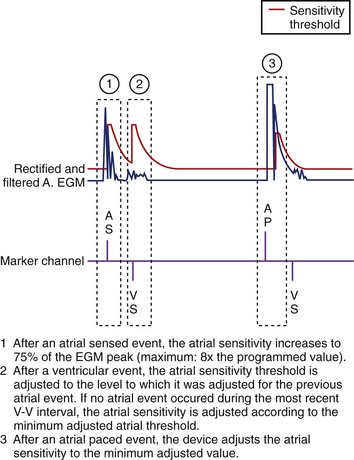
Single-lead VDD pacing combines a traditional ventricular pacing lead with atrial-sensing electrodes that make passive contact with atrial tissue and allow for atrial sensing without atrial pacing capabilities. The ACC/AHA/NASPE (American College of Cardiology/American Heart Association/National Association for Sport and Physical Education) guidelines state that VDD pacemakers are indicated for patients with AV block and normal sinus node function, and no need for atrial pacing.29 These leads are an attractive option for pediatric patients because they provide AV sequential pacing without the need for two leads in a potentially small vascular space. The most obvious disadvantage is that atrial pacing is not available in patients with coexisting sinus node dysfunction or bradycardia related to antiarrhythmic drug therapy. Atrial undersensing can also be a problem because a dedicated atrial lead is lacking. However, problems with atrial undersensing affect only 2% to 5% of these patients and rarely produce clinical symptoms. Data on the extractability of these leads, as compared with others, are not available.
Specific Challenges in Congenital Heart Defects
Transposition of the Great Arteries
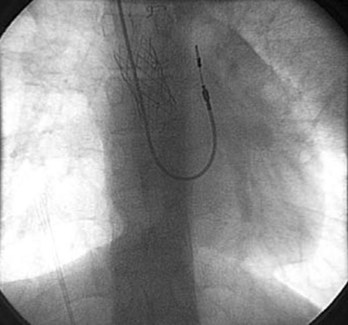
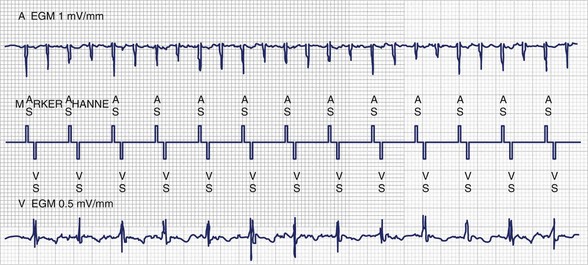
Patients who have undergone intra-atrial baffle repair (Mustard, Senning procedures) often have sinus node dysfunction and atrial flutter as adolescents and adults related to their extensive atrial surgery. At implantation, the atrial lead must be secured to the superior aspect of the anatomic left atrium, now in the physiological right or systemic venous atrium (Figure 77-1). Care must be taken to avoid phrenic nerve stimulation when pacing in this area. Because the systemic venous ventricle is a morphologic left ventricle with smooth walls and small trabeculae, an active fixation lead is preferred.
These patients often develop obstruction of the superior vena cava (SVC), which precludes the transvenous approach to pacing; hence, venography before implantation is important to delineate venous anatomy. Placing a stent in the atrial baffle may open the obstructed area and allow passage of the transvenous leads in some cases.30 The presence of right-to-left atrial or baffle shunts should also be identified and closed before placement of the transvenous pacing leads to prevent paradoxic emboli and strokes.
Single Ventricle
Several anatomic substrates result in single-ventricle physiology and are ultimately palliated with the Fontan operation, the most common being hypoplastic left heart syndrome. The systemic venous return is connected directly to the pulmonary artery and hence, access for transvenous ventricular pacing is lacking. Sinus node dysfunction is very common in this group, and atrial tachyarrhythmias, especially intra-atrial re-entrant tachycardia (IART) or focal atrial tachycardia, become more common as these patients approach adulthood. In single-ventricle patients with pure sinus node dysfunction with no AV nodal disease, a single-chamber atrial pacing system can sometimes be placed via the transvenous approach, provided that no residual “fenestration” that would be a source of paradoxic emboli is present and that atrial tissue can be reached through the systemic venous connection (which may be impossible if the conduit is entirely extracardiac).31 Because of the low-flow state in the systemic venous atrium, thrombosis is a potential concern, and epicardial pacing may be chosen even when transvenous pacing is technically possible.
Atrial Arrhythmias
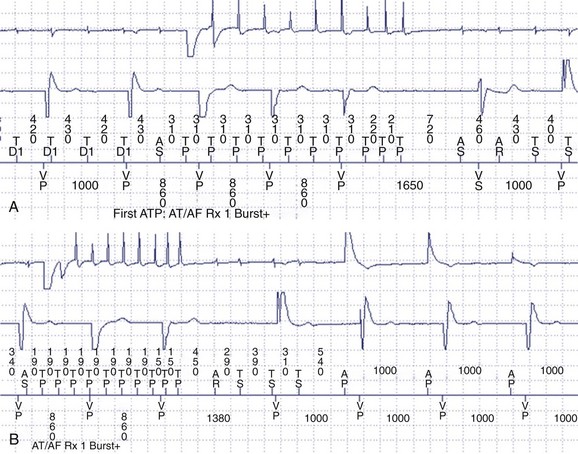
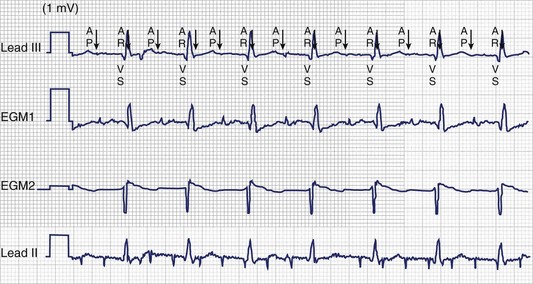
Atrial arrhythmias, particularly IART, are common in patients with single-ventricle physiology or complex congenital repairs such as the Mustard or Senning operation. IART is often refractory to medical therapy, and pacemakers with atrial ATP capabilities can be useful to detect and terminate atrial arrhythmias in this population (Figure 77-2).7 ATPs have certain drawbacks. To ensure proper arrhythmia detection, bipolar leads are required. Since many patients with congenital heart disease have pre-existing unipolar epicardial leads, “upgrading” to a device with ATP features may require replacement of the leads entirely. Furthermore, the risk of an atrial arrhythmia being accelerated to a faster arrhythmia does exist during an attempt to provide therapy.7 Another drawback is that the devices generally will not deliver therapy for atrial tachycardias that have 1 : 1 AV conduction, which is not unusual in patients with congenital heart disease with slow atrial cycle lengths during tachycardia.32
Implantable Cardioverter-Defibrillators In Young Patients
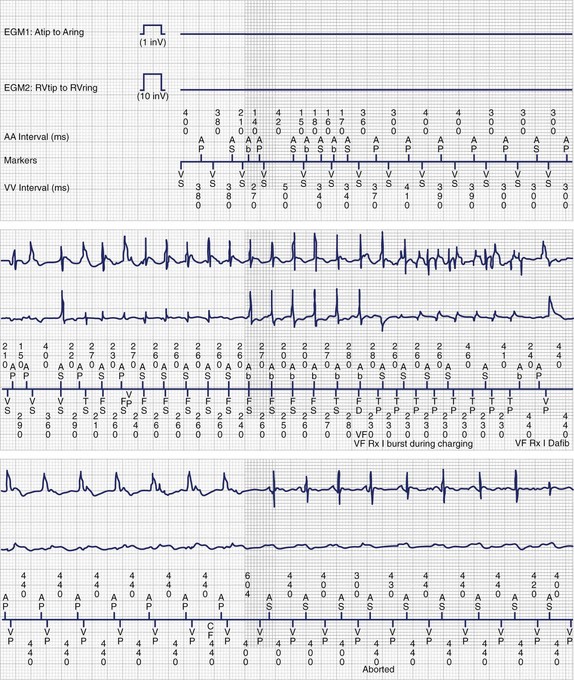
As indications expand and implantation becomes technically more feasible, more children are becoming candidates for life-saving ICD therapy (Figure 77-3). Still, pediatric patients and those with congenital heart disease comprise less than 1% of the total ICD population. Implementing therapy with devices that were manufactured and designed for older patients with an entirely different disease substrate is challenging. Indications for ICD implantation in children are still poorly defined and primarily include aborted sudden cardiac arrest and symptomatic ventricular arrhythmias (secondary prevention guidelines) and other high-risk factors without documented life-threatening arrhythmias (primary prevention guidelines). The last decade has seen a surge in the total number of pediatric ICD recipients and the percentage of patients implanted for primary prevention indications. Despite significant technological advances, inappropriate shocks occur frequently, and lead-related and device-related complication rates are significant. Furthermore, unique social and emotional issues are involved, and the psychosocial impacts of ICD implantation are especially important in this young population and should be addressed in the case of each recipient.
Indications
Primary and secondary indications for ICD implantation have evolved rapidly. Current recommendations for ICD implantation in young patients and survivors of congenital heart disease are summarized in Box 77-2.
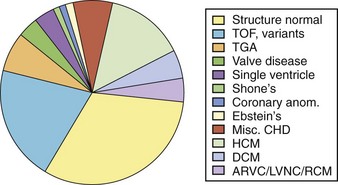
Data from two recent multi-center, retrospective pediatric ICD registries revealed that tetralogy of Fallot, hypertrophic cardiomyopathy, and LQTS were the most common diseases in pediatric ICD recipients.33,34 In both studies, about half the ICDs had been placed for primary prevention.
Pediatric sudden cardiac death (SCD) constitutes a small fraction of the total SCD population. However, the cumulative lifetime risk of SCD in high-risk young patients makes the ICD an excellent option in this patient population. Moreover, long-term antiarrhythymic therapy may be unreliable because of significant toxicity and low compliance rates, which decrease its therapeutic efficacy. Prospective identification of high-risk young patients is very important, since a very low percentage of children resuscitated from out-of-hospital cardiac arrest survive to hospital discharge.35
The etiologies for sudden cardiac arrest (SCA) or SCD in young patients are congenital heart disease, cardiomyopathies, and inherited arrhythmia syndromes.36 Small nonrandomized studies support the recommendation that young patients who have been resuscitated from SCA should undergo implantation of an ICD.36–38 Other class I indications include spontaneous sustained VT or unexplained syncope in a patient with congenital heart disease with hemodynamically significant VT induced on electrophysiological study (EPS). It is important to exclude hemodynamic abnormalities that can be surgically corrected, other treatable causes for syncope such as bradycardia or Wolff-Parkinson-White (WPW) syndrome, or both before proceeding with ICD implantation.36
The recommendations for device therapy for primary prevention of SCD are based on limited clinical experience, general consensus of experts, and extrapolation of adult studies. The risk of SCD is not the same at all ages. For example, individuals with LQTS are more likely to have torsades de pointes and SCD in adolescence than in childhood. Certain factors used to risk-stratify adults, including markedly prolonged QTc values or severe left ventricular outflow tract (LVOT) obstructions in hypertrophic cardiomyopathy (HCM), are applicable across a range of ages.39
In the patient population with congenital heart disease, the heterogeneity of the substrate and the lack of prospective studies prevent the formulation of any generalized strategy for risk stratification in postoperative patients to guide decisions for ICD implantation. Although several attempts have been made to identify the risk factors in individual anatomic entities, most commonly in tetralogy of Fallot, no absolute guidelines exist. These issues are discussed in the chapters on arrhythmias in congenital heart disease. One large retrospective study reviewed patients with tetralogy of Fallot who underwent ICD implantation (68 for primary prevention and 53 for secondary prevention) and found that higher left ventricular end-diastolic pressure and nonsustained VT were independent predictors of appropriate ICD shocks.40
Similar studies have been undertaken in other diseases with an increased risk of SCD and arrhythmias such as D-transposition of the great vessels after intra-atrial repairs.2,41 Presently, no good predictive factors are available to identify patients with congenital heart disease who would benefit from a primary prevention strategy.
Technical Challenges
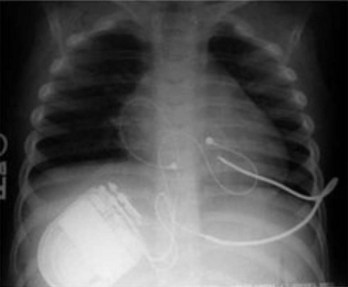
Novel approaches to ICD implantation in children and patients with congenital heart disease have been developed in response to the problems seen with standard epicardial ICD systems. These newer approaches do not incorporate trans-venous shocking coils or epicardial patches and still provide acceptable defibrillation thresholds. Several groups have reported the use of subcutaneous arrays or coils alone (that were initially approved for adjunctive use to lower defibrillation thresholds) with an epicardial or transvenous lead for pacing and sensing (Figure 77-4).42,43
ICD systems that use entirely subcutaneous sensing and defibrillation leads are currently being developed.44 These systems have clear advantages for pediatric patients because they can be placed without any intravascular or intracardiac hardware. However, these ICDs have several disadvantages. These “leadless” ICDs would not be able to provide pacing during bradycardia. Sensing would not be as precise without a dedicated bipolar intracardiac lead, which results in a decreased ability to identify sinus tachycardia and supraventricular arrhythmias and possibly an increased incidence of inappropriate shocks.
Implantable Cardioverter-Defibrillator Programming and Follow-up
Children should be monitored closely after ICD implantation to ensure optimal function of the system during periods of growth and as activity and lifestyle changes occur through the years. Despite careful attention to technical and programming factors at implantation, the risk of inappropriate shocks is significantly higher in younger patients, with reports of inappropriate shocks in 14% to 25% at mid-term follow-up.34,36,40,45 Patients are typically seen every 6 months for an in-office interrogation, with trans-telephonic transmissions between visits three or more additional times each year, depending on the age of the generator battery.
Supraventricular Arrhythmias
Supraventricular tachycardia (SVT) is common in pediatric ICD recipients and is another source of inappropriate shocks. In one study, 15% of pediatric ICD patients received an inappropriate shock for SVT.46 By adding a second zone for VT, the programmer may activate “SVT discriminators,” which are features designed to withhold therapy for sinus tachycardia and supraventricular arrhythmias even when heart rates exceed the threshold for the VT zone. In addition, the programmer can select ATP as the first-line therapy, which will defer defibrillation in that zone. ATP has been shown in large adult trials (Pravastatin or Atorvastatin Evaluation and Infection Therapy [PROVE-IT] and Pacing Fast VT Reduces Shock Therapies [PainFREE Rx]) to be a safe and effective way to terminate ventricular arrhythmias and results in a significant decrease in the number of “appropriate” shocks in primary and secondary prevention populations.47,48 In addition, SVT episodes that are misclassified as VT may be terminated painlessly with ATP (Figure 77-5).
Lead Fracture
Epicardial ICD and pacemaker leads have higher failure rates compared with their transvenous counterparts, and some studies have shown that pediatric patients have higher lead fracture rates than adults.20,33 Lead fracture is a leading cause of inappropriate shocks, and it is important to closely follow lead performance with in-office and transtelephonic transmissions.
Electrical Storm
The term electrical storm indicates a state of cardiac electrical instability manifested by several episodes of ventricular tachyarrhythmias within a short time.49 For patients with an ICD, electrical storm is often defined by the occurrence of two to three VT detections with therapy within a 24-hour period. This occurred in 5% of the patients in one large multi-center pediatric ICD study.33 Electrical storm is a particular concern in pediatric patients with catecholaminergic polymorphic VT (CPVT) and LQTS because the pain of subsequent shocks leads to sympathetic surge and continued arrhythmias. In patients with Brugada syndrome, VT storm has been successfully treated with isoproterenol infusion, oral quinidine, or both.50
Cardiac Resynchronization Therapy
CRT has been shown to be an effective intervention in adult patients who do not improve after optimal medical therapy.51 The initial adult trials showed that prolonged QRS duration and decreased left ventricular ejection fraction (LVEF) predicted which patients would respond to CRT.52,53 The value of CRT is less well established in pediatric patients and those with congenital heart disease. Many pediatric patients with heart failure have systemic right ventricles or right bundle branch block (RBBB) and right ventricular dysfunction rather than left bundle branch block (LBBB) and left ventricular dysfunction typical of adult CRT responders.54
Some data on CRT efficacy in pediatric patients and those with CHD are now available. Small studies have demonstrated the acute hemodynamic benefits of CRT after cardiac surgery, and three larger studies have now been published on this topic.55,56
A single pediatric center (Boston Children’s Hospital) retrospectively reviewed 60 patients implanted over a 5-year period with a mean follow-up close to 1 year.57 Overall, 77% had congenital heart disease, and 23% had dilated cardiomyopathy. That study reported an improvement in functional status in 87% of patients. The authors concluded that CRT is acutely beneficial in children and in patients with congenital heart disease, but implantation required unconventional approaches, often using the pericardial route.
A larger multi-center retrospective study evaluated 103 patients with short-term follow-up (mean, 4 months).58 In that study, 71% had congenital heart disease, 16% had cardiomyopathy, and 13% had complete congenital AV block. A significant improvement was observed in the mean ejection fraction overall, and 3 of 18 patients listed for transplantation improved enough to be removed from the list.
A second European multi-center retrospective study looked at 109 patients with a longer follow-up time (mean, 7.5 months).59 That study had 80% with congenital heart disease, 11% with congenital AV block, and 9% with dilated cardiomyopathy. Overall, 77% of the patients in that study had ventricular dyssynchrony associated with pacing. The authors reported an overall improvement in ejection fraction as well as New York Heart Association (NYHA) class.
Congestive heart failure and dilated cardiomyopathy occur in a small but significant subset of children after chronic right ventricular pacing.60 In children with congenital heart block related to maternal lupus antibodies, it is debated whether dilated cardiomyopathy develops as a consequence of chronic right ventricular pacing or as a natural progression of that disease.61 Heart failure associated with chronic ventricular pacing appears to be the strongest indication for CRT in pediatric and congenital heart disease patients.59,61 In the European study, patients with systemic left ventricular dyssynchrony and pacing-associated ventricular dyssynchrony demonstrated major clinical improvement and reverse left ventricular remodeling.
The second large group of patients in these studies had CHD with systemic right ventricles, wide QRS complexes, mechanical dyssynchrony, and heart failure. Results varied, and in the Boston series, patients with systemic right ventricle and single-ventricle physiology fared much worse compared with those with systemic left ventricles.57 The first multi-center study showed that patients with systemic right ventricles responded in a similar manner to those with systemic left ventricles.58 This was in contrast to the European multi-center experience, which found that the presence of a systemic left ventricle was the strongest multivariable predictor of improvement.59
Presently, the best candidates for CRT appear to be patients with dilated cardiomyopathy, left ventricular dysfunction, and LBBB (similar to adult criteria), or congenital AV block with dyssynchrony in the setting of chronic right ventricular pacing.59,61 Further studies are needed to determine the role of CRT in patients with systemic right ventricular failure and also in patients with single-ventricle physiology.
Long-Term Outcomes of Pacemaker and Implantable Cardioverter-Defibrillator Leads
Lead performance remains an ongoing issue of concern in young patients, especially in the era of increased need for lead extractions using multiple modalities such as radiofrequency or laser energy.62 Several studies have examined lead performance in pediatric patients in an attempt to identify factors associated with lead failure.20,22,63,64 The principal factor appears to be epicardial lead location. The 2-year survival rates for epicardial leads are relatively constant in multiple studies (84% to 95%) and do not vary much from that of transvenous leads (72% to 97%).20,64 The situation dramatically changes at the 5-year follow-up with 85% survival for transvenous leads and only 58% survival for steroid-eluting epicardial leads.20 Patient factors, which may be associated with early lead failure, include younger age at implantation and the presence of congenital heart disease.20,22
Lead Extraction in Pediatric Patients
With improved surgical techniques, pediatric patients and those with congenital heart disease have an increasingly positive prognosis and prolonged life expectancy. Consequently, lead failure is encountered more often and is an inevitable complication in many patients. Complete guidelines and indication for lead extraction in adults have been published and generally apply to pediatric patients as well.65 Some of the more common indications for extraction are lead infection and venous thrombosis that precludes implantation of necessary additional leads. For pediatric patients expected to live for decades, some recommend extraction of all inactive or failed transvenous leads to reduce the lifelong risk of vascular complications and also because the risks of extraction-related complications are higher with older leads.62,66
Lead extraction is not without risk. Data on adults suggest that major complications occur in 1% of procedures and include respiratory arrest, stroke, cardiac or vascular avulsion leading to hemorrhage, and death. Venous thrombosis is a concern, and the risk of thrombosis is increased in smaller patients because the transvenous lead occupies a larger proportion of the venous diameter.27 In the case of defibrillator leads, in particular, fibrosis can form on the lead body and at the shocking coils, making extraction more difficult and predisposing to tearing of the blood vessel where the lead adheres to the SVC. Some newer ICD leads have a polytetrafluoroethylene coating, which may prevent tissue ingrowth and improve extractability.67 Another strategy is to implant ICD leads with no SVC coil and a single shocking coil in the right ventricle.
Answer
Answer
Key References
Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.
Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51:1685-1691.
Cecchin F, Atallah J, Walsh EP, Triedman JK, Alexander ME, Berul CI. Lead extraction in pediatric and congenital heart disease patients. Circ Arrhythm Electrophysiol. 2010;3:437-444.
Cohen MI, Bush DM, Vetter VL, et al. Permanent epicardial pacing in pediatric patients: Seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585-2590.
Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: An international multicenter study. J Am Coll Cardiol. 2005;46:2277-2283.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1-e62.
Gross GJ, Chiu CC, Hamilton RM, Kirsh JA, Stephenson EA. Natural history of postoperative heart block in congenital heart disease: Implications for pacing intervention. Heart Rhythm. 2006;3:601-604.
Janousek J, Gebauer RA, Abdul-Khaliq H, et al. Cardiac resynchronisation therapy in paediatric and congenital heart disease: Differential effects in various anatomical and functional substrates. Heart. 2009;95:1165-1171.
Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363-370.
Pires LA, Abraham WT, Young JB, Johnson KM. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: Results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. Am Heart J. 2006;151:837-843.
Stephenson EA, Batra AS, Knilans TK, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41-46.
Stephenson EA, Casavant D, Tuzi J, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92:871-876.
Villain E. Indications for pacing in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S17-S20.
Villain E, Coastedoat-Chalumeau N, Marijon E, Boudjemline Y, Piette J-C, Bonnet D. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48:1682-1687.
Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534-545.
1 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-e62.
2 Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534-545.
3 Cohen MI, Rhodes LA, Wernovsky G, et al. Atrial pacing: An alternative treatment for protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 2001;121:582-583.
4 Benson DW, Wang DW, Dyment M, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest. 2003;112:1019-1028.
5 Kelly AM, Porter C-BJ, McGoon MD, et al. Breath-holding spells associated with significant bradycardia: Successful treatment with permanent pacemaker implantation. Pediatrics. 2001;108:698-702.
6 Gelatt M, Hamilton M, Robert M, et al. Arrhythmia and mortality after the mustard procedure: A 30-year single-center experience. J Am Coll Cardiol. 1997;29:194-201.
7 Rhodes LA, Walsh EP, Gamble WJ, et al. Benefits and potential risks of atrial antitachycardia pacing after repair of congenital heart disease. Pacing Clin Electrophysiol. 1995;18:1005-1016.
8 Stephenson EA, Casavant D, Tuzi J, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92:871-876.
9 Lillehei CW, Sellers RD, Bonnabeau RC, Eliot RS. Chronic postsurgical complete heart block, with particular reference to prognosis, management, and a new P-wave pacemaker. J Thorac Cardiovasc Surg. 1963;46:436-456.
10 Gross GJ, Chiu CC, Hamilton RM, et al. Natural history of postoperative heart block in congenital heart disease: Implications for pacing intervention. Heart Rhythm. 2006;3:601-604.
11 Banks MA, Jenson J, Kugler JD. Late development of atrioventricular block after congenital heart surgery in Down syndrome. Am J Cardiol. 2001;88:86-89.
12 Villain E, Ouarda F, Beyler C, et al. Predictive factors for late complete atrio-ventricular block after surgical treatment for congenital cardiopathy [translation]. Arch Mal Coeur Vaiss. 2003;96:495-498.
13 Silvetti MS, Drago F, Grutter G, et al. Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace. 2006;8:530-536.
14 Sachweh JS, Vazquez-Jimenez JF, Schöndube FA, et al. Twenty years experience with pediatric pacing: Epicardial and transvenous stimulation. Eur J Cardio-Thorac Surg. 2000;17:455-461.
15 Villain E, Coastedoat-Chalumeau N, Marijon E, et al. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48:1682-1687.
16 Moss A, Liu J, Gottlieb S, et al. Efficacy of permanent pacing in the management of high-risk patients with long QT syndrome. Circulation. 1991;84:1524-1529.
17 Costello JM, Alexander ME, Greco KM, et al. Lyme carditis in children: Presentation, predictive factors, and clinical course. Pediatrics. 2009;123:e835-e841.
18 Silver E, Pass RH, Kaufman S, et al. Complete heart block due to Lyme carditis in two pediatric patients and a review of the literature, Congenital. Heart Dis. 2007;2:338-341.
19 Gillette PC. Implantation technique for pediatric cardiac pacing. In: Gillette PC, editor. Pediatric cardiac pacing. Armonk, NY: Futura, 1995.
20 Fortescue E, Berul C, Cecchin F, et al. Comparison of modern steroid-eluting epicardial and thin transvenous pacemaker leads in pediatric and congenital heart disease patients. J Interv Card Electrophysiol. 2005;14(1):27-36.
21 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: A multicenter study. Circulation. 2006;113:2391-2397.
22 Spotnitz HM, Spotnitz MD, Weinberg AD, et al. Decreased endocardial lead survival in infants. In: Tse HF, Lee KL, Lau CP, editors. Clinical cardiac pacing and electrophysiology. Bologna, Italy: Monduzzi Editore, 2003.
23 Campbell RM, Raviele AA, Hulse EJ, et al. Experience with a low profile bipolar, active fixation pacing lead in pediatric patients. Pacing Clin Electrophysiol. 1999;22:1152-1157.
24 Gheissari A, Hordof AJ, Spotnitz HM. Transvenous pacemakers in children: Relation of lead length to anticipated growth. Ann Thorac Surg. 1991;52:118-121.
25 Silvetti MS, Drago F, Marcora S, Ravà L. Outcome of single-chamber, ventricular pacemakers with transvenous leads implanted in children. Europace. 2007;9:894-899.
26 Esposito M, Kennergren C, Holmstrom N, et al. Morphologic and immunohistochemical observations of tissues surrounding retrieved transvenous pacemaker leads. J Biomed Mater Res. 2002;63:548-558.
27 Figa FH, McCrindle BW, Bigras J-L, et al. Risk factors for venous obstruction in children with transvenous pacing leads. Pacing Clin Electrophysiol. 1997;20:1902-1909.
28 Gillette PC, Edgerton J, Kratz J, Zeigler V. The subpectoral pocket: The preferred implant site for pediatric pacemakers. Pacing Clin Electrophysiol. 1991;14:1089-1092.
29 Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices–summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). J Am Coll Cardiol. 2002;40:1703-1719.
30 Emmel M, Sreeram N, Brockmeier K, Bennink G. Superior vena cava stenting and transvenous pacemaker implantation (stent and pace) after the Mustard operation. Clin Res Cardiol. 2007;96(1):17-22.
31 Takahashi K, Cecchin F, Fortescue E, et al. Permanent atrial pacing lead implant route after Fontan operation. Pacing Clin Electrophysiol. 2009;32:779-785.
32 Batra A. Patient-activated antitachycardia pacing to terminate atrial tachycardias with 1 : 1 atrioventricular conduction in congenital heart disease. Pediatr Cardiol. 2008;29(4):851-854.
33 Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51:1685-1691.
34 Von Bergen NH, Atkins DL, Dick MII, et al. Multicenter study of the effectiveness of implantable cardioverter defibrillators in children and young adults with heart disease. Pediatr Cardiol. 2011;32(4):399-405.
35 Eisenberg M, Bergner L, Hallstrom A. Epidemiology of cardiac arrest and resuscitation in children. Ann Emerg Med. 1983;12:672-674.
36 Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.
37 Silka MJ, Kron J, Dunnigan A, Dick MII. Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. The Pediatric Electrophysiology Society. Circulation. 1993;87:800-807.
38 Hamilton RM, Dorian P, Gow RM, Williams WG. Five-year experience with implantable defibrillators in children. Am J Cardiol. 1996;77:524-526.
39 Maron BJ. Risk stratification and role of implantable defibrillators for prevention of sudden death in patients with hypertrophic cardiomyopathy. Circ J. 2010;74:2271-2282.
40 Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363-370.
41 Kammeraad JAE, van Deurzen CHM, Sreeram N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44:1095-1102.
42 Kaltman JR, Gaynor JW, Rhodes LA, et al. Subcutaneous array with active can implantable cardioverter defibrillator configuration: A follow-up study. Congenit Heart Dis. 2007;2:125-129.
43 Stephenson EA, Batra AS, Knilans TK, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41-46.
44 Saksena S. The leadless defibrillator or the return of the subcutaneous electrode: Episode III in the ICD saga? J Interv Card Electrophysiol. 2005;13:179-180.
45 Bonney WJ, Spotnitz HM, Liberman L, et al. Survival of transvenous ICD leads in young patients. Pacing Clin Electrophysiol. 2010;33:186-191.
46 Love BA, Barrett KS, Alexander ME, et al. Supraventricular arrhythmias in children and young adults with implantable cardioverter defibrillators. J Cardiovasc Electrophysiol. 2001;12:1097-1101.
47 Wathen MS, DeGroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591-2596.
48 Saeed M, Neason CG, Razavi M, et al. Programming antitachycardia pacing for primary prevention in patients with implantable cardioverter defibrillators: Results from the PROVE trial. J Cardiovasc Electrophysiol. 2010;21:1349-1354.
49 Israel CW, Barold SS. Electrical storm in patients with an implanted defibrillator: A matter of definition. Ann Noninvasive Electrocardiol. 2007;12:375-382.
50 Jongman JK, Jepkes-Bruin N, Ramdat Misier AR, et al. Electrical storms in Brugada syndrome successfully treated with isoproterenol infusion and quinidine orally. Neth Heart J. 2007;15:151-155.
51 Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873-880.
52 Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845-1853.
53 Pires LA, Abraham WT, Young JB, Johnson KM. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: Results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. Am Heart J. 2006;151:837-843.
54 Greene EA, Berul CI. Pacing treatment for dilated cardiomyopathy: Optimization of resynchronization pacing in pediatrics. Curr Opin Cardiol. 2010;25:95-101.
55 Bacha EA, Zimmerman FJ, Mor-Avi V, et al. Ventricular resynchronization by multisite pacing improves myocardial performance in the postoperative single-ventricle patient. Ann Thorac Surg. 2004;78:1678-1683.
56 Blom NA, Bax JJ, Ottenkamp J, Schalij MJ. Transvenous biventricular pacing in a child after congenital heart surgery as an alternative therapy for congestive heart failure. J Cardiovasc Electrophysiol. 2003;14:1110-1112.
57 Cecchin F, Frangini PA, Brown DW, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: Five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20:58-65.
58 Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: An international multicenter study. J Am Coll Cardiol. 2005;46:2277-2283.
59 Janousek J, Gebauer RA, Abdul-Khaliq H, et al. Cardiac resynchronisation therapy in paediatric and congenital heart disease: Differential effects in various anatomical and functional substrates. Heart. 2009;95:1165-1171.
60 Moak JP, Hasbani K, Ramwell C, et al. Dilated cardiomyopathy following right ventricular pacing for AV block in young patients: Resolution after upgrading to biventricular pacing systems. J Cardiovasc Electrophysiol. 2006;17:1068-1071.
61 Villain E. Indications for pacing in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S17-S20.
62 Cecchin F, Atallah J, Walsh EP, et al. Lead extraction in pediatric and congenital heart disease patients. Circ Arrhythm Electrophysiol. 2010;3:437-444.
63 Stojanov PL, Savic DV, Zivkovic MB, Calovic ZR. Permanent endovenous pediatric pacing: Absence of lead failure—20 years follow-up study. Pacing Clin Electrophysiol. 2008;31:1100-1107.
64 Cohen MI, Bush DM, Vetter VL, et al. Permanent epicardial pacing in pediatric patients: Seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585-2590.
65 Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6:1085-1104.
66 Zartner PA, Wiebe W, Toussaint-Goetz N, Schneider MB. Lead removal in young patients in view of lifelong pacing. Europace. 2010;12:714-718.
67 Di Cori A, Bongiorni MG, Zucchelli G, et al. Transvenous extraction performance of expanded polytetrafluoroethylene covered ICD leads in comparison to traditional ICD leads in humans. Pacing Clin Electrophysiol. 2010;33:1376-1381.