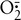Oxygen, Helium, and Nitric Oxide Therapy
I General Characteristics of Oxygen
E Density at standard temperature and pressure: 1.43 g/L
F Boiling point at 1 atm: ° C (—297.4° F)
G Melting point at 1 atm: −216.6° C (—361.1° F)
H Critical temperature: −118.4° C (—181.1° F)
I Critical pressure: 736.9 pounds per square inch absolute (psia)
J Triple point: −218.7° C (—361.89° F) at 0.2321 psia
K Forms oxides with all elements except inert gases
L Constitutes approximately 20.95% of atmosphere
M Used at the cellular level as the final electron acceptor in electron transport chain located in mitochondria of cell
II Hypoxia: Inadequate Quantities of Oxygen at the Tissue Level
A Decreased carrying capacity of blood for oxygen (anemic hypoxia)
B Decreased cardiac output, resulting in increased systemic transit time (stagnant hypoxia)
C Inability of tissue to use available oxygen (histotoxic hypoxia)
D Decrease in diffusion of oxygen across alveolar capillary membrane (hypoxemic hypoxia)
III Hypoxemia: Inadequate Quantities of Oxygen in the Blood
1. Normal: Pao2 80 to 100 mm Hg
2. Mild hypoxemia: Pao2 <80 mm Hg
3. Severe hypoxemia: Pao2 <60 mm Hg
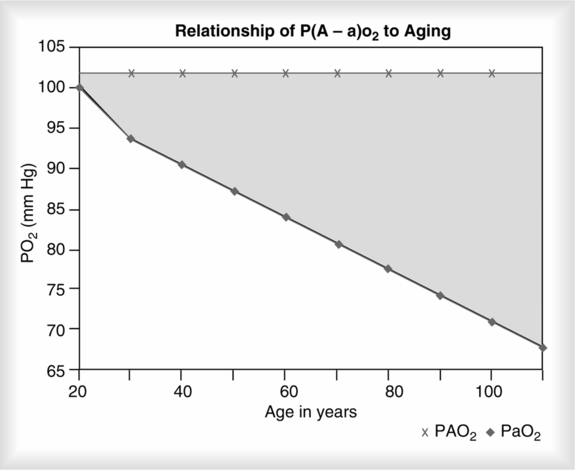
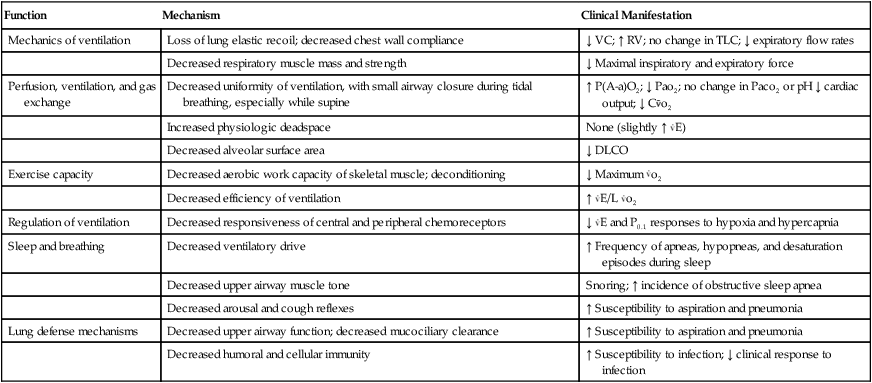
4. The lower level of acceptable Pao2 decreases with age because the normal aging process of the lung affects respiratory functions (Table 34-1).
TABLE 34-1
Changes in Respiratory Function with Aging
| Function | Mechanism | Clinical Manifestation |
| Mechanics of ventilation | Loss of lung elastic recoil; decreased chest wall compliance | ↓ VC; ↑ RV; no change in TLC; ↓ expiratory flow rates |
| Decreased respiratory muscle mass and strength | ↓ Maximal inspiratory and expiratory force | |
| Perfusion, ventilation, and gas exchange | Decreased uniformity of ventilation, with small airway closure during tidal breathing, especially while supine | ↑ P(A-a)O2; ↓ Pao2; no change in Paco2 or pH ↓ cardiac output; ↓ C o2 o2 |
| Increased physiologic deadspace | None (slightly ↑  E) E) |
|
| Decreased alveolar surface area | ↓ DLCO | |
| Exercise capacity | Decreased aerobic work capacity of skeletal muscle; deconditioning | ↓ Maximum  o2 o2 |
| Decreased efficiency of ventilation | ↑  E/L E/L  o2 o2 |
|
| Regulation of ventilation | Decreased responsiveness of central and peripheral chemoreceptors | ↓  E and P0.1 responses to hypoxia and hypercapnia E and P0.1 responses to hypoxia and hypercapnia |
| Sleep and breathing | Decreased ventilatory drive | ↑ Frequency of apneas, hypopneas, and desaturation episodes during sleep |
| Decreased upper airway muscle tone | Snoring; ↑ incidence of obstructive sleep apnea | |
| Decreased arousal and cough reflexes | ↑ Susceptibility to aspiration and pneumonia | |
| Lung defense mechanisms | Decreased upper airway function; decreased mucociliary clearance | ↑ Susceptibility to aspiration and pneumonia |
| Decreased humoral and cellular immunity | ↑ Susceptibility to infection; ↓ clinical response to infection |
From Pierson DJ, Kacmarek RM: Foundations of Respiratory Care. New York, Churchill Livingstone, 1993. Churchill Livingstone
a. The lower limit of “normal” Pao2 is decreased approximately 4 mm Hg per decade in later life.
b. Because the lung changes with age there is a progressive increase in the alveolar-arterial O2 partial pressure difference (Figure 34-1).
c. Acceptable lower limits of Pao2 for older adults can be estimated using the following formula.
B Causes of hypoxemia (see Chapters 8 and 15)
C Responsive versus refractory hypoxemia
1. Refractory hypoxemia is hypoxemia demonstrating a negligible increase in the Pao2 with the application of an acceptable level of oxygen.
a. If the FIO2 is >0.40 to 0.50 and the Pao2 is <60 mm Hg (Sao2 <90%), the hypoxemia is refractory.
b. If a 0.20 increase in the FIO2 results in a <10-mm Hg increase in the Pao2, the hypoxemia is refractory.
2. Responsive hypoxemia is hypoxemia that demonstrates a significant response to an increase in the FIO2.
D Clinical manifestations of hypoxemia
1. Rapid respiratory rate and/or large tidal volume (Vt)
3. Tachycardia and hypertension
4. Peripheral vasoconstriction with moderate to severe hypoxemia
5. Constriction of pulmonary vascular bed leading to pulmonary hypertension
6. Development of cyanosis if hypoxemia is severe
IV Indications for Oxygen Therapy, Modified from AARC Clinical Practice Guidelines on Oxygen Therapy, 2002.
V General Goals of Oxygen Therapy
A Maintain adequate tissue oxygenation and minimize cardiopulmonary work
1. Increasing alveolar Po2 increases pressure gradient for oxygen diffusion into bloodstream. This may increase Pao2.
2. An increase in Pao2 may reduce stimulation of peripheral chemoreceptors and reduce work of breathing.
3. Oxygen therapy may correct hypoxemia and decrease the stimulus to increase cardiac output.
A Retinopathy of prematurity (ROP) (see Chapter 27)
1. Occurs primarily in premature infants (<34 weeks gestation) with incomplete vascularization of the retina
2. Increased oxygen exposure (Sao2 96% to 99%) is just one of many factors involved in the pathogenesis of ROP.
1. A series of reversible pathophysiologic inflammatory changes of lung tissue that can produce a progressive and lethal form of lung injury similar to acute respiratory distress syndrome (ARDS)
2. Free radical theory of oxygen toxicity
a. The following free radicals of oxygen can be produced at the cellular level.
b. The following enzymes are important cellular defenses against oxygen free radicals.
(1) Superoxide dismutase (SOD), which converts 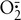 to O2
to O2
(2) Catalase (CAT), which converts H2O2 to H2O and O2
(3) Additional intracellular antioxidants that provide defense against oxygen free radicals include
c. The quantity of oxygen free radicals depends on Pao2. The greater the Pao2, the greater the quantity of free radicals.
3. Pathophysiology of oxygen toxicity
a. Cellular susceptibility to hyperoxia (100% O2)
(1) Pulmonary capillary endothelium (most susceptible)
(2) Alveolar type I epithelial cells
b. With continued exposure to 100% O2, type I alveolar cells are destroyed and replaced by type II cells.
c. Early or acute exudative phase is characterized by perivascular, interstitial, and intraalveolar edema with destruction and necrosis of endothelial cells. Alveolar congestion and fibrinous exudation (hyaline membrane) develop.
d. Late or chronic proliferative phase is characterized by a progressive reabsorption of the exudate and a thickening of the alveolar septa.
4. Susceptibility and risk factors associated with the development of oxygen toxicity
a. The balance between oxidant and antioxidant activity determines the severity of tissue injury.
b. Exposure to FIO2 levels >0.40 for lengthy periods
c. Previous development of severe acute pulmonary disease, which decreases the risk of toxicity. The acute disease is believed to induce the production of SOD and glutathione, thus reducing the oxygen free radical levels.
5. Prevention: Judicious use of oxygen therapy. Use only enough to maintain adequate tissue oxygenation.
a. When possible limit exposure to 100% oxygen to <24 hours. Decrease to level maintaining acceptable Pao2 >60 mm Hg as soon as possible.
6. Treatment: Appropriate use of positive end-expiratory pressure therapy, diuretics, and fluids while reducing the FIO2 as soon as possible.
C Oxygen-induced hypoventilation
1. This is infrequently observed in patients with chronic CO2 retention or central nervous system depression (see Chapter 20).
2. The increased Pao2 decreases or eliminates the hypoxic drive, inducing greater levels of hypoventilation. Although rare it can be potentially life threatening.
3. Intermittent use of oxygen therapy may cause arterial Po2 to decrease below pretreatment levels.
4. If oxygen is indicated it should never be withheld because of the risk of ventilatory depression.
D Absorption atelectasis (Figure 34-2)
1. Nitrogen is metabolically inactive and constitutes 80% of alveolar gas. Nitrogen is essential to maintain alveolar stability.
2. Administration of high FIO2 (>0.50) washes out nitrogen.
3. In poorly ventilated alveoli more oxygen is removed per unit time by the perfused blood than can be replaced by normal ventilation.
4. This results in a decrease in alveolar size.
5. As alveoli reach their critical volume, collapse and atelectasis occur.
6. This commonly occurs in patients with low Vt or poor distribution of ventilation because of partial airway obstruction.
A Delivery systems generally are divided into two categories: high flow and low flow (Table 34-2).
TABLE 34-2
Classification of Oxygen Delivery System
| Low-flow systems | Cannula |
| Simple mask | |
| Partial rebreathing mask | |
| Nonrebreathing mask* | |
| High-flow systems† | Venturi masks |
| Aerosol systems | |
| Large-volume humidifier systems |
*The disposable nonrebreathing mask is not tight fitting; thus entrainment of room air normally occurs.
†For a system to be considered high flow, it must provide ≥40 L/min flow.
1. The patient’s entire inspired gas volume is consistently and predictably delivered by the system.
2. To maintain a consistent FIO2 the apparatus flow must exceed the peak inspiratory flow of the patient.
3. Peak inspiratory flows are difficult to measure but may be approximated by delivering a total flow at least four times the patient’s measured minute volume (Ve).
a. Normal peak inspiratory flows are approximately four times the patient’s measured Ve.
b. This flow usually will provide adequate volume in the face of a changing ventilatory pattern.
a. Air entrainment masks: Deliver a specific FIO2 level up to 0.50 (Table 34-3; Figure 34-3).
TABLE 34-3
Entrainment Ratios and Outputs of Specific Air Entrainment Systems*
| System | FIO2 | Entrainment Ratio | Flow at which Operated | Total Flow (L/min) |
| Ventimasks | 0.24 | 1–25 | 4 | 104 |
| 0.28 | 1–10 | 4 | 44 | |
| 0.31 | 1–7 | 6 | 48 | |
| 0.35 | 1–5 | 8 | 48 | |
| 0.40 | 1–3 | 8 | 32 | |
| 0.50 | 1–1.7 | 12 | 32 | |
| Mechanical aerosol generators | 0.60 | 1–1 | 12 | 24 |
| 0.70 | 1–0.6 | 12 | 19 |
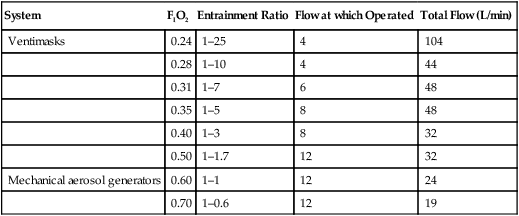
*Clinical trials indicate some variation in FIO2 levels provided by air entrainment masks.
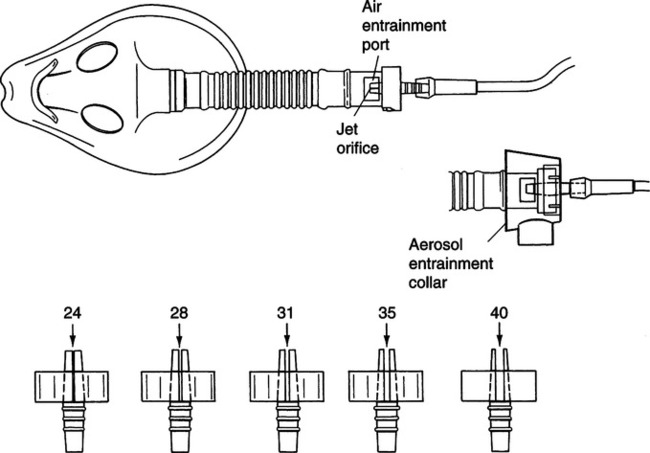
(1) Care should be taken to ensure the air entrainment mask provides sufficient flow to meet a patient’s need. This is especially true with masks delivering higher FIO2 levels.
b. Large-volume mechanical aerosol systems: Set up singly or in tandem to deliver high humidity along with a specific FIO2 level (see Table 34-3; Figure 34-4).
(1) FIO2 varies by amount of gas entrainment.
(2) Most nondisposable units have fixed air entrainment ports, whereas most disposable units offer a wide spectrum of available FIO2 levels.
(3) Some units adapt to heaters and provide temperature control and extra humidification.
(4) Always ensure sufficient flow, and if heated, unit should be monitored for temperature.
c. Wick-type passover humidification systems
(1) Volume and concentration of gas are determined by titration of compressed air and oxygen or the use of an oxygen blender (Figure 34-5).
(2) Virtually any FIO2 level is available.
(3) Virtually any flow is available.
(4) Systems are extremely versatile and may be applied to a patient via an aerosol mask, continuous positive airway pressure (CPAP)-type mask, or standard artificial airway attachment.
(5) Most systems are able to deliver controlled heated humidification for a wide range of flow rates.
d. Determinations of FIO2 with any system combining gas flows
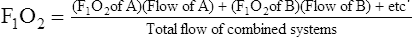 (2)
(2)e. High-flow systems can be attached to patients by a variety of devices in addition to the typical air entrainment mask (Figure 34-6).
1. The apparatus does not deliver the total  E.
E.
2. The FIO2 delivered to the patient depends on
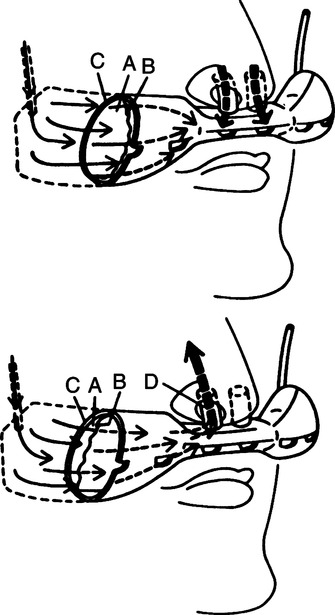
b. Patient anatomic reservoir (oral and nasal cavity)
(1) Normal anatomic reservoir in adults is approximately 50 ml.
(2) Normal end-expiratory pause may allow for filling of anatomic reservoir with 100% oxygen.
d. Patient respiratory rate, Vt, and  E
E
(1) The FIO2 delivered with any low-flow system is extremely variable and unpredictable.
(2) If the patient’s  E were to increase on a particular low-flow system, the FIO2 would decrease. The patient would entrain a larger percentage of room air in the
E were to increase on a particular low-flow system, the FIO2 would decrease. The patient would entrain a larger percentage of room air in the  E.
E.
(3) If the patient’s  E were to decrease on a particular low-flow system, the FIO2 would increase. The patient would entrain a smaller percentage of room air in the
E were to decrease on a particular low-flow system, the FIO2 would increase. The patient would entrain a smaller percentage of room air in the  E.
E.
e. An example of the calculations used to estimate the FIO2 delivered by a low-flow oxygen therapy system and the effect on the FIO2 when there is a change in the respiratory pattern can be seen in Table 34-4.
TABLE 34-4
Estimation of FIO2 from Low-Flow Systems
| Cannula | 6 L/min | VT | 500 ml | |
| Mechanical reservoir | None | I:E ratio | 1:2 | |
| Anatomic reservoir | 50 ml | Rate | 20/min | |
| 100% O2 provided per sec | 100 ml | Inspiratory time | 1 sec | |
| Volume inspired O2* | ||||
| Automatic reservoir | 50 ml | |||
| Flow/sec | 100 ml | |||
| Inspired room air* | 70 ml | |||
| O2 inspired | 220 ml | |||
 |
||||
| If VT is decreased to 250 ml | ||||
| Volume inspired O2 | ||||
| Anatomic reservoir | 50 ml | |||
| Flow/sec | 100 ml | |||
| Inspired room air† | 20 ml | |||
| O2 inspired | 170 ml | |||
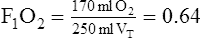 |
||||
| If VT increased to 1000 ml | ||||
| Volume inspired O2 | ||||
| Anatomic reservoir | 50 ml | |||
| Flow/sec | 100 ml | |||
| Inspired room air‡ | 170 ml | |||
| O2 inspired | 320 ml | |||
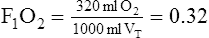 |
*Because 150 ml of 100% O2 is inspired, the remainder of VT is room air (350 ml), 20% of 350 ml = 70 ml – amount of O2 in room air that is inspired.
f. It must be kept in mind that the FIO2 listed for each low-flow system is purely speculative and that the FIO2 is totally dependent on the patient’s ventilatory pattern. The values provided should be used only as gross guidelines rather than as the exact FIO2 delivered.
(1) Calculations similar to those in Table 34-4 have been used to determine the approximate FIO2 levels for various low-flow systems (Figure 34-7). The approximate FIO2 at specific flows with an oxygen cannula are:
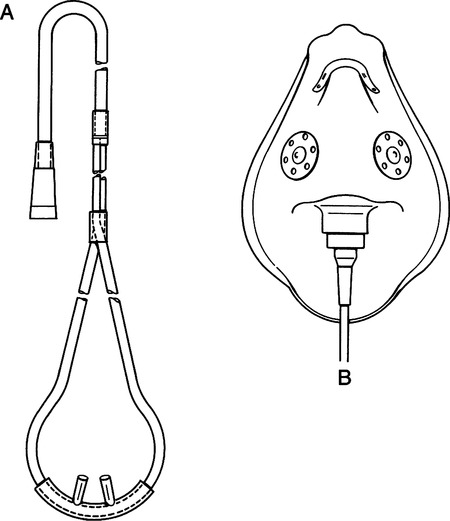
3. Simple oxygen mask: Generally a minimum flow of 5 L/min should be used to flush the mask of carbon dioxide and prevent rebreathing. FIO2 levels vary greatly depending on the ventilatory pattern. Flows between 5 and 12 L/min will generally provide an FIO2 between 0.3 and 0.6 (see Figure 34-7).
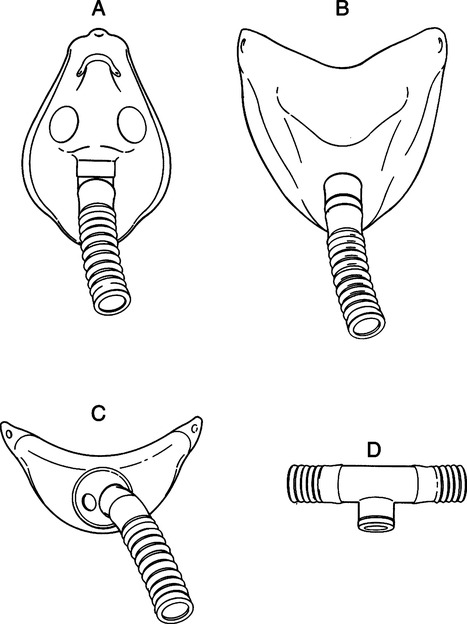
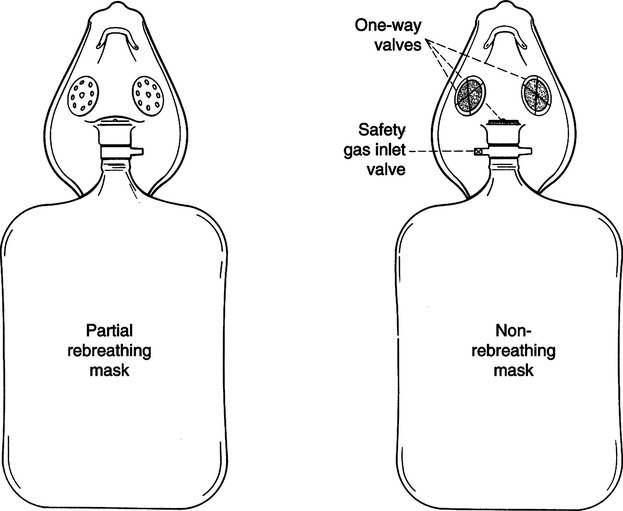
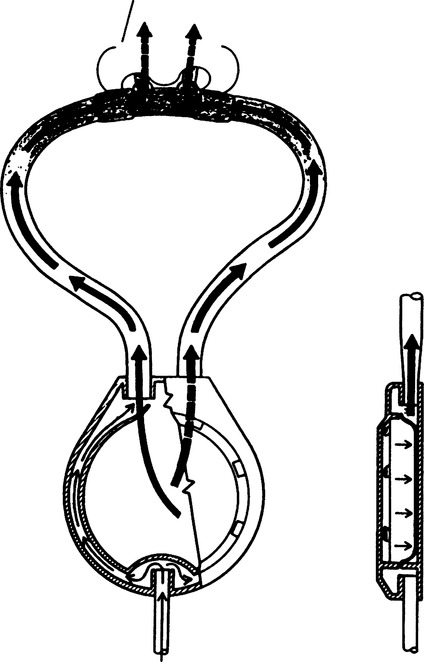
4. Partial rebreathing mask uses a simple mask connected to a bag reservoir with no valve between the bag and mask (Figure 34-8).
a. Called a partial rebreathing mask because the first one third of exhalation enters the bag, mixes with source oxygen, and is consumed during the next inhalation. Because it comes from anatomic deadspace it is primarily oxygen with a negligible amount of carbon dioxide.
b. Flow must be sufficient to prevent the bag from deflating no more than one third to one half during inspiration to ensure that no CO2 accumulates during exhalation. This is usually a minimum of 8 L/min flow.
c. Flows of 8 to 15 L/min will deliver an FIO2 concentration between 0.35 and 0.60 or more, depending on the patient’s ventilatory pattern.
5. Nonrebreathing mask: Variation of a partial rebreathing mask with a one-way valve between the mask and the reservoir bag, as well as a one-way valve over one or both exhalation ports on the mask body (see Figure 34-8).
a. Must have sufficient flow to prevent the reservoir bag from collapsing on inspiration.
b. If functioning properly, flows of 10 to 15 L/min will produce an FIO2 of 0.6 to 0.8 or more, depending on the patient’s ventilatory pattern.
6. Hi-Ox oxygen mask (Respironics Inc., Merryville, PA) is a variation of a nonrebreathing mask using a manifold and valve system to connect the reservoir bag and mask (Figure 34-9).
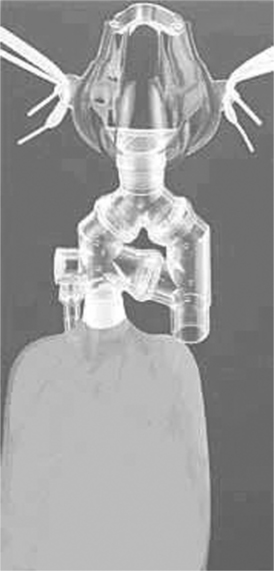
a. According to the manufacturer an FIO2 of ≥0.80 can be achieved at a flow of 8 L/min, depending on the patient’s ventilatory pattern (Vt cannot exceed 0.75 L).
b. As with other nonrebreathing masks the flow must be sufficient to prevent the bag from collapsing.
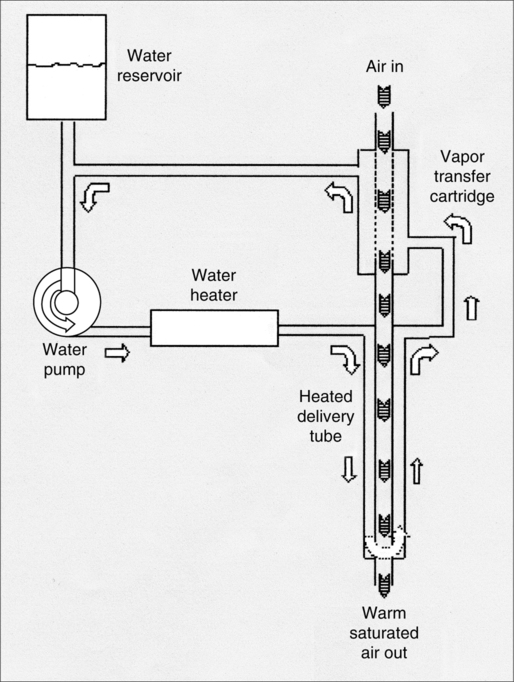
7. Vapotherm humidification device (Vapotherm Inc., Annapolis, MD) is a mechanical unit that provides high flow (up to 40 L/min) of heated humidified oxygen via nasal cannula, simple mask, or partial rebreathing mask (Figure 34-10).
D Criteria for use of high- and low-flow oxygen delivery systems
1. Whenever a consistent and predictable FIO2 is required, a high-flow system should be used.
2. A low-flow system provides relatively stable FIO2 levels if the patient’s
1. These devices either use a reservoir designed into an oxygen cannula system or provide pulsed flow of oxygen based on the patient demand.
2. Reservoir cannula (Figure 34-11)
a. This device incorporates two reservoir bags in the body of the cannula.
b. On exhalation the reservoir bags fill with oxygen.
c. Thus a larger volume of 100% oxygen is available on the next inspiration.
3. Pendant cannula (Figure 34-12)
a. It functions on the same principle as the reservoir cannula.
b. Its reservoir is located in the pendant setting on the patient’s chest.
c. It may reduce oxygen use from 50% to 75%.
4. Pulse dose oxygen or demand oxygen delivery system
a. These systems provide delivery of oxygen only during inspiration.
b. Negative pressure generated by the patient triggers gas delivery.
c. Two general types exist: Variable pulsed volume and variable ratio of pulsed breaths to no oxygen delivery breaths.
(1) Variable pulsed volume devices alter the volume of oxygen from approximately 10 to 40 ml/breath.
(2) Variable ratio devices vary delivery of a fixed volume every breath to a fixed volume every fourth breath.
d. Adequate oxygenation is maintained because only that gas at the lung parenchymal level participates in gas exchange.
e. Only the first one third of inspiration reaches the lung parenchyma.
f. These devices may conserve 50% to 75% of the oxygen used.
g. They are designed primarily for home use.
h. Their function with every patient should be carefully evaluated.
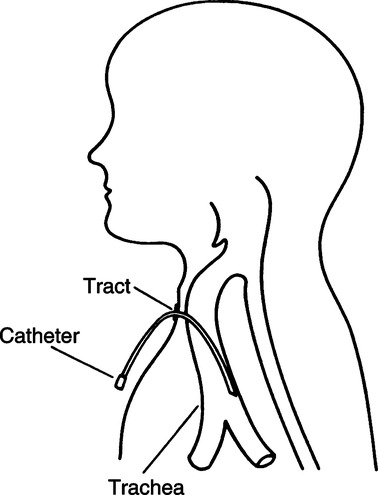
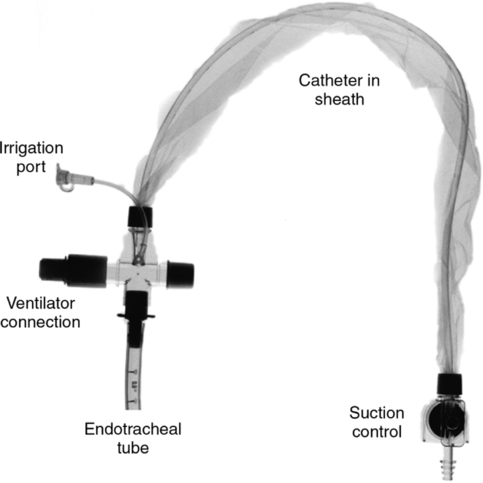
5. Transtracheal oxygen catheter (Figure 34-13)
a. With this system an 8-French catheter is inserted between the second and third tracheal rings and extended to approximately 2 cm above the carina.
b. Continuous delivery of oxygen is provided via the catheter.
c. Because oxygen is directly delivered into the trachea, oxygen use can be decreased by 50%.
d. In some patients refractory to oxygen therapy because of severe pulmonary fibrosis, transtracheal oxygen therapy has improved arterial oxygenation.
(1) Need for improved mobility
(2) Compliance with nasal cannula (poor)
(3) Complications with nasal cannula (high)
f. Generally it is used for long-term oxygen therapy in the home.
VIII Selection of Oxygen System for Adults (see Chapters 26, 27, and 29 for guidance regarding infants and children)
A Patients with artificial airways
1. Only high-flow systems are adaptable to artificial airways.
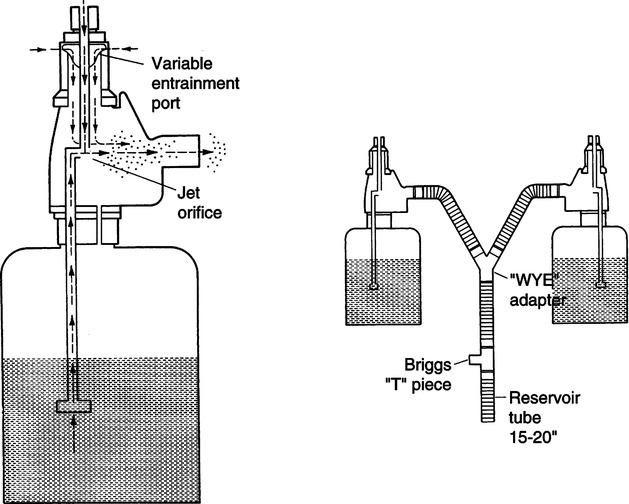
2. With an endotracheal tube a Briggs T piece can be used.
a. A Briggs T piece is used if a consistent FIO2 level is essential.
b. If humidity is the primary concern a tracheotomy mask or tracheotomy collar is more comfortable and produces less torque on the trachea.
c. At lower FIO2 levels (≤0.40) aerosol generators provide sufficient flow.
d. If the FIO2 level needed is >0.40, a wick-type large-volume passover humidifier system may be necessary, which can deliver high flows at high FIO2.
B Patients without artificial airways
1. Any system discussed may be used.
2. The low-flow system, especially the O2 cannula, is normally the best tolerated of all systems and has the best compliance. If it can provide adequate oxygenation it should be the first choice.
3. In the emergency room a simple oxygen mask or partial rebreathing mask should be used if the cannula does not provide sufficient FIO2.
4. In the recovery room an aerosol generator, normally unheated, with a face mask is usually sufficient.
A The use of 100% oxygen may result in
B Despite these possibilities short-term 100% oxygen should always be used during
X Monitoring of Oxygen Therapy
E It is important to evaluate the work of breathing and the work of the myocardium to determine the overall effectiveness of an increase in the FIO2. A minor change in the Po2 may be accompanied by a decrease in the work of breathing and the work of the myocardium, indicating the effectiveness of the FIO2 increase.
A General characteristics of nitric oxide (NO·)
8. Gaseous diatomic free radical
9. Universally present in nature
10. Short acting: Biologic half-life <5 seconds
11. Atmospheric concentration: 10 to 100 parts per billion (ppb); as by-product of combustion (Box 34-1)
12. NO· in the atmosphere rapidly changes to nitrogen dioxide, a toxic substance.
13. NO· binds to the iron atoms of the hemoglobin molecule.
14. NO· causes vascular smooth muscle relaxation.
15. NO· is present in cigarette smoke, 400 to 1000 parts per million (ppm).
a. Reaction of sulfur dioxide with nitric acid
b. Ammonia oxidized at high temperatures
c. After manufacture NO· is purified and mixed with nitrogen.
2. Biologic synthesis: Naturally produced in many human tissues
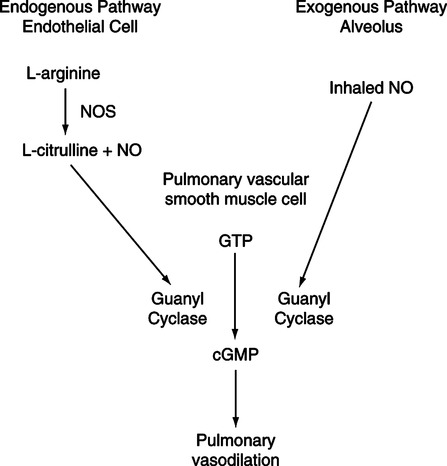
1. NO· diffuses into pulmonary vascular smooth muscle, where it activates guanyl cyclase, which converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), resulting in smooth muscle relaxation (see Figure 34-14).
2. Inhaled NO· is considered a selective pulmonary vascular dilation (Figure 34-15).
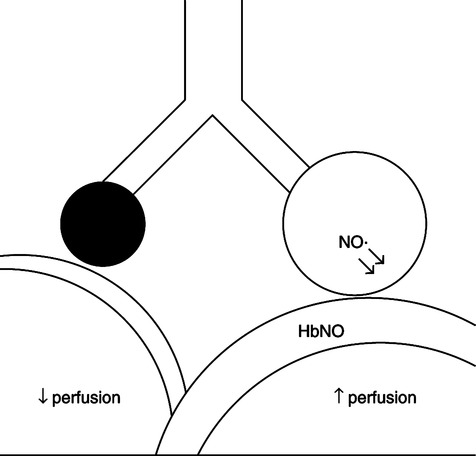
a. NO· inhaled in low concentrations (5 to 80 ppm) is a selective pulmonary vasodilator only in ventilated areas of the lung because of the high affinity of hemoglobin for NO·.
b. Systemic vasodilatation does not occur because NO· is rapidly inactivated by hemoglobin to produce methemoglobin (met-Hb).
c. Inhaled NO· improves blood flow to ventilated alveoli.
d. Reduces intrapulmonary shunt, thus improving  /
/
1. Food and Drug Administration–approved indication
2. Inhaled NO· is used off label in many centers for the treatment of
1. INOvent nitric oxide delivery system (INOtherapeutics, Clinton, NJ) (Figure 34-16)

b. Delivers 0 to 80 ppm inhaled NO·
c. 800 ppm NO· in large cylinders
d. Injection module measures flow and injects NO· proportional to changes in flow.
e. Gas monitoring unit for O2, NO·, and NO2
f. Continuous analysis is mandatory because of potential toxic effect of NO· and NO2.
2. Electrochemical nitric oxide analyzer affected by humidity
F Complications/precautions of NO· therapy
1. NO2 is produced when NO· reacts with oxygen.
a. Conversion rate is determined by NO· concentration, oxygen concentration, and residence time.
b. The Occupational Safety and Health Administration has set safety limits for NO2 at 5 ppm and NO· at 25 ppm for 8 hours (time-weighted average).
c. Airway and parenchymal lung injury has been reported with inhalation of NO2as low as 2 ppm.
d. Ventilator exhaust does not need to be scavenged because environmental contamination does not exceed 150 ppb when delivering NO· concentrations ≤80 ppm at a minute ventilation of 15 L/min.
e. The INOvent delivery system should be flushed before each use to ensure no residual NO2 remains in the system.
A General characteristics of helium
1. Mixture of oxygen and helium
a. Need at least 20% oxygen in mixture
b. Gas mixture has a density less than that of air or oxygen.
2. Low density produces greater tendency for laminar flow
a. Turbulent flow is density dependent.
b. Lower density of Heliox has a greater tendency for laminar flow.
c. Laminar flow is more energy efficient; thus less pressure is needed to move gas through constricted airways, resulting in decreased work of breathing.
3. Heliox (80% helium/20% oxygen) diffuses at a rate 1.8 times faster than oxygen.
1. Partial upper airway obstruction
2. Used for patients with acute asthma to decrease turbulent flow in constricted airways
1. Mix with oxygen to provide adequate FIO2.
a. FIO2 >0.5 limits clinical benefit of helium.
b. Oxygenation should be monitored to ensure adequate FIO2 is delivered.
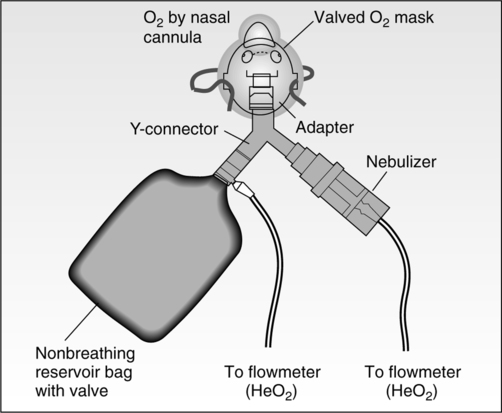
2. Heliox delivery to spontaneous breathing patients (Figure 34-17)
3. Mechanical ventilation: Use with caution; many ventilators do not function with Heliox.

 E, Expired minute ventilation; DLCO, diffusing capacity for carbon monoxide;
E, Expired minute ventilation; DLCO, diffusing capacity for carbon monoxide;  o2, O2 consumption; P0.1, mouth occlusion pressure; C
o2, O2 consumption; P0.1, mouth occlusion pressure; C o2, mixed venous O2 content; P(A-a)O2, alveolar-arterial PO2 difference.
o2, mixed venous O2 content; P(A-a)O2, alveolar-arterial PO2 difference.