Chapter 44 Overweight and Obesity
Body Mass Index
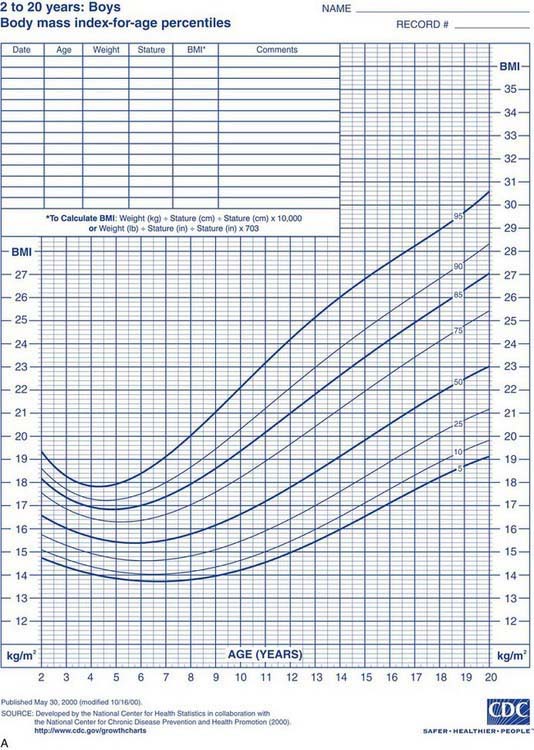
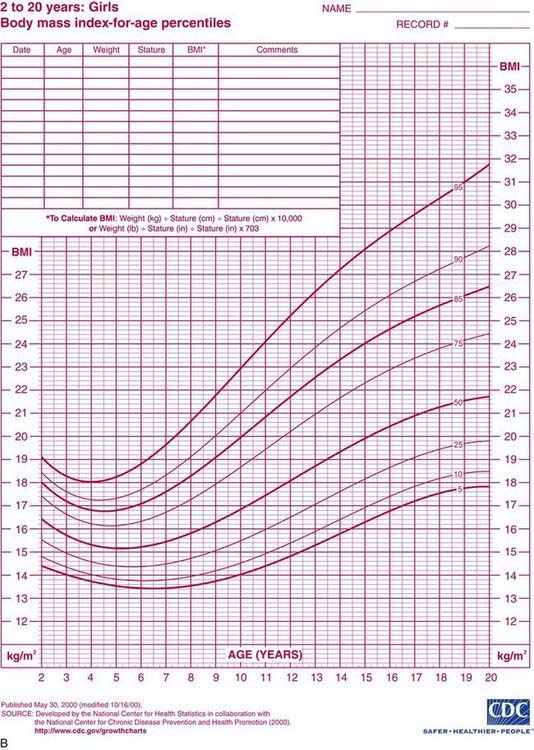
Health care professionals define obesity or increased adiposity using the body mass index (BMI), which is an excellent proxy for more direct measurement of body fat. BMI = weight in kg/(height in meters)2. Adults with a BMI ≥30 meet the criterion for obesity, and those with a BMI 25-30 fall in the overweight range. During childhood, levels of body fat change beginning with high adiposity during infancy. Body fat levels decrease for approximately 5.5 yr until the period called adiposity rebound, when body fat is typically at the lowest level. Adiposity then increases until early adulthood (Fig. 44-1). Consequently, obesity and overweight are defined using BMI percentiles; children >2 yr old with a BMI ≥95th percentile meet the criterion for obesity, and those with a BMI between the 85th and 95th percentiles fall in the overweight range. The terminology used for pediatric obesity previously was “overweight” and “risk for overweight.” This terminology has changed to improve consistency with the adult criteria and international definitions of pediatric obesity.
Etiology
Genetics
The rapid rise in obesity prevalence relates to dramatic environmental changes, but genetic determinants may be important for individual susceptibility. Rare single-gene disorders resulting in human obesity are known, including FTO (fat mass and obesity) and INSIG2 (insulin-induced gene 2) mutations as well as leptin deficiency and pro-opiomelanocortin deficiency. In addition, other genetic disorders associated with obesity such as Prader-Willi syndrome have long been recognized (Table 44-1). It is likely that genes are involved in behavioral phenotypes related to appetite regulation and preference for physical activity. More than 600 genes, markers, and chromosomal regions have been associated with human obesity.
| DISEASE | SYMPTOMS | LABORATORY |
|---|---|---|
| ENDOCRINE | ||
| Cushing syndrome | Central obesity, hirsutism, moon face, hypertension | Dexamethasone suppression test |
| Growth hormone deficiency | Short stature, slow linear growth | Evoked GH response, IGF-1 |
| Hyperinsulinism | Nesidioblastosis, pancreatic adenoma, hypoglycemia, Mauriac syndrome | Insulin level |
| Hypothyroidism | Short stature, weight gain, fatigue, constipation, cold intolerance, myxedema | TSH, FT4 |
| Pseudohypoparathyroidism | Short metacarpals, subcutaneous calcifications, dysmorphic facies, mental retardation, short stature, hypocalcemia, hyperphosphatemia | Urine cAMP after synthetic PTH infusion |
| GENETIC | ||
| Alstrom syndrome | Cognitive impairment, retinitis pigmentosa, diabetes mellitus, hearing loss, hypogonadism, retinal degeneration | ALMS1 gene |
| Bardet-Biedl syndrome | Retinitis pigmentosa, renal abnormalities, polydactyly, hypogonadism | BBS1 gene |
| Biemond syndrome | Cognitive impairment, iris coloboma, hypogonadism, polydactyly | |
| Carpenter syndrome | Polydactyly, syndactyly, cranial synostosis, mental retardation | Mutations in the RAB23 gene, located on chromosome 6 in humans |
| Cohen syndrome | Mid-childhood-onset obesity, short stature, prominent maxillary incisors, hypotonia, mental retardation, microcephaly, decreased visual activity | Mutations in the VPS13B gene (often called the COH1 gene) at locus 8q22 |
| Deletion 9q34 | Early-onset obesity, mental retardation, brachycephaly, synophrys, prognathism, behavior and sleep disturbances | Deletion 9q34 |
| Down syndrome | Short stature, dysmorphic facies, mental retardation | Trisomy 21 |
| ENPP1 gene mutations | Insulin resistance, childhood obesity | Gene mutation on chromosome 6q |
| Frohlich syndrome | Hypothalamic tumor | |
| Leptin or leptin receptor gene deficiency | Early-onset severe obesity, infertility (hypogonadotropic hypogonadism) | Leptin |
| Melanocortin 4 receptor gene mutation | ||
cAMP, cyclic adenosine monophosphate; FT4, free throxine; GH, growth hormone; IGF, insulin-like growth factor; PTH, parathyroid hormone; TSH, thyroid-stimulating hormone.
Endocrine and Neural Physiology
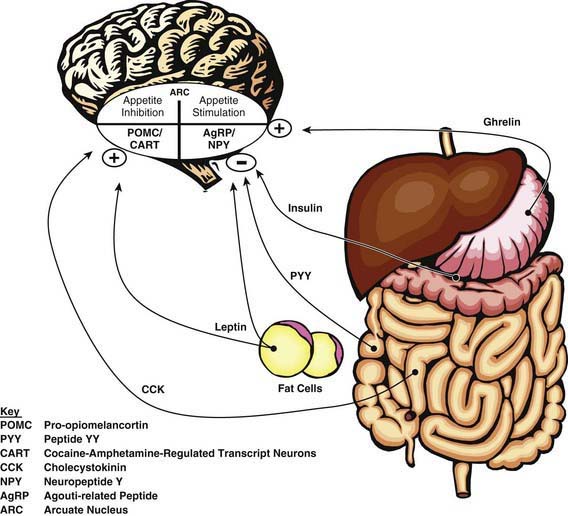
Monitoring of “stored fuels” and short-term control of food intake (appetite and satiety) occurs through neuroendocrine feedback loops linking adipose tissue, the gastrointestinal (GI) tract, and the central nervous system (Fig. 44-2). GI hormones, including cholecystokinin, glucagon-like peptide-1, peptide YY, and vagal neuronal feedback promote satiety. Ghrelin stimulates appetite. Adipose tissue provides feedback regarding energy storage levels to the brain through hormonal release of adiponectin and leptin. These hormones act on the arcuate nucleus in the hypothalamus and on the solitary tract nucleus in the brainstem and in turn activate distinct neuronal networks. Adipocytes secrete adiponectin into the blood, with reduced levels in response to obesity and increased levels in response to fasting. Reduced adiponectin levels are associated with lower insulin sensitivity and adverse cardiovascular outcomes. Leptin is directly involved in satiety, as low leptin levels stimulate food intake and high leptin levels inhibit hunger in animal models and in healthy human volunteers. Adiposity correlates to serum leptin levels among children and adults, with the direction of effect remaining unclear.
Comorbidities
Complications of pediatric obesity occur during childhood and adolescence and persist into adulthood. An important reason to prevent and treat pediatric obesity is the increased risk for morbidity and mortality later in life. The Harvard Growth Study found that boys who were overweight during adolescence were twice as likely to die from cardiovascular disease as those who had normal weight. More immediate comorbidities include type 2 diabetes, hypertension, hyperlipidemia, and nonalcoholic fatty liver disease (Table 44-2). Insulin resistance increases with increasing adiposity and independently affects lipid metabolism and cardiovascular health. Nonalcoholic fatty liver disease occurs in 10-25% of obese adolescents and can progress to cirrhosis.
| DISEASE | POSSIBLE SYMPTOMS | LABORATORY CRITERIA |
|---|---|---|
| CARDIOVASCULAR | ||
| Dyslipidemia | HDL <40, LDL >130, total cholesterol >200 | Fasting total cholesterol, HDL, LDL, triglycerides |
| Hypertension | SBP >95% for sex, age, height | Serial testing, urinalysis, electrolytes, blood urea nitrogen, creatinine |
| ENDOCRINE | ||
| Type 2 diabetes mellitus | Acanthosis nigrans, polyuria, polydipsia | Fasting blood glucose >110, hemoglobin, A1c, insulin level, C-peptide, oral glucose tolerance test |
| Metabolic syndrome | Central adiposity, insulin resistance, dyslipidemia, hypertension, glucose intolerance | Fasting glucose, LDL and HDL cholesterol |
| Polycystic ovary syndrome | Irregular menses, hirsutism, acne, insulin resistance, hyperandrogenemia | Pelvic ultrasound, free testosterone, LH, FSH |
| GASTROINTESTINAL | ||
| Gallbladder disease | Abdominal pain, vomiting, jaundice | Ultrasound |
| Nonalcoholic fatty liver disease (NAFLD) | ||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LH, luteinizing hormone; MRI, magnetic resonance imaging; Peds QL, Pediatric Quality of Life Inventory.
Some complications of obesity are mechanical, including obstructive sleep apnea and orthopedic complications. Orthopedic complications include Blount disease and slipped femoral capital epiphysis (Chapters 669, 670.4).
Evaluation
Consideration of possible medical causes of obesity is essential, even though endocrine and genetic causes are rare (see Table 44-1). Growth hormone deficiency, hypothyroidism, and Cushing syndrome are examples of endocrine disorders that can lead to obesity. In general, these disorders manifest with slow linear growth. Because children who consume excessive amounts of calories tend to experience accelerated linear growth, short stature warrants further evaluation. Genetic disorders associated with obesity can have coexisting dysmorphic features, cognitive impairment, vision and hearing abnormalities, or short stature. In some children with congenital disorders such as myelodysplasia or muscular dystrophy, lower levels of physical activity can lead to secondary obesity. Some medications can cause excessive appetite and hyperphagia, resulting in obesity. Atypical antipsychotic medications most commonly have this dramatic side effect. Rapid weight gain in a child or adolescent taking one of these medications might require a discontinuation of that medication. Poor linear growth and rapid changes in weight gain are indications for evaluation of possible medical causes.
Initial assessment of the overweight or obese child includes a complete review of bodily systems focusing on the possibility of comorbid conditions (see Table 44-2). Developmental delay and visual and hearing impairment can be associated with genetic disorders. Difficulty sleeping, snoring, or daytime sleepiness suggests the possibility of sleep apnea. Abdominal pain might suggest nonalcoholic fatty liver disease. Symptoms of polyuria, nocturia, or polydipsia may be the result of type 2 diabetes. Hip or knee pain can be caused by secondary orthopedic problems, including Blount disease and slipped capital femoral epiphysis. Irregular menses may be associated with polycystic ovary syndrome. Acanthosis nigricans can suggest insulin resistance and type 2 diabetes (Fig. 44-3).
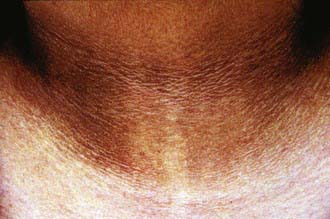
Figure 44-3 Acanthosis nigricans.
(From Gahagan S: Child and adolescent obesity, Curr Probl Pediatr Adolesc Health Care 34:6–43, 2004.)
Physical examination should be thorough, focusing on possible comorbid conditions (see Table 44-2). Careful screening for hypertension using an appropriately sized blood pressure cuff is important. Systematic examination of the skin can reveal acanthosis nigricans, suggesting insulin resistance, or hirsutism, suggesting polycystic ovary syndrome. Tanner staging can reveal premature adrenarche secondary to advanced sexual maturation in overweight and obese girls.
Laboratory testing for fasting plasma glucose, triglycerides, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, and liver function tests are recommended as part of the initial evaluation for newly identified pediatric obesity (Table 44-3). Overweight children (BMI 85th-95th percentile) who have a family history of diabetes mellitus or signs of insulin resistance should also be evaluated with a fasting plasma glucose test. Other laboratory testing should be guided by history or physical examination findings.
| LABORATORY TEST | NORMAL VALUE |
|---|---|
| Glucose | <110 mg/dL |
| Insulin | <15 mU/L |
| Hemoglobin A1c | <5.7% |
| AST 2-8 yr | <58 U/L |
| AST 9-15 yr | <46 U/L |
| AST 15-18 yr | <35 U/L |
| ALT | <35 U/L |
| Total cholesterol | <170 mg/dL |
| LDL | <110 mg/dL |
| HDL | <35 mg/dL |
| Triglycerides 2-15 yr | <100 mg/dL |
| Triglycerides 15-19 yr | <125 mg/dL |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
From Children’s Hospital of Wisconsin: The NEW (nutrition, exercise and weight management) kids program (PDF file). www.chw.org/display/displayFile.asp?docid=33670&filename=/Groups/NEWKids/08_Referral_Form.pdf. Accessed February 2, 2011.
Intervention
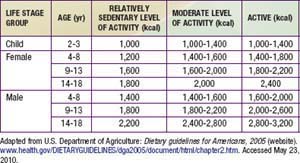
It is important to begin with clear recommendations about appropriate caloric intake for the obese child (Table 44-4). Working with a dietitian is very helpful. Meals should be based on fruits, vegetables, whole grains, lean meat, fish, and poultry. Prepared foods should be chosen for their nutritional value, with attention to calories and fat. Foods that provide excessive calories and low nutritional value should be reserved for infrequent treats. Because many obese children are consuming calories greatly in excess of their needs, it is often impossible to achieve an immediate reduction to the recommended daily calorie level. Instead, a gradual approach is recommended. A 10 yr old child who requires 2000 kcal/day and consumes 3500 kcal/day could reduce his typical daily intake by 280 kcal by forgoing two cans of high-carbohydrate beverage and drinking water instead. Although this dietary change will not result in weight loss, it will probably result in slightly slower weight gain. Once this change has been successfully incorporated, the child could make another change such as cutting out a snack, thereby eliminating an additional 300 kcal.
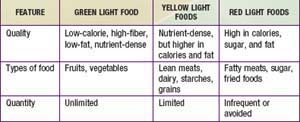
Psychological strategies are helpful. The “traffic light” diet groups foods into those that can be consumed without any limitations (green), in moderation (yellow), or reserved for infrequent treats (red) (Table 44-5). The concrete categories are very helpful to children and families. This approach can be adapted to any ethnic group or regional cuisine. Motivational interviewing, a strategy with proven efficacy for decreasing tobacco and substance use, shows promise for assisting patients in changing their nutritional patterns. This approach begins with assessing how ready the patient is to make important behavioral changes. The professional then engages the patient in developing a strategy to take the next step toward the ultimate goal of healthy nutritional intake. This method allows the professional to take the role of a coach, helping the child and family reach their goals. Other behavioral approaches include family rules about where food may be consumed—for example, “not in the bedroom.” Evidence-based strategies can be used to tailor interventions, given individual and environmental differences.
Pediatric providers should assist families to develop goals to change nutritional intake and physical activity. They can also provide the child and family with needed information. The family should not expect immediate lowering of BMI percentile related to behavioral changes but can instead count on a gradual decrease in the rate of BMI percentile increase until it stabilizes, followed by a gradual decrease in BMI percentile. Referral to multidisciplinary, comprehensive pediatric weight-management programs is ideal for obese children whenever possible. As part of a comprehensive program, adolescents may receive adjunctive pharmacologic therapy. In adults, the addition of antiobesity drugs to comprehensive lifestyle modification can produce more weight loss than lifestyle modification alone, with a BMI-lowering effect of 4%. Sibutramine, a norepinephrine and serotonin reuptake inhibitor, and Orlistat, an intestinal lipase inhibitor, is as effective as adjunctive therapy to behavior modification for weight loss in overweight adolescents (Table 44-6). The effect on long-term weight maintenance is not yet known. The pediatric provider also makes referrals to specialists to treat comorbid conditions, including type 2 diabetes, hypertension, nonalcoholic fatty liver disease, and orthopedic disorders.
| DRUG | MECHANISM OF ACTION | SIDE EFFECTS |
|---|---|---|
| Sibutramine*† | Appetite suppressant: combined norepinephrine and serotonin reuptake inhibitor | Modest increases in heart rate and blood pressure, nervousness, insomnia |
| Phentermine*† | Appetite suppressant: sympathomimetic amine | Cardiovascular, gastrointestinal |
| Diethylpropion*† | Appetite suppressant: sympathomimetic amine | Palpitations, tachycardia, insomnia, gastrointestinal |
| Orlistat* | Lipase inhibitor: decreased absorption of fat | Diarrhea, flatulence, bloating, abdominal pain, dyspepsia |
| Bupropion | Appetite suppressant: mechanism unknown | Paresthesia, insomnia, central nervous system effects |
| Fluoxetine | Appetite suppressant: selective serotonin reuptake inhibitor | Agitation, nervousness, gastrointestinal |
| Sertraline | Appetite suppressant: selective serotonin reuptake inhibitor | Agitation, nervousness, gastrointestinal |
| Topiramate | Mechanism unknown | Paresthesia, changes in taste |
| Zonisamide | Mechanism unknown | Somnolence, dizziness, nausea |
* Approved by the U.S. Food and Drug Administration for weight loss.
† Drug Enforcement Administration schedule IV.
From Snow V, Barry P, Fitterman N, et al: Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians, Ann Intern Med 142:525–531, 2005.
Prevention
Prevention of child and adolescent obesity is essential for public health in the USA and most other countries (Tables 44-7 and 44-8). Efforts by pediatric providers can supplement national- and community-level public health programs. The National Institutes of Health (NIH) and Centers for Disease Control and Prevention (CDC) recommend a variety of initiatives to combat the current obesigenic environment, including promotion of breast-feeding, access to fruits and vegetables, walkable communities, and 60 min/day of activity for children. The USDA sponsors programs promoting 5.5 cups of fruits and vegetables per day. Incentives for the food industry to promote consumption of healthier foods should be considered. Marketing of unhealthy foods to children has begun to be regulated. We expect to see changes in federal food programs including commodity foods, the Women, Infant, and Children Supplemental Food Program (WIC), and school-lunch programs to meet the needs of today’s children.
Table 44-7 PROPOSED SUGGESTIONS FOR PREVENTING OBESITY
PREGNANCY
POSTPARTUM AND INFANCY
FAMILIES
SCHOOLS
COMMUNITIES
HEALTH CARE PROVIDERS
INDUSTRY
GOVERNMENT AND REGULATORY AGENCIES
From Speiser PW, Rudolf MCJ, Anhalt H, et al: Consensus statement: childhood obesity, J Clin Endocrinol Metabol 90:1871–1887, 2005.
Table 44-8 ANTICIPATORY GUIDANCE: ESTABLISHING HEALTHY EATING HABITS IN CHILDREN
Adapted from Benton D: Role of parents in the determination of food preferences of children and the development of obesity, Int J Obes Relat Metab Disord 28:858–869, 2004.
Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin. 2008;37:811-823.
American Heart Association. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117:544-559.
Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. 2009;374:1606-1616.
August GP, Caprios S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576-4599.
Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666-670.
Cecchini M, Sassi F, Lauer JA, et al. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376:1775-1784.
Cecil JE, Tavendale R, Watt P, et al. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558-2566.
Centers for Disease Control and Prevention. CDC grand rounds: childhood obesity in the United States. MMWR. 2011;60:42-46.
Centers for Disease Control and Prevention. Effect of switching from whole to low-fat/fat-free milk in public schools—New York City, 2004–2009. MMWR. 2010;59:70-73.
Centers for Disease Control and Prevention. Recommended community strategies and measurements to prevent obesity in the United States. MMWR. 2009;58:1-29.
Centers for Disease Control and Prevention. Obesity prevalence among low-income, preschool-aged children—United States, 1998–2008. MMWR. 2009;58:769-772.
Centers for Disease Control and Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults—United States, 2006–2008. MMWR. 2009;58:740-744.
Centers for Disease Control and Prevention. Availability of less nutritious snack foods and beverages in secondary schools—selected states, 2002–2008. MMWR. 2009;58:1102-1104.
Chan G, Chen CT. Musculoskeletal effects of obesity. Curr Opin Pediatr. 2009;21:65-70.
Daniels SR, Jacobson MS, McCrindle BW, et al. American Heart Association childhood obesity research summit report. Circulation. 2009;119:e489-e517.
Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroentero Reports. 2008;10:67-72.
Eckel RH, Alberti KGMM, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181-183.
Ford AL, Bergh C, Södersten P, et al. Treatment of childhood obesity by retraining eating behaviour: randomized controlled trial. BMJ. 2010;340:b5388.
Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485-492.
Glaser Pediatric Research Network Obesity Study Group. Metformin extended release treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2010;164:116-123.
Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010;375:1737-1746.
2010 Evidence for effective obesity treatment: Pediatricians on the right track!. Pediatrics. 2010;125:37-38.
Ibáñez L, Lopez-Bermejo A, Diaz M, et al. Pubertal metformin therapy to reduce total, visceral, and hepatic adiposity. J Pediatr. 2010;156:98-102.
Kavey REW. Treatment for obese children: a ray of hope? J Pediatr. 2010;157(3):357-359.
The Lancet. Childhood obesity: affecting choices. Lancet. 2010;375:611.
Lane MD, Cha SH, et al. Effect of glucose and fructose on food intake via malonyl-CoA signaling in the brain. Biochem Biophys Res Comm. 2009;382:1-5.
Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102-2113.
Lee IM, Djoussé L, Sesso HD, et al. Physical activity and weight gain prevention. JAMA. 2010;303:1173-1179.
Meyre D, Delphanque J, Chévre JC, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nature Genetics. 2009;41:157-159.
Murry R, Bttista M. Managing the risk of childhood overweight and obesity in primary care practice. Curr Prob Pediatr Adoles. 2009;39:145-166.
Nadler EP, Brotman LM, Miyoshi T, et al. Morbidity in obese adolescents who meet the adult National Institutes of Health criteria for bariatric surgery. J Pediatr Surg. 2009;44:1869-1876.
Nguyen NT, Slone JA, Nguyen XM, et al. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity. Ann Surg. 2009;250:631-641.
O’Brien PE, Sawyer SM, Laurie C, et al. Laparoscopic adjustable gastric banding in severely obese adolescents. JAMA. 2010;303:519-526.
Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242-249.
Paz-Priel I, Cooke DW, Chen AR. Cyclophosphamide for rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome. J Pediatr. 2011;158(2):337-339.
Plum L, Lin HV, Dutia R, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature Med. 2009;15:1195-1201.
Reinehr T, Hinney A, Toschke AM, Hebebrand J. Aggravating effect of INSIG2 and FTO on overweight reduction in a one-year lifestyle intervention. Arch Dis Child. 2009;94:965-967.
Ruiz JR, Labayen I, Ortega FB, et al. Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents. Arch Pediatr Adolesc Med. 2010;164:328-333.
Sacher PM, Kolotourou M, Chadwick PM, et al. Randomized controlled trial of the MEND program: a family-based community intervention for childhood obesity. Obesity. 2010;18:S62-S68.
Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-872.
Savoye M, Nowicka P, Shaw M, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402-410.
Spyropoulos C, Kehagias I, Panagiotopoulos S, et al. Revisional bariatric surgery. Arch Surg. 2010;145:173-177.
Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453-1456.
Taveras EM, Rifas-Shiman SL, Belfort MB, et al. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177-1183.
U.S. Department of Agriculture. Dietary guidelines for Americans, 2005 (website). www.health.gov/DIETARYGUIDELINES/dga2005/document/html/chapter2.htm. Accessed May 23, 2010
Van Cauter E, Knutson K. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159:S59-S66.
Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303:623-630.
Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179-188.
Vanselow MS, Pereira MA, Neumark-Sztainer D, Raatz SK. Adolescent beverage habits and changes in weight over time: finding from Project EAT. Am J Clin Nutr. 2009;90:1489-1495.
Weiss R, Kaufman F. Metabolic complications of childhood obesity: identifying and mitigating the risk. Diabetes Care. 2008;31:S310-S316.
Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661-1673.
Williams G. Withdrawal of sibutramine in Europe. BMJ. 2010;340:377-378.