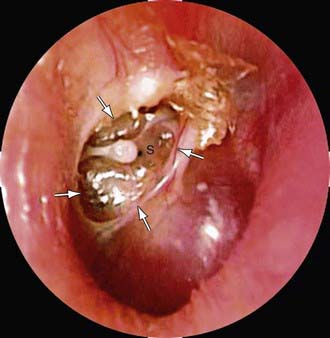
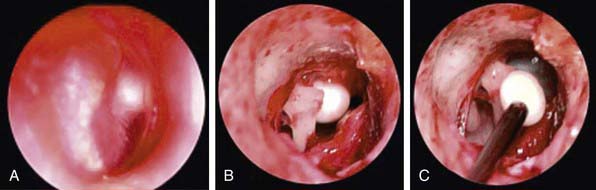
Chapter 632 Otitis Media
Accurate diagnosis of OM in infants and young children may be difficult (Table 632-1). Symptoms may not be apparent, especially in early infancy and in chronic stages of the disease. The eardrum may be obscured by cerumen, removal of which may be arduous and time-consuming. Abnormalities of the eardrum may be subtle and difficult to appreciate. In the face of these difficulties, both underdiagnosis and overdiagnosis occur. Once a diagnosis of OM has been established, its significance to the child’s health and well-being and its optimal method of management remain open to question and the subjects of continuing controversy. There is lack of consensus among authorities concerning benefit-risk ratios of available medical and surgical treatments; while OM can be responsible for serious infectious complications, middle- and inner-ear damage, hearing impairment, and indirect impairments of speech, language, cognitive, and psychosocial development, most cases of OM are not severe and are self-limiting.
Table 632-1 DEFINITION OF ACUTE OTITIS MEDIA
AOM, acute otitis media; MEE, middle-ear effusion; TM, tympanic membrane.
From Subcommittee on Management of Acute Otitis Media: Diagnosis and management of acute otitis media, Pediatrics 113:1451–1465, 2004.
Epidemiology
Congenital Anomalies
OM is universal among infants with unrepaired palatal clefts, and is also highly prevalent among children with submucous cleft palate, other craniofacial anomalies, and Down syndrome (Chapter 76). The common feature in these congenital anomalies is a deficiency in the functioning of the eustachian tubes, which predisposes these children to middle ear disease.
Vaccination Status
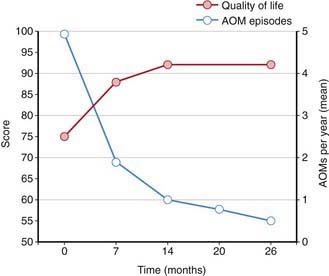
Streptococcus pneumoniae (Chapter 175) has historically been the most common pathogen identified in patients with acute OM. Vaccination of infants with a conjugated pneumococcal vaccine has a modest effect, lowering visits to physicians and antibiotic prescriptions for OM by only 6-8%. Vaccination does appear to have a somewhat more protective effect in limiting frequent OM episodes and the need for surgical intervention with tympanostomy tubes. Pneumococcal vaccination has decreased the overall rate of episodes of OM associated with pneumococcus and increased quality of life (Fig. 632-1). Annual influenza virus vaccination also results in a decrease in OM incidence.
Pathogenesis
Host Factors
The effectiveness of a child’s immune system in response to the bacterial and viral insults of the upper airway and middle ear during early childhood probably is the most important factor in determining which children are otitis prone. The maturation of this immune system during early childhood is most likely the primary event leading to the decrease in incidence of OM as children move through childhood. IgA deficiency is found in some children with recurrent AOM but the significance is questionable, inasmuch as IgA deficiency is also found not infrequently in children without recurrent AOM. Selective IgG subclass deficiencies (despite normal total serum IgG) may be found in children with recurrent AOM in association with recurrent sinopulmonary infection, and these deficiencies probably underlie the susceptibility to infection. Children with recurrent OM that is not associated with recurrent infection at other sites rarely have a readily identifiable immunologic deficiency. Nonetheless, evidence that subtle immune deficits play a role in the pathogenesis of recurrent AOM is provided by studies involving antibody responses to various types of infection and immunization; by the observation that breast milk feeding, as opposed to formula feeding, confers limited protection against the occurrence of OM in infants with cleft palate; and by studies in which young children with recurrent AOM achieved a measure of protection from intramuscularly administered bacterial polysaccharide immune globulin or intravenously administered polyclonal immunoglobulin. This evidence, along with the documented decrease incidence of upper respiratory tract infections and OM as children’s immune systems develop and mature is indicative of the importance of a child’s innate immune system in the pathogenesis of OM (Chapter 118).
Viral Pathogens
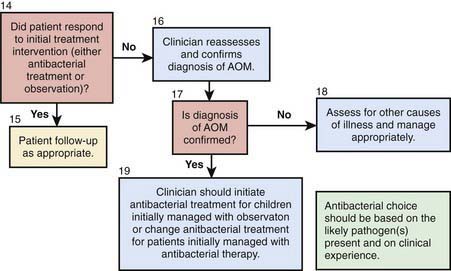
Although OM may develop and certainly may persist in the absence of apparent respiratory tract infection, many, if not most, episodes are initiated by viral or bacterial upper respiratory tract infection. In a study of children in group daycare, AOM was observed in approximately 30-40% of children with respiratory illness caused by RSV (Chapter 252), influenza viruses (Chapter 250), or adenoviruses (Chapter 254), and in approximately 10-15% of children with respiratory illness caused by parainfluenza viruses, rhinoviruses, or enteroviruses. Viral infection of the upper respiratory tract results in release of cytokines and inflammatory mediators, some of which may cause eustachian tube dysfunction. Respiratory viruses also may enhance nasopharyngeal bacterial colonization and adherence and impair host immune defenses against bacterial infection.
Clinical Manifestations
Symptoms of AOM are variable, especially in infants and young children. In young children, evidence of ear pain may be manifested by irritability or a change in sleeping or eating habits and occasionally, holding or tugging at the ear (see Table 632-1). Pulling at the ear has a low sensitivity and specificity. Fever may also be present. Rupture of the tympanic membrane with purulent otorrhea is uncommon. Systemic symptoms and symptoms associated with upper respiratory tract infections also occur; occasionally there may be no symptoms, the disease having been discovered at a routine health examination. OME often is not accompanied by overt complaints of the child but can be accompanied by hearing loss. This hearing loss may manifest as changes in speech patterns but often goes undetected if unilateral or mild in nature, especially in younger children. Balance difficulties or disequilibrium can also be associated with OME and older children may complain of mild discomfort or a sense of fullness in the ear (Chapter 628).
Examination of the Eardrum
Diagnosis
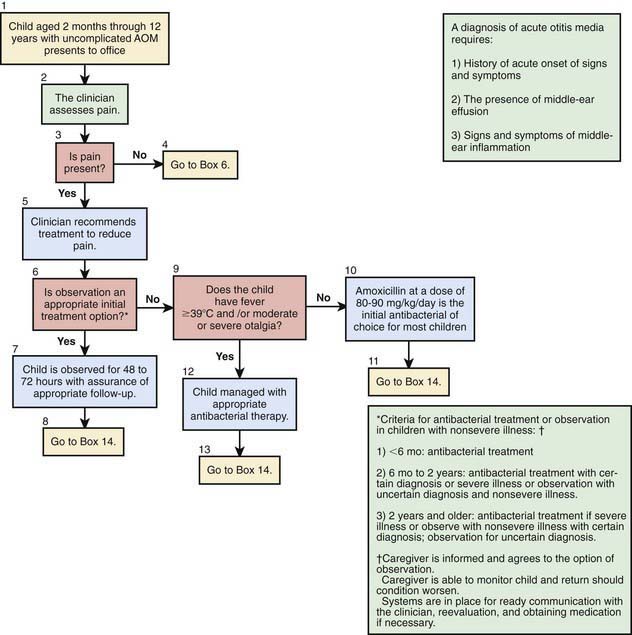
A certain diagnosis of OM should contain all of the following elements: (1) recent and usually acute onset of illness, (2) presence of MEE, and (3) signs and symptoms of middle-ear inflammation including erythema of the tympanic membrane or otalgia (see Table 632-1). A simplified differentiating schema establishes a diagnosis of AOM when, in addition to having MEE, a child gives evidence of recent, clinically important ear pain or the tympanic membrane shows marked redness or distinct fullness or bulging.
Distinguishing between AOM and OME on clinical grounds is straightforward in most cases, although each condition may evolve into the other without any clearly differentiating physical findings; any schema for distinguishing between them is to some extent arbitrary. In an era of increasing bacterial resistance, distinguishing between AOM and OME is important in determining treatment, because OME in the absence of acute infection does not require antimicrobial therapy. Purulent otorrhea of recent onset is indicative of AOM; thus, difficulty in distinguishing clinically between AOM and OME is limited to circumstances in which purulent otorrhea is not present. Both AOM without otorrhea and OME are accompanied by physical signs of MEE, namely, the presence of at least 2 of 3 tympanic membrane abnormalities: white, yellow, amber, or (rarely) blue discoloration; opacification other than that due to scarring; and decreased or absent mobility. Alternatively in OME, either air-fluid levels or air bubbles outlined by small amounts of fluid may be visible behind the tympanic membrane, a condition often indicative of impending resolution (Fig. 632-2).
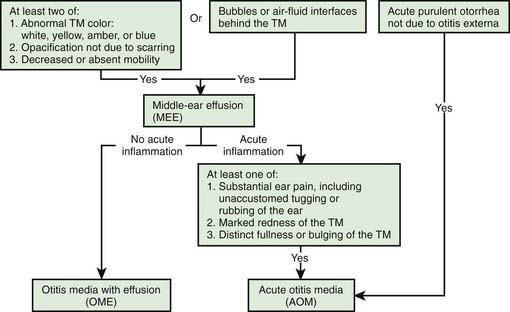
Figure 632-2 Algorithm for distinguishing between acute otitis media and otitis media with effusion. TM, tympanic membrane.
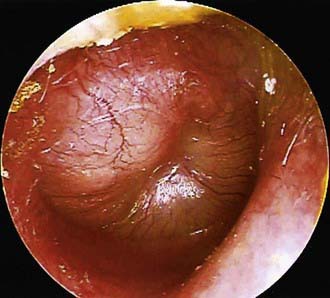
To support a diagnosis of AOM instead of OME in a child with MEE, distinct fullness or bulging of the tympanic membrane may be present, with or without accompanying erythema; or, at minimum, MEE should be accompanied by ear pain that appears clinically important. Unless intense, erythema alone is insufficient because erythema, without other abnormalities, may result from crying or vascular flushing. In AOM the malleus may be obscured, and the tympanic membrane may resemble a bagel without a hole but with a central depression (Fig. 632-3). Rarely the tympanic membrane may be obscured by surface bullae, or may have a cobblestone appearance. Bullous myringitis is a physical manifestation of AOM and not an etiologically discrete entity. Within days after onset, fullness of the membrane may diminish, even though infection may still be present.
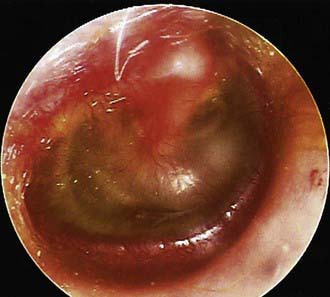
In OME, bulging of the tympanic membrane is absent or slight or the membrane may be retracted (Fig. 632-4); erythema also is absent or slight, but may increase with crying or with superficial trauma to the external auditory canal incurred in clearing the canal of cerumen. In children with MEE but without tympanic membrane fullness or bulging, the presence of unequivocal ear pain is usually indicative of AOM.
Tympanometry
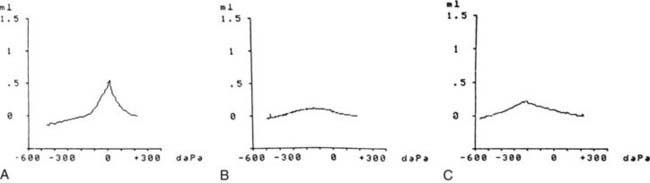
Tympanograms may be grouped into 1 of 3 categories (Fig. 632-5). Tracings characterized by a relatively steep gradient, sharp-angled peak, and middle-ear air pressure (location of the peak in terms of air pressure) that approximates atmospheric pressure (Fig. 632-5A) (type A curve) are assumed to indicate normal middle-ear status. Tracings characterized by a shallow peak or no peak and by negative or indeterminate middle-ear air pressure, and often termed “flat” or type B (Fig. 632-5B), usually are assumed to indicate the presence of a middle-ear abnormality that is causing decreased TM compliance. The most common such abnormality, by far, in infants and children is MEE. Tracings characterized by intermediate findings—somewhat shallow peak, often in association with a gradual gradient (obtuse-angled peak) or negative middle-ear air pressure, or combinations of these features (Fig. 632-5C)—may or may not be associated with MEE, and must be considered nondiagnostic or equivocal. In general, the shallower the peak, the more gradual the gradient, and the more negative the middle-ear air pressure, the greater the likelihood of MEE.
Conjunctivitis Otitis Media Syndrome
Simultaneous appearance of purulent and erythematous conjunctivitis with an ipsilateral OM is a well-recognized syndrome, due in most children to nontypeable H. influenzae (Chapter 186). The disease often is present in multiple family members and affects young children and infants. Topical ocular antibiotics are ineffective; therapy includes oral antibiotics (see later) effective against nontypeable H. influenzae.
Treatment
Management of Acute Otitis Media
Individual episodes of AOM have customarily been treated with antimicrobial drugs. Concern about increasing bacterial resistance has prompted some clinicians to recommend withholding antimicrobial treatment in some or most cases unless symptoms persist for 2 or 3 days, or worsen (Table 632-2). Three factors argue in favor of routinely prescribing antimicrobial therapy for children who have documented AOM using the diagnostic criteria outlined previously (see Table 632-1 and Fig. 632-3). First, pathogenic bacteria cause a large majority of cases. Second, symptomatic improvement and resolution of infection occur more promptly and more consistently with antimicrobial treatment than without, even though most untreated cases eventually resolve. Third, prompt and adequate antimicrobial treatment may prevent the development of suppurative complications. The sharp decline in such complications during the last half-century seems likely attributable, at least in part, to the widespread routine use of antimicrobials for AOM. In the Netherlands, where initial antibiotic treatment is routinely withheld from most children older than 6 mo of age, and where only approximately 30% of children with AOM receive antibiotics at all, the incidence of acute mastoiditis, although low (in children <14 yr, 3.8 per 100,000 person years), appears slightly higher than rates in other countries with higher antibiotic prescription rates by about 1-2 episodes per 100,000 person years. It is also the case that follow-up of children in the Netherlands may be generally more assiduous than is customary in other countries including the USA, where failure to improve or worsening symptoms might not be detected as promptly.
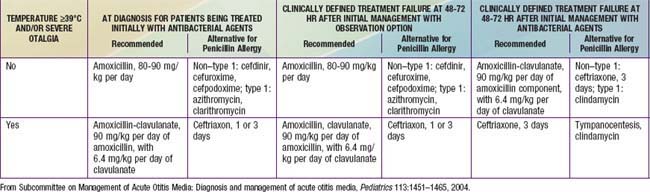
Table 632-2 CRITERIA FOR INITIAL ANTIBACTERIAL-AGENT TREATMENT OR OBSERVATION IN CHILDREN WITH AOM
| AGE | CERTAIN DIAGNOSIS | UNCERTAIN DIAGNOSIS |
|---|---|---|
| <6 mo | Antibacterial therapy | Antibacterial therapy |
| 6 mo-2 yr | Antibacterial therapy | Antibacterial therapy if severe illness; observation option* if nonsevere illness |
| ≥2 yr | Antibacterial therapy if severe illness; observation option* if nonsevere illness | Observation option* |
This table was modified with permission from the New York State Department of Health and the New York Region Otitis Project Committee.
* Observation is an appropriate option only when follow-up can be ensured and antibacterial agents started if symptoms persist or worsen. Nonsevere illness is mild otalgia and fever <39°C in the past 24 hr. Severe illness is moderate to severe otalgia or fever ≥39°C. A certain diagnosis of AOM meets all 3 criteria: (1) rapid onset; (2) signs of MEE; and (3) signs and symptoms of middle-ear inflammation.
From Subcommittee on Management of Acute Otitis Media: Diagnosis and management of acute otitis media, Pediatrics 113:1451–1465, 2004.
These considerations in treating AOM with antimicrobial therapy must be balanced against the continued increasing rates of bacterial antimicrobial resistance. In countries such as the Netherlands where use of antibiotics for OM is much less common, the antimicrobial resistance rates for the major pathogens in OM are substantially lower than those in countries that routinely treat AOM with antibiotics. Given that most episodes of OM will spontaneously resolve, consensus guidelines have been published by the American Academy of Pediatrics to assist clinicians who wish to consider a period of “watchful waiting” or observation prior to treating AOM with antibiotics (Tables 632-2 and 632-3; Fig. 632-6). The most important aspect of these guidelines is that close follow-up of the patient must be ensured to assess for lack of spontaneous resolution or worsening of symptoms and that patients should be provided with adequate analgesic medications (acetaminophen, ibuprofen) during the period of observation. When pursuing the practice of watchful waiting in patients with AOM, the certainty of the diagnosis, the patient’s age, and the severity of the disease should be considered. For younger patients, <2 yr of age, it is recommended to treat all confirmed diagnoses of AOM. In very young patients, <6 mo, even presumed episodes of AOM should be treated due to the increased potential of significant morbidity from infectious complications. In children between 6 and 24 mo who have a questionable diagnosis of OM but severe disease, defined as temperature of >102°F (>39°C), significant otalgia, or toxic appearance, antibiotic therapy is also recommended. Children in this age group with a questionable diagnosis and nonsevere disease can be observed for a period of 2-3 days with close follow-up. In children older than 2 yr of age, observation might be considered in all episodes of nonsevere OM or episodes of questionable diagnosis, while antibiotic therapy is reserved for confirmed, severe episodes of AOM.
First-Line Antimicrobial Treatment
Amoxicillin remains the drug of 1st choice for uncomplicated AOM under most circumstances because of its excellent record of safety, relative efficacy, palatability, and low cost (see Table 632-3). In particular, amoxicillin is the most efficacious of available oral antimicrobial drugs against both penicillin-susceptible and penicillin-nonsusceptible strains of S. pneumoniae. Increasing the dose from the traditional 40-45 mg/kg/24 hr to 80-90 mg/kg/24 hr will generally provide efficacy against penicillin-intermediate and some penicillin-resistant strains. This higher dose should be used particularly in children <2 yr of age, in children who have recently received treatment with β-lactam drugs, and in children who are exposed to large numbers of other children due to their increased likelihood of an infection with a nonsusceptible strain of S. pneumoniae. A limitation of amoxicillin is that it may be inactivated by the β-lactamases produced by many strains of nontypeable H. influenzae and most strains of M. catarrhalis. This factor has become increasingly important with data demonstrating an overall increase in frequency of H. influenzae as the primary pathogen in AOM secondary to widespread utilization of the conjugated pneumococcal vaccine in young children. Episodes of AOM caused by these pathogens often resolve spontaneously. Allergies to penicillin antibiotics should be categorized into type I hypersensitivity, consisting of urticaria or anaphylaxis, and those that fall short of type I reactions, such as rash formation. For children with a non–type I reaction in which cross reactivity with cephalosporins is less of a concern, first-line therapy with cefdinir would be an appropriate choice. In children with a type I reaction or known sensitivity to cephalosporin antibiotics, or in whom palatability or convenience of administration are of overriding importance, azithromycin is an appropriate alternative first-line drug. Resistance to trimethoprim-sulfamethoxazole (TMP-SMZ), by many strains of both H. influenzae and S. pneumoniae and a reported high clinical failure rate in children with AOM treated initially with this antimicrobial argue against its use as first-line treatment.
Second-Line Treatment
When treatment of AOM with a first-line antimicrobial drug has proven inadequate, a number of second-line alternatives are available (see Table 632-3). Drugs chosen for second-line treatment should be effective against β-lactamase–producing strains of H. influenzae and M. catarrhalis and against susceptible and most nonsusceptible strains of S. pneumoniae. Only 4 antimicrobial agents meet these requirements: amoxicillin-clavulanate, cefdinir, cefuroxime axetil, and intramuscular ceftriaxone. Because high-dose amoxicillin (80-90 mg/kg/24 hr) is effective against most strains of S. pneumoniae and because the addition of clavulanate extends the effective antibacterial spectrum of amoxicillin to include β-lactamase–producing bacteria, high-dose amoxicillin-clavulanate is particularly well-suited as a second-line drug for treating AOM. The 14 : 1 amoxicillin-clavulanate formulation contains twice as much amoxicillin as the previously available 7 : 1 formulation. Diarrhea, especially in infants and young children, is a common adverse effect, but may be ameliorated in some cases by feeding yogurt, and usually is not severe enough to require cessation of treatment. Cefdinir has demonstrated broad efficacy in treatment, is generally well tolerated with respect to taste and can be given as a once-daily regimen. The ability to also utilize cefdinir in children with mild type 1 hypersensitivity reactions has further added to its favorable selection as a second-line agent. Both cefuroxime axetil and intramuscular ceftriaxone have important limitations for use in young children. The currently available suspension of cefuroxime axetil is not palatable and its acceptance is low. Ceftriaxone treatment entails both the pain of intramuscular injection and substantial cost, and the injection may need to be repeated once or twice at 2-day intervals to achieve the desired degree of effectiveness. Nonetheless, use of ceftriaxone is appropriate in severe cases of AOM when oral treatment is not feasible, or in highly selected cases after treatment failure using orally administered second-line antimicrobials (i.e., amoxicillin-clavulanate or cefuroxime axetil), or when highly resistant S. pneumoniae is found in aspirates obtained from diagnostic tympanocentesis.
Management of Otitis Media with Effusion (Ome)
To distinguish between persistence and recurrence, examination should be conducted monthly until resolution; hearing should be assessed if effusion has been present for >3 mo (see Fig. 632-4). Management of OME depends on an understanding of its natural history and its possible complications and sequelae. Most cases of OME resolve without treatment within 3 mo. When MEE persists longer than 3 mo, consideration of surgical management with tympanostomy tubes is appropriate. In considering the decision to refer the patient for consultation the clinician should attempt to determine the impact of the OME on the child. Although hearing loss may be of primary concern, OME causes a number of other difficulties in children that should also be considered. These include predisposition to recurring AOM, pain, disturbance of balance, and tinnitus. In addition, long-term sequelae that have been demonstrated to be associated with OME include pathologic middle-ear changes; atelectasis of the tympanic membrane and retraction pocket formation; adhesive OM; cholesteatoma formation and ossicular discontinuity; and conductive and sensorineural hearing loss. Long-term adverse effects on speech, language, cognitive, and psychosocial development have also been demonstrated, although some studies have demonstrated that the long-term adverse impact of OME on development may be small. In considering the impact of OME on development, it is especially important to take into consideration the overall presentation of the child. Although it is unlikely that OME causing unilateral hearing loss in the mild range will have long-term negative effects on an otherwise healthy and developmentally normal child, even a mild hearing loss in a child with other developmental or speech delays certainly has the potential to compound this child’s difficulties (Table 632-4). At a minimum, children with OME persisting >3 mo deserve close monitoring of their hearing levels with skilled audiologic evaluation; frequent assessment of developmental milestones, including speech and language assessment; and attention paid to their rate of recurrent AOM.
Table 632-4 SENSORY, PHYSICAL, COGNITIVE, OR BEHAVIORAL FACTORS THAT PLACE CHILDREN WHO HAVE OME AT AN INCREASED RISK FOR DEVELOPMENTAL DIFFICULTIES (DELAY OR DISORDER)
From American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media with Effusion: Otitis media with effusion, Pediatrics 113(5):1412–1429, 2004, Table 3, p 1416.
Complications of Acute Otitis Media
Acute Mastoiditis
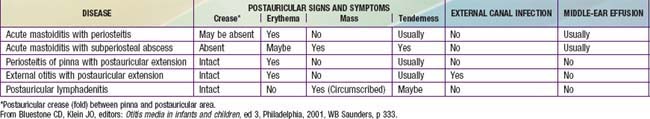
Technically, all cases of AOM are accompanied by mastoiditis by virtue of the associated inflammation of the mastoid air cells. However, early in the course of the disease, no signs or symptoms of mastoid infection are present, and the inflammatory process usually is readily reversible, along with the AOM, in response to antimicrobial treatment. Spread of the infection to the overlying periosteum, but without involvement of bone, constitutes acute mastoiditis with periosteitis. In such cases, signs of mastoiditis are usually present, including inflammation in the postauricular area, often with displacement of the pinna inferiorly and anteriorly (Table 632-5). Treatment with myringotomy and parenteral antibiotics, if instituted promptly, usually provides satisfactory resolution.
Table 632-5 DIFFERENTIAL DIAGNOSIS OF POSTAURICULAR INVOLVEMENT OF ACUTE MASTOIDITIS WITH PERIOSTEITIS/ABSCESS
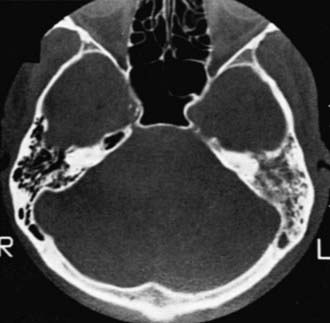
In acute mastoid osteitis, or coalescent mastoiditis, infection has progressed further to cause destruction of the bony trabeculae of the mastoid (Fig. 632-7). Frank signs and symptoms of mastoiditis are usually, but not always, present. In acute petrositis, infection has extended further to involve the petrous portion of the temporal bone. Eye pain is a prominent symptom, due to irritation of the ophthalmic branch of cranial nerve V. Cranial nerve VI palsy is a later finding suggesting further extension of the infectious process along the cranial base. Gradenigo syndrome is the triad of suppurative OM, paralysis of the external rectus muscle, and pain in the ipsilateral orbit. Rarely, mastoid infection spreads external to the temporal bone into the neck musculature that attaches to the mastoid tip, resulting in an abscess in the neck, termed a Bezold abscess.
When mastoiditis is suspected or diagnosed clinically, CT scanning of the temporal bones should be carried out to further clarify the nature and extent of the disease (see Fig. 632-7). Bony destruction of the mastoid must be differentiated from the simple clouding of mastoid air cells that is found often in uncomplicated cases of OM. The most common causative organisms in all variants of acute mastoiditis are S. pneumoniae and nontypeable H. influenzae. P. aeruginosa is also a causative agent, primarily in patients with CSOM. Children with acute mastoid osteitis generally require intravenous antimicrobial treatment and mastoidectomy, with the extent of the surgery dependent on the extent of the disease process. As imaging techniques have become more commonly employed in assessing children, early cases of mastoid osteitis are more frequently identified and may respond to myringotomy and parenteral antibiotics. Insofar as possible, choice of the antimicrobial regimen should be guided by the findings of microbiologic examination.
Acquired Cholesteatoma
Cholesteatoma is a cystlike growth originating in the middle ear, lined by keratinized, stratified squamous epithelium and containing desquamated epithelium and/or keratin (Chapter 630) (Fig. 632-8). Acquired cholesteatoma develops most often as a complication of long-standing chronic OM. The condition also may develop from a deep retraction pocket of the tympanic membrane or as a consequence of epithelial implantation in the middle-ear cavity from traumatic perforation of the tympanic membrane or insertion of a tympanostomy tube. Cholesteatomas tend to expand progressively, causing bony resorption, often extend into the mastoid cavity, and may extend intracranially with potentially life-threatening consequences. Cholesteatoma commonly presents as a chronically draining ear in a patient with a history of previous ear disease. Cholesteatoma should be suspected if otoscopy demonstrates an area of TM retraction or perforation with white, caseous debris persistently overlying this area. Along with otorrhea from this area, granulation tissue or polyp formation identified in conjunction with this history and presentation should prompt suspicion of cholesteatoma. The most common location for cholesteatoma development is in the superior portion of the TM, also called the pars flaccida. Most patients also present with conductive hearing loss on audiologic evaluation. When cholesteatoma is suspected, otolaryngology consultation should be sought immediately. Delay in recognition and treatment can have significant long-term consequences, including the need for more extensive surgical treatment, permanent hearing loss, facial nerve injury, labyrinthine damage with loss of balance function, and intracranial extension. The required treatment for cholesteatoma is tympanomastoid surgery.
Congenital Cholesteatoma
Congenital cholesteatoma is an uncommon condition generally identified in younger patients (Fig. 632-9). The etiology of congenital cholesteatoma is thought to be a result of epithelial implantation in the middle ear space during otologic development in utero. Congenital cholesteatoma most commonly presents in the anterior superior quadrant of the TM but can be found elsewhere. Congenital cholesteatoma appears as a discrete, white opacity in the middle ear space on otoscopy. Unlike patients with acquired cholesteatoma, there is generally not a strong history of OM or chronic ear disease, history of otorrhea, or changes in the TM anatomy such as perforation or retraction. Similar to acquired cholesteatoma many patients do have some degree of abnormal findings on audiologic evaluation, unless identified very early. Congenital cholesteatoma also requires surgical resection.
Intracranial Complications
Otitic hydrocephalus, a form of pseudotumor cerebri, is an uncommon condition that consists of increased intracranial pressure without dilatation of the cerebral ventricles, occurring in association with acute or chronic OM or mastoiditis (Chapter 597). The condition is commonly also associated with lateral sinus thrombosis, and the pathophysiology is thought to involve obstruction by thrombus of intracranial venous drainage into the neck, producing a rise in cerebral venous pressure and a consequent increase in cerebrospinal fluid pressure. Symptoms are those of increased intracranial pressure. Signs may include, in addition to evidence of OM, paralysis of 1 or both lateral rectus muscles and papilledema. MRI can confirm the diagnosis. Treatment measures include the use of antimicrobials and medications such as acetazolamide or furosemide to reduce intracranial pressure, mastoidectomy, repeated lumbar puncture, lumboperitoneal shunt, and ventriculoperitoneal shunt. If left untreated, otitic hydrocephalus may result in loss of vision secondary to optic atrophy.
Possible Developmental Sequelae
Permanent hearing loss in children has a significant negative impact on development, particularly in speech and language. The degree to which OM impacts long-term development in children is difficult to assess and there have been conflicting studies examining this question. Developmental impact is most likely to be significant in children that have greater levels of hearing loss, hearing loss that is sustained for longer periods of time, hearing loss that is bilateral and in those children that have other developmental difficulties or risk factors for developmental delay (see Table 632-4).
American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
American Family Physicians, American Academy of Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
Asher E, Dagan R, Greenberg D, et al. Persistence of pathogens despite clinical improvement in antibiotic-treated acute otitis media is associated with clinical and bacteriologic relapse. Pediatr Infect Dis J. 2008;27:296-300.
Bales CD, Sobol S, Wetmore R, et al. Lateral sinus thrombosis as a complication of otitis media: 10-year experience at the Children’s Hospital of Philadelphia. Pediatrics. 2009;123:709-713.
Barkai G, Leibovitz E, Givon-Lavi N, et al. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J. 2009;28:466-470.
Bauchner H, Marchant CD, Bisbee A, et al. Effectiveness of Centers for Disease Control and Prevention recommendations for outcomes of acute otitis media. Pediatrics. 2006;117:1009-1017.
Berkun Y, Nir-Paz R, Ami AB, et al. Acute otitis media in the first two months of life: characteristics and diagnostic difficulties. Arch Dis Child. 2008;93:690-694.
Bhattacharyya N, Shapiro NL. Air quality improvements and the prevalence of frequent ear infections in children. Otolaryngol Head Neck Surg. 2010;142:242-246.
Bluestone CD. Definitions, terminology, and classification. In: Rosenfeld RM, Bluestone CD, editors. Evidence-based otitis media. ed 2. Hamilton, Ontario: BC Decker Inc; 2008:120-135.
Bolt P, Barnett P, Babl FE, et al. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomized trial. Arch Dis Child. 2008;93:40-44.
Brook I, Gober AE. Bacteriology of spontaneously draining acute otitis media in children before and after the introduction of pneumococcal vaccination. Pediatr Infect Dis J. 2009;28:640-642.
Brouwer AR, Maillé M, Rovers R, et al. Effect of pneumococcal vaccination on quality of life in children with recurrent otitis media: a randomized, controlled trial. Pediatrics. 2005;115:273-279.
Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304-309.
Clegg AJ, Loveman E, Gospodarevskaya E, et al. The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation. Health Technol Assess. 2010;14:No. 28.
Coco A, Vernacchio L, Horst M, et al. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125:214-220.
Coker TR, Chan LS, Newberry SJ, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children. JAMA. 2010;304(19):2161-2168.
Dagan R, Schneider S, Givon-Lavi N, et al. Failure to achieve early bacterial eradication increases clinical failure rate in acute otitis media in young children. Pediatr Infect Dis J. 2008;27:200-206.
Garbutt J, Rosenbloom I, Wu J, et al. Empiric first-line antibiotic treatment of acute otitis in the era of the heptavalent pneumococcal conjugate vaccine. Pediatrics. 2006;117:e1087-e1094.
Grossman Z, Silverman BG, Porter B, et al. Implementing the delayed antibiotic therapy approach significantly reduced antibiotics consumption in Israeli children with first documented acute otitis media. Pediatr Infect Dis J. 2010;29:595-599.
Haggard M. Ventilation (tympanostomy) tubes for otitis media with effusion. BMJ. 2008;337:885-886.
Hall-Stoodley L, Hu FZ, Gieske A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
Hammaren-Malmi S, Saxen H, Tarkkanen J, et al. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics. 2005;116:185-189.
Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364(2):105-115.
Isaacson G. Diagnosis of pediatric cholesteatoma. Pediatrics. 2007;120:603-608.
Kadhim AL, Spilsbury K, Semmens JB, et al. Adenoidectomy for middle ear effusion: a study of 50,000 children over 24 years. Laryngoscope. 2007;117:427-433.
Kalu SU, Ataya RS, McCormick DP, et al. Clinical spectrum of acute otitis media complicating upper respiratory tract viral infection. Pediatr Infect Dis J. 2011;30(2):95-99.
Kaur R, Adlowitz DG, Casey JR, et al. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010;29(8):741-745.
Kerschner JE. Bench and bedside advances in otitis media. Curr Opin Otolaryngol Head Neck Surg. 2008;16:543-547.
Klein JO. Is acute otitis media a treatable disease? N Engl J Med. 2011;364(2):168-169.
Koch A, Homøe P, Pipper C, et al. Chronic suppurative otitis media in a birth cohort of children in Greenland. Pediatr Infect Dis J. 2011;30(l):25-29.
Kozyrskyj A, Klassen TP, Moffatt M, et al: Short-course antibiotics for acute otitis media, Cochrane Database Syst Rev (9):CD001095, 2010.
Laine MK, Tähtinen PA, Ruuskanen O, et al. Symptoms or symptom-based scores cannot predict acute otitis media at otitis-prone age. Pediatrics. 2010;125:e1154-e1161.
Le Monnier A, Jamet A, Carbonnelle E, et al. Fusobacterium necrophorum middle ear infections in children and related complications. Pediatr Infect Dis J. 2008;27:613-617.
Leibovitz E, Asher E, Piglansky L, et al. Is bilateral acute otitis media clinically different than unilateral acute otitis media? Pediatr Infect Dis J. 2007;26:589-592.
Leibovitz E, Serebro M, Givon-Levi N, et al. Epidemiologic and microbiologic characteristics of culture-positive spontaneous otorrhea in children with acute otitis media. Pediatr Infect Dis J. 2009;28:381-384.
Mandel EM, Doyle WJ, Winther B, et al. The incidence, prevalence and burden of OM in unselected children aged 1–8 years followed by weekly otoscopy through the “common cold” season. Int J Pediatr Otorhinolaryngol. 2008;72:491-499.
Noel GJ, Blumer JL, Pichichero ME, et al. A randomized comparative study of levofloxacin versus amoxicillin/clavulanate for treatment of infants and young children with recurrent or persistent acute otitis media. Pediatr Infect Dis J. 2008;27:483-489.
Ongkasuwan J, Valdez TA, Hulten KG, et al. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122:34-39.
Paradise JL, Feldman HM, Campbell TF, et al. Tympanostomy tubes and developmental outcomes at 9 to 11 years of age. N Engl J Med. 2007;356:248-261.
Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772-1778.
Pichichero ME, Casey JR, Hoberman A, et al. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003–2006. Clin Pediatr (Phila). 2008;4:901-906.
Porat N, Amit U, Givon-Lavi N, et al. Increasing importance of multidrug-resistant serotype 6A Streptococcus pneumoniae clones in acute otitis media in Southern Israel. Pediatr Infect Dis J. 2010;29:126-130.
Purzycki A, Thompson E, Argenta L, et al. Incidence of otitis media in children with deformational plagiocephaly. J Craniofac Surg. 2009;20:1407-1411.
Roland PS, Smith TL, Schwartz SR, et al. Clinical practice guideline: cerumen impaction. Otolaryngol Head Neck Surg. 2008;139:S1-S21.
Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130:S95-S118.
Rovers MM. The burden of otitis media. Vaccine. 2008;26(Suppl 7):G2-G4.
Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
Rovers MM, Schilder AG, Zielhuis GA, et al. Otitis media. Lancet. 2004;363:465-473.
Siegel RM. Acute otitis media guidelines, antibiotic use, and shared medical decision-making. Pediatrics. 2010;125:384-386.
Sox CM, Finkelstein JA, Yin R, et al. Trends in otitis media treatment failure and relapse. Pediatrics. 2008;121:674-679.
Tahtinen PA, Laine MK, Huovinen P, et al. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364(2):116-126.
Thompson PL, Gilbert RE, Long PF, et al. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the United Kingdom general practice research database. Pediatrics. 2009;123:424-430.
van der Veen EL, Rovers MM, Albers FWJ, et al. Effectiveness of trimethoprim/sulfamethoxazole for children with chronic active otitis media: a randomized, placebo-controlled trial. Pediatrics. 2007;119:897-904.
Williamson I, Benge S, Barton S, et al. Topical intranasal corticosteroids in 4–11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomized placebo controlled trial. BMJ. 2010;340:83.
Witsell DL, Stewart MG, Monsell EM, et al. The cooperative outcomes group for ENT: a multicenter prospective cohort study on the outcomes of tympanostomy tubes for children with otitis media. Otolaryngol Head Neck Surg. 2005;132:180-188.