Overview of Anaerobic Organisms
1. For each group of organisms listed, provide the general characteristics, including Gram stain reactions, colonial morphology, growth requirements (media, oxygen requirement, temperature), laboratory identification, and clinical significance.
2. Differentiate normal anaerobic bacteria from pathogenic bacteria isolated from clinical specimens.
3. Describe the pathogenesis and virulence factors associated with the Clostridium species C. perfringens, C. botulinum, C. difficile, and C. septicum.
4. Define and discuss the pathogenesis for anaerobic cellulitis, gas gangrene, clostridial gastroenteritis, pseudomembranous enterocolitis, botulism, actinomycosis, bacterial vaginosis, and enteritis necroticans.
5. Differentiate the three forms of botulism (food poisoning, wound botulism, and infant botulism).
6. Compare paralysis associated with botulism with tetanus.
7. Explain the procedure for spore isolation and growth using the ethyl alcohol shock procedure.
8. List the appropriate specimen collection, transport, and storage conditions for the recovery of anaerobic organisms.
9. Explain aerotolerance testing, including how to perform the test, what media is used, and the reason or reasons the media is important.
10. Identify the special potency antibiotics and explain the typical resistance patterns used to identify the various anaerobic groups (e.g., gram-positive cocci, gram-negative cocci).
11. Correlate disease signs and symptoms with laboratory data to identify the etiologic agent of infection.
As previously described in Chapter 41, the organisms in this chapter predominantly do not grow in the presence of oxygen.
Epidemiology
Most of the anaerobic bacteria that cause infections in humans are also part of our normal flora. The ecology of these organisms is such that various species and genera exhibit preferences for the body sites they inhabit (endogenous anaerobes) (Table 42-1). Other pathogenic anaerobes (e.g., Clostridium botulinum and Clostridium tetani) are soil and environmental inhabitants (exogenous anaerobes) and are not considered part of the normal human flora.
TABLE 42-1
Incidence of Anaerobes as Normal Flora of Humans
| Genus | Skin | Upper Respiratory Tract* | Intestine | External Genitalia | Urethra | Vagina |
| Gram-Negative Bacteria | ||||||
| Bacteroides | 0 | ± | 2 | ± | ± | ± |
| Prevotella | 0 | 2 | 2 | 1 | ± | 1 |
| Porphyromonas | 0 | 1 | 1 | U | U | ± |
| Fusobacterium | 0 | 2 | 1 | U | U | ± |
| Veillonella | 0 | 2 | 1 | 0 | U | 1 |
| Gram-Positive Bacteria | ||||||
| Finegoldia | 1 | 2 | 2 | 1 | ± | 1 |
| Parvimonas | 1 | 2 | 2 | 1 | ± | 1 |
| Clostridium | ± | ± | 2 | ± | ± | ± |
| Actinomyces | 0 | 1 | 1 | 0 | 0 | ± |
| Bifidobacterium | 0 | 1 | 2 | 0 | 0 | ± |
| Eubacterium | 0 | 1 | 2 | U | U | 1 |
| Lactobacillus | 0 | 1 | 1 | 0 | ± | 2 |
| Propionibacterium | 2 | 1 | ± | ± | ± | 1 |
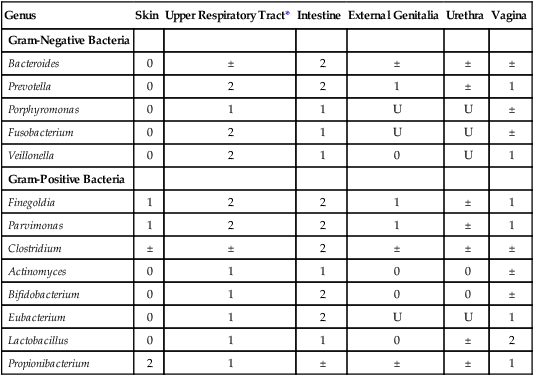
U, Unknown; 0, not found or rare; ±, irregular; 1, usually present; 2, present in large numbers.
*Includes nasal passages, nasopharynx, oropharynx, and tonsils.
Modified from Summanen PE, Baron EJ, Citron DM et al: Wadsworth anaerobic bacteriology manual, ed 5, Belmont, Calif, 1993, Star.
The ways in which anaerobic infections are acquired are summarized in Table 42-2. Person-to-person nosocomial spread of Clostridium difficile among hospitalized patients presents an enormous clinical and infection control dilemma; however, most anaerobic infections occur when a patient’s normal flora gains access to a sterile site as a result of disruption of some anatomic barrier.
TABLE 42-2
Acquisition of Anaerobic Infections and Diseases
| Mode of Acquisition | Examples |
| Endogenous strains of normal flora gain access to normally sterile sites, usually as result of one or more predisposing factors that compromise normal anatomic barriers (e.g., surgery or accidental trauma) or alter other host defense mechanisms (e.g., malignancy, diabetes, burns, immunosuppressive therapy, aspiration) | Wide variety of infections involving several anatomic locations, including bacteremia, head and neck infections, dental and orofacial infections, pneumonia and other infections of the thoracic cavity, intraabdominal and obstetric and gynecologic infections, bite wound and other soft tissue infections, and gangrene (i.e., clostridial myonecrosis). Organisms most commonly encountered in these infections include Bacteroides fragilis group, Prevotella spp., Porphyromonas spp., Fusobacterium nucleatum, Peptostreptococcus spp., and Clostridium perfringens. |
| Contamination of existing wound or puncture by objects contaminated with toxigenic Clostridium spp. | Tetanus (Clostridium tetani), gas gangrene (Clostridium perfringens and, less commonly, C. septicum, C. novyi, and others) |
| Ingestion of preformed toxins in vegetable- or meat-based foods | Botulism (Clostridium botulinum) and other clostridial food poisonings (C. perfringens) |
| Colonization of gastrointestinal tract with potent toxin-producing organism | Infant botulism (C. botulinum) |
| Person-to-person spread | Nosocomial spread of Clostridium difficile–induced diarrhea and pseudomembranous colitis; bite wound infections caused by a variety of anaerobic species |
Pathogenesis and Spectrum of Disease
The types of infections and diseases in humans caused by anaerobic bacteria span a wide spectrum. Certain species, such as C. botulinum and C. tetani, produce some of the most potent toxins known. In contrast, specific virulence factors for the organisms commonly encountered in infections (e.g., B. fragilis group, C. difficile) are not well understood (Table 42-3).
TABLE 42-3
Pathogenesis and Spectrum of Disease for Anaerobic Bacteria
| Organism | Virulence Factors | Spectrum of Disease and Infections |
| Clostridium perfringens | Produces several exotoxins; alpha-toxin, the most important, mediates destruction of host cell membranes; enterotoxin inserts and disrupts membranes of mucosal cells Beta-toxin—cytotoxin |
Gas gangrene (myonecrosis): Life-threatening, toxin-mediated destruction of muscle and other tissues after traumatic introduction of the organism. Food poisoning: Caused by release of the toxin after ingestion of large numbers of the organism. Usually self-limiting and benign; manifested by abdominal cramps, diarrhea, and vomiting. Enteritis necroticans (necrotizing enteritis; NEC): Life-threatening infection that causes ischemic necrosis of the jejunum. Often associated with immunocompromised patients (e.g., those with diabetes, alcohol-induced liver disease, or neutropenia). NEC, a gastrointestinal disease that causes bowel necrosis and inflammation, affects low-birth-weight, premature infants. |
| Clostridium sordellii | Produces a variety of bacterial proteases, phospholipases Produces up to seven exotoxins, including lethal toxin (LT), hemorrhagic toxin (HT), and enterotoxins A, B, and C |
Gas gangrene of the uterus as a result of abortion, normal delivery, or cesarean section. Patient presents with little or no fever, lack of purulent discharge, hypotension, peripheral edema, and an increased white blood cell (WBC) count. Infection is typically fatal, and death is rapid. |
| Clostridium tetani | Produces tetanospasmin (TeNT), a neurotoxic exotoxin that disrupts nerve impulses to muscles | Tetanus (commonly known as lockjaw). Organism establishes a wound infection and elaborates TeNT, a potent toxin that mediates generalized muscle spasms. If the disease goes untreated, spasms continue to be triggered by even minor stimuli, leading to exhaustion and, eventually, respiratory failure. |
| Clostridium botulinum | Produces an extremely potent neurotoxin (BoNT) | Foodborne botulism: Results from ingestion of preformed toxin in nonacidic vegetable or mushroom foodstuffs. Absorption of the toxin leads to nearly complete flaccid (rag doll) paralysis of respiratory and other essential muscle groups. Infant botulism: Occurs when the organism elaborates the toxin after it has colonized the gastrointestinal tract of infants (i.e., infant botulism). Wound botulism: Occurs when C. botulinum produces the toxin from an infected wound site |
| Clostridium difficile | Produces toxin A (TcdA), an enterotoxin, and toxin B (TcdB), a cytotoxin Both toxin A and toxin B are classified as large clostridial cytotoxins The toxins glycosylate guanosine triphosphate (GTP) signaling proteins, leading to a breakdown of the cellular cytotoxin and cell death |
Organism requires diminution of normal gut flora by the activity of various antimicrobial agents to become established in the gut of hospitalized patients. Once established, elaboration of one or more toxins results in antibiotic-associated diarrhea or potentially life-threatening inflammation of the colon. When the surface of the inflamed bowel is overlaid with a “pseudomembrane” composed of necrotic debris, white blood cells, and fibrin, the disease is referred to as pseudomembranous colitis. Only strains producing toxin A or toxin B (or both) cause infections. |
| Actinomyces spp., including A. israelii, A. meyeri, A. naeslundii, and A. odontolyticus | No well-characterized virulence factors. Infections usually require disruption of protective mucosal surface of the oral cavity, respiratory tract, gastrointestinal tract, and/or female genitourinary tract | Usually involved in mixed oral or cervicofacial, thoracic, pelvic, and abdominal infections caused by patient’s endogenous strains. Certain species (A. viscosus and A. naeslundii) also involved in periodontal disease and dental caries. Identified in a variety of soft tissue infections, including perianal, groin, ancillary, breast, and periaural abscesses. |
| Propionibacterium spp. | No definitive virulence factors known | Associated with inflammatory process in acne. Identified in systemic opportunistic infections, including endocarditis, central nervous system (CNS) infections, osteomyelitis, and arthritis. As part of normal skin flora, the organism is considered the most common anaerobic contaminant of blood cultures and is often ignored. |
| Atopobium spp. | No definitive virulence factors known | Isolated from various infections in the genital tract, including bacterial vaginosis. Considered normal flora of the female genital tract. |
| Bifidobacterium spp. | No definitive virulence factors known | Not commonly found in clinical specimens. Usually encountered in mixed infections of the pelvis or abdomen. |
| Eggerthella spp. | No definitive virulence factors known | Recovered from a variety of infections including intraabdominal and periabdominal infections. |
| Eubacterium spp. | No definitive virulence factors known | Usually associated with mixed infections of the oral cavity, abdomen, pelvis, or genitourinary tract. |
| Lactobacillus spp. | No definitive virulence factors known | Associated with advanced dental caries. Organism has also been identified in endocarditis and bacteremia. |
| Mobiluncus spp. | No definitive virulence factors known | Organisms are found in the vagina and have been associated with bacterial vaginosis, but their precise role in gynecologic infections is unclear. Rarely encountered in infections outside the female genital tract. |
| Bacteroides fragilis group, other Bacteroides spp., including B. gracilis and B. ureolyticus Prevotella spp. Porphyromonas spp. Fusobacterium nucleatum and other Fusobacterium spp. |
Anaerobic, gram-negative bacilli that produce capsules, endotoxin, and succinic acid, which inhibit phagocytosis, and various enzymes that mediate tissue damage. Most infections still require some breach of mucosal integrity that allows the organisms to gain access to deeper tissues |
Organisms most commonly encountered in anaerobic infections. Infections are often mixed with infections caused by other anaerobic and facultative anaerobic organisms. Infections occur throughout the body, usually as localized or enclosed abscesses, and may involve the cranium, periodontium, thorax, peritoneum, liver, and female genital tract. May also cause bacteremia, aspiration pneumonia, septic arthritis, chronic sinusitis, decubitus ulcers, and other soft tissue infections. The hallmark of most but not all infections is the production of a foul odor. In general, infections caused by B. fragilis group occur below the diaphragm; pigmented Prevotella spp., Porphyromonas spp., and F. nucleatum generally are involved in head and neck and pleuropulmonary infections. |
| Finegoldia magna Parvimonas micra |
No definitive virulence factors known P. micra has been shown to produce a variety of enzymes capable of tissue destruction, including collagenase, hemolysin, and elastase. |
Most often found mixed with other anaerobic and facultatively anaerobic bacteria in cutaneous, respiratory, oral, or female pelvic infections. |
| Peptostreptococcus anaerobius | No definitive virulence factors known | Most often isolated from polymicrobic infections, including abscesses. |
| Veillonella spp. | No definitive virulence factors known | May be involved in mixed infections. Organisms have been isolated in increasingly serious infections, including meningitis, osteomyelitis, endocarditis, bacteremia, and prosthetic infections. |
Gram-Positive, Spore-Forming Bacilli
The clostridia are the endospore-forming, obligately anaerobic (or aerotolerant), catalase-negative, gram-positive bacilli (Figure 42-1). The rods are pleomorphic and may be arranged in pairs or short chains. If spores are not present on Gram stain, the ethanol shock spore or heat shock spore test can separate this group from the non–spore-forming anaerobic bacilli (see Procedure 42-1 on the Evolve site). Some strains of C. perfringens, C. ramosum, and C. clostridioforme may not produce spores or survive a spore test, so it is important to recognize these organisms using other characteristics. Some clostridia typically stain gram negative, although they are susceptible to vancomycin on the disk test. Several species of clostridia grow aerobically (C. tertium, C. carnis, C. histolyticum, and occasional strains of C. perfringens), but they produce spores only under anaerobic conditions. C. perfringens may appear weakly catalase positive.
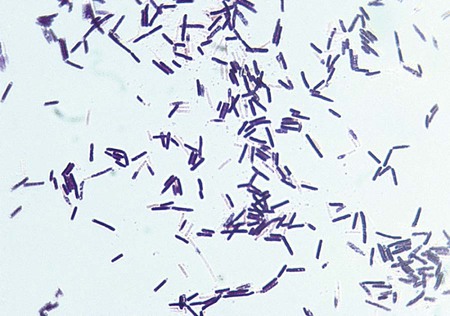
Clostridium species are widespread in nature because of their ability to form spores, referred to as endospores, in the mother cell (Table 42-4). In addition, they are present in large numbers as normal flora in the gastrointestinal tract of humans and animals, the female genital tract, and the oral mucosa.
TABLE 42-4
Characteristics of Clinically Significant Clostridium species
| Species | Spore Location | Gelatin | Lecithinase | Lipase | Indole | Esculin | Nitrate |
| C. argentinense | ST | + | − | − | − | − | − |
| C. baratii | ST | − | + | − | − | + | V |
| C. bifermentans | ST | + | + | − | + | V | − |
| C. bolteae | ST | − | − | − | − | V | − |
| C. botulinum Types A,B, and F | ST | + | − | + | − | + | − |
| Types B, E, and F-nonproteolytic | ST | + | − | + | − | − | − |
| Types C and D | T | + | V | + | V | − | − |
| C. butyricum | ST | − | − | − | − | + | − |
| C. cadaveris | T | + | – | – | + | − | − |
| C. canis | ST | − | − | − | − | + | − |
| C. clostridioforme | ST | − | − | − | − | + | − |
| C. difficile | ST(T) | + | − | − | − | + | − |
| C. glycolicum | ST | − | − | − | − | V | − |
| C. hastiforme | T | + | − | − | − | − | V |
| C. hathewayi | ST | − | − | − | − | + | − |
| C. histolyticum | ST | + | − | − | − | − | − |
| C. indolis | T | − | − | − | + | + | V |
| C. innocuum | T | − | − | − | − | + | − |
| C. limosum | ST | + | + | − | − | − | − |
| C. novyi A | ST | + | + | + | − | − | − |
| C. paraputrificum | T(ST) | − | − | − | − | + | V |
| C. perfringens | ST | + | + | − | − | V | V |
| C. putrificum | T(ST) | + | − | − | − | V | − |
| C. ramosum | T | − | − | − | − | + | − |
| C. septicum | ST | + | − | − | − | + | V |
| C. sordelli | ST | + | + | − | + | V | − |
| C. sphenoides | ST(T) | − | − | − | + | + | V |
| C. sporogenes | ST | + | − | + | − | + | − |
| C. subterminale | ST | + | V | − | − | V | − |
| C. symbiosum | ST | − | − | − | − | − | − |
| C. tertium | T | − | − | − | − | + | V |
| C. tetani | T | + | − | − | V | − | − |
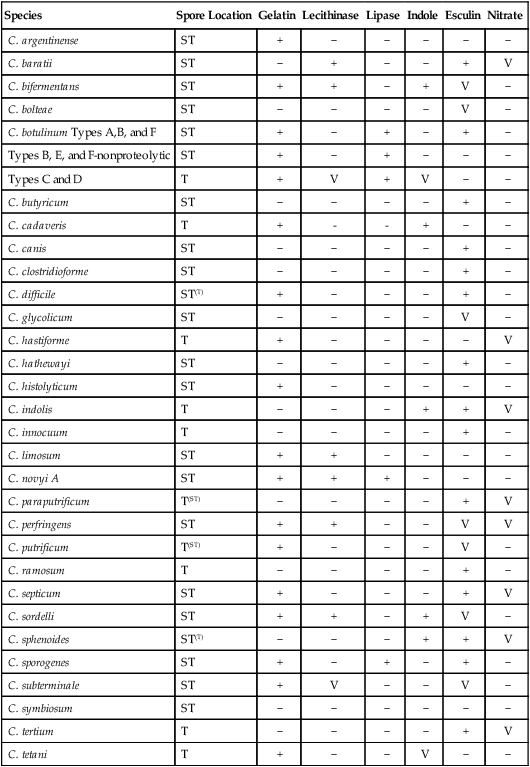
+, Positive reaction;—negative reaction; V, variable reaction; ST, subterminal; T, terminal; superscript indicates variability.
Modified from Versalovic J: Manual of clinical microbiology, ed 10, 2011, Washington, DC, ASM Press.
C. botulinum is listed by the Centers for Disease Control and Prevention (CDC) as a potential agent of bioterrorism (see Chapter 80). A diagnosis of botulism is made by the demonstration of botulinum neurotoxin in serum, feces, gastric contents, vomitus, or suspect food (food poisoning) or environmental specimen (potential bioterrorism incident). This means that most hospital laboratories must know how to package and ship such a specimen to the State Health Department or CDC. Isolation of C. botulinum is rarely seen in the clinical microbiology laboratory. Table 42-3 describes the pathogenesis of the frequently encountered Clostridium spp.
Laboratory Diagnosis and Specimen Collection
As stated in Chapter 41, the proper collection and transport of specimens for anaerobic culture cannot be overemphasized. General considerations are included in the discussion in Chapter 41. However, special collection instructions must be followed for some clostridial illnesses, specifically foodborne C. perfringens and C. botulinum, C. difficile pseudomembranous enterocolitis, and C. septicum neutropenic enterocolitis (NEC). Food and freshly passed fecal specimens must be sent to a public health laboratory for confirmation of C. perfringens food poisoning; these should be transported at 4° C. The specimens should be processed within 24 hours of collection. The clinical diagnosis of botulism is confirmed by demonstration of botulinum toxin in serum, feces, vomitus, or gastric contents, as well as by recovery of the organism from the stool of patients (Figure 42-2). Several methods are available, including cell culture assays, enzyme-linked immunosorbent assay (ELISA), and latex agglutination.
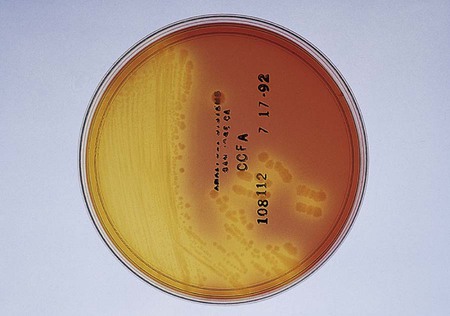
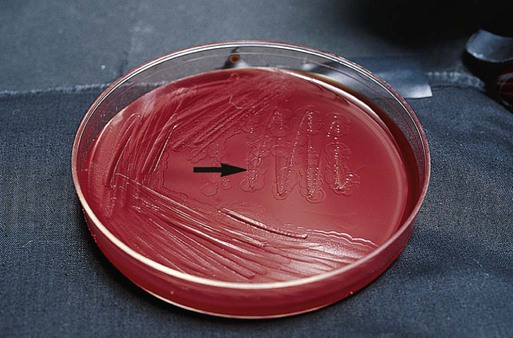
Suspected C. difficile infection (CDI) indicates collection of a freshly passed stool specimen for culture and toxin assays for both toxin A and toxin B. Only liquid or unformed stools should be processed for CDI to prevent the treatment of patients colonized with the bacterium. Formed stools or rectal swabs are adequate to detect carriers. Specimens should be cultured within 2 hours after collection. Figure 42-3 demonstrates the isolation of C. difficile on cycloserine cefoxitin fructose agar (CCFA) and on anaerobic blood agar. Specimens may be stored in anaerobic transport bags at 4° C for up to 48 hours; however, this reduces the recovery rate of viable organisms in culture. Specimens for toxin assays may be stored at 4° for 72 hours or frozen at –70° C if a longer delay is expected.
A variety of immunoassays are commercially available for the identification of C. difficile enterotoxin. In addition, a variety of molecular-based assays have been developed for the amplification of the toxin A (tcdA) and toxin B (tcdB) genes. Stool samples may be submitted for PCR amplification. The assays include amplification of the glutamate dehydrogenase (GDH) gene or 16srRNA as internal control housekeeping genes. Detection of the GDH and 16s rRNA genes without the presence of a toxin gene would indicate a nonpathogenic strain or carrier state. A new molecular assay is currently available: Illumigene C. difficile, (Meridian Bioscience, Inc., Memphis, TN), using LAMP isothermal amplification. See Chapter 8 for information on LAMP methodology. Cell culture cultivation is still recommended for molecular stain typing and epidemiologic studies.
The specimens of choice for neutropenic enterocolitis involving C. septicum are three different blood cultures, stool, and lumen contents or tissue from the involved ileocecal area; a muscle biopsy sample should also be collected if myonecrosis (death of muscle tissue) is suspected. Table 42-5 provides an identification scheme for representative anaerobic organisms.
TABLE 42-5
Differentiation of Representative Gram-Negative Bacilli and Gram-Positive Cocci
| GRAM-NEGATIVE BACILLI | |||
| B. fragilis | B. thetaiotaomicron | F. nucleatum | |
| Test Method | |||
| Arabinose | Neg | Pos | Neg |
| Bile 20%, growth | Pos | Pos | Neg |
| Catalase | Pos | Pos | Neg |
| Colistin (Col) | R | R | S |
| Esculin hydrolysis | Pos | Pos | Neg |
| Gelatinase | 0 | 0 | 0 |
| Indole | Neg | Pos | Pos |
| Kanamycin (Km) | R | R | S |
| Nitrate | Neg | Neg | Neg |
| Vancomycin (Van) | R | R | R |
| GRAM-POSITIVE BACILLI | |||
| P. anaerobius | P. asaccharolyticus | F. magna | |
| Test Method | |||
| Indole | Neg | Pos | Neg |
| Nitrate | Neg | Neg | Neg |
| Sodium polyanethol sulfonate (SPS) | S | R | R |
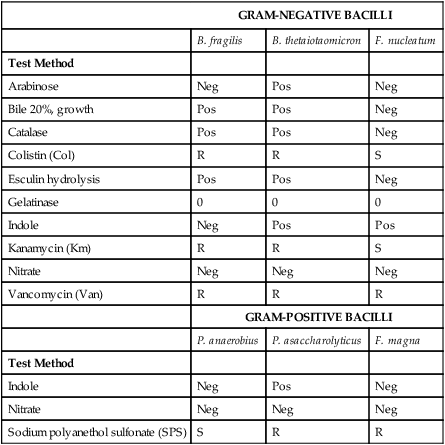
Neg, Negative reaction; Pos, positive reaction; R, resistant; S, sensitive; V, variable.
>10 mm = sensitive for Km, Van, Col; >12 mm = sensitive for SPS.
From Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
Gram-Positive, Non–spore-Forming Bacilli
The genera Actinomyces (anaerobic and aerotolerant) and Mobiluncus (strictly anaerobic) include species that show non–acid-fast, gram-positive, pleomorphic branching rods or coccobacilli. Direct examination and the macroscopic presence in purulent exudate of “sulfur granules,” which reveal gram-positive filaments when crushed, is diagnostic for an infection with Actinomyces spp. Mobiluncus spp., a cause of bacterial vaginosis, usually is diagnosed on Gram staining of vaginal secretions by observation of gram-variable, curved rods with tapered ends. It is rarely isolated in the clinical laboratory, because vaginal secretions are not acceptable specimens for anaerobic culture. Propionibacterium spp. are anaerobic and aerotolerant, pleomorphic, gram-positives rods. The bacterium produces propionic acid from glucose. Bifidobacterium spp. are strictly anaerobic or microaerophilic, gram-positive, pleomorphic rods that appear as rods or as branched or club shaped. Lactobacillus spp. contain microaerophilic, catalase-negative, gram-positive rods capable of producing lactic acid from glucose fermentation. The genus Eubacterium remains poorly characterized, although its species are commonly isolated from oral infections. The pathogenic mechanisms and the spectrum of diseases associated with these organisms are included in Table 42-3.
Laboratory Diagnosis
Differentiation of the gram-positive, non–spore forming anaerobes is based on colony and Gram stain morphology. Follow-up tests to identify the organism are determined by the interpretation of the initial Gram stain results. Additional tests include an aerotolerance test (see Procedure 42-2 on the Evolve site) or growth in 5% CO2, followed by routine screening of special-potency antibiotic susceptibility patterns. The gram-positive organisms typically are resistant to colistin (10 µg), susceptible to vancomycin (5 µg), and have variable sensitivity to kanamycin (1 mg). Additional rapid testing includes a 15% catalase test, production of indole, and nitrate reduction. Although currently no rapid molecular amplification tests are available in the clinical laboratory, isolates can be submitted to reference laboratories for 16s RNA sequence analysis. Table 42-5 provides an identification scheme for representative anaerobic organisms.
Gram-Negative Rods
Bacteroides Fragilis Group
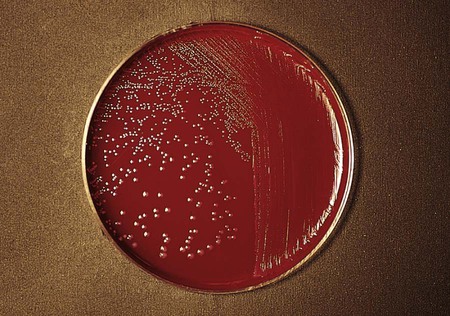
The anaerobic gram-negative rods typically are isolated from the mucosal surfaces of the human oral cavity and gastrointestinal tract (Figure 42-4). (Table 42-3 presents an overview of the pathogenesis and infections associated with these organisms.) The Bacteroidaceae family consists of the saccharolytic, bile-resistant, nonpigmented Bacteroides fragilis group. B. fragilis is the most common organism isolated from clinical specimens, followed by B. thetaiotaomicron and B. ovatus. These organisms have been associated with a variety of infections.
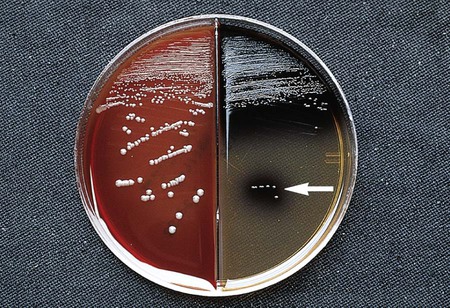
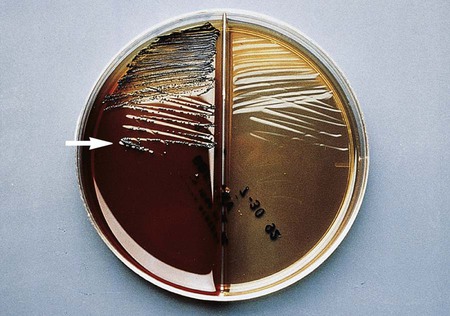
The gram-negative Bacteroides fragilis group grows in 20% bile, and the organisms are almost always resistant to all three special-potency antibiotic disks (Figures 42-5 and 42-6). Rare strains of B. fragilis are susceptible to colistin.
Pigmented Porphyromonas and Prevotella spp.
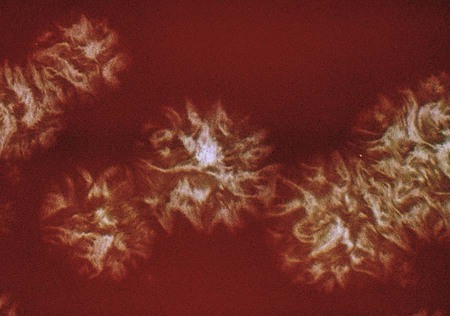
The Porphyromonadaceae family comprises five genera, including the genera, Parabacteroides, Porphyromonas, Tannerella, Odoribacter, and Barnesiella. Porphyromonas generally is considered the pathogenic genus in the Porphyromonadaceae family. Most Porphyromonas spp. are asaccharolytic and pigmented. The Prevotellaceae includes saccharolytic organisms that have been isolated from a variety of body sites, including the oral cavity and feces. Colonies that fluoresce brick red or produce brown to black pigment are placed among the pigmented Prevotella (Figure 42-7) and Porphyromonas spp. (Figure 42-8). Some species appear coccobacillary on Gram staining.
Bacteroides ureolyticus
Bacteroides ureolyticus is asaccharolytic, reduces nitrate, and requires formate and fumarate for growth in broth culture. Its disk pattern is the same as for the fusobacteria; however, the colony morphology is different. B. ureolyticus forms small, translucent to transparent colonies that may corrode the agar (Figure 42-9), whereas the Fusobacterium colony generally is larger and more opaque (Figure 42-10). B. ureolyticus formerly was grouped with organisms that have been transferred to the genus Campylobacter (C. gracilis, C. concisus, C. recta, and C. curva). The campylobacters are all microaerophiles, not anaerobes, and are discussed in Chapter 34. Curved or motile organisms that grow anaerobically but not in 5% CO2 should be retested in a microaerophilic atmosphere with approximately 6% oxygen.
Fusobacteriaceae
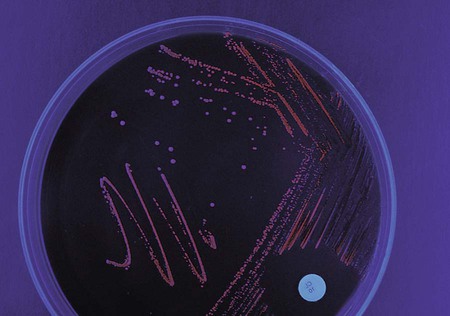
The Fusobacteriaceae family includes the genera Fusobacterium, Leptotrichia, and Sneathia. These organisms typically are isolated from the oral cavity as integral components of dental biofilms (Figure 42-11). The gram-negative Fusobacterium spp. are sensitive to kanamycin, and most strains fluoresce chartreuse. Different species have characteristic cell and colony morphologies (Figure 42-12).
Anaerobic Gram-Positive and Gram-Negative Cocci
As stated earlier in this chapter and in Chapter 41, the proper collection and transport of specimens for anaerobic culture cannot be overemphasized. General considerations are included in the discussion in Chapter 41. The gram-positive anaerobic cocci typically are found as part of the normal flora of the oral cavity, upper respiratory tract, gastrointestinal tract, female genitourinary tract, and the skin. The anaerobic cocci are non-spore forming and may appear slightly elongated. The cells vary in size and may be arranged in tetrads, chains, clusters, pairs, or clumps. Carbohydrate utilization varies among the genera. In addition, organisms typically classified as aerobic, such as Staphylococcus epidermidis, include strictly anaerobic strains. Staphylococcus saccharolyticus and Staphylococcus aureus subsp. anaerobius grow under anaerobic conditions, although after subculture they may develop aerotolerance. If a gram-positive coccus demonstrates resistance to metronidazole (5 µg) after 48 hours of incubation, it is likely a Streptococcus species.
Laboratory Diagnosis
Direct examination of clinical specimens reveals gram-positive or gram-negative cocci in chains, pairs, or singly. Follow-up tests to identify the organism are determined by interpretation of the initial Gram stain results. Organisms typically are isolated on anaerobic blood agar, and they can be differentiated using the special-potency antibiotic disks previously described in this chapter. Gram-positive cocci are sensitive to vancomycin and resistant to colistin. Gram-negative cocci typically are resistant to vancomycin. Peptostreptococcus anaerobius and Parvimonas micra demonstrate sensitivity to sodium polyanethol sulfonate (SPS). P. micra also produces a milky halo around the colonies on blood agar. Interpretation and identification of either gram-positive or gram-negative cocci from a clinical specimen should be reported with caution and should correlate with the patient’s signs and symptoms. Table 42-5 provides an identification scheme for representative anaerobic organisms.