Outflow Tract Ventricular Tachyarrhythmias
Mechanisms, Clinical Features, and Management
Idiopathic ventricular tachycardias (VTs) typically occur in the absence of structural heart disease and can originate from multiple anatomic regions, including the left and right ventricular outflow tracts (OTs), the left fascicular system, the mitral and tricuspid annuli, and the papillary muscles, or they can develop perivascularly in the epicardial space. More than half of all idiopathic VTs originate from the OTs, and of these, approximately 80% originate from the right ventricular outflow tract (RVOT).1 The remainder originate from the left ventricular outflow tract (LVOT), which is located immediately posterior to the RVOT and includes the aortic sinuses of Valsalva, the ventricular myocardium just inferior to the aortic valve, the aortomitral continuity (AMC), the superior basal septum (ventricular summit), and the epicardial OT surface.2–7 OT arrhythmias can present as isolated premature ventricular complexes (PVCs), as nonsustained VTs, or as sustained VTs.8 Symptoms of OT VTs vary widely and may include palpitations, presyncope, and syncope. Since the time that they were initially described, advances in our understanding of the mechanism and pathophysiology of OT VTs have been significant. This chapter reviews the anatomy, epidemiology, mechanisms, diagnosis, prognosis, and management of OT PVCs and VTs.
Anatomy
RVOT
The RVOT is generally divided into rightward (free wall), anterior, leftward (septal), and posterior portions (Figure 81-1).9 The RVOT courses anterior and leftward of the LVOT with the distal RVOT and pulmonic valve located leftward of the distal LVOT and aortic valve, respectively. The pulmonary valve is superior to the aortic valve, such that the posteroseptal RVOT is immediately anterior and adjacent to the aortic valve, specifically the right and left coronary cusps (RCCs and LCCs).9 Its inferior and most rightward portion is continuous with the tricuspid annulus and the interventricular septum, where the bundle of His and the proximal right bundle branch are located, whereas its most superior and leftward portion is above the anterior pulmonary valve leaflet (Figure 81-2). Although the RVOT is muscular throughout, it is relatively thin in the rightward, anterior, and subpulmonary valve portions, and it is thicker in the proximal posterior portion, where it is directly opposite the LVOT and the anterior interventricular septum.
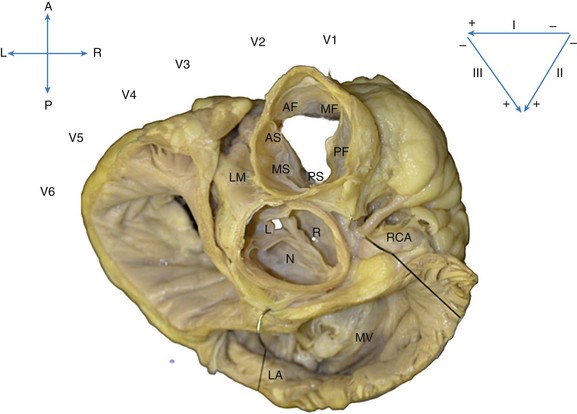
Figure 81-1 Superior View of the Human Heart With the Atria, Pulmonary Artery, and Aorta Cut Away
The heart is oriented with the anterior (A) aspect of the chest at the top, posterior (P) at the bottom, and right (R) and left (L) sides in the appropriate orientation. The location of the ECG leads on the chest is shown. The most anterior and superior part of the ventricles is the right ventricular free wall just below the pulmonic valve, which is separated into anterior (AF), mid (MF), and posterior (PF) free-wall regions. Just posterior to the free wall is the septal aspect of the RVOT, separated into anterior (AS), mid (MS), and posterior (PS) septal regions. Immediately posterior and slightly inferior to the RVOT septum lies the aortic root. The right coronary cusp (R) lies immediately posterior to the PS RVOT, and the left coronary cusp (L) lies posterior to the AS RVOT. The noncoronary cusp (N) overlies the atrial septum and therefore is a useful location for mapping atrial tachycardia but not OT VTs. Posterior to the aortic root lies the mitral annulus (MA), below the left atrium (LA).
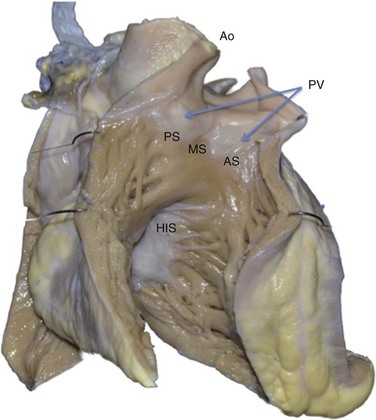
Figure 81-2 Anterior View of the Human Heart With the Free Wall of the Right Ventricle Cut Open
This view shows the anteroseptal (AS), midseptal (MS), and posteroseptal (PS) aspects of the RVOT just below the pulmonic valve (PV). Immediately posterior to the RVOT lies the aortic root (Ao). Inferior and posterior to the RVOT lies the para-Hisian region (His), a less common site of origin of idiopathic PVCs. This region at the superior aspect of the tricuspid valve has been termed the tricuspid “inflow tract.”
Relationship to Coronary Arteries
The left main coronary artery arises from the LCC, adjacent and immediately posterior to the RVOT below the pulmonary valve; therefore the potential for left main arterial damage exists during ablation both in the LCC and in the septal and posterior regions of the RVOT (see Figure 81-1). The left main coronary artery courses laterally relative to the RVOT as it branches into the left circumflex (LCx) and left anterior descending (LAD) arteries. Because the RVOT courses leftward, the right coronary artery (RCA), arising from the RCC, is closest to the proximal RVOT near the tricuspid annulus.
Aortic and Pulmonary Valve Cusps
The ventricular myocardium extends beyond the semilunar valves into the proximal pulmonary artery and the aortic valve cusps,10 and can be found both between and within the cusps.11 Some cases of myocardial sleeves in the region of the coronary artery have also been reported.12,13 Because the pulmonary valve is superior and leftward of the aortic valve, the RVOT beneath the pulmonary valve is at the same level as the aortic cusps. The RCC and the junction between the LCC and the RCC lie immediately adjacent to the posterior RVOT, beneath the pulmonary valve. Myocardial sleeves are found most often in the RCC and less commonly in the LCC.10 The NCC lies posterior and immediately adjacent to the interatrial septum; therefore its myocardial sleeves may be a site of origin for atrial tachycardias but not for idiopathic VTs.14
Epicardial and Perivascular Sites
Approximately 9% of idiopathic VTs originate epicardially, most often near coronary venous sites: the great cardiac vein (GCV), the anterior interventricular vein (AIV), and the middle cardiac vein (MCV).15 Immediately distal (in the retrograde direction to blood flow) to the coronary sinus (CS) os, the CS gives rise to the MCV, which courses in the posterior interventricular sulcus. The CS courses in the inferior aspect of the atrioventricular groove parallel to the mitral valve annulus, and ends at the valve of Vieussens in the region of obtuse marginal arteries. Beyond the valve of Vieussens, the vein continues as the GCV, coursing epicardially directly overlying the lateral mitral annulus. The junction of the AIV and the GCV is immediately lateral to the LCC. Proximal to distal, the AIV is adjacent to the posterolateral subvalvar RVOT, or the epicardial lateral RVOT, and the anterior epicardial space. Transpericardial mapping of the origin of epicardial idiopathic VTs has confirmed their propensity for origin near these perivascular structures.15
Left Ventricular Summit
OT VTs may also originate from the region of the LV summit, the most superior LV epicardial region at the top of the interventricular septum. The LV summit lies between the LAD and LCx arteries, near the junction of the GCV and the AIV, and superior to the aortic valve cusps.16 One series reported that 12% of idiopathic LV VTs originate from the LV summit.16 The superior-most region of the LV summit is close to the LAD and LCx arteries, along with prominent overlying epicardial fat, thus is inaccessible to epicardial catheter ablation; the lateral region is accessible via the GCV.
Epidemiology
OT VTs have accounted for approximately 10% of all patients referred for evaluation of VT.17 OT VTs may present from the spectrum of premature ventricular complexes, nonsustained VT, and sustained monomorphic VT.8 OT VT typically occurs in young to middle-aged patients, with mean age at presentation lower than that of patients with VT secondary to structural heart disease.18,19 Although some have noted a higher incidence in women,2 others have reported no gender predominance.8
Symptoms
Palpitations, chest pain, dyspnea, and light-headedness during episodes are common, and rarely syncope can occur; some patients may be entirely asymptomatic.18
Triggers
Episodes are often triggered by caffeine, emotional stress, or exercise, typically during the recovery period after exercise.18,20,21 In women, hormonal flux (e.g., premenstrual, perimenopausal) may be a particular trigger.21 Circadian variation with morning and late afternoon peaks of occurrence of outflow tract PVCs has also been reported.22 These suggest the importance of sympathetic activity and circulating catecholamines in the mechanism of OT VTs.
Mechanism
The mechanism of OT VTs is triggered activity due to cyclic adenosine monophosphate (cAMP)-mediated delayed afterdepolarizations (DADs).1,23 Increased cAMP can occur with beta-adrenergic receptor stimulation (e.g., during exercise), resulting in increased intracellular calcium and DADs, triggering OT VT.
Consistent with this mechanism, in the electrophysiology (EP) laboratory, OT PVCs and VTs typically are induced by burst atrial or ventricular pacing rather than by programmed stimulation (which would be more characteristic of monomorphic VT due to reentrant mechanism) and are facilitated by isoproterenol or epinephrine infusion.18,24 These VTs cease with adenosine, verapamil, and β-blockers, all of which interfere with the cAMP-mediated calcium flux.25,26
Diagnosis
Recognition and localization of OT arrhythmias are ideally suited to the 12-lead electrocardiogram (ECG)18 because they originate from a focal source and usually occur in a structurally normal heart. The classic ECG pattern consists of left bundle branch block morphology in V1 with an inferior axis (Figure 81-3). This occurs because the PVC/VT originates from the superior-most aspect of the ventricle, just at or below the level of the semilunar valves. Recognizing the typical ECG pattern is important for establishing the correct diagnosis and for discussing the prognosis with the patient. More detailed ECG localization is helpful when treatment options and the risks of catheter ablation are discussed, and when a starting point is identified from which more detailed intracardiac mapping can be performed in the EP laboratory.
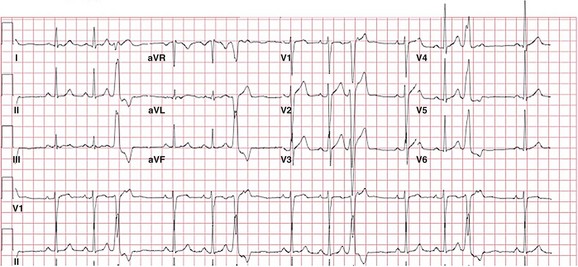
Figure 81-3 12-Lead ECG of a Patient With a Typical RVOT PVC
The PVC has a left bundle branch block pattern in V1 with an rS—a transition at lead V4 that is typical of RVOT PVCs. The PVC has a marked inferior axis and is multiphasic in lead I, suggesting a midseptal origin. Although notching in the inferior leads is more commonly seen in PVCs of free-wall origin, it can also be seen in PVCs of septal origin.
RVOT
The most common origin of OT VT is the septal aspect of the RVOT, just inferior to the pulmonic valve (see Figure 81-2).27 Because the PVC/VT exits from the superior-most region of the right ventricle, the inferior leads in stereotypical ECG morphology are markedly positive and narrow (see Figure 81-3). Leads aVR and aVL are markedly negative with a QS pattern. All RVOT PVCs/VT have left bundle branch block morphology in lead V1 (rS or QS; see Figure 81-3). The precordial lead transition from net negative to net positive (from r<S to R>s) is critical for distinguishing RVOT from LVOT origin. The transition for PVCs of RV origin typically occurs at or later than lead V3. Lead I is typically of low amplitude and is useful for differentiating posteroseptal (positive) from midseptal (multiphasic) or anteroseptal (negative) origin (Figure 81-4). The more leftward location of the anterior RVOT compared with the posterior RVOT accounts for the differences in lead I polarity (see Figures 81-1 and 81-4). The most common site of PVC origin is the anteroseptal or midseptal region.27 Less than 10% of RVOT PVCs originate from the free wall.28 For free-wall RVOT PVCs, the QRS remains inferiorly directed but is wider and notched compared with those of septal origin (see Figure 81-4). In the study by Dixit and colleagues,29 no QRS width cutoff clearly separated free-wall from septal origin; however, relative QRS widening with free-wall compared with septal origin was clearly noted within each patient. Because the RV free wall is more anteriorly located, the precordial transition is seen later than that of VT with septal origin, nearly always at or later than lead V4 (see Figure 81-4). Similar to septal PVCs, lead I is useful for differentiating anterior (negative) from posterior (positive) free-wall origin. Because a free-wall origin is very uncommon in patients with a structurally normal heart, the presence of PVCs of free-wall morphology should elicit a more thorough search for underlying structural heart disease, in particular arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D).
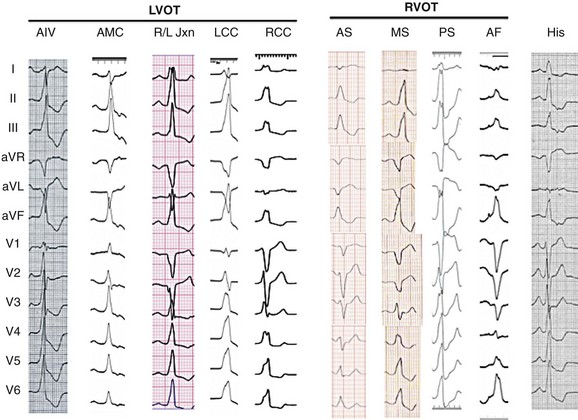
Figure 81-4 Typical 12-Lead ECG Morphologies of the OT VTs, All from Patients With the Origin Confirmed With Intracardiac Mapping and Ablation
From right to left, para-Hisian PVCs are not really located in the outflow tract but do have a left bundle inferior axis; the hallmark is the isoelectrical or “w” pattern in lead aVL and the more positive R wave in lead I. Next, an anterior free-wall (AF) PVC is shown, with the characteristic lower amplitude and notched inferior leads and a late precordial transition. The more typical septal origin PVCs are shown next; lead I distinguishes anteroseptal (AS, negative) from midseptal (MS, multiphasic) or posteroseptal (PS, positive) origin. The coronary cusp PVCs all have left bundle branch block (LBBB) morphology in V1 with an earlier precordial transition. More posteriorly located is the region of aortomitral continuity (AMC), which has an inferior axis and a signature qR pattern in lead V1. See text for a more detailed discussion.
PVCs may also originate lower in the OT, further beneath the pulmonic valve. As the origin becomes more inferior, a series of changes to the ECG occurs: The inferior leads become less positive (first lead III, then lead aVF, and finally lead II), lead aVL becomes less negative, and lead I becomes more positive (as the origin becomes more posterior toward the tricuspid valve; Figure 81-2). An ECG morphology that is slightly inferiorly directed (positive in II and aVF and biphasic or positive in III), flat, or w-shaped in lead aVL and is more positive in lead I than in the typical posteroseptal PVC is typical for a PVC that originates from the RV inflow tract at the level of the His bundle30 (see Figure 81-4). These para-Hisian PVCs are less common than OT PVCs but do occur in structurally normal hearts. The origin may be just superior or free-wall to the His bundle region, and careful mapping is critical to avoid heart block during ablation.
Even more infrequently, PVCs may originate superior to the pulmonic valve from myocardial fibers that extend into or just above the pulmonary valve.31 Few ECG features are helpful in distinguishing pulmonary artery (PA) from RVOT PVCs, other than a markedly inferior axis, earlier precordial transition (>R/S ratio in lead V2), and the presence of a small atrial and ventricular potential on the mapping catheter at the site of successful ablation.32 Pulmonary artery origin can be confirmed with right ventriculography or intracardiac echocardiography (ICE).
LVOT
Recognition that OT PVCs can also originate from the LVOT is increasing.33 The most common origin is from myocardial fibers within or adjacent to the aortic root,34,35 but PVCs may also originate from the left ventricular (LV) myocardium just below the aortic valve, from the LV summit at the top of the interventricular septum, from the region of the AMC, or from the LV outflow epicardium.36 LVOT PVCs also have a left bundle branch block pattern in lead V1 (usually rS) with an inferior axis. Because the aortic root is immediately posterior to the RVOT (see Figure 81-1), LVOT PVCs have an earlier precordial transition, typically at or before lead V3.11,37 Further analysis can be performed to identify the origin within the aortic root. PVCs from the LCC region typically have an rS in lead I and an earlier precordial transition (lead V1 or V2) than those from the RCC (see Figure 81-4). PVCs from the RCC typically are positive and notched in lead I, with a precordial transition at lead V2 or V3.38 The region at the junction of the RCC and LCC is a common site of aortic root PVC origin and has a signature “notch” or qrS pattern in lead V1 or V239,40 (see Figure 81-4).
As was already mentioned, PVCs with a precordial transition before lead V3 typically originate in the LVOT, and those with a transition after V3 typically originate in the RVOT. PVCs with a transition at lead V3 are therefore challenging to localize.41 In addition, cardiac rotation or precordial lead misplacement can confound most ECG localization algorithms. One study found that comparing the precordial transition of the PVC with that in sinus rhythm can be helpful in localizing PVCs with a V3 transition. If the PVC transition occurs after the sinus rhythm transition, the origin is RVOT with 93% specificity.42 Other algorithms are available for distinguishing RVOT from LVOT arrhythmias43,44 (Table 81-1, Distinguishing ECG Features of LVOT vs. RVOT PVCs).
Rarely, PVCs can originate just below the aortic valve at the AMC—the region between the aortic and mitral valves. These PVCs have a marked inferior axis with a “signature” qR pattern in lead V1 and a positive QRS complex in all precordial leads (see Figure 81-4).45
Several criteria can be used to identify an epicardial exit to OT PVCs. The typical morphology is a left bundle branch block/inferior axis with an early precordial transition, delayed or “slurred” intrinsicoid deflection, and a QS pattern in lead I. A criterion called the maximal deflection index (MDI) takes advantage of the slower intrinsicoid deflection for arrhythmias of epicardial origin.15 The MDI measures the time from onset to the latest R wave peak in any precordial lead divided by the QRS duration; an MDI greater than 0.55 suggests an epicardial origin that is best approached via the coronary venous system or epicardium rather than via the aortic cusps. The typical PVC morphology originating closest to the GCV or the AIV has an early transition (V1 or V2) with a slurred initial r wave in V1 and an rS or QS in lead I.46 Another common morphology for an epicardial OT PVC is a right bundle branch block pattern in V1, where a “pattern break” occurs from V1 to V3 (i.e., an R in V1 and V3 and an Rs or rS in V2).
One should always be aware of the location of the ECG electrodes on the chest when analyzing a 12-lead ECG of OT VT, particularly in the EP laboratory, where other patches on the chest may affect usual ECG lead placement. Limb lead placement in the anterior chest during a stress test or precordial lead placement at the wrong interspace may lead to a significant change in ECG morphology that may confound any PVC localization algorithm.47
Prognosis
In contrast to many ventricular arrhythmias that occur in the setting of structural heart disease, OT PVCs and VTs are generally considered benign, with long-term studies demonstrating that a vast majority of patients do not develop structural heart disease or sudden cardiac death.18 Of course, OT VT can also occur in patients with underlying structural heart disease—it is important to identify the typical OT morphology in these patients, as the presence of VT does not have the same implications as the presence of ventricular arrhythmias that are associated with scar. Two notable instances when OT VT may have more serious implications involve patients with “short coupled” PVCs triggering polymorphic VT and patients with a PVC-induced cardiomyopathy.
A malignant variant of “short-coupled” PVCs triggering torsade de pointes VT was first described in the 1990s.48 Initial reports identified a high mortality risk in these patients, despite medical treatment with traditional β-blockers or calcium channel blockers. Noda and Viskin then reemphasized the phenomenon of short-coupled OT PVCs that triggered torsade de pointes VT and resulted in syncope or sudden death.49,50 Although no absolute coupling interval cutoff identifies potentially “malignant” PVCs, the coupling interval that triggered polymorphic VT was generally shorter than that of benign OT PVCs, even within the same patient.51 Catheter ablation should be strongly considered in patients with short-coupled OT PVCs with syncope or runs of torsade de pointes VT,49 and an implanted cardioverter-defibrillator (ICD) may be required if the PVC trigger cannot be completely eliminated or if the patient has a history of cardiac arrest.
The syndrome of PVC-triggered torsade should be differentiated from inherited arrhythmia syndromes such as catecholaminergic polymorphic VT (CPVT). Both may be triggered by exercise. CPVT has a younger age of onset, a more malignant clinical history with frequent syncope, and a family history of sudden death. Genetic testing for CPVT can be performed. In some patients, PVCs triggering runs of polymorphic VT during extreme exertion may present in older adulthood. The prognosis in these patients has not been clearly defined but may be better than those with younger onset.52
A small percentage (5% to 7%) of patients with very frequent OT PVCs and VTs may develop a tachycardia-mediated cardiomyopathy.53,54 The threshold of ectopy required to produce left ventricular dysfunction has ranged from 17,000 to 30 000 PVCs/day,55 or 16% to 24% of daily heartbeats.53,54 Bogun and colleagues described a cohort of patients with cardiomyopathy and frequent PVCs who had near-complete resolution of the cardiomyopathy after PVC ablation.56 The mechanism of this cardiomyopathy is not well understood, but multiple subsequent studies have demonstrated significant improvement in LV function after PVC ablation in this cohort of patients. In the Bogun trial, a cardiomyopathy was never seen in patients with less than 10% PVC burden and occurred most frequently in patients with more than 20% PVCs.
In some patients with underlying structural heart disease and frequent PVCs, a significant improvement in LV function can be noted after PVC ablation. Among patients with residual PVCs after ablation, those with a burden reduced to less than 5% generally fared well, with LV ejection fraction (EF) improvement of 10% to 15%.57 Therefore, in nearly any patient with frequent PVCs (>20% or 20 000/day) and reduced LV function, medical or catheter-based treatment of PVCs should be considered. Patients who are asymptomatic with normal LV size and function should undergo yearly echocardiograms and are often treated with β-blockers, although limited data support this. At the first sign of LV dilatation or decreased LV function, more aggressive treatment with antiarrhythmic medication or catheter ablation should be undertaken. It should be noted that even after complete resolution of a tachycardia-mediated cardiomyopathy, some residual LV structural changes and risk of malignant arrhythmias may occur, particularly with any recurrent tachycardia.58
A common inherited arrhythmia syndrome that is also associated with OT PVCs is ARVD/C. This inherited disorder of the myocardial desmosome leads to fibrofatty replacement of the RV myocardium and is associated with syncope, left bundle branch (LBB) morphology PVCs/VT, and sudden death. Screening tests for ARVC include genetic testing, echocardiography, and magnetic resonance imaging (MRI). Treatment of PVCs associated with ARVC is different from treatment of idiopathic PVCs, as an implantable defibrillator is nearly always required and ablation typically requires an epicardial approach. Therefore, distinguishing patients with benign OT PVCs from those with ARVC is important. In general, young patients with a single-morphology PVC of typical origin from the RV septum or the LVOT and no family history of sudden death do not require screening beyond transthoracic echocardiography. Patients with multiple PVC morphologies or PVCs originating from an unusual location, such as the RV free wall, may require a screening MRI. ECG criteria have been developed that help to distinguish PVCs/VT in ARVC from benign idiopathic VT.59 These include a late precordial transition at or beyond lead V5, QRS notching in multiple leads, and a lead I QRS duration of 120 milliseconds or longer.
Finally, in the inherited arrhythmia syndromes such as Brugada syndrome or long QT syndrome, PVCs can serve as initiators of life-threatening arrhythmias by creating long-short coupling intervals that may predispose to torsade de pointes VT. Ablation of PVCs in patients with these inherited syndromes and frequent ICD shocks has been associated with a marked reduction in ICD therapy.60
Management
Medical Therapy
OT VTs generally respond well to β-blockers or calcium channel blockers. These medications are typically first-line treatment because they have mild side effect profiles as compared with other antiarrhythmic medications, along with success rates in the range of 65%.18 Side effects such as fatigue may limit patient adherence. Calcium channel blockers may be tried in patients with asthma or intolerance to β-blockers but are typically less effective.
For patients who do not respond to β-blockers or calcium channel blockers, the addition or substitution of a class IC antiarrhythmic drug (flecainide, propafenone) may be useful.19,61 In refractory patients, class III drugs (sotalol or amiodarone) may also be considered.61 Because class I medications are contraindicated in the setting of ischemic heart disease, coronary artery disease should be excluded in patients with intermediate or higher risk before administration of such medications is considered. The efficacy of drug therapy can be monitored by patient symptoms, Holter monitoring, or stress testing. We typically perform a routine stress test every other year in patients on class IC antiarrhythmic drugs.
Mapping and Catheter Ablation
Mapping of PVCs in the EP laboratory requires fluoroscopy and an electrophysiological recording system that is typically coupled with an electroanatomical mapping system. For cases of aortic root or LVOT arrhythmia, phased-array ICE can be invaluable. PVCs can be mapped with pace mapping or activation mapping. If PVCs occur spontaneously and frequently, activation mapping can be used to identify the earliest bipolar electrogram preceding the surface QRS. For typical OT PVCs, a bipolar activation time longer than 20 to 25 milliseconds before QRS is usually sufficient for successful ablation. A QS pattern on the unipolar electrogram can also be recorded at the site of origin of the PVC/VT. Pace mapping should also be performed at the earliest site—recording of a pace map that does not match the clinical PVC at the earliest recorded site suggests that the PVC may originate from an adjacent cardiac chamber (e.g., LVOT when recording from posterior RVOT or epicardium). In cases of infrequent PVCs, pace mapping alone can be used to localize PVC origin. Pacing output should be set to twice diastolic threshold to avoid far-field or anodal capture. Pace mapping should begin in the area of suspected VT origin based on the 12-lead ECG and the catheter manipulated to achieve a 12/12 lead pace-map match with clinic VT (Figure 81-5). The first beat of sustained VT can sometimes have a slightly different morphology than the sustained run as the result of repolarization changes during initiation; therefore the morphology during sustained VT should be targeted. In addition, sometimes a pace map can appear similar over a sizable distance (1 to 3 cm2,62,63). Attempts to match notching and QRS amplitude in all 12 leads will yield better ablation success.
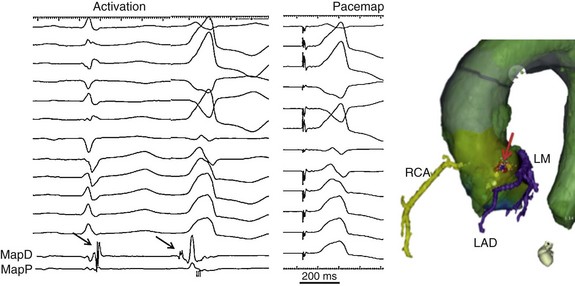
Figure 81-5 Left panel, Surface ECG leads and intracardiac electrograms recorded from the distal (MapD) and proximal (MapP) mapping electrodes in a patient with PVC originating from the left sinus of Valsalva. Note the rS in lead I, the notch in lead V1, and early precordial transition. On the bipolar MapD catheter is a small potential that is late during sinus rhythm but precedes the QRS during the PVC by 22 milliseconds (black arrow). Middle panel, A perfect pace map from this location is shown. Reversal of potentials with the catheter in the aortic cusps confirms a supra-aortic valve PVC origin, and ablation at this site was successful in eliminating the PVC. Right panel, Registration of a computed tomographic image with the electroanatomical mapping system allows delineation of the proximal coronary arteries so that ablation can be performed without a coronary angiogram (as long as registration is confirmed to be accurate). Here the ablation sites are denoted by red tags (arrow) in the left coronary cusp, near the junction of the left and right cusps—a safe distance from the left main ostium (LM) and the left anterior descending coronary artery (LAD, colored purple). The right coronary artery (RCA) is colored yellow. The yellow tags represent sites of pace mapping.
For nearly all patients, mapping of the RVOT should occur first, given the low risk and high prevalence of RVOT origin PVCs. When the RVOT is mapped, positioning a catheter at the RV apex helps to delineate septal from free-wall RVOT location in the left anterior oblique projection. Typically the site of earliest activation will also have a perfect 12-lead pace-map match; if activation is longer than 20 ms before QRS with qS on the unipolar electrogram, then radiofrequency (RF) energy should be applied. A standard nonirrigated 4-mm RF energy ablation catheter can be used in the RVOT. Because the origin of these PVCs is superficial, powers of 25 to 30 watts are usually sufficient. An early “flurry” of PVCs during ablation is often considered a good sign, although this may occur with catheter ablation in any region. Very high-power lesions should be used cautiously, given the proximity of the superior RVOT, and particularly the proximal pulmonary artery, to the coronary arteries.64 If the earliest activation is less than 20 milliseconds before QRS, if a small initial r wave is present on the unipolar electrogram at the earliest site, and/or if the pace map has a later precordial transition than the clinical PVC, mapping of the LVOT should be performed. Because the aortic valve plane is inferior to the plane of the pulmonic valve (Figure 81-6), when PVC activation is earliest in the low posterior RVOT and is less than 20 milliseconds before QRS, mapping of the aortic cusps should be performed. Typically the RCC is directly posterior to the inferoposterior RVOT, and the LCC is posterior to the anterior RVOT (see Figure 81-1). Unsuccessful ablation in these RVOT regions should lead to exploration of the corresponding aortic sinus regions. If after ablation in the superior RVOT, a change in PVC morphology is noted with a more inferior axis, the septal aspect of the pulmonary artery should be explored.65 Coronary angiography should be performed before ablation in the PA. Cryoablation of RVOT and of pulmonary artery PVCs has been described but offers few advantages over RF ablation unless one is close to the His bundle.66 Cryoablation may have a role in para-Hisian PVCs, although before ablation is performed in the para-Hisian region, the RCC should be explored, because this region lies just above the His region and may serve as a safer location for catheter ablation.67
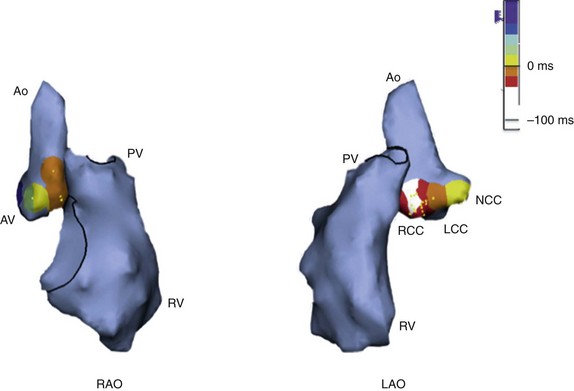
Figure 81-6 Electroanatomical Map of RVOT and LVOT in a Right Anterior Oblique (RAO) and a Left Anterior Oblique (LAO) Projection During an Ablation Procedure
Right ventricle (RV), aorta (Ao), pulmonic valve (PV), and aortic valve (AV) locations are shown. Note that the aortic root is located inferiorly to the level of the pulmonic valve. The relationship of the right coronary sinus of Valsalva and the bundle of His region, at the superoseptal tricuspid annulus, can also be appreciated in this figure. PVC activation is superimposed on the aortic root, with the site of earliest activation depicted in white at the right coronary cusp (RCC), which lies inferior and immediately posterior to the posteroseptal RVOT. Ablation at the RCC was successful in eliminating the PVC. LCC, Left coronary cusp; NCC, noncoronary cusp.
For LVOT mapping catheter localization, unless the operator has experience with aortography for identifying the coronary cusps, ICE is extremely useful for identifying the aortic cusps and the catheter position within the cusps (Figure 81-7). Fluoroscopy alone can often be misleading. The aortic root is typically mapped using the retrograde aortic approach, and it should be mapped as a separate cardiac chamber, with multiple activation points and pace maps taken throughout each coronary cusp at the valve level and up to 2 cm above the valve. The NCC overlies the left atrium and has never been associated with PVC origin in our experience. However, sites throughout the RCC, R/LCC junction, and LCC have been described. In contrast to the RVOT, pace maps in the aortic root are often unreliable because pace mapping can capture distant structures rather than the thin sleeves of myocardium in the aortic root that may be the source of PVCs. The precordial transition will often be a closer match than the RVOT, and although a poor pace map should not dissuade ablation, a perfect pace map is a useful guide for suggesting an aortic root origin.
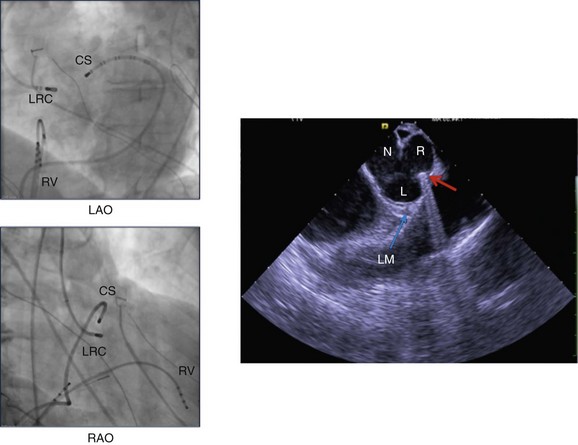
Figure 81-7 The left panels demonstrate fluoroscopic views of catheter positions in the RAO and LAO projections during an ablation procedure. The coronary sinus catheter (CS) has been advanced distally to the anterior interventricular vein (AIV) for mapping purposes. The ablation catheter is taking a retrograde aortic route to the junction of the right and left coronary cusps (RLC). The exact location of the ablation catheter tip is difficult to confirm using fluoroscopy. In the right panel, a phased-array ICE image is shown, confirming the catheter tip location at the junction of the left (L) and right (R) coronary cusps (red arrow). The location of the left main coronary artery (LM) is also shown (blue arrow). N, Noncoronary cusp.
The primary tool used for mapping in the aortic root is activation mapping. As can be seen with RVOT sites, early sites are typically greater than 20 milliseconds before QRS. In the aortic root, “reversal” of a sharp potential from late during sinus rhythm to early during a PVC confirms the origin as aortic root and often suggests a successful ablation site68 (see Figure 81-5). Because of the proximity of the aortic valve region to the coronary arteries, coronary angiography is recommended before ablation is performed in the aortic root. If ICE is being used by an experienced operator, coronary angiography may be omitted if the site of earliest activation at the R/LCC junction or at the LCC if the coronary artery can be visualized farther than 1 cm away from the left main coronary artery. Registering the electroanatomical map with another imaging modality such as computed tomography (CT) angiography can facilitate identification of the proximal coronary arteries (see Figure 81-5). Visualizing the RCA with ICE is difficult; therefore coronary angiography should be performed before ablation within the right cusp. If ablation at an early site is ineffective, changing the angle of the catheter or looping the catheter into the LV cavity and back up into the aortic root can sometimes help. Mapping in the aortic root should be performed under systemic heparinization, and ablation should be performed with the use of irrigated ablation catheters, because any thrombus can potentially embolize down a coronary artery. Power usually is initiated at 15 to 20 watts and is slowly titrated up to 30 watts. Higher powers usually are not needed, although for an intramyocardial or epicardial focus, powers of up to 50 watts may be used. The impedance drop should be carefully observed and power discontinued at any sign of a rise in impedance.
The aortic cusps are the most common sites of LVOT PVCs; other sites include the LVOT myocardium just beneath the aortic valve and the LVOT epicardium, which can be approached via the aortic root or the coronary venous system or by subxiphoid epicardial access. Mapping and ablation of the LVOT endocardium beneath the aortic valve can be undertaken using conventional activation and pace mapping as described for the RVOT, although catheter stability is often challenging. For PVCs with a suggested epicardial exit by ECG or mapping, we often first explore the coronary venous system (GCV or AIV) and the left aortic sinus before considering epicardial access, because epicardial fat and proximity to the coronary arteries often limit the ability to perform epicardial ablation. A coronary sinus venogram can be performed to delineate the caliber and anatomy of the distal AIV. We place a small coronary sinus catheter as far distally as possible in the AIV for all LV outflow tract PVCs, to compare activation timing during the PVC with the use of catheters in the RVOT and the aortic root (see Figure 81-7). The main limitations of ablation within the GCV/AIV include the following: (1) the small size may not allow access with the ablation catheter; (2) low flow within a small vein may limit the power delivered, even with irrigated RF ablation; and (3) the proximity to the left main and LAD coronary arteries (Figure 81-8). Coronary angiography should always be performed before ablation is begun in the GCV/AIV so that closeness to the LAD coronary artery may be noted and ablation avoided if the catheter tip is less than 5 mm from the coronary artery. If activation is noted as equally early in AIV and LCC, ablation in the LCC can be safer and more effective because higher power can be safely delivered.69 In some cases, the AIV has been the only site in which PVC elimination has occurred.46,70
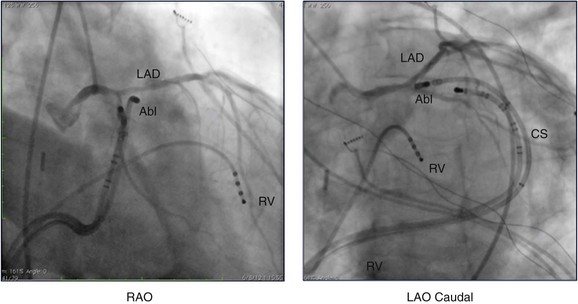
Figure 81-8 Coronary angiogram of the left coronary system shown in the right anterior oblique (RAO) and left anterior oblique (LAO) caudal projections during an ablation procedure. The ablation catheter (Abl) tip has been advanced via the coronary sinus (CS) distal to the beginning of the anterior interventricular vein and is located at the site of earliest activation. The angiogram demonstrates that the ablation catheter tip is just below the left anterior descending (LAD) coronary artery—a common challenge at this location. In this case, the ablation catheter was moved slightly more inferiorly, and then successful ablation was performed. RV, Right ventricle.
Percutaneous subxiphoid access can also be used to gain transpericardial access to map the epicardial surface of the OT. Although this is often limited by epicardial fat and proximity to the epicardial coronary arteries, a safe site that results in PVC elimination can be identified occasionally. It should be emphasized that even in expert hands, some PVCs of epicardial or intramyocardial origin near a major coronary artery cannot be eliminated with catheter ablation. Cryoablation has been performed for lesions near epicardial coronary arteries or the His bundle.71 In theory the warm blood pool would protect the coronary arteries, making cryoablation safer than RF energy when near a coronary vessel. However, animal studies have demonstrated that chronic coronary artery damage can occur after cryoablation directly on top of coronary arteries.72 In cases of unsuccessful ablation, treatment with drug therapy, including 1C agents, sotalol, or, if necessary, amiodarone, can then be undertaken. Minimally invasive surgical cryoablation under direct visualization has also been described for malignant PVCs originating near a coronary artery.73
References
1. Iwai, S, Cantillon, DJ, Kim, RJ, et al. Right and left ventricular outflow tract tachycardias: Evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol. 2006; 17:1052–1058.
2. Callans, DJ, Menz, V, Schwartzman, D, et al. Repetitive monomorphic tachycardia from the left ventricular outflow tract: Electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997; 29:1023–1027.
3. Sekiguchi, Y, Aonuma, K, Takahashi, A, et al. Electrocardiographic and electrophysiologic characteristics of ventricular tachycardia originating within the pulmonary artery. J Am Coll Cardiol. 2005; 45:887–895.
4. Yamauchi, Y, Aonuma, K, Takahashi, A, et al. Electrocardiographic characteristics of repetitive monomorphic right ventricular tachycardia originating near the his-bundle. J Cardiovasc Electrophysiol. 2005; 16:1041–1048.
5. Yamada, T, McElderry, HT, Okada, T, et al. Idiopathic left ventricular arrhythmias originating adjacent to the left aortic sinus of valsalva: Electrophysiological rationale for the surface electrocardiogram. J Cardiovasc Electrophysiol. 2010; 21:170–176.
6. Alasady, M, Singleton, CB, McGavigan, AD. Left ventricular outflow tract ventricular tachycardia originating from the noncoronary cusp: Electrocardiographic and electrophysiological characterization and radiofrequency ablation. J Cardiovasc Electrophysiol. 2009; 20:1287–1290.
7. Kumagai, K, Fukuda, K, Wakayama, Y, et al. Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 2008; 19:495–501.
8. Kim, RJ, Iwai, S, Markowitz, SM, et al. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol. 2007; 49:2035–2043.
9. Asirvatham, SJ. Correlative anatomy for the invasive electrophysiologist: Outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol. 2009; 20:955–968.
10. Hasdemir, C, Aktas, S, Govsa, F, et al. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin Electrophysiol. 2007; 30:534–539.
11. Asirvatham, SJ. Correlative anatomy and electrophysiology for the interventional electrophysiologist: Right atrial flutter. J Cardiovasc Electrophysiol. 2009; 20:113–122.
12. Kanagaratnam, L, Tomassoni, G, Schweikert, R, et al. Ventricular tachycardias arising from the aortic sinus of valsalva: An under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol. 2001; 37:1408–1414.
13. Srivathsan, KS, Bunch, TJ, Asirvatham, SJ, et al. Mechanisms and utility of discrete great arterial potentials in the ablation of outflow tract ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2008; 1:30–38.
14. Yamada, T, Huizar, JF, McElderry, HT, et al. Atrial tachycardia originating from the noncoronary aortic cusp and musculature connection with the atria: Relevance for catheter ablation. Heart Rhythm. 2006; 3:1494–1496.
15. Daniels, DV, Lu, YY, Morton, JB, et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of valsalva: Electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006; 113:1659–1666.
16. Yamada, T, McElderry, HT, Doppalapudi, H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: Anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol. 2010; 3:616–623.
17. Brooks, R, Burgess, JH. Idiopathic ventricular tachycardia. A review. Medicine (Baltimore). 1988; 67:271–294.
18. Buxton, AE, Waxman, HL, Marchlinski, FE, et al. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation. 1983; 68:917–927.
19. Mont, L, Seixas, T, Brugada, P, et al. The electrocardiographic, clinical, and electrophysiologic spectrum of idiopathic monomorphic ventricular tachycardia. Am Heart J. 1992; 124:746–753.
20. Lemery, R, Brugada, P, Bella, PD, et al. Nonischemic ventricular tachycardia: Clinical course and long-term follow-up in patients without clinically overt heart disease. Circulation. 1989; 79:990–999.
21. Marchlinski, FE, Deely, MP, Zado, ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J. 2000; 139:1009–1013.
22. Hayashi, H, Fujiki, A, Tani, M, et al. Circadian variation of idiopathic ventricular tachycardia originating from right ventricular outflow tract. Am J Cardiol. 1999; 84:99–101.
23. Yamawake, N, Nishizaki, M, Hayashi, T, et al. Autonomic and pharmacological responses of idiopathic ventricular tachycardia arising from the left ventricular outflow tract. J Cardiovasc Electrophysiol. 2007; 18:1161–1166.
24. Niroomand, F, Carbucicchio, C, Tondo, C, et al. Electrophysiological characteristics and outcome in patients with idiopathic right ventricular arrhythmia compared with arrhythmogenic right ventricular dysplasia. Heart. 2002; 87:41–47.
25. Lerman, BB, Stein, K, Engelstein, ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation. 1995; 92:421–429.
26. Sung, RJ, Keung, EC, Nguyen, NX, et al. Effects of beta-adrenergic blockade on verapamil-responsive and verapamil-irresponsive sustained ventricular tachycardias. J Clin Invest. 1988; 81:688–699.
27. Movsowitz, C, Schwartzman, D, Callans, DJ, et al. Idiopathic right ventricular outflow tract tachycardia: Narrowing the anatomic location for successful ablation. Am Heart J. 1996; 131:930–936.
28. Tada, H, Ito, S, Naito, S, et al. Prevalence and electrocardiographic characteristics of idiopathic ventricular arrhythmia originating in the free wall of the right ventricular outflow tract. Circulation. 2004; 68:909–914.
29. Dixit, S, Gerstenfeld, EP, Callans, DJ, et al. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: Distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2003; 14:1–7.
30. Yamauchi, Y, Aonuma, K, Takahashi, A, et al. Electrocardiographic characteristics of repetitive monomorphic right ventricular tachycardia originating near the his-bundle. J Cardiovasc Electrophysiol. 2005; 16:1041–1048.
31. Timmermans, C, Rodriguez, LM, Medeiros, A, et al. Radiofrequency catheter ablation of idiopathic ventricular tachycardia originating in the main stem of the pulmonary artery. J Cardiovasc Electrophysiol. 2003; 13:281–284.
32. Sekiguchi, Y, Aonuma, K, Takahashi, A, et al. Electrocardiographic and electrophysiologic characteristics of ventricular tachycardia originating within the pulmonary artery. J Am Coll Cardiol. 2005; 45:887–895.
33. Callans, DJ, Menz, V, Schwartzman, D, et al. Repetitive monomorphic tachycardia from the left ventricular outflow tract: Electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997; 29:1023–1027.
34. Kanagaratnam, L, Tomassoni, G, Schweikert, R, et al. Ventricular tachycardias arising from the aortic sinus of valsalva: An under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol. 2001; 37:1408–1414.
35. Ouyang, F, Fotuhi, P, Ho, SY, et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: Electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002; 39:500–508.
36. Tada, H, Nogami, A, Naito, S, et al. Left ventricular epicardial outflow tract tachycardia: A new distinct subgroup of outflow tract tachycardia. Japanese Circulation J. 2001; 65:723–730.
37. Hachiya, H, Aonuma, K, Yamauchi, Y, et al. How to diagnose, locate, and ablate coronary cusp ventricular tachycardia. J Cardiovasc Electrophysiol. 2002; 13:551–556.
38. Lin, D, Ilkhanoff, L, Gerstenfeld, E, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008; 5:663–669.
39. Yamada, T, Yoshida, N, Murakami, Y, et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of valsalva in the aorta: The activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm. 2008; 5:184–192.
40. Bala, R, Garcia, FC, Hutchinson, MD, et al. Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm. 2010; 7:312–322.
41. Tanner, H, Hindricks, G, Schirdewahn, P, et al. Outflow tract tachycardia with r/s transition in lead v3: Six different anatomic approaches for successful ablation. J Am Coll Cardiol. 2005; 45:418–423.
42. Betensky, BP, Park, RE, Marchlinski, FE, et al. The v(2) transition ratio: A new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011; 57:2255–2262.
43. Yang, Y, Saenz, LC, Varosy, PD, et al. Using the initial vector from surface electrocardiogram to distinguish the site of outflow tract tachycardia. Pacing Clin Electrophysiol. 2007; 30:891–898.
44. Kumagai, K, Fukuda, K, Wakayama, Y, et al. Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 2008; 19:495–501.
45. Shimoike, E, Ohba, Y, Yanagi, N, et al. Radiofrequency catheter ablation of left ventricular outflow tract tachycardia: Report of two cases. J Cardiovasc Electrophysiol. 1998; 9:196–202.
46. Hirasawa, Y, Miyauchi, Y, Iwasaki, YK, et al. Successful radiofrequency catheter ablation of epicardial left ventricular outflow tract tachycardia from the anterior interventricular coronary vein. J Cardiovasc Electrophysiol. 2005; 16:1378–1380.
47. Anter, E, Frankel, DS, Marchlinski, FE, et al. Effect of electrocardiographic lead placement on localization of outflow tract tachycardias. Heart Rhythm. 2012; 9:697–703.
48. Leenhardt, A, Glaser, E, Burguera, M, et al. Short-coupled variant of torsade de pointes: A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994; 89:206–215.
49. Noda, T, Shimizu, W, Taguchi, A, et al. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol. 2005; 46:1288–1294.
50. Viskin, S, Rosso, R, Rogowski, O, et al. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: A not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005; 16:912–916.
51. Shimizu, W. Arrhythmias originating from the right ventricular outflow tract: How to distinguish “malignant” from “benign”? Heart Rhythm. 2009; 6:1507–1511.
52. Tan, JH, Scheinman, MM. Exercise-induced polymorphic ventricular tachycardia in adults without structural heart disease. Am J Cardiol. 2008; 101:1142–1146.
53. Baman, TS, Lange, DC, Ilg, KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010; 7:865–869.
54. Hasdemir, C, Ulucan, C, Yavuzgil, O, et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: The incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011; 22:663–668.
55. Yarlagadda, RK, Iwai, S, Stein, KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005; 112:1092–1097.
56. Bogun, F, Crawford, T, Reich, S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007; 4:863–867.
57. Mountantonakis, SE, Frankel, DS, Gerstenfeld, EP, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: Effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011; 8:1608–1614.
58. Nerheim, P, Birger-Botkin, S, Piracha, L, et al. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004; 110:247–252.
59. Hoffmayer, KS, Machado, ON, Marcus, GM, et al. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2011; 58:831–838.
60. Haissaguerre, M, Extramiana, F, Hocini, M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003; 108:925–928.
61. Ritchie, AH, Kerr, CR, Qi, A, et al. Nonsustained ventricular tachycardia arising from the right ventricular outflow tract. Am J Cardiol. 1989; 64:594–598.
62. Azegami, K, Wilber, DJ, Arruda, M, et al. Spatial resolution of pacemapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2005; 16:823–829.
63. Bogun, F, Taj, M, Ting, M, et al. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008; 5:339–344.
64. Vaseghi, M, Cesario, DA, Mahajan, A, et al. Catheter ablation of right ventricular outflow tract tachycardia: Value of defining coronary anatomy. J Cardiovasc Electrophysiol. 2006; 17:632–637.
65. Tada, H, Tadokoro, K, Miyaji, K, et al. Idiopathic ventricular arrhythmias arising from the pulmonary artery: Prevalence, characteristics, and topography of the arrhythmia origin. Heart Rhythm. 2008; 5:419–426.
66. Kurzidim, K, Schneider, HJ, Kuniss, M, et al. Cryocatheter ablation of right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2005; 16:366–369.
67. Yamada, T, McElderry, HT, Doppalapudi, H, et al. Catheter ablation of ventricular arrhythmias originating in the vicinity of the his bundle: Significance of mapping the aortic sinus cusp. Heart Rhythm. 2008; 5:37–42.
68. Srivathsan, KS, Bunch, TJ, Asirvatham, SJ, et al. Mechanisms and utility of discrete great arterial potentials in the ablation of outflow tract ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2008; 1:30–38.
69. Jauregui Abularach, ME, Campos, B, Park, KM, et al. Ablation of ventricular arrhythmias arising near the anterior epicardial veins from the left sinus of valsalva region: ECG features, anatomic distance, and outcome. Heart Rhythm. 2012; 9:865–873.
70. Obel, OA, d’Avila, A, Neuzil, P, et al. Ablation of left ventricular epicardial outflow tract tachycardia from the distal great cardiac vein. J Am Coll Cardiol. 2006; 48:1813–1817.
71. Di Biase, L, Al-Ahamad, A, Santangeli, P, et al. Safety and outcomes of cryoablation for ventricular tachyarrhythmias: Results from a multicenter experience. Heart Rhythm. 2011; 8:968–974.
72. D’Avila, A, Aryana, A, Thiagalingam, A, et al. Focal and linear endocardial and epicardial catheter-based cryoablation of normal and infarcted ventricular tissue. Pacing Clin Electrophysiol. 2008; 31:1322–1331.
73. Frey, B, Kreiner, G, Fritsch, S, et al. Successful treatment of idiopathic left ventricular outflow tract tachycardia by catheter ablation or minimally invasive surgical cryoablation. Pacing Clin Electrophysiol. 2000; 23:870–876.