Chapter 73 Osteoporosis After Anterior Cruciate Ligament Reconstruction?
Anterior Cruciate Ligament Injuries and their Treatment
Anterior cruciate ligament (ACL) injuries are usually sustained by a young and active population, often under heavy pressure to continue heavy labor or sports activities at a competitive or recreational level. The natural history of an ACL injury still remains unclear. Conservative (nonsurgical) treatment has been reported to produce unsatisfactory results, such as chronic instability, muscle weakness, and osteoarthritis (OA), but also to render acceptable function for some patients.1 It is a common algorithm that surgical intervention is recommended if the patient requests a return to high-risk pivoting sports or if symptoms of giving way are persistent after a conservative program. To our knowledge, however, this has not been scientifically proven in randomized controlled studies.
Peak Bone Mass and Natural Bone Losses
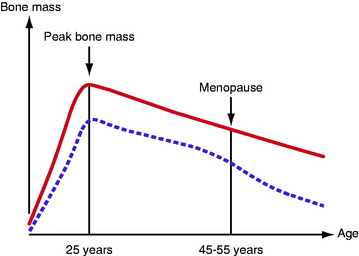
We start adult life with a peak bone mass, 80% of which is determined by genetic factors. This is also the case for fat and muscle tissue, 65% to 80% of which are genetically determined. Men have a higher peak bone mass than women (Fig. 73-1). During childhood and adolescence, it is possible to increase bone mass with sufficient nutrition and physical activity. However, the positive effect of training during childhood and adolescence appears to be lost with the cessation of a sporting career.2
From the peak bone mass at the age of 25 years, there is an annual loss of approximately 0.5% to 1%. The loss for women is accelerated in connection with menopause and, during the subsequent 10 years, the annual loss is as much as 2% to 4% (see Fig. 73-1). The bone metabolism turnover rate is 4 to 10 times higher in trabecular bone compared with cortical bone. The content of trabecular bone in the calcaneus is 95%; in the lumbar vertebrae, 40%; and in the neck of the femur, 40%. In a period of 8 years, the bone tissue is completely replaced. The potential as an adult to compensate for a low peak bone mass or significantly to increase bone mass by strenuous exercise or an excessive intake of calcium/vitamin D appears to be virtually nonexistent. However, the female athlete triad, a disorder in young females characterized by eating disturbances, amenorrhea, and osteoporosis, could be an exception. For these individuals, there is still the potential for “catch-up” in bone mass in the third decade of life if the condition is reversed. The normal physiological bone loss seen during pregnancy and breastfeeding also appears to be reversible.
Osteoporosis
Osteoporosis and its consequences, with an increasing fracture incidence among the aging population in Western countries, have become a challenge to general public health and the welfare system. There are many known risk factors for osteoporosis and/or fractures. They can be divided into (1) nontreatable risk factors such as older age, previous fracture, female gender, menopausal age, heredity, ethnicity, and body height and (2) treatable risk factors such as physical inactivity, low body weight/low body mass index (BMI), cortisone treatment, low bone density, tendency to fall, tobacco smoking, alcohol consumption, low exposure to sunlight, impaired vision, and calcium and vitamin D deficiency.3 Older age and a history of previous fractures are regarded as by far most important risk factors.
Bone Loss and Musculoskeletal Injuries
Local and generalized bone loss is reported in the literature to be associated with musculoskeletal injury and physical inactivity. The bone loss caused by inactivity might be prevented at least in the early phase of inactivity through increased weight bearing, exercise, or medication. Fractures in the lower extremities are reported to cause bone loss. Andersson and Nilsson4 reported a loss of 25% in the proximal tibia and fibula 1 year after tibial shaft fractures. The loss was found irrespective of treatment in a below-knee brace with weight bearing or a long leg plaster without weight bearing. Nine years after tibial fractures, Kannus et al reported approximately 10% lower bone mineral density in the spine and in the knee region in the injured leg compared with the noninjured leg.5 Andersson and Nilsson6 also found a loss of bone mineral in the knee region after knee ligament injuries. The average loss after 1 year was 10% in the nonoperated group treated with a knee bandage for a short time and 18% in the operated group treated with a plaster cast for 5 weeks.
Anterior Cruciate Ligament Surgery and the Effect on Bone Tissue
Kannus et al reported a 3% to 9% lower bone mineral density in the knee region compared with the noninjured knee 10 to 11 years after ACL injuries treated surgically. The injured knee had a reduction of 6% in the distal femur, 9% in the patella, and 3% in the proximal tibia. However, no differences were found in the calcaneus and femoral neck. When examining patients with medial collateral ligament injuries, no reduction was found compared with the noninjured side, although the immobilization period was the same (cast for 6–7 weeks). The authors speculated that using rehabilitation braces and early weight bearing instead of immobilization in a plaster cast after ACL reconstruction could prevent osteoporosis.7
In a cross-sectional study, Kartus et al8 reported that male patients with unilateral ACL injury had a significantly lower bone mass in the calcaneus on the injured side compared with the noninjured side before primary reconstruction, 2 years after primary reconstruction, and 2 years after revision surgery. Furthermore, the time period between the injury and the index operation did not correlate with the bone mass. However, a high level of activity correlated with the bone mass on both the injured and the uninjured side 2 years after primary reconstruction.
Leppala et al9 found a bone loss of 14% to 21% in the knee region in the affected extremity 1 year after ACL reconstruction. In a conservatively treated group (complete or partial ACL tears), they found a small yet significant bone loss in the patella (3%) and proximal tibia (2%) on the affected side. The “healthy” extremity was not measured, however.
Ejerhed et al10 reported a bone loss of approximately 17% in both calcanei 2 years after ACL reconstruction using the arthroscopic technique, bone–patellar tendon–bone (BPTB) grafts, and aggressive rehabilitation. The patients underwent surgery 1 year after ACL injury, and by that time they had a low activity level but still had bone mineral values above those of the normal population. Two years after the reconstruction, the patients had regained most of their desired activity level more than 1 year earlier, but with bone mineral values far below the normal (Fig. 73-2). The previously proposed explanation for excessive bone loss due to inactivity therefore appeared to be of minor importance, at least in that study.
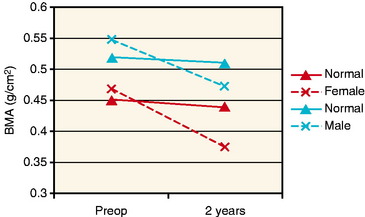
Fig. 73-2 A schematic representation of the bone mineral loss found by Ejerhed et al10 in the calcanei from the preoperative assessment and 2 years after anterior cruciate ligament (ACL) reconstruction (dotted lines). The unbroken lines show the expected loss due to aging for males and females, respectively.
It might be that bone mineral decreases in the knee region as a result of the surgical trauma to the knee during reconstruction. The technique used for the reconstruction, BPTB, removes a substantial amount of bone tissue in the knee region (femoral condyle, patella, and tibial condyle). Another proposed explanation for the localized bone loss in the knee region is that harvesting the central third of the patellar tendon causes a weakening in an intact tendon. This leads to reduced forces acting on this region, with a subsequent bone loss in the patella and the proximal tibia.11
Surgery: A Risk Factor for Osteoporosis?
Alfredson et al reported that a group of patients selected for operative treatment of chronic Achilles tendinosis had a bone loss of 16.4% on the injured side compared with the noninjured side, 12 months postoperatively. In a group of patients selected for heavy-loaded eccentric calf-muscle training, there was no side-to-side difference in bone mass in the calcanei 9 months after the start of the training program. Both groups had good clinical results with recovery in muscle strength on both the injured and noninjured sides.12
The study by Leppala et al9 is the only study found in the literature relating to bone loss after ACL injuries treated either surgically or conservatively. The authors found bone loss in the patella of 17% versus 2% and in the proximal tibia of 17% versus 3% in the surgically and nonsurgically treated groups, respectively. This represents a fivefold to eightfold difference in the loss of bone between the study groups. The explanations proposed by the authors were the different treatments (either surgical or conservative). The patients had different types of injury and rehabilitation; in the conservatively treated group there were also some partial tears, and in the surgically treated group, a longer non–weight-bearing period.
The finding in the study by Ejerhed et al that the bone loss was 16% and 17% in both calcanei 2 years after ACL reconstruction is unexpected.10 In spite of the relatively long period of reduced activity level before reconstruction, the aggressive rehabilitation program, and significantly higher activity level postoperatively, the authors were not able to find any restoration of bone mass during the follow-up period of 2 years. The strength of this study was that the bone mass was measured in a prospective manner on both the injured and noninjured sides. Frequently in previous studies, a comparison has been made between the injured side and the “healthy” contralateral side. By doing so, there is an obvious risk of drawing the wrong conclusion if small or no differences are found. It is easy to miss a progressive process. For instance, if the 2-year assessment had just been made in this study, a difference of only 2.5% would have been found between the injured and noninjured sides.
1 Casteleyn PP, Handelberg F. Non-operative management of anterior cruciate ligament injuries in the general population. J Bone Joint Surg. 1996;78B:446-451.
2 Karlsson MK, Linden C, Karlsson C, et al. Exercise during growth and bone mineral density and fractures in old age. Lancet. 2000;355:469-470.
3 SBU. Bone density measurement—a systematic review. A report from SBU, the Swedish Council on Technology Assessment in Health Care. J Intern Med Suppl. 1997;739:1-60.
4 Andersson SM, Nilsson BE. Post-traumatic bone mineral loss in tibial shaft fractures treated with a weight-bearing brace. Acta Orthop Scand. 1979;50:689-691.
5 Kannus P, Järvinen M, Sievanen H, et al. Osteoporosis in men with a history of tibial fracture. J Bone Miner Res. 1994;9:423-429.
6 Andersson SM, Nilsson BE. Changes in bone mineral content following ligamentous knee injuries. Med Sci Sports. 1979;11:351-353.
7 Kannus P, Sievanen H, Järvinen M, et al. A cruciate ligament injury produces considerable, permanent osteoporosis in the affected knee. J Bone Miner Res. 1992;7:1429-1434.
8 Kartus J, Stener S, Nilsen R, et al. Bone mineral assessments in the calcaneus after anterior cruciate ligament injury. An investigation of 92 male patients before and two years after reconstruction or revision surgery. Scand J Med Sci Sports. 1998;8:449-455.
9 Leppala J, Kannus P, Natri A, et al. Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: a prospective one-year follow-up study. Calcif Tissue Int. 1999;64:357-363.
10 Ejerhed L, Kartus J, Nilsen R, et al. The effect of anterior cruciate ligament surgery on bone mineral in the calcaneus: a prospective study with a 2-year follow-up evaluation. Arthroscopy. 2004;20:352-359.
11 Rittweger J, Maffulli N, Maganaris CN, et al. Reconstruction of the anterior cruciate ligament with a patella-tendon-bone graft may lead to a permanent loss of bone mineral content due to decreased patellar tendon stiffness. Med Hypotheses. 2005;64:1166-1169.
12 Alfredson H, Nordström P, Pietila T, et al. Bone mass in the calcaneus after heavy loaded eccentric calf-muscle training in recreational athletes with chronic achilles tendinosis. Calcif Tissue Int. 1999;64:450-455.