CHAPTER 321 Osteoporotic Fractures
Evaluation and Treatment with Vertebroplasty and Kyphoplasty
The impact of osteoporosis is increasingly being recognized as the population ages.1 One of the primary adverse manifestations of osteoporosis is vertebral compression fractures. Indeed, an estimated 700,000 osteoporotic vertebral compressions fractures occur annually in the United States.2,3 Vertebral compression fractures are a significant problem associated with pain and functional impairment.4–7 The antiquated perception that these fractures are benign and self-limited is being replaced as our understanding evolves and our current treatment options have expanded.
History
The first vertebroplasty was performed in France in 1984 by Galibert and colleagues, with its subsequent appearance in the literature 3 years later.8 At that time, PMMA was used to successfully treat a painful hemangioma of the C2 vertebra. One year after the technique was first described, it was reported for the treatment of osteoporotic vertebral compression fractures.9 Almost a decade later, in 1993, the first vertebroplasty was performed in North America at the University of Virginia and was subsequently reported in 1997.10 Kyphoplasty, a modification of the established procedure vertebroplasty, was first reported in 1998, and shortly thereafter data began to accumulate supporting its use.11–13
Indications and Contraindications
Vertebroplasty and kyphoplasty are indicated in patients with osteoporotic vertebral body fractures associated with significant pain. These osteoporotic vertebral body fractures include compression fractures, as well as selected burst fractures,14 specifically those with no or limited canal compromise and an intact posterior cortex and posterior longitudinal ligament.
The timing of treatment with vertebroplasty and kyphoplasty is evolving. It has generally been suggested that vertebroplasty and kyphoplasty were indicated only after a failed 4- to 6-week trial of conventional medical therapy consisting of bed rest and analgesics, a protocol that has been used in numerous clinical studies.10,15 Although some have anecdotally reported improvement in pain after treatment years after the initial injury,16 late treatment (>6 months after injury) is less likely to be successful.17 Others have advocated earlier treatment for those unable to ambulate secondary to pain or for individuals requiring parenteral narcotics and hospital admission.18
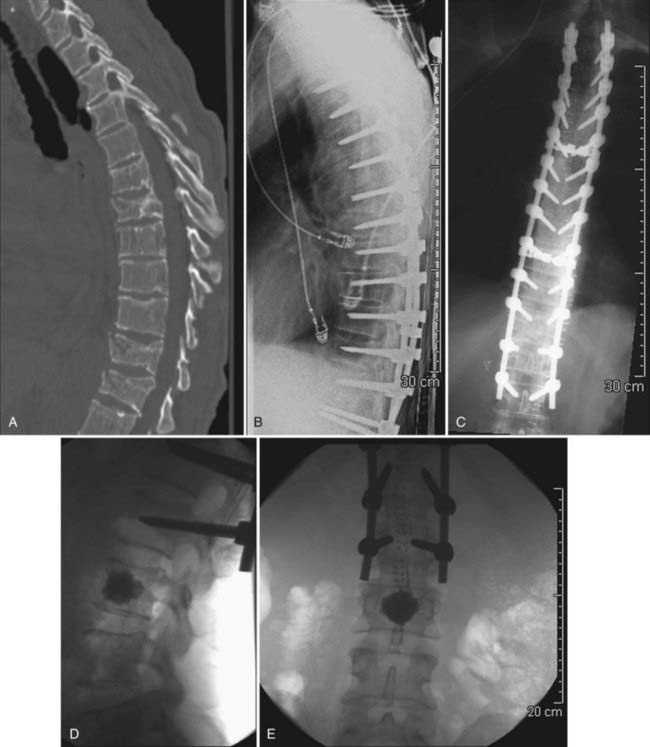
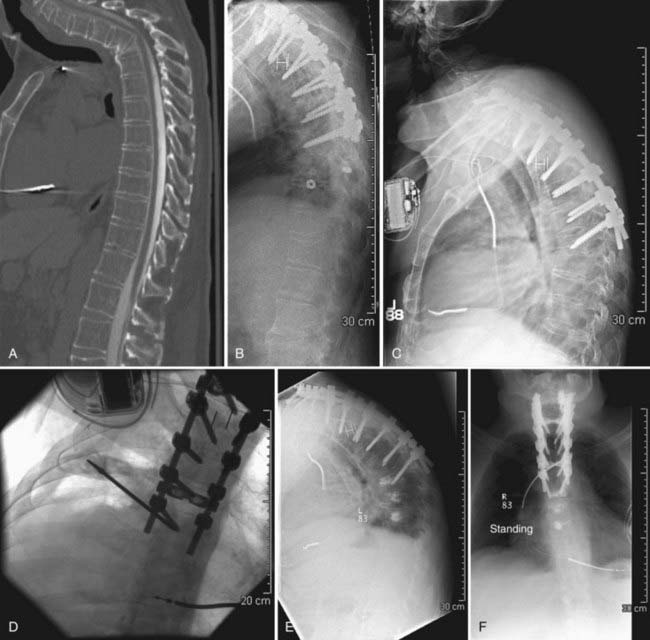
Although there may be a role for prophylactic vertebroplasty and kyphoplasty in patients who are at high risk for compression fractures, prophylactic treatment is not widely accepted or approved as an indication, even though there has been recent interest in investigating this issue.19–21 One exception may be patients who have undergone long-segment posterior spinal instrumentation procedures and are at high risk for failure at the cranial or caudal limits of the fusion (Figs. 321-1 and 321-2).
Patient Evaluation
To determine a patient’s suitability for vertebral body augmentation, one must obtain appropriate images before performing the procedure. The initial evaluation often consists of a plain radiograph followed by computed tomography (CT), magnetic resonance imaging (MRI), or a bone scan. For patients who have a documented new compression fracture seen on plain films, it may be unnecessary to acquire additional studies if they are clinically appropriate for vertebroplasty.22 Additionally, plain films document overall sagittal and coronal balance and provide some indication of bone quality and the degree of vertebral body collapse.
If it is not clear whether the fracture in question is acute or whether multiple compression fractures are present, MRI is often helpful to determine which level or levels may best be treated by vertebroplasty or kyphoplasty. It has been suggested that the finding of edema on MRI may predict a favorable outcome with vertebroplasty, although the absence of signal change on MRI does not necessarily obviate one from having such a response with treatment.22,23 For obvious reasons, MRI should be considered mandatory if the diagnosis of a vertebral body metastatic lesion is being entertained.
Bone scintigraphy has been proposed as an adjunctive technique for facilitating both the selection of patients and which levels to treat when multiple fractures are present in a given patient. Bone scintigraphy is particularly useful when multiple fractures of different age are present. Levels at which increased uptake of tracer are identified generally respond well to vertebroplasty or kyphoplasty in terms of pain relief.24–26
Technique
Vertebroplasty
Vertebroplasty is typically an outpatient procedure that can be performed in a location with appropriate imaging equipment, including the operating room and interventional radiology suite. We perform vertebroplasty in a neurointerventional suite with high-quality biplanar fluoroscopy. Alternatively, CT guidance has been used effectively for the performance of vertebroplasty.27–31 Vertebroplasty can usually be performed with a moderate amount of conscious sedation and local anesthesia. Even though conscious sedation may be adequate, certain individuals, such as patients unable to lie still because of pain, may require general anesthesia. Once sedated, the patient is placed in the prone position with the arms raised above the head to minimize interference with fluoroscopy. This position increases extension of the targeted vertebral bodies, and treatment in this position is thought to reduce the kyphotic deformity associated with the fracture.32
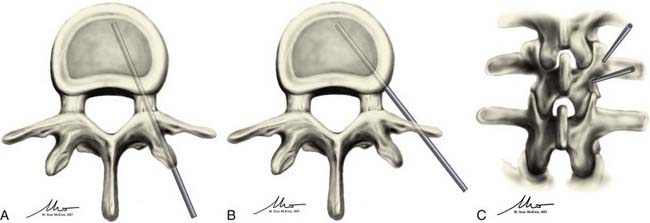
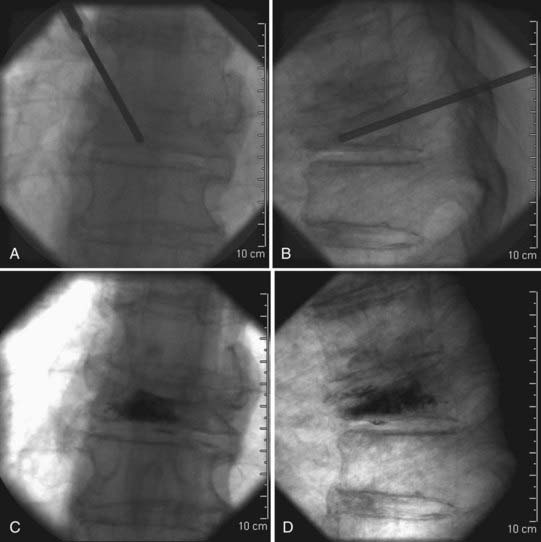
Once adequately positioned, the affected vertebrae and their respective landmarks are identified with biplanar fluoroscopy. After confirming that the target vertebrae are suitable for injection of cement, the needle trajectory is planned. Needle placement can be accomplished from either a transpedicular or parapedicular approach (Fig. 321-3). Factors that guide selection include pedicle size, vertebral body size, and whether a unipedicular or bipedicular approach is being contemplated. For the lumbar and thoracic spine, where pedicle size is larger, a transpedicular approach may be preferred. In the thoracic spine, where pedicle size is smaller, a parapedicular approach may be preferable. As mentioned, a unipedicular or bipedicular approach may be chosen. Early reports of vertebroplasty described a bipedicular approach in which cement was injected through each needle located in their respective hemivertebrae.10,22,33,34 The bipedicular approach was undertaken to maximize the volume of filling within the vertebral body. However, a unipedicular approach was proposed and implemented as a means of decreasing procedure time, radiation exposure, discomfort for the patient, and difficulties associated with monitoring the second injection, particularly on the lateral plane, when there was already preexisting cement from the first injection.35 This approach entailed the insertion of a single needle into the midline of the vertebral body near the anterior cortex of the vertebral body. With such needle placement, filling of the central vertebral body from pedicle to pedicle can be achieved. There does not appear to be any difference in clinical outcome between a unipedicular and a bipedicular approach for vertebroplasty.35 We have generally adopted a unipedicular approach.
When using a unipedicular approach, we prefer to insert the needle on the patient’s right side (the operators stand on the patient’s left side). This places the physician further away from the radiation source and is less cumbersome than an ipsilateral approach through the left pedicle. A straight anteroposterior (AP) image is obtained, and the AP tube is then moved obliquely approximately 20 degrees laterally until the Scotty dog profile is seen over the pedicle. For the thoracic spine, an oblique orientation of the tube may make visualization of the pedicle more difficult because the pedicles are less angulated in the AP plane. However, if the pedicle is carefully tracked from the straight AP to the oblique position, it can be visualized; alternatively, the AP tube can be oriented obliquely laterally until the pedicle projects over the medial fifth of the vertebral body.22 The needle for local anesthetic is then inserted until it rests on the periosteum at the proposed site where the vertebroplasty trocar will be inserted into the pedicle. Local anesthetic is then infused into the periosteum and infiltrated into the tissues as the needle is withdrawn to the skin surface. A wheal at the skin surface is raised just before the needle is withdrawn.
In the unilateral transpedicular approach, the needle is advanced until it docks on the intended pedicle. This should occur at the superior lateral quadrant of the pedicle to minimize the risk of damaging the exiting nerve root, a risk that increases with more inferior and medial positioning. This also creates a trajectory that is directed toward the center of the vertebral body. Once at the appropriate location on the pedicle, the needle is advanced in 1- to 2-mm increments through the pedicle into the vertebral body under biplanar fluoroscopic guidance. The needle tip should ideally end in the anterior half of the vertebral body and should be midline when seen on an AP view (see Fig. 321-3A and C). The target may be manipulated slightly according to the location of the fracture; however, the majority of treatments can be accomplished by placing the needle tip in this final location. If using the bipedicular approach, the contralateral side may be cannulated in similar fashion. If multiple levels are being treated, they are typically all cannulated before the injection. If the levels to be treated are contiguous and the distance between levels is relatively small, the side of needle placement can be alternated.
The parapedicular approach takes a more lateral trajectory to the vertebral body and begins by advancing the needle until it is docked on the transverse process. Once at the transverse process, the needle is walked caudal to the transverse process and passed anteromedially to the entry point. The ideal entry point into the vertebral body is at the junction between the pedicle and the vertebral body and is visualized on the lateral projection as being just anterior to the posterior end plate and on the lateral wall of the vertebral body on the AP projection. Once at this point, the needle is advanced into the middle of the vertebral body and should have a ending point similar to that for the transpedicular approach (see Fig. 321-3B and C).
Both the parapedicular and transpedicular approaches have their advantages and disadvantages. The transpedicular approach does offer more protection for the postganglionic nerve roots during passage of the needle and for the surrounding soft tissues from retrograde extravasation of cement along the needle’s path.36 However, because of its course within the pedicle, bilateral needle insertion may be required for injection of sufficient cement, with the accompanying risk associated with twice as many needle insertions as needed for the parapedicular approach. Additionally, its path within the pedicle requires the pedicle to be of sufficient width, a requirement that may make this technique less suitable above the midthoracic level.
The parapedicular approach exposes the patient to a higher risk for bleeding and pneumothorax37; however, the vertebroplasty is almost always performed through a unilateral approach. It also has no reliance on finding a suitable pedicle and can therefore be performed regardless of pedicle size or the angle at which the pedicle meets the vertebral body. This allows management of fractures that may be considered untreatable with the transpedicular approach. In our practice, with proper radiographic guidance, a transpedicular approach is usually sufficient and excellent filling can be achieved through a unilateral approach. When the size of the pedicle is small and particularly in the mid and upper thoracic spine, the needle insertion point can be at the junction of the rib head and pedicle interface, thereby allowing placement of the needle in the middle of the vertebral body while staying lateral to the medial aspect of the pedicle regardless of pedicle size.
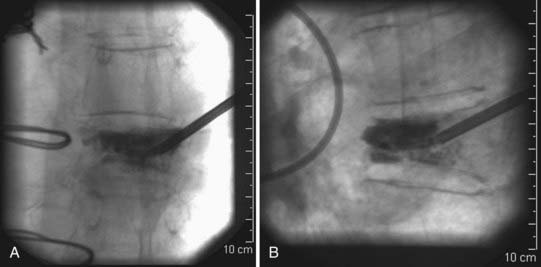
Some adjunctive techniques may allow salvage of an initially suboptimal needle trajectory. If the needle is nearing an end plate, especially when collapse of the vertebral body is severe, a beveled inner cannula can replace the initial pointed inner cannula and may help direct the needle away from the end plate that it is close to. Another helpful strategy that can be used to overcome suboptimal needle placement involves the use of a curved inner cannula for delivery of cement. This may allow administration of cement throughout the vertebral body even when the needle is not placed precisely where it was intended. The curved inner cannula may also facilitate delivery of cement directly to areas where the fractures are located (Fig. 321-4).
After proper placement, injection of the cement may be performed (Fig. 321-5). Some groups advocate injecting a small amount of contrast material into the vertebral body before injection of cement because it may eliminate the risk of injection into a large venous sinus.10,38 However, this risk has been shown to be negligible when vertebroplasty is performed by experienced operators, and therefore performing the procedure without an antecedent venogram may reduce contrast-related complications without a significant difference in clinical outcomes.39 The cement used for the procedure is PMMA. To fully monitor placement of the PMMA, barium sulfate is added to allow direct visualization of the cement as it is injected under fluoroscopic guidance. It is important to recognize that contrast material is often included in commercial PMMA preparations; however, it may be necessary to add additional sterile barium to the mixture. The PMMA is mixed until it reaches the consistency of toothpaste, at which time it is ready for injection. Injection should be performed under constant fluoroscopic visualization in the lateral projection and with intermittent visualization along the AP trajectory. Constant imaging on the lateral plane helps the surgeon prevent what is perhaps the most devastating potential complication of vertebral augmentation, posterior leakage with subsequent spinal cord compromise. Intermittent views in the AP plane allow monitoring to ensure that cement has not leaked out through lateral venous tributaries. We perform injections with an injector device. This places the surgeon further away from the x-ray source and facilitates AP views because the operator’s hands remain away from the x-ray field. As mentioned, injection is performed under constant lateral fluoroscopic guidance. The injector is then depressurized by turning the injector crank in the opposite direction so that the AP view can be examined. Once the cement has filled the anterior two thirds of the vertebral body, the procedure is complete. The patient is kept prone for 15 minutes to ensure that the cement has hardened sufficiently. Patients are most often discharged home on the day of the procedure.
Kyphoplasty
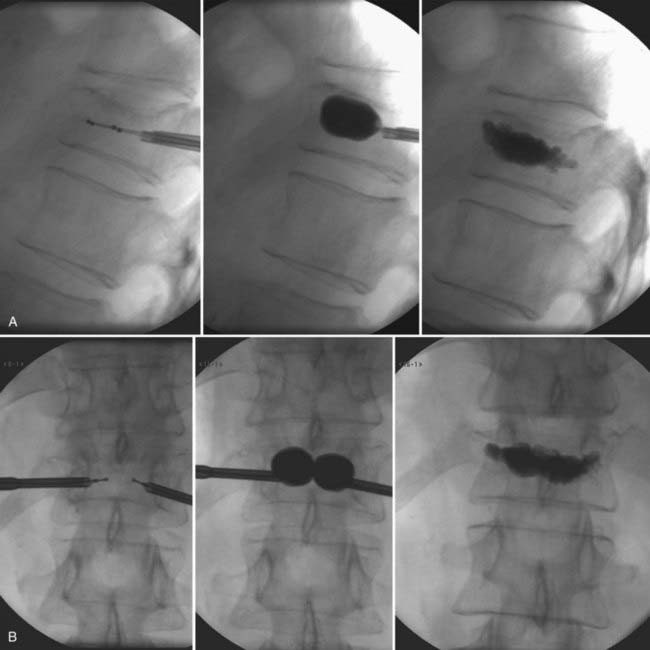
Insertion of the needle into the vertebral body is performed in a fashion similar to that for vertebroplasty (see earlier). Generally, a bipedicular approach is performed, although there are reports of successful kyphoplasty from a unipedicular approach.40–42 Once the needle is in place through the pedicle within the posterior aspect of the vertebral body, the stylet of the needle is removed. After this is accomplished, a hand-controlled manual drill is used to create a pathway for insertion of the balloon tamp. Ideal final positioning of the drill bit should be roughly 4 mm posterior to the anterior cortex of the vertebral body when seen on a lateral projection and roughly midline on an AP projection. Once the path has been created, the drill is removed and the inflatable tamp is inserted. The tamp is inserted until the marker signifying the posterior aspect of the balloon is at least 3 to 4 mm anterior to the tip of the cannula. Once in place, the balloon is inflated with contrast material. This is done while continuously transducing the pressure inside the balloon with a manometer that has a digital pressure gauge and while monitoring with fluoroscopy. The balloon can typically be inflated to a maximum pressure of 220 psi or until the balloon is fully inflated, but it is not always necessary to reach this maximum pressure. Occasionally, the kyphosis associated with the fracture can be corrected, or the balloon reaches one of the cortical margins without maximizing the balloon pressure, at which point inflation is ceased. Once this occurs, the balloon is deflated in preparation for injection of cement. The cement is hand-injected through smaller cannulas under continuous fluoroscopic visualization until the cement reaches the posterior third of the vertebral body. This can be repeated on the contralateral side if desired, although if planned, it is preferable to cannulate both sides before initial cement injection. It is not always necessary, however, to perform bilateral injections, as mentioned earlier, because unilateral injections have been shown to be sufficient for kyphoplasty.43 If the decision is made to perform bilateral injections, the balloons are typically inflated by alternately injecting contrast solution in small increments. This should aid in achieving appropriate fracture correction. In the rare cases in which loss of reduction occurs once the balloons are deflated, it may be possible to inject cement through one cannula while the contralateral balloon remains inflated. This cement can then be allowed to harden before deflating the remaining balloon and injecting cement contralaterally. Once the cement injection is complete, the inner cannulas are removed. Patients may remain prone for a short period and are often able to be discharged the same day (Fig. 321-6).
Vertebroplasty and Kyphoplasty of the Cervical Spine
Although less commonly performed, vertebroplasty and kyphoplasty can be performed for pathology of the cervical spine.8,44–49 Hemangiomas or neoplastic lesions of C2 can be treated through a transoral approach. Cement augmentation of the lower cervical vertebrae can be performed through image-guided transpedicular or anterolateral approaches.17,45
Sacroplasty
Sacral insufficiency fractures are spontaneous fractures of the sacrum and often occur as a result of osteoporosis. They typically cause buttock or low back pain, with or without associated radicular symptoms,50–52 and treatment has traditionally consisted of bed rest, pain medications, and the use of corsets.51,53,54 Despite the use of these treatment options, resolution of symptoms can take as long as 9 to 12 months, with an increase in associated risks such as pulmonary emboli, pneumonia, and pressure ulcers.50,51
After evidence of successful treatment of vertebral body pathology began to accumulate, the application of these techniques to sacral pathology was investigated. Sacroplasty was initially described in 2000 for the treatment of symptomatic metastatic lesions of the sacrum.55,56 Since that time, its indications have expanded to include sacral insufficiency fractures, with good success in relieving pain and returning the patient to a functioning state.50,57,58
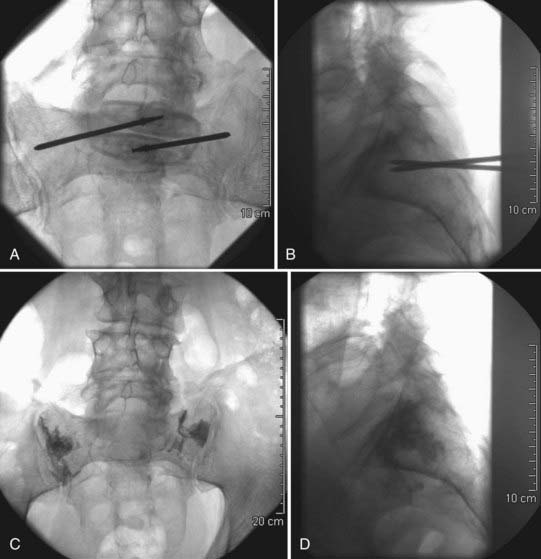
The technical aspects of sacroplasty are quite similar to those for vertebroplasty. The patient setup is unchanged; the patient is placed prone and biplanar fluoroscopy is used. Insertion of the needles takes place approximately midway between the sacral foramina and the sacroiliac joint on the side of the fracture. The needles should then be angled laterally approximately 45 degrees toward the sacroiliac joint. The ideal final positioning of the needle tip should be at the midpoint of the sacrum when viewed on a lateral projection and 3 to 4 mm medial to the sacroiliac joint on an AP projection. Once in appropriate position, the cement is injected. Approximately 4 to 8 mL of cement is typically injected, and again careful attention must be paid to avoid leakage medially toward the nerve roots (Fig. 321-7).
In addition to the risk for sacral nerve root compromise, sacroplasty carries with it the unique risk of injection into the sacroiliac joint.59 Because of the risks associated with cement leakage from sacroplasty, it has been advocated that sacroplasty be used only for zone 1 sacral fractures.50
Complications
As is the case with any procedure or surgery, vertebroplasty and kyphoplasty have potential complications associated with them. Pain and tenderness at the site of needle insertion are the most common complications associated with these procedures and are often attributable to small hematomas or bruising within the first 72 hours.17 Occasionally, patients will experience more painful radicular symptoms after undergoing treatment, a complication that is typically ameliorated with nonsteroidal anti-inflammatory drugs, oral steroids, or rarely, epidural steroid injections.17,18
The more serious complications seen with kyphoplasty or vertebroplasty are usually secondary to extravasation of cement. With extravasation into the basivertebral vein, the epidural veins, or the epidural space, compression of the spinal cord or nerve roots can result.38,60–62 Similarly, extravasation into the venous plexuses of the vertebral body can result in systemic emboli to the pulmonary or even the cerebral vasculature.10,63–66 Although some have advocated the use of antecedent venography to decrease the incidence of these complications,10,38 later studies have found that venography does not alter the effectiveness or safety of vertebroplasty when performed by experienced operators.39 In addition to extravasation into vascular channels, cement can extravasate along fracture lines, with resultant compression of the spinal cord or nerve roots, depending on the location of the fracture. This is typically avoided by temporary cessation of the injection as soon as extravasation is noted to allow the cement to harden. Once the previously injected cement is thought to be hardened, further injection can proceed. If extravasation is observed again, the procedure is aborted.
In addition to cement-related complications, complications from improper needle placement are very real concerns, most notably pneumothorax during treatment of thoracic levels, although the potential exists for injury to occur from the spinal cord to the aorta. These injuries should easily be avoidable by well-trained individuals; however, concern for such disastrous complications should always be present. Although infection is rare with vertebroplasty or kyphoplasty, cases of osteomyelitis requiring corpectomy have been reported after vertebral body augmentation.67
Outcomes
Despite the widespread use of vertebroplasty and kyphoplasty, as well as their similarities, studies evaluating and comparing their outcomes are limited, with the majority of data being derived from case series or retrospective analyses. Recent meta-analyses comparing vertebroplasty with kyphoplasty have helped elucidate the differences in outcomes between the two procedures68–70; however, the need for prospective randomized controlled trials remains.
Despite the lack of prospective, randomized, controlled trials comparing kyphoplasty with vertebroplasty, two such trials comparing vertebroplasty with placebo have recently been published,71,72 and their results have called into question the utility of vertebroplasty. Buchbinder and coworkers performed a multicenter, randomized, double-blind, placebo-controlled trial in which they enrolled a total of 78 patients with painful osteoporotic vertebral fractures.71 Patients were randomized to either vertebroplasty or a sham procedure. Although they found significant reductions in pain in both study groups, vertebroplasty did not confer any beneficial effect at multiple time points up to 6 months after treatment. At the same time, Kallmes and coauthors published the results of a multicenter, randomized, controlled trial called the Investigational Vertebroplasty Safety and Efficacy Trial (INVEST).72 In this trial, 131 patients with painful osteoporotic vertebral fractures were randomized to undergo either vertebroplasty or a simulated procedure without injection of PMMA. Similar to the results of Buchbinder and colleagues, both groups showed immediate improvement in pain after the procedure. Although they saw a trend toward an increased rate of clinically relevant improvement in pain, this was not statistically significant, which led to the conclusion that improvements in pain and pain-related disability were similar in both the vertebroplasty group and the control group. Although these two studies represent the highest level of data on the subject to date, they have led to considerable discussion on the matter, with a variety of differing opinions regarding their true significance.73–75
Because pain is the main indicator for both kyphoplasty and vertebroplasty, perhaps the most important factor when deciding which modality to use is pain relief. In the largest meta-analysis to date, Eck and associates reviewed 83 studies and looked at pain scores for either vertebroplasty or kyphoplasty.68 Even though they found that both these treatment options resulted in considerable improvement in pain scores on a visual analog scale (VAS), vertebroplasty was with statistically significantly associated greater improvement in VAS scores than kyphoplasty was, although the authors questioned whether this difference was clinically significant.
In addition to pain scores, they also evaluated complications associated with vertebroplasty and kyphoplasty. They saw no significant difference between kyphoplasty and vertebroplasty for the majority of the observed complications; however, several key differences were noted. Perhaps the most striking disparity was seen with extravertebral cement leakage, with vertebroplasty having an incidence of 19.7% versus 7.0% for kyphoplasty.68 This difference was more pronounced in two other smaller meta-analyses.69,70 A statistically significant difference was also seen for symptomatic cement leakage, 1.6% for vertebroplasty versus 0.3% for kyphoplasty.68 These findings can be partially explained by the decreased viscosity of the cement often injected for vertebroplasty, as well as the increased pressure needed to inject the cement, which is largely relieved by inflation of the balloon in kyphoplasty.
The prevalence of new compression fractures was also slightly higher with vertebroplasty, a finding possibly attributed to extravasation of cement into the intervertebral disks.68,70,76 Kyphoplasty was shown to have a higher prevalence of myocardial infarction, which occurred in 0.5% of patients.68
Several studies have also looked at restoration of height and correction of kyphotic angles with kyphoplasty,12,77,78 and studies have been conducted to compare kyphoplasty with vertebroplasty for these parameters.79,80 Although the data suggest that kyphoplasty is more effective in restoring vertebral body height and decreasing kyphotic deformity, the significance of this finding is not clear. In their series, Pradhan and colleagues showed that the correction in kyphotic angle with kyphoplasty was essentially limited to the affected vertebra, with no improvement in overall sagittal alignment with one-level procedures.81 It is also unclear whether the correction in height seen with kyphoplasty is clinically significant, as evidenced by the relative improvement in pain seen with vertebroplasty as opposed to kyphoplasty.
Conclusion
Percutaneous vertebral body augmentation with vertebroplasty or kyphoplasty has now gained widespread acceptance for the treatment of osteoporotic vertebral body fractures, as well as a number of other pathologic conditions. Principally used for their effectiveness in pain relief and subsequent acceleration of patients’ functional status, they have proved to be significant advancements in the treatment of patients with osteoporotic compression fractures. It would be negligent, however, to compare kyphoplasty with vertebroplasty without noting the significant cost difference—kyphoplasty is currently close to eight times as expensive as vertebroplasty. However, it is not necessarily a cut-and-dry decision because despite evidence that vertebroplasty is more effective in relieving pain, several meta-analyses have reported a decreased rate of cement-related complications with kyphoplasty.68–70 Nevertheless, in the hands of experienced operators, these risks are thought to be minimized to an acceptable rate. Further prospective randomized trials are needed before definitive conclusions can be made regarding the superiority of one treatment modality over the other; however, until that time, both options appear to provide reasonable benefit in appropriately selected patients.
Bascoulergue Y, Duquesnel J, Leclercq R, et al. Percutaneous injection of methyl methacrylate in the vertebral body for the treatment of various diseases: percutaneous vertebroplasty [abstract]. Radiology. 1988;169(suppl):372.
Bono CM, Heggeness M, Mick C, et al. North American Spine Society: newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J. 2010;10:238.
Boszczyk BM, Bierschneider M, Schmid K, et al. Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J Neurosurg. 2004;100:32.
Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557.
Butler CL, Given CA2nd, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol. 2005;184:1956.
Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S.
Crandall D, Slaughter D, Hankins PJ, et al. Acute versus chronic vertebral compression fractures treated with kyphoplasty: early results. Spine J. 2004;4:418.
Eck JC, Nachtigall D, Humphreys SC, et al. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488.
Frey ME, Depalma MJ, Cifu DX, et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J. 2008;8:367.
Furtado N, Oakland RJ, Wilcox RK, et al. A biomechanical investigation of vertebroplasty in osteoporotic compression fractures and in prophylactic vertebral reinforcement. Spine. 2007;32:E480.
Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166.
Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511.
Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983.
Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897.
Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215.
Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569.
Kallmes DF, Jensen ME. Percutaneous vertebroplasty. Radiology. 2003;229:27.
Kaufmann TJ, Jensen ME, Schweickert PA, et al. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2001;22:1860.
Kim AK, Jensen ME, Dion JE, et al. Unilateral transpedicular percutaneous vertebroplasty: initial experience. Radiology. 2002;222:737.
Lieberman IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631.
Lourie H. Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA. 1982;248:715.
Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5:244.
Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793.
Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26:E300.
Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. 2006;31:2747.
Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28:555.
Zhou JL, Liu SQ, Ming JH, et al. Comparison of therapeutic effect between percutaneous vertebroplasty and kyphoplasty on vertebral compression fracture. Chin J Traumatol. 2008;11:42.
1 Tarantino U, Cannata G, Lecce D, et al. Incidence of fragility fractures. Aging Clin Exp Res. 2007;19:7.
2 Melton LJ3rd, Kan SH, Frye MA, et al. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129:1000.
3 Wasnich RD. Vertebral fracture epidemiology. Bone. 1996;18:179S.
4 Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S.
5 Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215.
6 Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793.
7 Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261.
8 Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166.
9 Bascoulergue Y, Duquesnel J, Leclercq R, et al. Percutaneous injection of methyl methacrylate in the vertebral body for the treatment of various diseases: percutaneous vertebroplasty [abstract]. Radiology. 1988;169(suppl):372.
10 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897.
11 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511.
12 Lieberman IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631.
13 Togawa D, Lieberman IH. Vertebroplasty and kyphoplasty, 2nd ed. Benzel EC, editor. Spine Surgery: Techniques, Complication Avoidance, and Management, vol 2. Philadelphia: Elsevier. 2005:1309.
14 Chen JF, Wu CT, Lee ST. Percutaneous vertebroplasty for the treatment of burst fractures. Case report. J Neurosurg Spine. 2004;1:228.
15 Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28:555.
16 Kaufmann TJ, Jensen ME, Schweickert PA, et al. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2001;22:1860.
17 Stallmeyer MJ, Zoarski GH, Obuchowski AM. Optimizing patient selection in percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14:683.
18 Cyteval C, Sarrabere MP, Roux JO, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol. 1999;173:1685.
19 Furtado N, Oakland RJ, Wilcox RK, et al. A biomechanical investigation of vertebroplasty in osteoporotic compression fractures and in prophylactic vertebral reinforcement. Spine. 2007;32:E480.
20 Hart RA, Prendergast MA, Roberts WG, et al. Proximal junctional acute collapse cranial to multi-level lumbar fusion: a cost analysis of prophylactic vertebral augmentation. Spine J. 2008;8:875.
21 Oakland RJ, Furtado NR, Wilcox RK, et al. The biomechanical effectiveness of prophylactic vertebroplasty: a dynamic cadaveric study. J Neurosurg Spine. 2008;8:442.
22 Kallmes DF, Jensen ME. Percutaneous vertebroplasty. Radiology. 2003;229:27.
23 Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22:373.
24 Karam M, Lavelle WF, Cheney R. The role of bone scintigraphy in treatment planning, and predicting pain relief after kyphoplasty. Nucl Med Commun. 2008;29:247.
25 Masala S, Schillaci O, Massari F, et al. MRI and bone scan imaging in the preoperative evaluation of painful vertebral fractures treated with vertebroplasty and kyphoplasty. In Vivo. 2005;19:1055.
26 Maynard AS, Jensen ME, Schweickert PA, et al. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol. 2000;21:1807.
27 Caudana R, Renzi Brivio L, Ventura L, et al. CT-guided percutaneous vertebroplasty: personal experience in the treatment of osteoporotic fractures and dorsolumbar metastases. Radiol Med (Torino). 2008;113:114.
28 Kim JH, Park KS, Yi S, et al. Real-time CT fluoroscopy (CTF)-guided vertebroplasty in osteoporotic spine fractures. Yonsei Med J. 2005;46:635.
29 Pitton MB, Herber S, Bletz C, et al. CT-guided vertebroplasty in osteoporotic vertebral fractures: incidence of secondary fractures and impact of intradiscal cement leakages during follow-up. Eur Radiol. 2008;18:43.
30 Pitton MB, Herber S, Koch U, et al. CT-guided vertebroplasty: analysis of technical results, extraosseous cement leakages, and complications in 500 procedures. Eur Radiol. 2008;18:2568.
31 Vogl TJ, Proschek D, Schwarz W, et al. CT-guided percutaneous vertebroplasty in the therapy of vertebral compression fractures. Eur Radiol. 2006;16:797.
32 Teng MM, Wei CJ, Wei LC, et al. Kyphosis correction and height restoration effects of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2003;24:1893.
33 Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18:311.
34 Deramond H, Depriester C, Toussaint P, et al. Percutaneous vertebroplasty. Semin Musculoskelet Radiol. 1997;1:285.
35 Kim AK, Jensen ME, Dion JE, et al. Unilateral transpedicular percutaneous vertebroplasty: initial experience. Radiology. 2002;222:737.
36 Eskey CJ. Vertebroplasty and Kyphoplasty, 5th ed. Schmidek HH, Roberts DW, editors. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, vol 2. Philadelphia: WB Saunders. 2006:2032.
37 Mathis JM, Wong W. Percutaneous vertebroplasty: technical considerations. J Vasc Interv Radiol. 2003;14:953.
38 Martin JB, Jean B, Sugiu K, et al. Vertebroplasty: clinical experience and follow-up results. Bone. 1999;25:11S.
39 Gaughen JRJr, Jensen ME, Schweickert PA, et al. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol. 2002;23:594.
40 Chung HJ, Chung KJ, Yoon HS, et al. Comparative study of balloon kyphoplasty with unilateral versus bilateral approach in osteoporotic vertebral compression fractures. Int Orthop. 2008;32:817.
41 Hoh BL, Rabinov JD, Pryor JC, et al. Balloon kyphoplasty for vertebral compression fracture using a unilateral balloon tamp via a uni-pedicular approach: technical note. Pain Physician. 2004;7:111.
42 Hu MM, Eskey CJ, Tong SC, et al. Kyphoplasty for vertebral compression fracture via a uni-pedicular approach. Pain Physician. 2005;8:363.
43 Ortiz AO, Zoarski GH, Beckerman M. Kyphoplasty. Tech Vasc Interv Radiol. 2002;5:239.
44 Anselmetti GC, Regge D, Sardo E, et al. Minimally invasive treatment of C2 odontoid traumatic fracture with transoral percutaneous vertebroplasty. Eur Radiol. 2007;17:850.
45 Dang D, Baig MN, Christoforidis G, et al. C2/C3 pathologic fractures from polyostotic fibrous dysplasia of the cervical spine treated with percutaneous vertebroplasty. Eur Spine J. 2007;16(suppl 3):250.
46 Monterumici DA, Narne S, Nena U, et al. Transoral kyphoplasty for tumors in C2. Spine J. 2007;7:666.
47 Reddy AS, Hochman M, Loh S, et al. CT guided direct transoral approach to C2 for percutaneous vertebroplasty. Pain Physician. 2005;8:235.
48 Rodriguez-Catarino M, Blimark C, Willen J, et al. Percutaneous vertebroplasty at C2: case report of a patient with multiple myeloma and a literature review. Eur Spine J. 2007;16(suppl 3):242.
49 Tan HQ, Li MH, Wu CG, et al. Percutaneous vertebroplasty for eosinophilic granuloma of the cervical spine in a child. Pediatr Radiol. 2007;37:1053.
50 Frey ME, Depalma MJ, Cifu DX, et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J. 2008;8:367.
51 Gotis-Graham I, McGuigan L, Diamond T, et al. Sacral insufficiency fractures in the elderly. J Bone Joint Surg Br. 1994;76:882.
52 Lourie H. Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA. 1982;248:715.
53 Grasland A, Pouchot J, Mathieu A, et al. Sacral insufficiency fractures: an easily overlooked cause of back pain in elderly women. Arch Intern Med. 1996;156:668.
54 Lin JT, Lane JM. Sacral stress fractures. J Womens Health (Larchmt). 2003;12:879.
55 Dehdashti AR, Martin JB, Jean B, et al. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000;23:235.
56 Marcy PY, Palussiere J, Descamps B, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer. 2000;8:500.
57 Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol. 2002;13:1265.
58 Butler CL, Given CA2nd, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol. 2005;184:1956.
59 Zaman FM, Frey M, Slipman CW. Sacral stress fractures. Curr Sports Med Rep. 2006;5:37.
60 Chiras J, Depriester C, Weill A, et al. [Percutaneous vertebral surgery. Technics and indications]. J Neuroradiol. 1997;24:45.
61 Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine. 2002;27:E419.
62 Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26:E300.
63 Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford). 2000;39:1410.
64 Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine. 2002;27:E416.
65 Padovani B, Kasriel O, Brunner P, et al. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20:375.
66 Scroop R, Eskridge J, Britz GW. Paradoxical cerebral arterial embolization of cement during intraoperative vertebroplasty: case report. AJNR Am J Neuroradiol. 2002;23:868.
67 Walker DH, Mummaneni P, Rodts GEJr. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus. 2004;17(6):E6.
68 Eck JC, Nachtigall D, Humphreys SC, et al. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488.
69 Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983.
70 Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. 2006;31:2747.
71 Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557.
72 Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569.
73 Bono CM, Heggeness M, Mick C, et al. North American Spine Society: newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J. 2010;10:238.
74 Buchbinder R, Kallmes DF. Vertebroplasty: when randomized placebo-controlled trial results clash with common belief. Spine J. 2010;10:241.
75 Weinstein JN. Balancing science and informed choice in decisions about vertebroplasty. N Engl J Med. 2009;361:619.
76 Komemushi A, Tanigawa N, Kariya S, et al. Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol. 2006;29:580.
77 Crandall D, Slaughter D, Hankins PJ, et al. Acute versus chronic vertebral compression fractures treated with kyphoplasty: early results. Spine J. 2004;4:418.
78 Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5:244.
79 Boszczyk BM, Bierschneider M, Schmid K, et al. Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J Neurosurg. 2004;100:32.
80 Zhou JL, Liu SQ, Ming JH, et al. Comparison of therapeutic effect between percutaneous vertebroplasty and kyphoplasty on vertebral compression fracture. Chin J Traumatol. 2008;11:42.
81 Pradhan BB, Bae HW, Kropf MA, et al. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine. 2006;31:435.