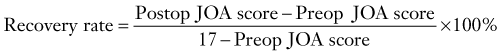CHAPTER 282 Ossification of the Posterior Longitudinal Ligament and Other Enthesopathies
Background and Epidemiology
OPLL is characterized by growth of the posterior longitudinal ligament with the development of ossification centers and eventual calcification and mature ectopic bone formation. OPLL was first reported in 1838; however, the disorder gained recognition in the 1960s when brought to attention by the Japanese spine surgery community. OPLL is a relatively common cause of cervical myelopathy in middle-aged and elderly Japanese and is therefore believed to occur with higher frequency in the Asian population. Epidemiologic studies have revealed the prevalence of OPLL in asymptomatic Japanese adults to be 2.0% as opposed to 0.95% in Koreans and 0.17% to 0.20% in white individuals.1 OPLL thus represents a major public health problem in East Asia.
Recently, better understanding of OPLL with improved radiographic imaging suggests that it may be more prevalent in even the non-Japanese population. A computed tomography (CT) and magnetic resonance imaging (MRI) study revealed that in symptomatic patients with myelopathy or radiculopathy, OPLL or some variant may be identified in as many as 25% of U.S. patients as compared with 27% of Japanese patients.2 However, this study probably reflected a bias, with the higher proportion of OPLL patients being due to referral patterns.
Because it is believed that North American Indians are originally descended from humans who migrated across the Bering Strait, North American Hispanics may have a higher prevalence of OPLL than the general North American population, in whom cervical spondylotic myelopathy is frequently encountered. Recent study of OPLL variants found the distribution of compressive morphologies to be similar to that reported in East Asians.3
Pathophysiology
Genetic Factors
Familial surveys and genetic studies reveal that OPLL has a strong hereditary component with an autosomal dominant pattern of inheritance. OPLL occurs in 26% of parents and 29% of siblings of patients in whom OPLL is diagnosed.4 Genetic linkage analysis reveals an association between the HLA haplotype and OPLL. Patients with two concurrent HLA strands have a 53% rate of expression of OPLL as opposed to a 24% rate in those with only one concurrent strand.5
Further genetic analysis of 91 sibling pairs of patients with OPLL suggests that the genetic locus for OPLL may be situated close to the HLA region on chromosome 6p.6,7 Patients with DISH, who commonly have OPLL, also test positive for the same HLA antigen. The COL11A2 gene, which is located within the class II major histocompatibility complex region of chromosome 6, is believed to be the gene responsible for OPLL. Patients with OPLL have a significantly higher incidence of genetic abnormalities in the COL11A2 region.8 COL11A2 encodes the α2 chain of type XI collagen, which is a minor collagen in the cartilage matrix and may be involved in controlling the growth of type II collagen. Overproduction of type XI collagen has been seen in histologic studies of some surgical specimens of OPLL.9
Other genetic loci associated with OPLL include the collagen 6A1 gene (COLA1). COLA1 is located on chromosome 21q, and genome-wide linkage and linkage disequilibrium analysis of 142 affected sibling pairs has identified this gene locus as the candidate gene for the pathogenesis of OPLL.10 COLA1 encodes the α1 chain of type VI collagen.11 Overproduction of type VI collagen may serve as a scaffold for chondrocyte infiltration and endochondral bone growth.
The animal model of OPLL, the tiptoe walking (ttw) mouse, demonstrates progressive calcification of spinal ligaments. A single-base mutation in the nucleotide pyrophosphate gene (NPPS) is thought to be responsible for the development of ligament ossification in this model. NPPS encodes for a protein that inhibits the mineralization and calcification of cartilaginous tissue. Loss of the NPPS protein may cause unregulated ossification of the spinal ligaments. In humans, NPPS has three isoforms, with NPP1 being located on chromosome 6. A single-base polymorphism in NPP1 is more common in patients with cervicothoracic OPLL than in those with only cervical OPLL, perhaps indicative of more severe and progressive disease.12
Growth Factors
The posterior longitudinal ligament in patients with OPLL demonstrates an enhanced potential for osteogenesis, thus suggesting that intrinsic growth factors may be integral in the pathogenesis of OPLL. Bone morphogenetic proteins (BMPs) are a class of growth factors involved in the induction of new bone formation. The nonossified ligaments of OPLL patients and their relatives are found to have elevated concentrations of BMP.13 Histologic evaluation of OPLL surgical specimens demonstrates the presence of BMP-2 in the ossified matrix, chondrocytes, and fibroblasts in cartilaginous areas adjacent to the OPLL.14,15 Furthermore, BMP-2 mRNA was isolated from the spinal ligaments of patients with OPLL and not from control subjects.15 Zinc finger protein 145, a regulator of BMP expression and an early initiator of osteoblastic differentiation, is also upregulated in OPLL patients. Comparatively, the MSX2 gene, which expresses a protein that inhibits osteoblastic differentiation and mineralization of fibroblasts, is downregulated in OPLL patients.
Other growth factors associated with OPLL include transforming growth factor-β, which is present in the ossified matrix and chondrocytes in cartilaginous tissue adjacent to OPLL. Osteocalcin synthesis, which reflects the osteoblastic phenotype of cells, is observed in the supernatant of the posterior longitudinal ligament of patients with OPLL and not from patients with non-OPLL cervical spondylosis.16 Insulin-like growth factor, connective tissue growth factor, parathyroid hormone, platelet-derived growth factor, retinoid, estrogens, and interleukin-1 are also associated with the pathogenesis of OPLL.
Metabolic Factors
OPLL is associated with a high incidence of adult-onset obesity and impaired glucose tolerance.17,18 Among 535 patients with OPLL, 28% were diabetic and 18% qualified as borderline diabetic.19 In patients with known OPLL, the extent of ossification is significantly correlated with the fasting serum insulin level.17 In a study from the Japan Collaborative Epidemiological Study Group for Evaluation of Ossification of the Posterior Longitudinal Ligament of the Spine Risk,20 adult-onset obesity and non–insulin-dependent diabetes were identified as independent risk factors for OPLL. When 69 eligible patients with OPLL were compared with control patients without spinal disorders matched for age and gender, the OPLL group had a significantly higher proportion of patients with diabetes and a higher body mass index.
From the same study, additional dietary habits associated with increased risk for the development of OPLL included frequent consumption of pickled foods. Alternatively, diets rich in chicken and soy products were correlated with a decreased risk for OPLL. No association between cigarette or alcohol consumption and OPLL was observed. Other reports have suggested that hypoparathyroidism and hypophosphatemic rickets are related to a higher incidence of OPLL.19
Other Enthesopathies
The enthesopathies are characterized by progressive inflammation of tendons and ligaments and their association with bone. OPLL is of primary concern to neurosurgeons and orthopedic spine surgeons because of the location of the posterior longitudinal ligament within the spinal canal and the risk for cord compression with hypertrophy and calcification. Other major spinal enthesopathies include DISH, OALL, and OLF. DISH is an ossifying condition that affects the axial and appendicular skeleton and is commonly also known as ankylosing hyperostosis. With spinal involvement, the anterolateral vertebral column is primarily affected. DISH most commonly occurs in adults, and it affects 3% of those older than 40 years and 15% of those older than 65 years.21,22 Because ossification occurs outside the spinal canal, patients are generally asymptomatic; however, an extensive calcified cervical mass may cause dysphagia or thoracic outlet syndrome and require surgical resection.23 DISH and OPLL may be genetically linked, with the same HLA antigen that tests positive in patients with OPLL occurring in those with DISH as well. Fifty percent of patients with DISH concurrently have OPLL, whereas 24% of those with OPLL also demonstrate DISH.19
AS is an immune-mediated inflammatory condition that results in progressive erosion of the intervertebral disks and apophyseal joints. Joint destruction is eventually replaced by ankylosis and subsequent autofusion of the spine. Patients with AS also suffer from diffuse osteoporosis, which leads to an increased susceptibility to fractures after minor trauma and a high risk for spinal deformity. AS is genetically linked to the HLA-B27 antigen. OPLL reportedly occurs in 3.5% to 29% of patients with AS.24,25 AS, an important entity for those concerned with spinal disease, is discussed separately in Chapter 284.
Clinical Findings
The cervical myelopathy attributable to OPLL is classified according to either the Nurick grade or the Japanese Orthopedic Association (JOA) score. The Nurick classification is composed of six grades (0 to 5) based on the severity of radiculopathy and myelopathy. A patient who is neurologically intact or has only mild radiculopathy is grade 0; grade 6 correlates with severe myelopathy or quadriplegia. The JOA classification is a 17-point composite score in which points are assigned based on hand and leg motor function, upper and lower extremity sensory function, trunk sensory function, and bladder continence.26 A modification of the JOA score incorporates a manual muscle test of shoulder and elbow strength, and a western modification has also been developed because the scoring of upper extremity function is based in part on the ability to use chopsticks.
Diagnosis
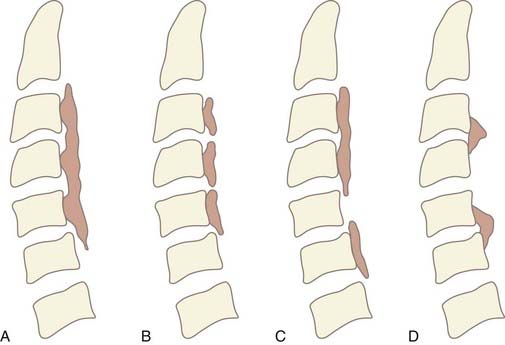
OPLL is diagnosed by the presence of an ossified ligament on radiographic imaging. It is often identified on lateral plain radiographs as a longitudinal hyperdense area of calcification along the dorsal margin of the vertebral body. Based on lateral radiographs, OPLL is classified into one of four subtypes (Fig. 282-1A to D). The continuous type is characterized by a single contiguous ossified ligament that spans two or more adjacent vertebrae. The segmental type is the most common form and has fragmented lesions located behind the vertebral bodies that do not cross the disk space. The mixed type is a combination of both continuous and segmental lesions. Rarely, OPLL is a localized variant that is confined to only the area posterior to the disk space.
Lateral plain films are used to determine the occupying ratio and the space available for the spinal cord (SAC). The occupying ratio is the maximum anteroposterior thickness of the ossified ligament divided by the diameter of the spinal canal at the corresponding level. An occupying ratio of greater than 40% is associated with a high risk for myelopathy. In a clinical series of patients with OPLL, Matsunaga and colleagues found that all patients with greater than 60% stenosis suffered myelopathy.27 Alternatively, in patients with less than 60% stenosis, there was no correlation between the occupying ratio and Nurick grade. The SAC is determined by measuring the anteroposterior distance between the dorsal aspect of the ossified lesion and the posterior margin of the lamina at a constant tube-to-film distance of 150 cm. In one clinical series, all patients with a SAC of less than 6 mm had myelopathy, whereas no patients with a SAC of 14 mm or greater demonstrated myelopathy.28 For patients with a SAC of between 6 and 14 mm, there was no correlation between SAC and the degree of neurological compromise.
Natural History
The natural history of OPLL is characterized by slowly progressive and variable growth of the ossified lesion. In a 2-year radiographic follow-up study of 13 patients with OPLL managed conservatively, the growth rate of the ossified lesion varied highly from patient to patient; however, mean growth was 4.07 mm longitudinally and 0.67 mm in thickness per year.29 The natural history clinically appears to be dependent on the initial neurological status at diagnosis. Matsunaga and coworkers studied 167 patients in whom OPLL was diagnosed and treated conservatively for an average of 11 years.28 Of the 140 patients who were initially asymptomatic, in only 18% did myelopathy subsequently develop during the study period. Alternatively, of the 27 patients who were initially myelopathic and refused to undergo surgery, 44% experienced worsening of their neurological status during the study period. Of all 167 patients managed conservatively, myelopathy either developed or worsened in only 22% despite the fact that 42% demonstrated radiographic growth of their ossified lesion during the study period.
In a subsequent study with a minimum of 10 years’ follow-up, Matsunaga and associates evaluated 304 patients with OPLL who were managed conservatively for 10 to 30 years.27 Of the patients who were initially asymptomatic, only 17% became myelopathic during the study period. The Kaplan-Meier estimate of the myelopathy-free rate in patients without myelopathy at initial evaluation was 71% at 30 years. Of patients who were myelopathic initially, however, 64% deteriorated neurologically during the follow-up period. Radiographic evaluation revealed that patients with minimal stenosis on initial assessment rarely progressed to greater than 60% stenosis during follow-up examination. The results of these studies suggest that in only a small percentage of patients in whom OPLL is diagnosed and is initially asymptomatic will myelopathy eventually develop during their lifetime.
Management
Severely compromised patients, however, may not benefit from surgical treatment. In a study of patients with a minimum of 10 years’ follow-up, surgery was no more effective than conservative measures in patients with a poor Nurick grade 5 (wheelchair bound or bedridden).27 Surgical decompression proved better than conservative management only in patients with a moderate Nurick grade 3 or 4 (able to ambulate with assistance but unable to work). However, this lack of improvement may be due to the spinal cord already being permanently damaged from traumatic compression or ischemia. Additionally, posterior surgery alone instituted in severely compromised states may not provide adequate neural decompression.
Some contend that prophylactic surgery may be indicated in younger patients with mild symptoms but severe stenosis radiographically. With a longer life expectancy, younger patients have a greater risk of suffering even minor cervical trauma that may result in permanent neurological injury. Patients with a narrowed SAC of less than 10 mm have a higher risk for the development of myelopathy after trauma, with up to 23% of patients with OPLL becoming acutely or increasingly symptomatic after a minor event.30 In a study of patients with OPLL manifested as acute cervical cord injury, the level of the injury was frequently at the caudal edge of the ossified lesion, probably secondary to hyperextension of the neck with minor trauma.31
Once the decision to proceed with surgical intervention is made, an appropriate surgical approach must be selected. A variety of anterior and posterior procedures have been described for direct or indirect decompression of the spinal cord in the setting of OPLL (Fig. 282-2). Anterior cervical approaches consist of direct decompression by either surgical resection or “floating” of the ossified lesion, along with reconstruction of the anterior spinal column and fusion. Posterior cervical approaches achieve indirect decompression by increasing SAC through either laminectomy or laminoplasty without directly addressing the ossified lesion. Even though several studies have failed to demonstrate any significant difference in outcome between anterior and posterior surgery,32–35 anterior approaches are associated with longer operative time, increased blood loss, and more frequent complications.35
Anterior Surgery
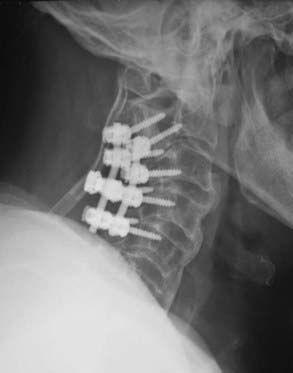
Anterior procedures for cervical OPLL allow definitive resection or mobilization of the ossified lesion and direct decompression of the spinal cord (Fig. 282-3A to I); however, they are frequently complicated by significant epidural bleeding, leakage of cerebrospinal fluid from ossified dural involvement, and potential injury to the spinal cord or nerve roots. Alternatively, an anterior floating method has been described in which a generous corpectomy is performed laterally such that the transverse dimensions extend at least 20 to 25 mm.34,36 Particular attention is directed toward identifying the uncinate processes to demarcate the extent of lateral resection. Once the ossified lesion is released at its lateral, cranial, and caudal margins with the use of a high-speed drill and a diamond bur, the calcified plaque rises ventrally to decompress the spinal cord. With the anterior floating method, the ossified lesion is not resected but instead is allowed to migrate anteriorly into the corpectomy defect. After decompression, either by resection of the OPLL or with the anterior floating method, anterior strut grafting with or without instrumentation is performed. Other complications described with anterior decompression include graft dislodgement, pseudarthrosis, and C5 nerve root palsy.
Posterior Surgery
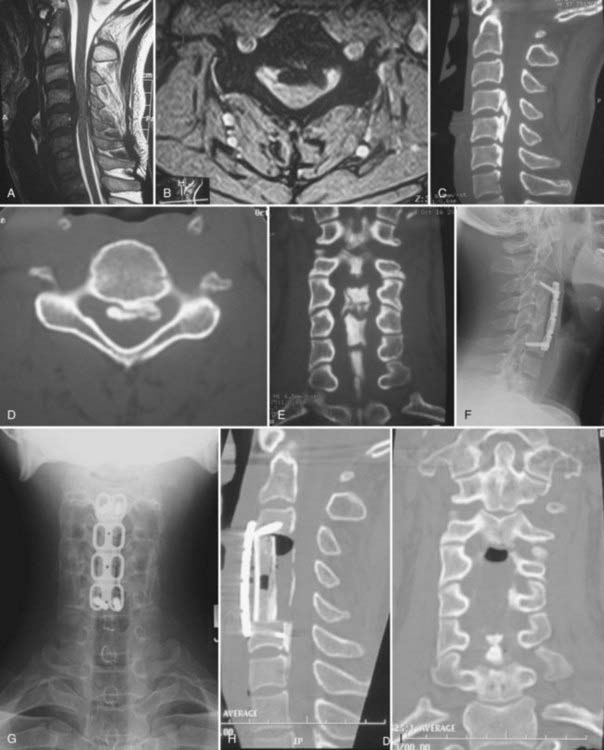
Cervical laminectomy without fusion may be performed in patients with a stable spinal column; however, particular attention to maintaining greater than 50% of the facet joint is necessary to preserve stability. Laminectomy with posterior fusion may be indicated for patients who require generous facetectomies or foraminotomies or for those who have questionable spinal stability (Fig. 282-4A to C). Complications associated with cervical laminectomy include postlaminectomy membrane and scar formation, delayed kyphosis, and C5 nerve root palsy.
Because patients with OPLL being treated surgically often have a significant degree of compression, en bloc laminectomy is recommended. With en bloc laminectomy or “lobster tail” removal of the lamina, bilateral gutters are drilled along the lamina-facet junction across the segments that require decompression. The ligamentum flavum is then resected along these lateral margins with a 2-mm Kerrison rongeur. Finally, disconnection of the interspinous ligaments and the ligamentum flavum at the cranial and caudal extents of the decompression allows en bloc removal of the intervening lamina and ligamentum (Fig. 282-4D). In so doing, one avoids repeatedly inserting the Kerrison rongeur into the central portion of the spinal canal, where the cord is the most compromised.
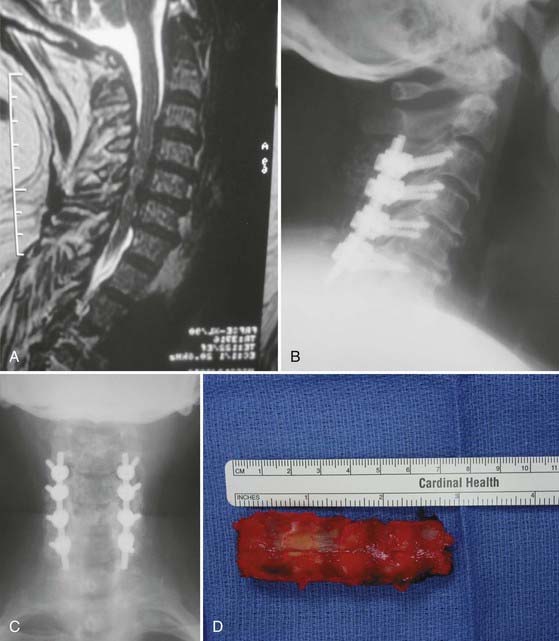
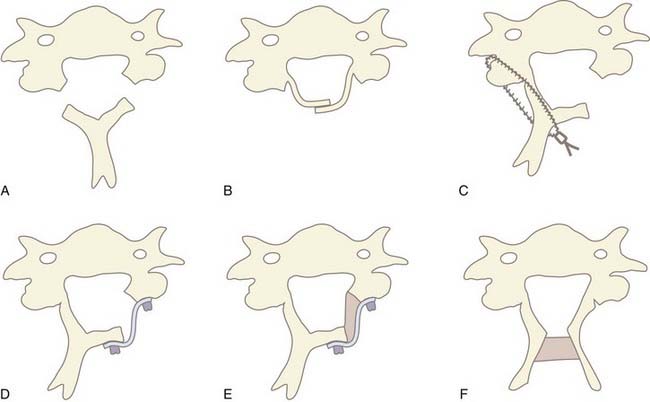
In 1971, Oyama and Hattori introduced an alternative posterior approach—the Z-plasty or laminoplasty.37 Laminoplasty achieves decompression by elevating without removing the posterior elements from the spinal cord, thereby allowing the cord to migrate dorsally. Shortly thereafter, Hirabayashi and coauthors reported a revised laminoplasty procedure, the expansile open-door or “single-door” laminoplasty.38,39 Several different techniques and subsequent modifications of cervical laminoplasty have been developed (Fig. 282-5A to F). The expansile open-door laminoplasty technique has since been augmented with graft spacers or the addition of hardware (or both) for maintaining the “open door.” The “French door” laminoplasty creates an enlargement of the median canal by splitting the spinous process, elevating the lamina bilaterally, and interposing a graft spacer to maintain the “open double door.”
The benefit of laminoplasty over laminectomy is maintenance of the dorsal tension band and preservation of the posterior elements as a barrier against postlaminectomy membrane formation. For adequate decompression, 4 to 5 mm of canal expansion in the anteroposterior diameter or a 50% increase in overall canal area is necessary. This corresponds to greater than 3 mm of dorsal cord migration, which correlates with optimal clinical outcomes.40 Despite these theoretical advantages of laminoplasty, studies have failed to demonstrate any conclusive benefit of any laminoplasty technique over cervical laminectomy.41
Complications associated with laminoplasty include a 5% to 10% risk for postoperative C5 nerve root palsy.33,42,43 The development of C5 nerve root palsy is probably due to dorsal migration of the spinal cord and subsequent traction on the exiting nerve roots. With the C5 nerve root being at the apex of the lordosis, it is susceptible to greater tethering and tension. A variable rate of axial neck and shoulder pain has been described after laminoplasty. This pain is frequently reported as affecting the ipsilateral shoulder of the hinge side of a single-door laminoplasty, which may be due to subsequent intersegmental fusion. Cervical laminoplasty is associated with a significant decrease in range of neck motion postoperatively in most series.41 Although delayed kyphosis develops less frequently after laminoplasty than after laminectomy, a significant percentage of patients have a relative loss of lordosis postoperatively.41
Surgical Decision Making: Anterior versus Posterior Approach
Ultimately, the surgical treatment of symptomatic patients with OPLL requires selecting the appropriate technique, whether it be an anterior or posterior approach. Both methods have been demonstrated in the literature to be effective in achieving surgical decompression with a high rate of neurological improvement.33,34,42–47 However, the paucity of the literature directly comparing anterior and posterior decompression poses an oftentimes difficult decision in selecting the appropriate procedure for a given patient. General guidelines that factor in specific patient characteristics can be applied on a case-by-case basis to facilitate providing patients with a reasonable surgical option that optimizes clinical results while minimizing surgical morbidity (Table 282-1).
TABLE 282-1 Factors Involved in Selecting Anterior versus Posterior Decompression for Treatment of Ossification of the Posterior Longitudinal Ligament
| ANTERIOR APPROACH | POSTERIOR APPROACH | |
|---|---|---|
| Canal compromise | >60% | <60% |
| Age | Younger Higher lifetime risk for progression of disease |
Older Lower lifetime risk for progression of disease |
| Medical comorbidities | Prefer patients with low surgical risk | More amenable for patients with higher surgical risk |
| Number of levels involved | Focal or short-segment disease | Multilevel disease |
| Cervical alignment | Kyphotic, neutral, or lordotic | Lordotic |
| Previous surgery | Difficult if previous anterior surgery | Amenable to patients with previous anterior surgery |
| Positioning | Amenable to patients who are unable to be positioned prone | Only acceptable for patients who can tolerate prone positioning |
| Axial neck pain | Amenable to patients with existing neck pain | Prefer patients without preexisting neck pain |
| Osteoporosis | Prefer patients with adequate bone density | Amenable to patients with osteoporosis |
| Cosmesis | May result in a visible scar | Generally a well-concealed scar |
| Voice | Relatively contraindicated in singers, speakers | Amenable to singers, speakers |
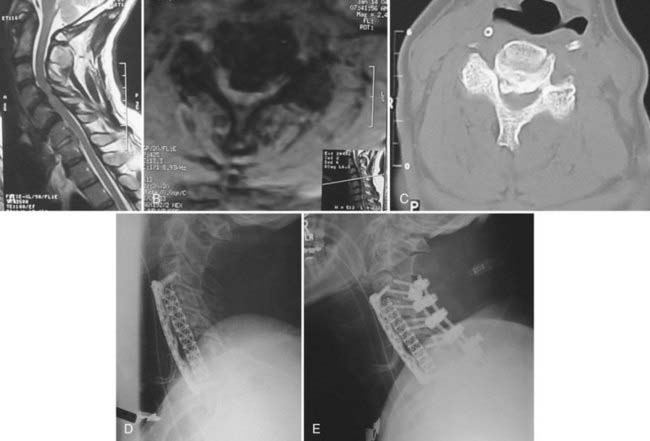
Anterior procedures are primarily of benefit by providing the ability to perform direct surgical resection of the OPLL. Therefore, anterior surgery is recommended for patients who will gain from total removal of the offending lesion, including in particular those with greater than 60% canal compromise (Fig. 282-6A to E),48 as well as younger patients, who may enjoy a longer lifetime benefit with a reduced risk for progression of OPLL at the operated levels. Anterior surgery is likewise recommended for patients with preexisting cervical kyphosis because these patients fare poorly with posterior indirect decompression. Anterior decompression with reconstruction and fusion may also help restore cervical lordosis and spinal alignment.
Surgical Results
Anterior Surgery
Anterior cervical decompression plus fusion in patients with OPLL provides excellent outcomes in both short- and long-term evaluation. In a series of 107 patients with at least 2 years of follow-up, 89% of those with myelopathy exhibited significant postoperative improvement in neurological status.45 The fusion rate was 97% despite often performing multilevel procedures. The rate of cerebrospinal fluid leakage was high (20%), and it was the primary complication. Two patients required reoperation for growth of OPLL at a nonoperated site. In only 1 patient did postoperative C5 nerve root palsy develop. In a long-term study of 30 patients monitored for a minimum of 10 years after anterior cervical decompression, 53% demonstrated excellent improvement in ambulatory function (based on Okamoto’s classification of walking disability).46 Twenty-seven percent had good recovery, 17% were unchanged, and only 3% had a poor outcome. Postoperative neurological deterioration occurred in 13%; however, no cases were due to continued growth of OPLL at the operated site.
Matsuoka and colleagues studied 63 patients who underwent the anterior floating method with a minimum of 10 years’ follow-up.34 Overall, the average JOA score improved from 8.3 preoperatively to a peak of 14.2 at 5 years’ follow-up. The average postoperative JOA score was maintained for up to 10 years; however, a slight decline to 13.5 occurred at final follow-up. Accordingly, the recovery rate, which is calculated as
peaked at 68.5% at 5 years and was stable for up to 10 years. At last evaluation, the recovery rate had decreased slightly to 59.3%. Radiographic studies have revealed that between 4 and 8 weeks postoperatively, the ossified lesion had successfully migrated anteriorly away from the spinal cord.
Posterior Surgery
Laminectomy
Kato and colleagues studied 44 patients for an average of 14 years after laminectomy.49 Overall, the average JOA score improved from 7.6 preoperatively to 11.5 at 1 year postoperatively. JOA scores remained stable for up to 5 years, but they declined to 10.3 at last examination. Accordingly, the recovery rate improved to 40% at 1 and 5 years postoperatively but decreased to 30% at final follow-up. Late neurological deterioration occurred in 19% of patients and was predominantly due to minor trauma or the development of thoracic OPLL or OLF. Only 1 patient (2%) had a decrease in neurological function because of growth of OPLL at the operated levels.
Laminoplasty
Several studies have evaluated the long-term clinical results (5 to 14 years postoperatively) in patients who underwent laminoplasty for OPLL.33,42–44,47 Average JOA scores improved from 8.3 to 9.2 preoperatively to 13.5 to 14.2 at 3 to 10 years postoperatively. Studies that evaluated patients beyond 10 years observed a slight decline in JOA score to 12.3 to 13.1 at last examination. Accordingly, recovery rates peaked at 59% to 63.1% at 3 to 10 years postoperatively and subsequently declined to 47.9% to 54% at last examination when monitored for more than 10 years.
In the study by Chiba and associates, late neurological deterioration, defined as greater than a 1-point decrease in JOA score, was observed in 30% of patients. Patients who met the criteria for late neurological deterioration had an average age of 77 years.42 Iwasaki and colleagues33 and Ogawa and coauthors43 reported that 15% to 16% demonstrated a late decrease in JOA score of greater than 2 points, with only 4% of patients in both series deteriorating because of progression of OPLL at the operated level. Primarily, the decreased neurological function was due to the de novo development of thoracic OPLL or OLF. Concordant with these findings, Chiba and coauthors reported that patients maintained a stable degree of canal expansion from 1 year postoperatively until final examination. The authors observed that the canal increased from 13.5 mm before surgery to 18.3 mm at 1 year and was sustained at 17.9 mm at last radiographic evaluation, thus suggesting that late deterioration was not due to progression of disease at the operated segments.42
Surgical complications included a 5% to 10% incidence of C5 or C6 nerve root palsy.33,42,43 Axial neck pain occurred in 25% to 31% of patients, with 41% to 53% complaining of shoulder pain. Other complications encountered included cerebrospinal fluid leaks, postoperative hematoma, and infection. Several studies observed that patients exhibited a significant loss of range of motion after laminoplasty.42–44 Decreased cervical lordosis was also seen after laminoplasty42; however, it remains unclear whether the change in cervical alignment correlated with diminished recovery of neurological function.43,44 The development of kyphosis, though, does appear to be related to a higher incidence of postoperative shoulder pain.43
Factors correlating with better clinical outcome included younger age at the time of surgery and higher preoperative JOA score, particularly for patients with severe myelopathy at initial evaluation.33,43 Patients with moderate preoperative myelopathy fared better with a shorter duration between the onset of symptoms and initiation of surgery.43 In 2007, Iwasaki and colleagues reanalyzed their case series and found that the morphology of the ossified lesion, particularly a hill-shaped ossification, correlated with worse clinical outcomes.50 Despite comparable preoperative JOA scores, patients with a preoperative occupying ratio of less than 60% had higher postoperative JOA scores than did those with an occupying ratio of greater than 60%. Segmental versus nonsegmental OPLL was not found to affect postoperative JOA scores or the degree of neurological recovery.51
Anterior versus Posterior Surgery
Few studies have directly compared anterior and posterior surgery for the treatment of cervical OPLL. Unfortunately, these studies are generally retrospective in nature and reflect a change in institutional preference rather than a randomized comparison between two techniques. Wada and coworkers performed a long-term study of patients treated with anterior subtotal corpectomy versus laminoplasty for cervical spondylotic myelopathy.52 They found no significant difference in neurological recovery at 1 and 5 years postoperatively and at last examination. The laminoplasty group had a higher incidence of axial neck pain and greater loss of range of motion. The corpectomy group suffered from a longer operative time, increased blood loss, and pseudarthrosis (26%). The anterior cervical fusion group also demonstrated a 54% rate of radiographically evident adjacent segment degeneration; however, in only 4% did clinical symptoms develop as a result.
Iwasaki and associates studied anterior decompression and fusion versus laminoplasty for an average of 6 years in patients with OPLL.48 They observed no significant difference in postoperative JOA score or recovery rate between anterior and posterior procedures in patients with an occupying ratio of less than 60%. However, patients with an occupying ratio of 60% or greater fared significantly better after anterior decompression than after laminoplasty. The anterior surgical group had a maximum recovery rate of 64%, which decreased slightly to 54% at final examination. Comparatively, the laminoplasty group had a maximum recovery rate of just 34%, which significantly deteriorated to 14% at last evaluation. Of note, 26% of patients in the anterior decompression group did require an additional operation because of either graft-related complications, need for posterior stabilization, or subsequent laminoplasty for late neurological deterioration. Only one patient (2%) in the laminoplasty group required a second surgery, which was performed because of postoperative epidural hematoma.
Progression of Disease
A major issue in clinical outcome after either conservative or surgical intervention is the long-term potential for progression of the ossified lesion. Matsunaga and coworkers studied 167 patients with OPLL who were treated conservatively with an average follow-up of 11.2 years.28 Radiographic evidence of axial progression of OPLL occurred in 42% of patients, with longitudinal growth occurring in 86%. Of the 70 patients with OPLL growth, de novo myelopathy or clinical worsening of their initial neurological status developed in 53%.
The reported incidence of OPLL progression after anterior decompression is variable and ranges from 36% to 64%.34,53 In a study of patients monitored for more than 10 years after undergoing anterior interbody fusion or subtotal corpectomy for OPLL, 87% demonstrated longitudinal growth of the OPLL, and 50% had an increase in thickness of the OPLL.46 In a long-term study of patients who underwent the anterior floating method, only 8% demonstrated postoperative progression of OPLL at the operated level.34 The low rate of OPLL progression may be due to disruption of the vascular supply to the ossified mass or elimination of intersegmental motion after fusion. At the levels adjacent to the operated field, however, 36% showed progression of OPLL either longitudinally or in thickness. Thirty percent had longitudinal growth adjacent to the operated level, with 25% demonstrating increased thickness of the OPLL adjacent to the operated segment.
OPLL progression is of particular concern in patients undergoing posterior indirect decompression. Because the ossified lesion is not resected, further growth of OPLL may lead to late neurological deterioration. Some have questioned whether posterior decompression surgery actually increases the rate of OPLL growth. Takatsu and colleagues radiographically studied OPLL progression in patients undergoing laminectomy, laminoplasty, and conservative treatment.54 They found no significant difference in OPLL growth in the laminectomy and laminoplasty groups. OPLL progression, however, was more rapid in both surgically treated groups than in patients treated conservatively. Postsurgical changes in the microcirculatory and biomechanical environment may cause stimulation of OPLL development. However, patients in this series who were surgically treated may also have had more aggressive disease than those treated conservatively. Another study performed by Matsunaga and associates found that the rate of OPLL progression after laminoplasty was in fact comparable to that in patients treated nonsurgically.27
Several studies have evaluated the risk for progression of OPLL after long-term follow-up of patients who underwent laminectomy or laminoplasty.33,49,51,55–58 Radiographic evidence of OPLL progression occurred in 70% to 73% of patients who were evaluated for a minimum of 10 years after posterior decompression.33,49,58 In a multicenter study using a computer-assisted method for measuring OPLL progression, the incidence of OPLL growth at 1 and 2 years was 38.9% and 56.5%, respectively.55 OPLL progression occurred more commonly in younger patients, with the average age of patients with OPLL growth being 52 to 54 years56–58 as compared with 60 years in those who did not demonstrate significant OPLL growth.58 Several studies have observed that OPLL progression occurs more commonly in the continuous and mixed types than in segmental OPLL.33,51,56–58 Gender and type of posterior decompression were not correlated with increased risk for OPLL progression.55
With regard to longitudinal growth, 64% to 75% of patients demonstrated longitudinal progression of OPLL after laminoplasty over 5 to 13 years of follow-up, with the average total growth being 9 to 13 mm.33,56,58 Using computer-assisted radiographic analysis, Chiba and colleagues found that OPLL growth had occurred 1.5 mm rostrally and 1.3 mm caudally 1 year postoperatively.55 At 2 years after surgery, the OPLL had progressed by 2.4 mm rostrally and 2.4 mm caudally. The OPLL had increased in thickness in 22% to 53% of patients at 5 to 13 years postoperatively.33,57,58 The average total increase in thickness in these patients ranged from 1.3 to 3.5 mm during the follow-up period. Computer-assisted radiographic analysis found that OPLL thickness had increased by 1.1 mm at 1 year postoperatively and by 1.4 mm at 2 years.55
Despite a 70% or greater rate of OPLL progression after posterior decompression, less than 10% of patients demonstrated worsening myelopathy or late neurological deterioration after surgery because of OPLL growth.43,49 The delayed neurological deterioration occurred predominantly as a result of trauma or the development of OPLL or OLF at a site other than the originally operated levels. Consequently, studies have failed to demonstrate an association between OPLL growth and the postoperative score-based recovery rate.57,58 Kawaguchi and coworkers found that 73% of patients after en bloc cervical laminoplasty had radiographic evidence of OPLL progression; however, only 7% of patients worsened as a result of increased thickness of the OPLL.58 Other studies have observed that OPLL growth causes late neurological deterioration in only 3% to 4% of patients after posterior decompression.33,43 These findings suggest that although OPLL may continue to grow after posterior decompression, neurological recovery does not appear to be clinically affected by the slow progression.
Thoracic Disease
Laminectomy for indirect posterior decompression of thoracic OPLL was studied by Tokuhashi and colleagues.59 They performed intraoperative ultrasonography after laminectomy to assess for the presence of cerebrospinal fluid ventral to the spinal cord as an indication that the spinal cord had migrated posteriorly and was decompressed. In patients with positive ultrasound evidence of effective posterior decompression, the JOA score improved from 4.3 preoperatively to 8.6 at final follow-up, with a recovery rate of 62.7%. Alternatively, 67% of patients with ultrasonography that showed ineffective decompression demonstrated significant postoperative neurological deterioration.
Alternatively, Kawahara and colleagues described a method for circumspinal decompression of thoracic OPLL.60 They suggested that anterior decompression is the most effective measure for relieving ventral compression of the thoracic cord; however, anterior decompression is technically challenging and is not sufficient in patients with concomitant OLF. Kawahara and coworkers described an initial posterior decompression in which laminectomy is performed at least one level above and below the length of the thoracic OPLL. Parallel gutters are then drilled on either side of the thecal sac ventrally into the vertebral bodies approximately 1 cm deep. Posterior segmental instrumentation is then placed and connected to rods that are underbent approximately 15 to 20 degrees less than the patient’s natural kyphosis to reduce the kyphosis angle and facilitate dorsal migration of the spinal cord.
Comparing various approaches for the treatment of thoracic OPLL, Yamazaki and coauthors evaluated patients who underwent laminectomy alone, laminectomy with posterior instrumented fusion, and OPLL extirpation via either thoracotomy or posterior resection of OPLL.61 The three groups had comparable mean preoperative JOA scores (3.4 to 3.6) and postoperative JOA scores at 1 year (7.7 to 7.9). However, a significant percentage of patients in the group that underwent laminectomy alone (39%) demonstrated late neurological deterioration, with a mean JOA score at final follow-up of 6.5. The groups that underwent OPLL extirpation and laminectomy with posterior fusion remained stable at final follow-up with a JOA score of 8 and no evidence of late deterioration. Both the laminectomy-alone and OPLL extirpation groups had patients in whom paralysis developed postoperatively. In the laminectomy-alone group, two of the three patients recovered with a secondary posterior instrumentation procedure to stabilize the spine. In the OPLL extirpation group, the three patients with postoperative paralysis had already been severely weak and wheelchair bound preoperatively. In no patients in the group that underwent laminectomy with posterior fusion did postoperative paralysis develop.
In analysis of their results, Yamazaki and colleagues recommended that laminectomy alone should not be performed for thoracic OPLL given the considerable risk for postoperative paralysis and late neurological deterioration.61 They suggest that patients who are amenable to posterior decompression should also undergo supplemental stabilization with a posterior instrumented fusion. Patients treated by OPLL extirpation demonstrated a high degree of neurological recovery that remained stable during follow-up, and this should be the primary surgical modality for patients who are myelopathic. In severely myelopathic patients who are nonambulatory, the authors recommend posterior decompression with instrumented fusion because they found that these patients are at high risk for postoperative paralysis from OPLL extirpation. Laminectomy with posterior fusion may also be considered for patients who are at high surgical risk when undergoing an anterior procedure.
Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998.
Chiba K, Yamamoto I, Hirabayashi H, et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine. 2005;3:17.
Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354.
Hirabayashi K, Toyama Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35.
Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament thicken following cervical laminoplasty? Spine. 2007;32:E551.
Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament progress after cervical laminoplasty? Spine. 2006;31:2807.
Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96:180.
Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32:654.
Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: clinical results and limitations of laminoplasty. Spine. 2007;32:647.
Kato Y, Iwasaki M, Fuji T, et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217.
Kawahara N, Tomita K, Murakami H, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2008;33:39.
Koga H, Sakou T, Taketomi E, et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998;62:1460.
Matsunaga S, Sakou T, Taketomi E, et al. Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg. 2004;100:245.
Matsunaga S, Yamaguchi M, Hayashi K, et al. Genetic analysis of ossification of the posterior longitudinal ligament. Spine. 1999;24:937.
Matsuoka T, Yamaura I, Kurosa Y, et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine. 2001;26:241.
Ogawa Y, Toyama Y, Chiba K, et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2004;1:168.
Okamoto K, Kobashi G, Washio M, et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); findings from a case-control study in Japan. J Bone Miner Metab. 2004;22:612.
Onari K, Akiyama N, Kondo S, et al. Long-term follow-up results of anterior interbody fusion applied for cervical myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2001;26:488.
Sakou T, Matsunaga S, Koga H. Recent progress in the study of pathogenesis of ossification of the posterior longitudinal ligament. J Orthop Sci. 2000;5:310.
Sodeyama T, Goto S, Mochizuki M, et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine. 1999;24:1527.
Takatsu T, Ishida Y, Suzuki K, et al. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1999;12:271.
Tokuhashi Y, Matsuzaki H, Oda H, et al. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine. 2006;31:E26.
Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine. 2001;26:1443.
Yamaura I, Kurosa Y, Matuoka T, et al. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res. 1999;359:27.
Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine. 2006;31:1452.
1 Izawa K. Comparative roentgenographic study on the incidence of ossification of the posterior longitudinal ligament and other degenerative changes of the cervical spine among Japanese, Koreans, Americans and Germans (author’s transl). Nippon Seikeigeka Gakkai Zasshi. 1980;54:461.
2 Epstein NE, Yonenobu K. Ossification of the posterior longitudinal ligament, 2nd ed. Benzel EC, editor. Spine Surgery: Techniques and Complication Avoidance, and Management, vol. 2. Philadelphia: Elsevier. 2005:729.
3 Wang MY. Ossification of the posterior longitudinal ligament in non-Asians: demographic, clinical, and radiographic findings. Paper presented at the Annual Meeting of the Congress of Neurological Surgeons, San Diego, California; 2001.
4 Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine. 1989;14:1184.
5 Matsunaga S, Yamaguchi M, Hayashi K, et al. Genetic analysis of ossification of the posterior longitudinal ligament. Spine. 1999;24:937.
6 Koga H, Hayashi K, Taketomi E, et al. Restriction fragment length polymorphism of genes of the alpha 2(XI) collagen, bone morphogenetic protein-2, alkaline phosphatase, and tumor necrosis factor-alpha among patients with ossification of posterior longitudinal ligament and controls from the Japanese population. Spine. 1996;21:469.
7 Koga H, Sakou T, Taketomi E, et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998;62:1460.
8 Sakou T, Matsunaga S, Koga H. Recent progress in the study of pathogenesis of ossification of the posterior longitudinal ligament. J Orthop Sci. 2000;5:310.
9 Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027.
10 Tanaka T, Ikari K, Furushima K, et al. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 2003;73:812.
11 Tsukahara S, Miyazawa N, Akagawa H, et al. COL6A1, the candidate gene for ossification of the posterior longitudinal ligament, is associated with diffuse idiopathic skeletal hyperostosis in Japanese. Spine. 2005;30:2321.
12 Tahara M, Aiba A, Yamazaki M, et al. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine. 2005;30:877.
13 Yonemori K, Imamura T, Ishidou Y, et al. Bone morphogenetic protein receptors and activin receptors are highly expressed in ossified ligament tissues of patients with ossification of the posterior longitudinal ligament. Am J Pathol. 1997;150:1335.
14 Kawaguchi H, Kurokawa T, Hoshino Y, et al. Immunohistochemical demonstration of bone morphogenetic protein-2 and transforming growth factor-beta in the ossification of the posterior longitudinal ligament of the cervical spine. Spine. 1992;17:S33.
15 Tanaka H, Nagai E, Murata H, et al. Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology (Oxford). 2001;40:1163.
16 Epstein NE, Grande DA, Breitbart AS. In vitro characteristics of cultured posterior longitudinal ligament tissue. Spine. 2002;27:56.
17 Akune T, Ogata N, Seichi A, et al. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am. 2001;83:1537.
18 Shingyouchi Y, Nagahama A, Niida M. Ligamentous ossification of the cervical spine in the late middle-aged Japanese men. Its relation to body mass index and glucose metabolism. Spine. 1996;21:2474.
19 Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;184:71.
20 Okamoto K, Kobashi G, Washio M, et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); findings from a case-control study in Japan. J Bone Miner Metab. 2004;22:612.
21 Julkunen H, Heinonen OP, Knekt P, et al. The epidemiology of hyperostosis of the spine together with its symptoms and related mortality in a general population. Scand J Rheumatol. 1975;4:23.
22 Resnick D, Guerra JJr, Robinson CA, et al. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am J Roentgenol. 1978;131:1049.
23 Kritzer RO, Rose JE. Diffuse idiopathic skeletal hyperostosis presenting with thoracic outlet syndrome and dysphagia. Neurosurgery. 1988;22:1071.
24 Kim TJ, Kim TH, Jun JB, et al. Prevalence of ossification of posterior longitudinal ligament in patients with ankylosing spondylitis. J Rheumatol. 2007;34:2460.
25 Ramos-Remus C, Russell AS, Gomez-Vargas A, et al. Ossification of the posterior longitudinal ligament in three geographically and genetically different populations of ankylosing spondylitis and other spondyloarthropathies. Ann Rheum Dis. 1998;57:429.
26 Yonenobu K, Abumi K, Nagata K, et al. Interobserver and intraobserver reliability of the Japanese Orthopaedic Association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26:1890.
27 Matsunaga S, Sakou T, Taketomi E, et al. Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg. 2004;100:245.
28 Matsunaga S, Kukita M, Hayashi K, et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96:168.
29 Sato M, Turu M, Yada K. The anteroposterior diameter of the cervical spinal canal in the ossification of the posterior longitudinal ligament (author’s transl). No Shinkei Geka. 1977;5:511.
30 Katoh S, Ikata T, Hirai N, et al. Influence of minor trauma to the neck on the neurological outcome in patients with ossification of the posterior longitudinal ligament (OPLL) of the cervical spine. Paraplegia. 1995;33:330.
31 Koyanagi I, Iwasaki Y, Hida K, et al. Acute cervical cord injury associated with ossification of the posterior longitudinal ligament. Neurosurgery. 2003;53:887.
32 Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13:774.
33 Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96:180.
34 Matsuoka T, Yamaura I, Kurosa Y, et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine. 2001;26:241.
35 Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty versus subtotal corpectomy. A comparative study of results in multisegmental cervical spondylotic myelopathy. Spine. 1992;17:1281.
36 Yamaura I, Kurosa Y, Matuoka T, et al. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res. 1999;359:27.
37 Oyama M, Hattori S. A new method of posterior decompression. Central Jpn J Orthop Traumac Surg (Chubuseisaisi). 1973;16:792.
38 Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354.
39 Wang MY, Green BA. Open-door cervical expansile laminoplasty. Neurosurgery. 2004;54:119.
40 Sodeyama T, Goto S, Mochizuki M, et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine. 1999;24:1527.
41 Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98:230.
42 Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998.
43 Ogawa Y, Toyama Y, Chiba K, et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2004;1:168.
44 Hirabayashi K, Toyama Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35.
45 Mizuno J, Nakagawa H. Outcome analysis of anterior decompressive surgery and fusion for cervical ossification of the posterior longitudinal ligament: report of 107 cases and review of the literature. Neurosurg Focus. 2001;10(4):E6.
46 Onari K, Akiyama N, Kondo S, et al. Long-term follow-up results of anterior interbody fusion applied for cervical myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2001;26:488.
47 Wang MY, Shah S, Green BA. Clinical outcomes following cervical laminoplasty for 204 patients with cervical spondylotic myelopathy. Surg Neurol. 2004;62:487.
48 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32:654.
49 Kato Y, Iwasaki M, Fuji T, et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217.
50 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: clinical results and limitations of laminoplasty. Spine. 2007;32:647.
51 Ogawa Y, Chiba K, Matsumoto M, et al. Long-term results after expansive open-door laminoplasty for the segmental-type of ossification of the posterior longitudinal ligament of the cervical spine: a comparison with nonsegmental-type lesions. J Neurosurg Spine. 2005;3:198.
52 Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine. 2001;26:1443.
53 Iwasaki M, Yonenobu K. Ossification of the posterior longitudinal ligament, 5th ed. Herkowitz HN, Garfin SR, Eismont FJ, et al, editors. Rothman-Simeone The Spine, vol. 2. Philadelphia: WB Saunders. 2006:896.
54 Takatsu T, Ishida Y, Suzuki K, et al. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1999;12:271.
55 Chiba K, Yamamoto I, Hirabayashi H, et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine. 2005;3:17.
56 Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament progress after cervical laminoplasty? Spine. 2006;31:2807.
57 Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament thicken following cervical laminoplasty? Spine. 2007;32:E551.
58 Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798.
59 Tokuhashi Y, Matsuzaki H, Oda H, et al. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine. 2006;31:E26.
60 Kawahara N, Tomita K, Murakami H, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2008;33:39.
61 Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine. 2006;31:1452.