88 Osteomyelitis and Other Bone and Joint Infections
Osteomyelitis
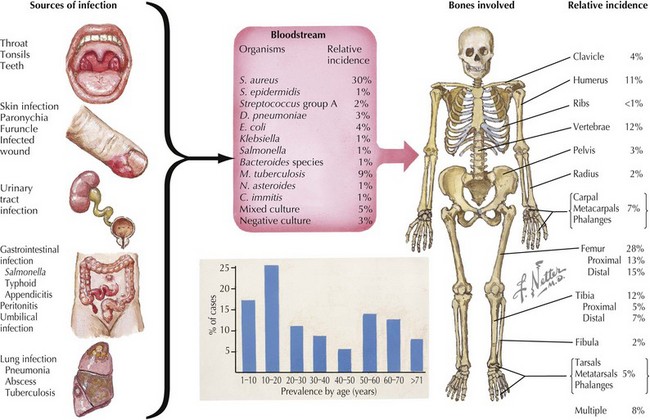
Osteomyelitis is inflammation of bone caused by bacterial or, less often, fungal infection. Osteomyelitis is categorized both by the mechanism of pathogen transmission to the bone (hematogenous, direct extension) and by the clinical presentation (acute, subacute, or chronic). In children, hematogenous spread of bacteria to the bone is the most common mode of transmission (Figure 88-1). Less often, osteomyelitis is the result of contiguous spread from a soft tissue infection or direct inoculation by penetration, such as after trauma or surgery. Vascular insufficiency is a rare cause of osteomyelitis in children. Eighty-five percent of cases of osteomyelitis occur in children younger than 16 years of age (50% in children younger than 5 years of age), with a male-to-female ratio of 2 : 1, except within the first year of life, when both genders are affected equally. Long bones (femur, tibia, humerus, in that order of frequency) followed by bones of the hands and feet and pelvis are the most common sites involved (see Figure 88-1). Approximately 5% of patients have multiple foci.
Etiology
Isolation of a bacterial source of osteomyelitis occurs in 50% to 80% of patients when both blood and bone are cultured. The bacteria responsible for osteomyelitis in children vary by age and underlying condition (see Figure 88-1). Staphylococcus aureus is the most common pathogen in any age group (70%-90% of cases), with community-acquired methicillin-resistant S. aureus (CA-MRSA) becoming more prevalent in recent years. In infants younger than 2 months of age, group B streptococci and gram-negative enteric bacteria are seen in addition to S. aureus. In children younger than 5 years of age, S. aureus, Streptococcus pyogenes, Streptococcus pneumoniae, and Kingella kingae are leading causes of osteomyelitis. Children older than 5 years of age are most commonly infected by S. aureus or S. pyogenes. Neisseria gonorrhoeae may be the etiologic agent in sexually active adolescents.
Pathogenesis
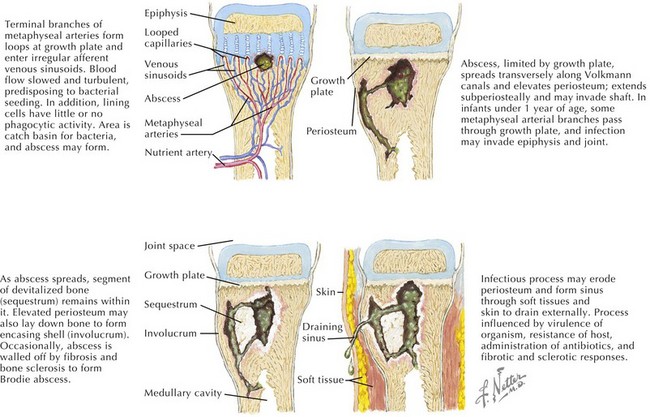
The pathogenesis of acute hematogenous osteomyelitis is dictated by the anatomy of growing bone in young children. The rich vascular supply of the metaphysis and its sluggish flow allow deposition of bacteria that enter via the nutrient artery (Figure 88-2). An absence of macrophages in the metaphyseal capillary loops allows bacterial replication, inducing an inflammatory response that may lead to abscess formation, infarction of bone, and necrosis. Acute hematogenous osteomyelitis is preceded by minor trauma to the site of infection in one-third of cases; resultant small hematomas may make the underlying metaphysis more favorable for bacterial deposition.
Diagnosis
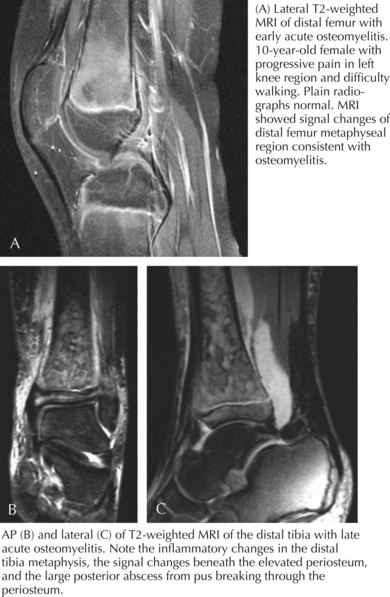
Plain radiographs show soft tissue swelling within 3 days of symptom onset; osteolytic lesions and periosteal elevation are apparent after 10 to 20 days; and after 1 month of symptoms, sclerosis of bone can be seen. Technetium-99 bone scanning has a sensitivity of 80% to 100% for osteomyelitis and can be helpful early on when plain films are normal and can identify multiple sites of infection, when present. Because the radionuclide bone scan can be falsely negative in 5% to 20% of children in the first few days of illness, magnetic resonance imaging (MRI) may be performed. MRI has a sensitivity of 92% to 100% for osteomyelitis and is effective in distinguishing soft tissue infection (e.g., cellulitis) from osteomyelitis. Pelvic and vertebral osteomyelitis are best imaged by MRI. Additionally, chronic osteomyelitis may have a different appearance from acute osteomyelitis on MRI (Figure 88-3).
Septic Arthritis
Pathogenesis
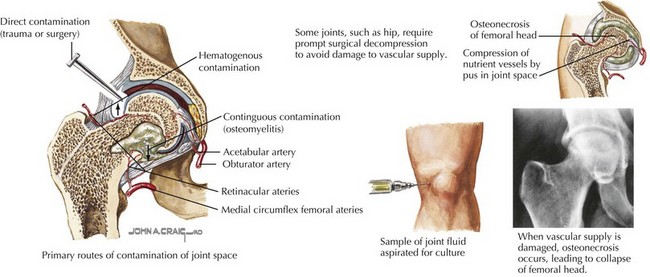
Similar to acute osteomyelitis, hematogenous spread of bacteria to the vascular synovium is the most common cause of septic arthritis in children (Figure 88-4). The precise mechanism by which the presence of bacteria leads to joint destruction is unknown, but the resultant inflammatory response causes activation of host leukocytes, cytokine secretion, and chondrolysis. Degradation of the joint begins as early as 8 hours after infection and can lead to irreversible synovial fibrosis and bony ankylosis.
Diagnosis
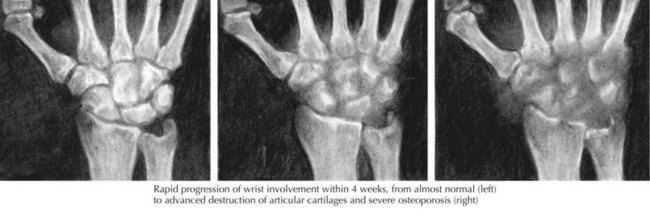
Plain radiographs in septic arthritis may be negative early on, but joint space widening and capsular distension can be seen (Figure 88-5); erosion of subchondral bone is evident 2 to 4 weeks into infection. Other pathologies such as fracture, osteomyelitis, and malignancy can be identified on the plain radiograph. With septic arthritis of the hip, plain films may again be negative, but the characteristic finding early on is swelling of the hip capsule and lateral displacement of the gluteal fat planes; later, there is upward and lateral displacement of the femoral head. Ultrasound of the hip can be performed to detect fluid in the joint and to guide arthrocentesis. Bone scan, CT scan, and MRI are typically not performed for diagnosis of septic arthritis, but can be useful for evaluating deep joints, such as the sacroiliac joint and identifying osteomyelitis.
The differential diagnosis of septic arthritis includes transient (toxic) synovitis, viral arthritis, tuberculous arthritis, fungal arthritis, reactive arthritis, Reiter’s syndrome, juvenile idiopathic arthritis, rheumatic fever, trauma, malignancy, Legg-Calvé-Perthes disease, and a slipped capital femoral epiphysis (Table 88-1).
| Condition | Differential Diagnosis from Septic Arthritis |
|---|---|
| Appendicitis | Possible psoas muscle irritation that may result in flexion of the right hip, abdominal tenderness |
| Bursitis | No joint effusion, swelling in the area of affected bursa |
| Cellulitis | No joint effusion, marked erythema of skin |
| Gout | Intense pain, great toe common involvement, crystals seen on joint aspiration |
| Hemarthrosis | History of trauma, bloody fluid on aspiration |
| Juvenile rheumatoid arthritis | Gradual onset of symptoms, morning stiffness, better joint motion |
| Lyme disease | Indolent onset, boggy synovium |
| Osteomyelitis | No joint effusion, better range of motion |
| Rheumatic fever | Less effusion, arthralgia |
| Reactive arthritis | Less pain and effusion; joint fluid white blood cell count, 10,000 to 20,000 per mm3 |
| Transient synovitis | Hip involvement only, no systemic symptoms, better range of motion, may require aspiration to be differentiated from septic arthritis |
Reprinted with permission from Greene W: Netter’s Orthopaedics. Philadelphia, Saunders, Elsevier, 2006, p 157.
Gutierrez K. Bone and joint infections in children. Pediatr Clin North Am. 2005;52(3):779-784.
Kaplan SL. Osteomyelitis in children. Infect Dis Clin North Am. 2005;19(4):787-797.
Peltola H, Pääkkönen M, Kallio P, Kallio MJ, Osteomyelitis-Septic Arthritis (OM-SA) Study Group. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin Infect Dis. 2009;48:1201-1210.
Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668-1674.
Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642.