12 Osteoporosis and the Aging Spine: Diagnosis and Treatment
KEY POINTS
Osteoporosis is a systemic debilitating disease of the skeleton, characterized by significantly decreased bone mass in combination with the deterioration of bone microarchitecture. This process results in weakened bone with a great propensity for fracture with low-energy stress. As the average life expectancy and median age of the population rises, fractures secondary to underlying osteoporosis are becoming increasingly commonplace. More than 1.5 million osteoporotic fractures occur annually in the United States, the majority of which occur in the spine, hip, and wrist.1 Women are predominantly affected; a recent study estimates that as many as one in two women who are older than 50 years of age will suffer an osteoporotic fracture.2 These fractures can result in marked morbidity and mortality. For example, a single vertebral compression fracture in women is associated with a 1.2-fold increased age-adjusted mortality rate, and the presence of five fractures increases that risk to 2.3-fold.3 In addition, a vertebral fracture increases the risk of a second vertebral fracture by 5-fold, and a hip fracture by 2-fold. Among patients with osteoporotic hip fractures, only 25% of patients ever make a full recovery, while 20% die within the year secondary to complications. Thus, spine surgeons must be increasingly suspicious of this disease in certain patient demographics, achieve a firm understanding of the pathogenesis of osteoporotic bone and the conditions that result in bone fragility, and become familiar with the current strategies for diagnosis, prevention, and treatment of osteoporosis.
Physiology of Bone Remodeling and Bone Turnover
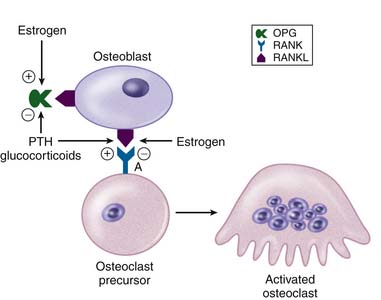
Bone remodeling is a complex process that is regulated both locally and systemically. As previously mentioned, RANKL/RANK interactions at the local level promote induction of osteoclast activity and subsequent remodeling. Conversely, osteoprotegerin (OPG) is a soluble receptor for RANKL that acts as an antagonist to decrease osteoclastic activation and thereby reduce the rate of bone resorption. Interestingly, there are a number of systemic signaling mechanisms that act through the RANKL/RANK/OPG pathway to regulate bone homeostasis.4 For example, parathyroid hormone (PTH) and the glucocorticoids both act to increase local expression of RANKL but decrease concomitant expression of OPG, resulting in a net increase in osteoclast activation and bone resorption. Alternatively, estrogens act to increase the local expression of OPG and decrease RANKL, which results in a net decrease in osteoclast activity and bone resorption (Figure 12-1). Derangement of these pathways can alter the delicate balance between bone resorption and bone formation, and may result in a net decrease of bone formation that contributes to the development of osteoporosis.
Diagnosis of Osteoporosis
Although a good clinical understanding of osteoporosis takes into account the pathophysiology of bone remodeling, mineralization changes, and variable bone quality of the patient, the diagnosis of osteoporosis until recently has relied upon a single criterion: the bone mineral density. The current gold standard of measuring BMD is dual-energy x-ray absorptiometry (DXA), which uses an x-ray beam to calculate the patient’s BMD. The most preferred skeletal sites for evaluation of BMD are the spine and hip, because these two locations provide the best data for correlating low BMD with the risk of future fracture BMD is reported as the T-score, which is a measurement of how many standard deviations the patient’s bone density is below the mean of young, healthy individuals at their peak bone mass. Based on this T-score, the World Health Organization (WHO) developed a classification system to define osteoporosis (Table 12-1).
| T-Score | Diagnosis |
|---|---|
| − 1.0 or above | Normal bone |
| Below −1.0 to above −2.5 | Osteopenia |
| − 2.5 or below | Osteoporosis |
| − 2.5 or below with fracture | Severe osteoporosis |
Secondary osteoporosis is defined by the presence of some preexisting disease process or other causative factor, which causes a secondary decline in BMD (Table 12-2). Forty-five percent of osteoporotic women and 66% of osteoporotic men have their osteoporosis secondary to some underlying condition. Therefore patients with secondary osteoporosis must be identified because definitive treatment of the underlying cause is necessary to prevent further bone loss, and thus lower the risk of fracture. In this regard, it is important to consider the patient’s BMD using the Z-score. The Z-score indicates how many standard deviations the patient’s BMD is below the expected value for his or her own age. The Z-score cannot be used to diagnose osteoporosis, but it is useful for screening the patient for secondary causes. A Z-score of −2.0 or lower should increase the index of suspicion that underlying medical problems, medications, or other factors may be responsible for the patient’s low BMD.6
TABLE 12-2 Causes of Secondary Osteoporosis5
Evaluation for Osteoporosis
Once diagnosed with osteoporosis, a complete medical history should be obtained with particular attention to the risk factors for osteoporosis. These include age of 65 years or older, a history of vertebral fracture or any fracture during childhood, a family history of hip fracture, low body weight (BMI < 21 or weight < 127 lb), cigarette smoking, and use of corticosteroids for more than 3 months.6 The physical examination should be performed particularly at the spine region. Height should be measured and compared with the greatest known height to determine height loss, which is an indicator of the presence of vertebral compression fractures. Balance and walking gait should be observed in each individual. The assessment of functional balance is performed by using the single limb stance test and the 6-minute walking test.
Screening for Osteoporosis with Bone Mineral Density Measurement
A number of risk factors for osteoporosis have been identified by the International Society for Clinical Densitometry (ISCD),7 and should be used to guide the screening process in a cost-effective manner. The current indications for BMD testing include any patient who is one or more of the following:
Laboratory Investigations for Osteoporosis
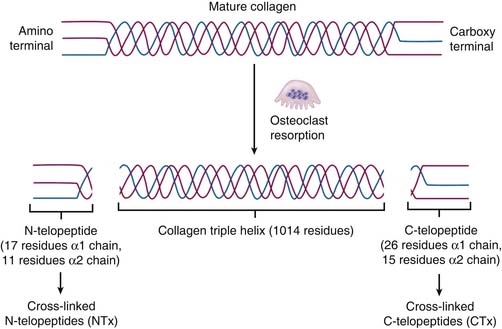
Bone markers can be classified into two groups: bone formation and bone resorption markers. Bone specific alkaline phosphatase and serum osteocalcin are both produced when bone is formed and can be used as markers for bone formation. On the other hand, during bone resorption, human collagen is broken down and released into the bloodstream and subsequently secreted into the urine. By assaying the amount of collagen breakdown products, such as the cross-linked amino-terminal telopeptides of bone collagen (NTx) in urine or blood, the rate that bone is being resorbed can be determined (Figure 12-2). Measurement of bone markers is helpful for following a patient’s response to treatment over time. Therefore it is advisable to get a baseline value as part of the initial workup. The goal of treatment of osteoporosis is to keep the NTx levels close to the normal range for premenopausal women.
Evaluation for Secondary Osteoporosis
When secondary osteoporosis is suspected on the basis of clinical findings or because the patient is relatively young and presented with fragility fracture, specific tests should be considered to evaluate contributing causes that may require additional medical attention. These include basic laboratory investigation of a complete blood count with differential, erythrocyte sedimentation rate, serum calcium and phosphate level, liver function tests, thyroid-stimulating hormone level, testosterone level in men, and a serum protein electrophoresis if myeloma is considered (Table 12-3). When abnormalities are detected, the patient should be referred to a specialist for further evaluation and specific treatment.
Table 12-3 Laboratory Investigations for Secondary Osteoporosis
| Medical Diseases | Diagnostic Study |
|---|---|
| Endocrine |
TSH = thyroid stimulating hormone; LH = luteinizing hormone; FSH = follicle stimulating hormone; CBC = complete blood count; ESR = erythrocyte sedimentation rate; CRP = c-reactive protein; BUN = blood urea nitrogen
Assess for Risk of Falls and Fractures
Certain comorbidities associated with the aging population, such as unsteady gait, use of sedative or hypnotic medications, and impaired visual or neuromuscular function may predispose a patient to falls. By identifying patients at particularly high risk for falls early in the course of treatment, it is possible to prevent a subsequent fracture. It is well recognized that the fracture rate is highest among patients with osteoporotic bones (T-score −2.5 or below). However, a much larger proportion of patients reside in the range of osteopenia (below −1.0 to above −2.5). Consequently, more total fractures occur in this osteopenic group (55% of hip fractures). To adjust for the disparity, a new vehicle called the Fracture Risk Assessment Tool (FRAX) has been developed that adds additional risk factors to the calculation, and offers a better assessment of fracture risk than DXA scanning alone. This instrument calculates the patient’s 10-year fracture risk based on age, sex, weight, height, previous fracture, parent with fractured hip, current smoking, use of glucocorticoids, presence of rheumatoid arthritis, secondary osteoporosis, alcohol use (>3 drinks/day), and BMD at the femoral neck area.
Each risk factor is weighted differently in the calculation depending on its importance. For example, in the absence of BMD measurements, a prior history of fracture is considerably more predictive of a patient’s future fracture risk than is a history of smoking, and is weighted as such in the FRAX calculation (Table 12-4). As the patient accrues more risk factors, the FRAX score increases in a predictable manner.8
TABLE 12-4 Ten-Year Probability for Osteoporotic Fracture Based on FRAX Calculations8
| Clinical Risk Factors∗ | 10-Year Fracture Probability (%)† |
|---|---|
| None | 8.6 |
| Current smoker | 9.2 |
| Alcohol (≥3 drinks/day) | 10.4 |
| Rheumatoid arthritis | 11.7 |
| Oral glucocorticoids | 13.7 |
| Parent with history of hip fracture | 16.0 |
| Previous fragility fracture | 16.4 |
* Assume in a female patient, aged 65 years, with BMI = 25 kg/m2.
Treatment in Osteoporosis
Nonpharmacologic Treatment
A multidisciplinary approach is critically important in the management of osteoporosis.9 Nonpharmacologic treatment is used concurrently with pharmacologic therapy to optimize fracture risk reduction. Thus, every patient should be considered for nonpharmacologic management. Commonly used nonpharmacologic treatments include, but are not limited to, calcium and vitamin D supplementation, fall prevention, and balance and exercise programs.
Pharmacologic Treatment
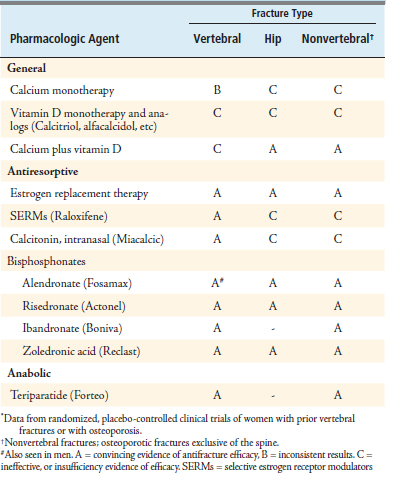
Osteoporosis has been divided into two categories, high-turnover and low-turnover osteoporosis. The pharmacologic agents currently available are commonly divided into antiresorptive and anabolic groups. Antiresorptive agents have been developed to address the high-turnover state. These include estrogen, selective estrogen receptor modulators (SERMs), calcitonin, and bisphosphonates. The anabolic agent, parathyroid hormone, provides active building of bone mass and has been suggested to treat the low-turnover state. Both antiresorptive and anabolic agents have demonstrated antifracture efficacy in many studies (Table 12-5).
Antiresorptive Agents
Estrogen is an antiosteoporotic agent that has been shown to increase bone mass and thus decrease the risk of vertebral and hip fracture by approximately 30% to 40% as compared with patients taking placebo. Estrogen, however, has been found to increase rates of stroke and deep vein thrombosis, whereas combined estrogen and progesterone therapy is associated with increased risks of cardiovascular disease, breast cancer, dementia, and gallbladder disease. As a consequence, estrogen is mainly used in the early postmenopausal period to treat postmenopausal syndrome and then lowered to the least effective dose to control symptoms. Because of the risks of estrogen formulations, this precludes their use as primary agents in the treatment of osteoporosis.
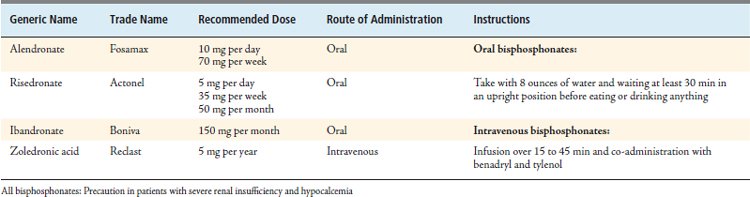
Bisphosphonates are a class of antiresorptive agents that have been shown to be extremely efficacious in high-turnover osteoporosis. Currently, four bisphosphonates have been approved by the U.S. Food and Drug Administration for the treatment of posmenopausal osteoporosis: alendronate (Fosamax), risedronate (Actonel), ibandronate (Boniva) and zoledronic acid (Reclast).10 These drugs differ in their potency, mode of administration, and dosing schedules. Alendronate and risedronate are administered orally, whereas zoledronic acid is administered by intravenous injection. Ibandronate is available in both oral and intravenous formulations (Table 12-6).
1. Keen R.W. Burden of osteoporosis and fractures. Curr. Osteoporos. Rep.. 2003;1:66-70.
2. Lane J.M., Gardner M.J., Lin J.T., van der Meulen M.C., Myers E. The aging spine: new technologies and therapeutics for the osteoporotic spine. Eur Spine. J. 2003;12(Suppl. 2):S147-S154.
3. Lin J.T., Lane J.M. Osteoporosis: a review. Clin. Orthop. Relat. Res. 2004 Aug;425:126-134.
4. Hadjidakis D.J: Androulakis II, Bone remodeling, Ann. N. Y. Acad. Sci, 1092, 385-396, 2006.
5. Stein E., Shane E. Secondary osteoporosis. Endocrinol Metab. Clin. North Am. 2003;32:115-134.
6. Kaufman J.D., Cummings S.R. Osteoporosis and prevention of fractures: practical approaches for orthopaedic surgeons. Instr. Course Lect. 2002;51:559-565.
7. International Society for Clinical Densitometry. 2007 Official Positions & Pediatric Positions of the International Society for Clinical Densitometry. Available at http://www.iscd.org/Visitors/pdfs/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed October 23, 2008.
8. Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008;19:385-397.
9. Bouxsein M.L., Kaufman J., Tosi L., Cummings S., Lane J., Johnell O. Recommendations for optimal care of the fragility fracture patient to reduce the risk of future fracture. J. Am. Acad. Orthop. Surg. 2004;12:385-395.
10. Gehrig L., Lane J., O’Connor M.I. Osteoporosis: management and treatment strategies for orthopaedic surgeons. J. Bone Joint Surg. Am. 2008;90:1362-1374.