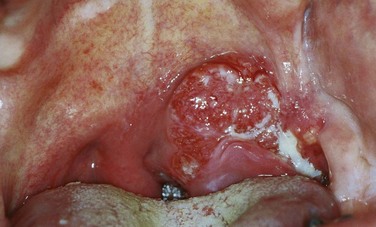
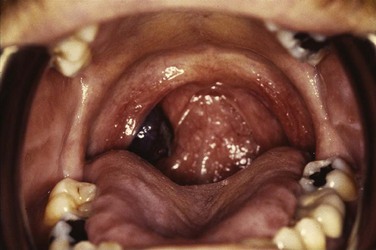
Chapter 31 Oropharyngeal Cancer
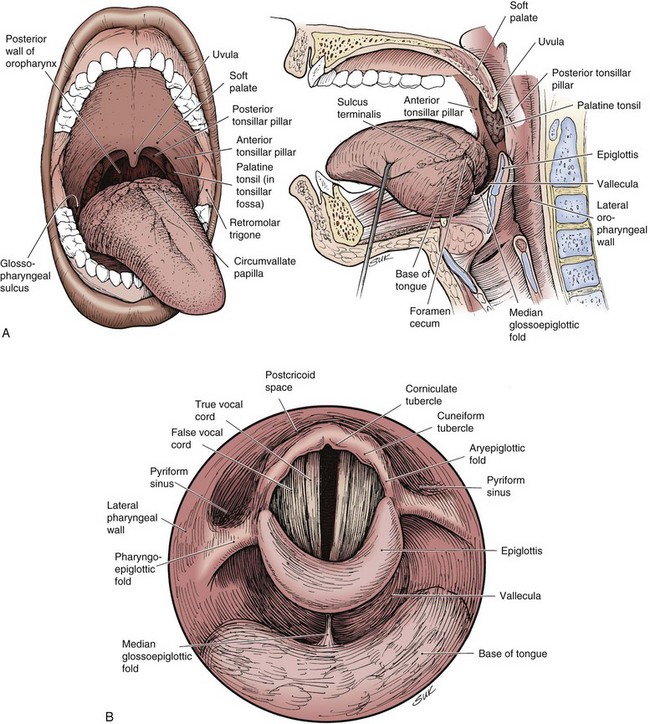
The oropharynx consists of the base of the tongue, tonsillar region, soft palate, and lateral and posterior oropharyngeal walls (Fig. 31-1). Determining the precise annual incidence for new cancers of the oropharynx is difficult because there is overlap with other head and neck sites within various reporting classifications. The cumulative incidence of cancers of the “oral cavity and pharynx,” comprised of the tongue, mouth, pharynx, and oral cavity, was estimated at 36,540 in the United States in 2010, with 25,420 male and 11,120 female patients.1 Malignant tumors within the oropharynx, defined as tumors arising from the base of tongue, tonsillar complex, soft palate, or lateral and posterior pharyngeal walls by the American Joint Committee on Cancer (AJCC) staging definition,2 are generally thought to represent approximately 25% of the total. This places the estimated total between 8000 and 9000 new oropharyngeal carcinomas per year in the United States, although some believe the rate is lower.3
The incidence of head and neck tumors has increased during the twentieth century, particularly in persons born after 1920. The higher cancer incidence over this time period is associated with the rise in alcohol and tobacco consumption in the United States and other westernized countries over the same era.4,5 This trend has reversed over the past two decades in the United States, with a small decline in squamous cell carcinomas of the head and neck, likely secondary to a reduction in tobacco use. Despite this general reduction in head and neck cancers and the diminished incidence rate seen for the neighboring oral cavity, the incidence of oropharyngeal carcinomas has continued to increase since the early 1980s, predominantly in men younger than age 60 years.6,7
The worldwide estimated annual incidence of tumors defined as arising either from the oral cavity or the pharynx excluding the nasopharynx, including oropharyngeal site tumors, was 404,585 in 2002. Over two thirds of the patients were male, and the mortality rate on average was less than half of the incidence rate.8 Such an analysis comes with the typical challenges in documenting such malignant diseases, particularly among the medically underserved in the world. Geographic variations in global rates reflect the prevalence of known risk factors, such as tobacco and alcohol use, within specific populations.
Etiology and Epidemiology
Tobacco and Alcohol Use
Squamous cell carcinoma of the oropharynx most commonly affects patients older than 50 years, and men more than women. Despite this generalization, over the past 30 years oropharyngeal cancer rates have been on the rise in both men and women under the age of 45 years.7 Both alcohol and tobacco use are long-known, well-defined risk factors for squamous cell carcinomas of the head and neck, including those arising within the oropharynx. Again, separating data regarding oral cavity tumors and oropharyngeal tumors within published series can be challenging given reporting methods, and results may overstate the role of tobacco and alcohol use in oropharyngeal malignant diseases. The two substances individually and together contribute to a field cancerization effect within the upper aerodigestive tract. The effect of alcohol consumption and cigarette smoking on oropharyngeal cancer risk is reported to be dependent on both the intensity and duration of use and is responsible for approximately 80% of oropharyngeal cancers, with a higher proportion in men than in women in one series.9
A population-based study in Sweden compared 605 patients with head and neck cancer with 756 controls. A hazard ratio (HR) of 8.4 was seen with current smokers; this figure was increased by an earlier starting age, longer duration of smoking, greater lifetime total consumption, and increased intensity of daily tobacco use.10 Increasing alcohol intake, particularly more than 50 g per day, was also associated with an increased relative risk of head and neck cancer. A compilation of case-control studies places the tobacco-associated odds ratio at 2.13 for tobacco users when compared with never users, with the ratio doubling in persons who smoke more than 30 cigarettes per day or those who have smoked for more than 30 pack-years.11 Involuntary or secondhand tobacco exposure may also be a risk factor, particularly when the person has been exposed for more than 15 years either at home or at work.12 Alcohol use increases the rate of oropharyngeal carcinomas in never smokers, prior smokers, and current smokers, and the risk decreases with cessation of alcohol use.13 Although use of either alcohol or tobacco elevates the risk of oral cavity and oropharyngeal cancer, there is a synergistic effect from the use of both simultaneously. The risk is calculated to be thirteenfold the expected risk of each used independently, creating an approximately fiftyfold greater risk for oral cavity and oropharyngeal cancer compared with that of nonsmokers and nondrinkers.13,14 Tobacco cessation decreased the risk of head and neck cancer by 40% in years 1 through 4, although the risk of head and neck cancers did not return to baseline until 20 years had elapsed after either tobacco or alcohol cessation.15
Human Papillomavirus Infection
Additional behavioral risk factors have been described besides the classic one of substance abuse, and they appear to be correlated with an elevated risk of oral human papillomavirus (HPV) infection. The role of HPV corresponds to an identified increase in head and neck cancer incidence seen in younger nonsmokers and nondrinkers. The increased incidence has been seen both in the United States and in European countries, despite the general trend of fewer head and neck cancers. This rise has been most marked among young patients in sites associated with HPV.7 HPV is a sexually transmitted disease recently recognized as a risk factor for squamous cell carcinoma of the head and neck, particularly in the oropharyngeal subsite, although the connection was initially suggested in 1983.16 HPV has been implicated in approximately 25% of head and neck cancers based on polymerase chain reaction (PCR) detection of HPV DNA. This prevalence seems to be site specific, with a significantly higher percentage of HPV-associated cancers in the oropharynx compared with the oral cavity or larynx.17 A prospective trial found the rate of HPV-positive tumors to be 60% in the oropharynx overall and even higher in the lingual or palatine tonsils.18 The increase in oropharyngeal cancers is not thought to be entirely attributable to the reduction in tonsillectomy frequency between the 1970s and the 1990s, because this trend would not be expected to alter the incidence of base of tongue primary tumors.19
The link between HPV and oropharyngeal cancers is severalfold and mimics the lines of evidence associating HPV and cervical cancer. First, HPV has been identified in tumor cells of head and neck squamous cell carcinomas.16,20 ![]()
The polymerase chain reaction has been frequently used for identification and subtyping of HPV, as has in situ hybridization via a signal amplification system. Identified prevalence rates have varied, depending on the tumor subsite, sensitivity of the HPV detection method, and geographic location, with associated ethnic variance.2
HPV-16 is the most common HPV subtype associated with oropharyngeal squamous cell carcinomas, seen in 85% to 90% of HPV-associated cancers.17,20 The remainder are associated with HPV subtypes 18, 31, 33, and 35, which play a role in the development of cervical cancer. Second, HPV-positive squamous cell carcinomas of the head and neck have viral characteristics concordant with HPV-associated carcinogenesis, including a high viral load and viral oncoprotein expression.21 The oncogenic function of these HPV-associated oncoproteins ultimately accounts for the different molecular alterations found in HPV-positive tumors compared with HPV-negative tumors.
Additional evidence for an HPV association includes significant links in case-control studies between HPV seropositivity and squamous cell carcinomas of the head and neck. A case-control study comparing 100 patients diagnosed with oropharyngeal cancer and 200 controls found oropharyngeal cancer to be closely associated with oral HPV-16 infection (odds ratio, 14.6), oral infection with any of the HPV types (odds ratio, 12.3), and seropositivity for the HPV-16 L1 capsid protein (odds ratio, 32.2), strongly associating oral HPV with oropharyngeal cancer.22 Behavioral risk factors associated with oral HPV infection have also been correlated with an increased risk of oropharyngeal cancer. They include a high lifetime number of vaginal-sex partners and oral-sex partners.22 This correlation between HPV DNA in tumor specimens and lifetime sex partners has also been identified by others,23 as has age at first intercourse and genital warts.24 ![]()
Patients with HPV-associated head and neck cancers also have an increased risk of anogenital cancers, of which cervical and anal cancers have been strongly associated with HPV infection, and vice versa.25 Again, this supports the influence of sexual behavior and HPV infection on oropharyngeal cancer risk.
Patients with HPV-associated oropharyngeal cancers tend to have distinct clinical and pathologic features compared with those with HPV-negative cancers (Table 31-1). Besides the history of high-risk sexual behavior described above, patients with HPV-associated oropharyngeal cancers are more typically light users or nonusers of alcohol and tobacco,26,27 although this lack of connection has not been universally identified.24 Despite the apparent lack of association between HPV-associated oropharyngeal cancers and tobacco use, there is a correlation with marijuana use and its intensity and duration.27 Individuals with HPV-positive squamous cell carcinoma of the head and neck tend to be younger by approximately 5 years compared with HPV-negative patients.21 Pathologically, HPV-positive cancers are more likely to be poorly differentiated with basaloid features.18
TABLE 31-1 Distinctive Clinicopathologic Characteristics for HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas
| HPV-Positive Tumors | HPV-Negative Tumors | |
|---|---|---|
| Anatomic site | Tonsil, base of tongue | All sites |
| Histology | Basaloid | Keratinized |
| Age | Younger | Older |
| Social economic status | High | Low |
| Risk factors | Sexual behavior | Alcohol and tobacco use |
| Survival | Improved | Worse |
| Incidence | Increasing | Decreasing |
| EGFR expression | Negative | Positive |
EGFR, endothelial growth factor receptor; HPV, human papillomavirus.
A meta-analysis of the prognostic impact of HPV in head and neck squamous cell carcinomas demonstrated that HPV-positive patients were likely to have improvements in rates of overall survival (OS) (HR, 0.85) and disease-free survival (DFS) (HR, 0.62). Similar improvements in OS and DFS were identified specifically within the oropharyngeal subsite.28 Improved outcomes with HPV positivity were confirmed in an Eastern Cooperative Oncology Group (ECOG) phase II multicenter trial in which higher response rates were seen after induction chemotherapy and after chemoradiation treatment. This led to statistically improved OS and a lower risk of progression.18 See the Expert Consult website for more information on HPV subtyping, association with cancers, and cancer survival. ![]()
Analysis of the subset of patients with stage III or IV oropharyngeal cancer in Radiation Therapy Oncology Group (RTOG) study 0129 found HPV status to be the major determinant of overall survival (OS), followed by duration of tobacco use and tumor stage.29 Locoregional recurrences were reduced at 3 years (13.6% vs. 35.1%) in HPV-positive tumors, and OS was 82.4% versus 57.1%. Although the bulk of the survival difference was attributable to HPV positivity, favorable prognostic features associated with the HPV-positive subgroup accounted for approximately 10% of the outcome difference.
HPV-positive patients also have a lower risk of second malignant tumors because such patients lack the field cancerization effect produced by alcohol and tobacco exposure.29,30 Given the different clinical, pathologic, and molecular characteristics, as well as the improved prognosis and treatment response, it has been proposed that HPV-positive squamous cell carcinomas of the oropharynx and elsewhere should be considered distinct disease entities from HPV-negative tumors.21,29
Other Risk Factors
Numerous other potential risk factors have been described for oropharyngeal cancer and head and neck cancer in general. A family history of head and neck cancer produces an odds ratio risk increase of 1.7, although this is only seen in subjects exposed to tobacco, suggesting that this factor may represent an environmental as much as a genetic influence.31 Diets low in raw vegetables and citrus and noncitrus fruits have increased the oral cancer risk in a majority of studies.19 These results seem to hold true even when studies have been controlled for alcohol and tobacco usage, although the effect on diet and nutritional deficiencies from heavy alcohol and tobacco abuse is substantial and such factors can be difficult to account for in case-control studies.
Nutritional deficiencies, as signified by a body mass index decline before diagnosis, have also been associated with oral cancer risk. This was again seen primarily among individuals with active or prior alcohol and tobacco use. Poor oral hygiene, as measured by surrogates such as tooth loss and infrequent dental visits, also correlates with an elevated rate of oral and oropharyngeal carcinomas.32 In short, the family history, diet, nutritional status, and oral hygiene may all play a role in oropharyngeal carcinoma predisposition, but defining the impact of each of these in the setting of the more dominant risk factors of alcohol and tobacco use is challenging, particularly given the potential interplay between such factors.
Prevention and Early Detection
Classic prevention for squamous cell carcinoma of the oropharynx, and head and neck cancer in general, has revolved around tobacco cessation and bringing alcohol use to more moderate levels. With the impact of HPV now better understood, ongoing HPV vaccination campaigns to lower the incidence of cervical cancer may simultaneously reduce HPV-related head and neck cancers. Two vaccines are currently available for HPV: one against HPV types 16 and 18, Cervarix (GlaxoSmithKline), and a quadrivalent vaccine against HPV types 16, 18, 6, and 11, Gardasil (Merck). Both vaccines have been advised for women between the ages of 13 and 26 years because they lead to a reduction in precancerous cervical lesions and cancer incidence related to HPV.33,34 Vaccine efficacy in nongynecologic cancers remains to be assessed, but there is optimism that a similar reduction in HPV-associated cancers will be seen in these sites, including the oropharynx.27
Routine examinations of the oral cavity and proximal oropharynx should be part of a standard medical history and physical and dental examination. Routine oral examinations in high-risk patients have the potential to reduce cancer-related deaths through the discovery of premalignant and early-stage malignant changes. Unfortunately, the same substance abuse conditions that predispose individuals to head and neck cancer correlate with underuse of health and dental services,32 often leading to late detection and diagnosis of head and neck cancers. Radiation oncologists can play a role in the early detection of secondary oral cavity and oropharyngeal cancers attributable to field cancerization in patients who have been treated for primary head and neck cancers and are undergoing follow-up in the radiation oncology clinic.
Potentially malignant lesions include erythroplakia, leukoplakia, erythroleukoplakia, oral submucous fibrosis, and lichen planus. Leukoplakia and erythroplakia are Greek terms translated as “white plaque” and “red plaque,” respectively. Leukoplakia is an exclusionary diagnosis and is currently defined as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer.”35 The worldwide prevalence of leukoplakia is estimated at approximately 2% of the general population,36 with the highest incidence in men from the fifth through the seventh decades; histologic findings show hyperplasia of the squamous epithelium. Most leukoplakia lesions do not progress to squamous cell carcinoma; the annual transformation rate is estimated at 1%.37 This rate is strongly dependent on the presence and severity of dysplasia.38 Dysplasia, carcinoma in situ, or carcinoma is frequently present within erythroplakia, which has only one-sixth of the prevalence of leukoplakia. Of the premalignant lesions, transformation to malignancy is highest in erythroplakia, particularly when dysplastic changes are present, with an annual transformation rate of at least 2.9%.39 Others have predicted that the vast majority of erythroplakias will ultimately undergo malignant transformation.37
Multiple chemopreventive regimens have been evaluated to prevent progression of dysplasia within the upper aerodigestive tract. At least one randomized study demonstrated a decreased rate of biopsy-proven dysplasia after the administration of isotretinoin.40 However, the toxicity was significant, with almost half of patients requiring dose reduction. In addition, most responders relapsed within the first 3 months after treatment. Larger randomized trials found no reduction in the rate of second primary tumors with isotretinoin.41–43
Biologic Characteristics and Molecular Biology
Significant research is ongoing to determine the genetic and molecular impact of HPV on associated oropharyngeal squamous cell carcinomas. Molecular profiles of HPV-positive tumors are different than those of HPV-negative tumors. The oncogenic function of viral E6 and E7 from HPV appears to propel the molecular abnormalities present in HPV-positive tumors. Inactivation of TP53 and PRB is seen with both types, but are the result of separate mechanisms. E6 inactivates TP53 function in HPV-positive tumors, whereas disruptive mutations of TP53 are more common in HPV-negative tumors.44 Besides TP53, disparate effects on 14-3-3σ, RASSF1A, cyclin D, PRB, and P16 have been identified between HPV-positive and HPV-negative tumors. Disruption of TP53 by alcohol- and tobacco-mediated damage seems to be a critical genetic alteration in HPV-negative tumors. A molecular impact of tobacco exposure has also been identified in cells with HPV present. Normal oral keratinocytes transfected with HPV and subsequently exposed to tobacco carcinogens are found to have enhanced E6 and E7, increased resistance to apoptosis, impaired DNA repair, and activated telomerase.44
Endothelial growth factor receptor (EGFR) expression in oropharyngeal cancers correlates inversely with HPV status.45,46 Not surprisingly, given its association with HPV-negative tumors, EGFR expression predicts for a lower rate of response to induction chemotherapy and inferior overall survival and disease-specific survival rates.45
A similar effect was seen in an analysis of the conventional arm of RTOG 90-03, in which 155 patients with adequate pathologic specimens were evaluated for expression of EGFR by immunohistochemical testing.47 EGFR expression did not correlate with T category, N category, or category grouping, but high EGFR-expressing tumors had higher local recurrence rates and worse disease-free and overall survival. Distant metastases did not differ between the two groups.
Pathology and Pathways of Spread
Pathologic Findings
Primary oropharyngeal tumors are almost exclusively squamous cell carcinomas (SCC) (≈95% of tumors). Alternative pathologic types include melanoma (web-only Fig. 31-1)![]() , primary lymphoid malignant tumors (web-only Fig. 31-2)
, primary lymphoid malignant tumors (web-only Fig. 31-2)![]() , minor salivary gland tumors, sarcomas, and other oddities. Given the preponderance of SCC in this site, this chapter will refer to the evaluation and treatment of SCC unless another type is specifically indicated.
, minor salivary gland tumors, sarcomas, and other oddities. Given the preponderance of SCC in this site, this chapter will refer to the evaluation and treatment of SCC unless another type is specifically indicated.
Web-Only Figure 31-1 Hyperpigmented mass arising from the right tonsillar fossa pathologically confirmed as a mucosal melanoma.
A substantial amount of literature has been generated evaluating the histologic subtypes of SCC of the head and neck, the appropriate nomenclature for these tumors, and their clinical impact. Specific subtypes include the spindle cell variant characterized by noncohesive spindle cells, which closely resembles sarcoma microscopically. The basaloid squamous variant is a distinct subtype traditionally correlated with aggressive behavior and poor patient outcomes.48 However, the recent correlation of HPV-associated malignant disease with the basaloid subtype has shown that the basaloid group is a mixture of both HPV-16-positive and HPV-16-negative carcinomas. Because the HPV status imparts a substantially favorable influence on outcome, the implication is that the basaloid phenotype alone does not confer a worse clinical outcome.49 Angiolymphatic invasion and perineural invasion are both associated with increased rates of cervical nodal metastases and a worse prognosis.50
The prototypical SCC of the head and neck, including the oropharynx, is moderately differentiated, with approximately 60% moderately differentiated, 20% well differentiated, and 20% poorly differentiated. Some studies have found tumor grade to be predictive of regional nodal metastasis and, subsequently, clinical outcome. The determination of tumor grade based on traditional cytologic and histologic features of differentiation is challenging to reproduce because of interobserver variability. As a result, the tumor grade seems to have limited prognostic value, particularly when compared with the clinical staging parameters.50 Undifferentiated carcinomas with a lymphoid stroma arising from the tonsil have biologic behavioral characteristics and an increased radiosensitivity that are more similar to undifferentiated nonkeratinizing nasopharyngeal carcinomas rather than conventional types of SCC.51
Pathways of Spread
The oropharynx makes up the central portion of the upper aerodigestive tract, encompassing the tongue base, palatine (faucial) tonsils, inferior aspect of the soft palate, and posterior and lateral oropharyngeal walls52 (see Fig. 31-1 and Figs. 31-2 and 31-3). The anterior margins are the anterior tonsillar pillars, the hard-soft palate junction, and the circumvallate papillae of the tongue, placing the posterior third of the tongue within the oropharynx. Superiorly, the oropharynx is bounded by the inferior aspect of the soft palate, posteroinferiorly by the pharyngoepiglottic folds that lie approximately at the level of the hyoid bone, and inferiorly by the glossoepiglottic fold and vallecula. The lateral and posterior borders of the oropharynx are defined by the pharyngeal constrictor muscles and their overlying mucosa. Understanding the anatomy of the oropharynx is necessary when identifying the typical pathways of spread by oropharyngeal neoplasms.53–55
Soft Palate
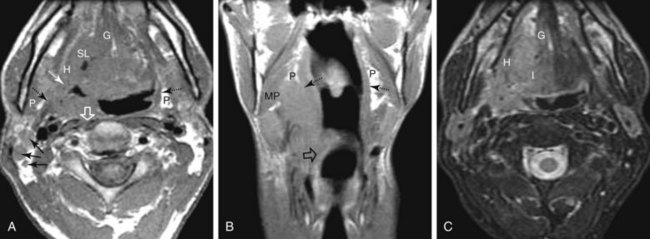
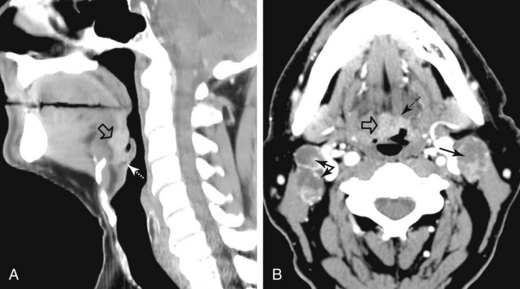
A soft palate tumor can spread anteriorly to involve the hard palate. There is also potential for perineural spread along the greater and lesser palatine branches of the maxillary nerve superiorly into the pterygopalatine fossa56,57 (Fig. 31-4). Further perineural spread from the pterygopalatine fossa can result in extension along branches of the maxillary division of the trigeminal nerve into the orbit via the inferior orbital fissure, into the central skull base via the foramen rotundum, and into the facial nerve and temporal bone via the nerve of the pterygoid canal (vidian nerve). This perineural spread can result in palsies of both the trigeminal and facial nerves. Patients with advanced disease and perineural spread may present with trismus resulting from involvement of the pterygoid muscles or branches of the trigeminal nerve.
Tonsillar Region
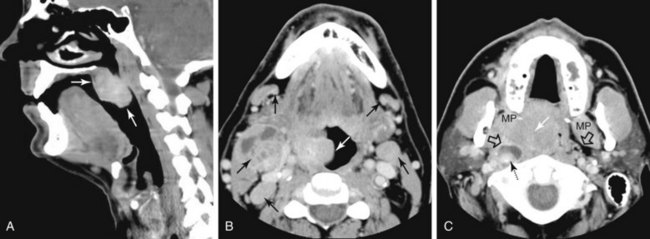
The palatine (faucial) tonsils consist of lymphoid tissue constrained within a fibrous capsule that projects into the oropharynx between the triangle formed by the anterior and posterior tonsillar pillars. The tonsillar fossa is bounded laterally by the superior pharyngeal constrictor muscle. The anterior and posterior tonsillar pillars consist of the mucosa overlying the palatoglossus and palatopharyngeal muscles, respectively. Squamous cell carcinoma is the most frequent neoplasm of the palatine tonsils, followed distantly by non-Hodgkin’s lymphoma. Tumors within the tonsillar region can spread superiorly to the soft palate along the vertically oriented palatoglossus and palatopharyngeal muscles, as well as inferiorly to the tongue base and posterolaterally to the oropharyngeal wall58 (Figs. 31-4 and 31-5).
The superior pharyngeal constrictor muscle, in addition to forming the posterior and lateral borders of the oropharynx, courses deep to the palatine tonsils as it extends anteriorly to insert on the pterygomandibular raphe.52 This fibrous raphe is a shared insertion site with the buccinator muscle, with the raphe extending to the medial pterygoid plates superiorly and the lingual cortex of the mandible inferiorly just posterior to the mylohyoid line. Tonsillar neoplasms that invade the superior pharyngeal constrictor muscle and involve the pterygomandibular raphe, therefore, can spread anteriorly to the buccinator muscle and space, superiorly to the medial pterygoid plate and masticator space, and inferiorly to the lingual cortex of the mandible. Lateral extension of tumor allows its entry into the parapharyngeal space (Fig. 31-6). Involvement of the parapharyngeal space by tonsillar neoplasms may result in spread to and along the styloglossus and stylopharyngeal muscles, which extend superiorly toward the skull base and attach to the styloid process. Lateral extension into the medial pterygoid muscle is often associated with involvement of the mandibular division (V3) of the trigeminal nerve. Involvement of V3 can allow perineural tumor spread inferiorly into the mandible along the inferior alveolar nerve and superiorly along the main trunk of V3 to the foramen ovale and cavernous sinus.57
Oropharyngeal Wall
The posterior and lateral walls of the oropharynx are formed by the superior pharyngeal constrictor muscle and overlying mucosa. The deep aspect of the superior constrictor muscle is separated from the prevertebral fascia by a thin layer of retropharyngeal fat. Tumors within the posterior aspect of the oropharynx can extend through this layer of fat and fascia and into the prevertebral space59,60 (see Fig. 31-6). Advanced tumors can ultimately involve the vertebral column, although such spread is typically inhibited by the multiple intervening fascial planes and the longus colli and longus capitis muscles. Tumors of the posterolateral oropharyngeal wall can extend into the nasopharynx and hypopharynx by way of either mucosal or submucosal spread (see Fig. 31-6).
Tongue Base
The tongue base is separated from the oral tongue anteriorly by the circumvallate papillae. It extends posteriorly and inferiorly to terminate near the level of the hyoid bone (see Figs. 31-2 and 31-3). Laterally, it is separated from the palatine tonsils by the glossotonsillar sulcus. The lingual tonsils lie along the posterior surface of the tongue base. The lingual tonsils are variable in size and may be quite large in the first two decades of life and in patients with concurrent head and neck infections.
Neoplasms of the tongue base spread along several pathways as they increase in size. They may infiltrate along the intrinsic muscles of the tongue into the oral tongue and deep tongue musculature. It is especially important to identify involvement of the extrinsic muscles of the tongue (i.e., the hyoglossus, styloglossus, and genioglossus muscles) because this indicates a T4 lesion.61 Tumor may track superolaterally along the mylohyoid muscle to invade the lingual surface of the mandible.62 Deep anterior extension may also involve the hypoglossal or lingual neurovascular bundles. Tumor can track posteriorly along these nerves between the superior and middle pharyngeal constrictor muscles into the parapharyngeal and masticator spaces.
Base of tongue cancer can also spread superficially along the mucosa. Posteroinferior extension can involve the vallecula, lingual surface of the epiglottis, and pharyngoepiglottic folds (Fig. 31-7). There may be invasion of the pre-epiglottic space whenever the vallecula or glossoepiglottic folds are involved.
Nodal Involvement
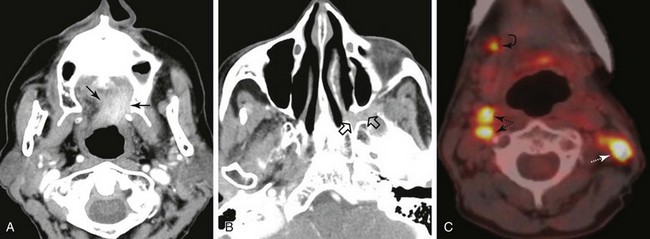
Nodal metastasis has been reported in 40% to 66% of patients presenting with soft palate carcinoma.63,64 Level II nodal involvement is most frequent, with extension into level III and IV nodes commonly seen. Level I, level V, and lateral retropharyngeal nodal involvement is uncommon.65 Lymph node metastasis from squamous cell cancer of the palatine tonsils is very common (69% to 74%) at the time of presentation.64,66 The incidence of nodal involvement at diagnosis in patients diagnosed with tongue base carcinoma has been reported to be between 64% and 78%.63,64 Bilateral nodal disease is commonplace with base of tongue cancer, especially in patients with tumors near or crossing the midline. Both tonsillar and base of tongue primary tumors can drain to retropharyngeal nodes, with implications for radiation treatment design. The incidence of nodal involvement in patients with oropharyngeal wall squamous carcinomas has been reported to be between 57% and 60%64,67 (see web-only Figs. 31-3 to 31-5)![]() .
.
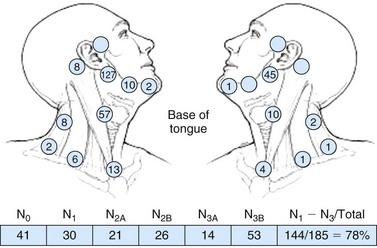
Web-Only Figure 31-3 Distribution of positive nodes in patients with squamous cell carcinoma of the base of the tongue on presentation to the M.D. Anderson Cancer Center, 1948 to 1965.
Data from Lindberg RD: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 29:1446, 1972, with permission.
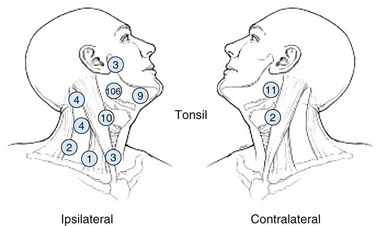
Web-Only Figure 31-4 Distribution of positive nodes in patients with squamous cell carcinoma of the tonsil on presentation to the M.D. Anderson Cancer Center, 1968 to 1979.
Data from Remmler D, Medina JE, Byers RM, et al: Treatment of choice for squamous carcinoma of the tonsillar fossa. Head Neck Surg 7:206-211, 1985.
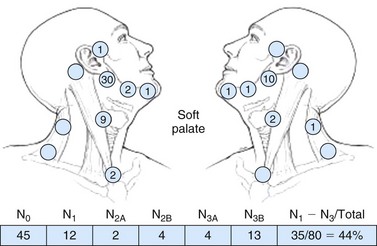
Web-Only Figure 31-5 Distribution of positive nodes in patients with squamous carcinoma of the soft palate on presentation to the M.D. Anderson Cancer Center, 1948 to 1965.
Data from Lindberg RD: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 29:1446, 1972, with permission.
Clinical Manifestations, Patient Evaluation, and Staging
Patient Evaluation
A comprehensive history with an emphasis on head and neck–related symptoms should be taken for all patients, and a detailed physical examination of the head and neck region should be performed (Table 31-2). The examination should include a direct inspection of the oral cavity and proximal oropharynx; an indirect (mirror) examination of the distal oropharynx, hypopharynx, nasopharynx, and larynx; careful palpation of the oral cavity and proximal oropharynx; and inspection of the cervical lymph nodes (Fig. 31-8). Relaxing the sternocleidomastoid muscle by tilting the head slightly to the ipsilateral side allows easier palpation of the jugulodigastric nodes. Flexible fiberoptic nasopharyngoscopy is used as indicated by examination findings and the quality of the indirect examination.
| General |
CT, computed tomography; MRI, magnetic resonance imaging.
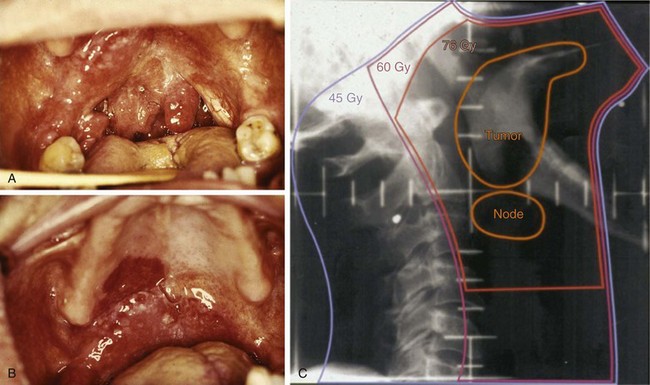
Additional staging information can be gleaned during the physical examination. Trismus, documented by measuring the distance between the upper and lower incisors, or alveolar ridges in edentulous patients, is a consequence of tumor invasion into the pterygoid musculature. Deviation of the tongue on protrusion suggests involvement of the deep or extrinsic muscles of the tongue or invasion or compression of the hypoglossal nerve (CN XII) (see web-only Fig. 31-6)![]() . A detailed physical examination is especially important with oropharyngeal cancers because treatment-altering findings may manifest themselves on visual inspection but not on radiographic evaluation, and can alter both radiation treatment coverage and staging. For example, subtle superficial mucosal extension of a tonsillar tumor onto the adjacent soft palate or retromolar trigone may only be apparent during direct inspection and must be documented. Such regions may be more apparent during radiation treatment at approximately 20 to 30 Gy because “tumoritis” causes increased erythema of these regions compared with the surrounding normal tissue (see web-only Fig. 31-7)
. A detailed physical examination is especially important with oropharyngeal cancers because treatment-altering findings may manifest themselves on visual inspection but not on radiographic evaluation, and can alter both radiation treatment coverage and staging. For example, subtle superficial mucosal extension of a tonsillar tumor onto the adjacent soft palate or retromolar trigone may only be apparent during direct inspection and must be documented. Such regions may be more apparent during radiation treatment at approximately 20 to 30 Gy because “tumoritis” causes increased erythema of these regions compared with the surrounding normal tissue (see web-only Fig. 31-7)![]() . With base of tongue tumors in particular, digital palpation is essential to determine the size and extent of local spread because these tumors are frequently not seen by or are underestimated by visual examination, especially if they are endophytic. All borders of the tumor must be determined precisely because this has a crucial impact on target volume delineation and treatment field arrangement. Precision in defining tumor borders becomes even more paramount when intensity-modulated radiation therapy (IMRT) is anticipated. Detailed examination of the remainder of the head and neck is also important because of field cancerization and the risk of synchronous primary tumors.
. With base of tongue tumors in particular, digital palpation is essential to determine the size and extent of local spread because these tumors are frequently not seen by or are underestimated by visual examination, especially if they are endophytic. All borders of the tumor must be determined precisely because this has a crucial impact on target volume delineation and treatment field arrangement. Precision in defining tumor borders becomes even more paramount when intensity-modulated radiation therapy (IMRT) is anticipated. Detailed examination of the remainder of the head and neck is also important because of field cancerization and the risk of synchronous primary tumors.
Web-Only Figure 31-6 Atrophy of the right hemitongue with rightward deviation on protrusion consistent with right CN 12 palsy.
Aside from a complete history and physical examination, the workup should include biopsy confirmation of the mass. Biopsy should be performed by the least invasive means possible and can include fine-needle aspiration of involved cervical nodes or biopsy of the primary site. In general, excisional biopsy of the primary site or involved lymph nodes is not recommended because of concerns about prolonged healing times that may delay definitive irradiation and surgery-associated hypoxic changes. Patients who present with malignant jugulodigastric nodes or, less commonly, midcervical nodes and no clear primary tumor are statistically most likely to have a primary tumor of the oropharynx, and a thorough examination of the oropharynx should be performed as part of the evaluation.68 Ipsilateral tonsillectomy is a recommended component of the evaluation for head and neck cancer patients presenting with upper cervical or midcervical nodal metastases without an apparent mucosal primary tumor.
Imaging Studies
Selection of Imaging Studies
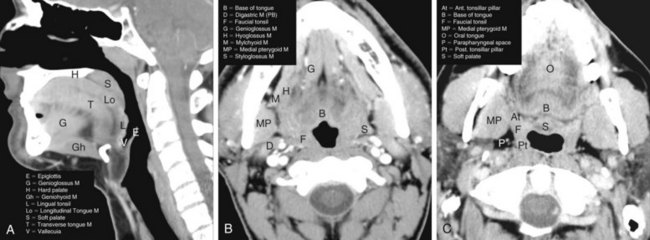
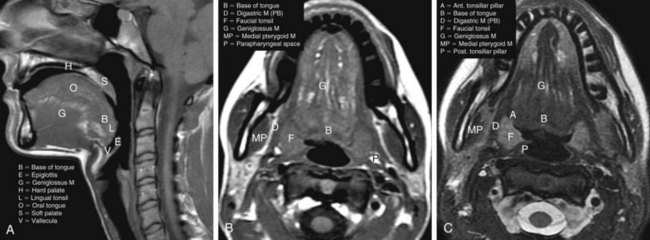
Recommended radiographic studies include CT or MRI scanning of the neck with contrast enhancement, chest imaging, and panoramic studies as indicated.69 CT is generally considered to be the examination of choice for the initial diagnostic evaluation of oropharyngeal neoplasms when compared with MRI and PET imaging.70,71,72 The benefits of CT include lower cost, faster acquisition speed, less motion artifact, better detection of bone invasion, higher spatial resolution, greater reproducibility on sequential examinations, and wide availability. MRI is superior to CT in specific situations, including delineating orbital or skull base involvement and defining intracranial perineural tumor spread. MRI also has slightly superior multiplanar imaging capabilities and is better able to identify subtle soft tissue involvement by tumor, deep osseous invasion and marrow replacement, perineural spread of disease, and extent of anterior spread of tongue base tumors. MRI is also useful for patients in whom dental amalgam artifact renders the CT examination suboptimal. MRI and PET/CT are used in most institutions as ancillary diagnostic studies for oropharyngeal cancers when CT imaging inadequately addresses specific clinical questions. Imaging modalities are often complementary, however, and both CT and MRI are useful in some instances. PET/CT studies can provide additive information relative to the extent of the primary tumor, nodal involvement, and metastatic disease.
Imaging is indispensable for assessing regional lymphatic spread.73 This is particularly true in patients in whom clinical assessment of nodal disease is difficult (because of a large or muscular neck), in whom there is occult involvement of normal-sized nodes, or in whom nodal chains are involved that cannot be palpated on physical examination (i.e., retropharyngeal nodes). Multiple criteria must be used to determine whether a specific lymph node is involved by tumor on CT or MRI studies. These criteria include the node’s size, homogeneity, distinctness of outer margin, focal areas of enhancement, location, loss of fatty hilum, and spherical shape and the presence of a clustering of lymph nodes. These criteria are best used as a whole and not considered in isolation. When imaging tests have been directly compared in the same patient population, PET and PET/CT studies have typically been found to be slightly more accurate in lymph node staging, followed by CT scanning and then by MRI.74,75 See the Expert Consult website for further information on lymph node staging![]() .
.
There is varied opinion regarding size criteria and how to best measure lymph nodes in order label them as radiographically involved. Measurements of 10 mm in the long axis and 8 mm in the short axis are often used as the upper limits of normal for nodes in most cervical levels. Upper level II nodes (jugulodigastric nodes) and level Ib nodes, however, may normally be slightly larger (12 to 15 mm). Lateral retropharyngeal nodes are considered abnormal if larger than 8 mm. Very young patients, those with severe dental disease, or those with concomitant head and neck infection may have reactive lymph nodes that are larger than the stated norm. Lymph node size should be considered as only one diagnostic criterion and must be modified in certain circumstances. One should specifically look for focal enhancement of a lymph node along its outer aspect where the afferent lymphatics enter the node. This is often the earliest evidence of lymphadenopathy and may even be seen in patients with normal-sized nodes. Assessment of lymphadenopathy on MRI scans can be more challenging than on CT scans.70,72 In addition to the aforementioned criteria, one should look for areas of decreased signal intensity on T2-weighted images, which typically represent intranodal tumoral deposits. Meta-analysis suggests that the mean sensitivity of a neck CT scan for the detection of cervical lymph node metastasis is 81% (95% CI, 68% to 90%), of an MRI scan is 81% (95% CI, 65% to 91%), and of a PET scan is 79% (95% CI, 72% to 85%). The specificity of CT scanning has been found to be 76% (95% CI, 62% to 87%); of MRI scanning, 63% (95% CI, 43% to 80%); and of PET scanning, 86% (95% CI, 83% to 89%).72,74 The quoted sensitivity and specificity for these studies depends greatly on the criteria that are used in declaring a lymph node positive. When imaging tests have been directly compared in the same patient population, PET and PET/CT studies have typically been found to be slightly more accurate in lymph node staging, followed by CT scanning and then by MRI.74
PET/CT and PET can serve a complementary role in the imaging evaluation of oropharyngeal primary tumors. Besides the slightly improved accuracy for nodal disease, PET/CT is particularly useful in evaluating for a primary site in patients with cervical nodal disease but an unidentified primary site. An oropharyngeal origin remains the most likely source of an occult primary tumor in patients with level II or III lymph node involvement. PET/CT scanning can also identify synchronous second primary tumors in patients with oropharyngeal squamous cell carcinomas.76 A PET/CT scan can also be used to screen for metastatic disease and excels at identifying atypical metastases outside of the standard metastatic search pattern, although metastatic spread at presentation is rare. The performance of PET/CT scanning can be improved with a dedicated head and neck protocol, which can also be incorporated into treatment planning. Although PET/CT in particular may have value in oropharyngeal cancer workup and staging, overuse of this expensive resource remains a persistent concern as more standard imaging modalities typically supply equivalent diagnostic information.
The overall incidence of pulmonary metastases has traditionally been placed at 1% to 2% in newly diagnosed patients with cancers of the head and neck. The standard practice is to obtain a chest radiograph to evaluate for pulmonary disease, but chest CTs allow better definition of the thoracic cavity and have a higher sensitivity for detection of pulmonary parenchymal metastases or mediastinal or hilar nodal disease. A review of the literature found a pooled prevalence of 7.9% for abnormalities on chest CT scans in patients with head and neck squamous cell carcinoma.77 Abnormal findings were more prevalent in patients with N2 or N3 nodal involvement or stage III or IV disease and less prevalent for oral cavity primary tumors. Not surprisingly, patients with recurrent disease had higher rates of positive chest CT scans (25.3%). These risk factors correlated closely with the clinical factors statistically associated with the development of distant metastases (T stage, N stage, gender, nodal level, and locoregional control).78 Although there is significant institutional variation regarding chest imaging, a measured approach would include chest CT scans for patients with advanced nodal involvement (N2b, N2c, and N3), locally advanced primary disease (T3 and T4), low cervical nodes, and other risk factors, including recurrent disease. A CT scan of the chest can be performed at the same time as the neck CT scan, and modern multislice scanners can provide contrast-enhanced images with the same contrast bolus.
Imaging Technique
The Expert Consult website contains information on imaging technique. ![]()
MRI protocols should be constructed in a similar manner to cover the region from the skull base and cavernous sinuses to the aortic arch. It is particularly important to obtain both high-quality non–contrast-enhanced T1- and T2-weighted images because these are often more helpful than contrast-enhanced images at defining the extent of the primary neoplasm and assessing nodal disease.21,22,23–27 Post-contrast-enhanced T1-weighted images obtained with fat suppression are helpful for identifying perineural tumor spread, skull base invasion, and deep spread to the masticator and parapharyngeal spaces, as well as for detecting intranodal tumor deposits. Because of the extensive pterygoid plexus of veins in the masticator space and normal enhancement of mucosal areas of the head and neck, contrast-enhanced scans are often challenging to interpret. These images must be carefully compared with non–contrast-enhanced images in the same plane.
Staging
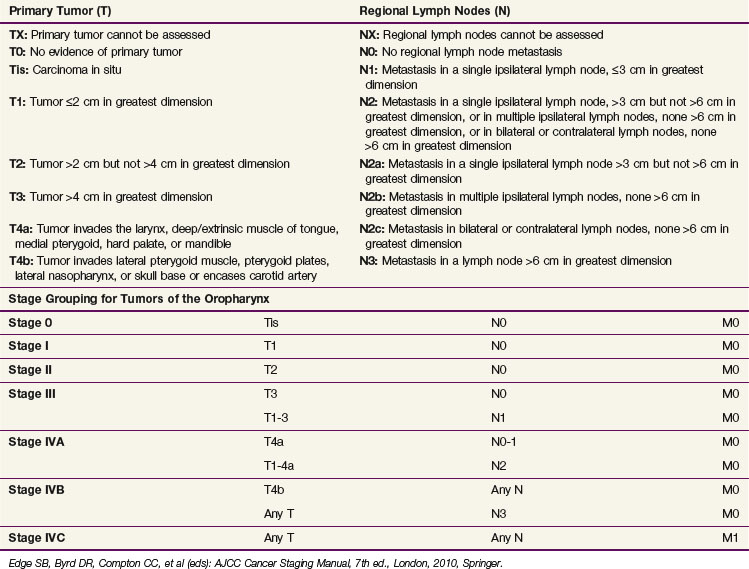
Table 31-3 shows the AJCC seventh edition staging system for oropharyngeal carcinomas.61 Endoscopic, radiographic, and physical examination findings are included and collated when determining the clinical stage. Pathologic staging may also be performed using the same criteria as clinical staging. Even when histologic evaluation of the disease is available, information obtained during clinical staging should be incorporated. Comparisons of surgical and radiation-based treatment outcomes should be based on clinical staging because up-staging and down-staging may occur with surgical intervention and both should be reported.
Primary Therapy
General Principles of Treatment
To date, only a single randomized trial evaluating primary surgery versus radiation therapy has been conducted for primary oropharyngeal squamous cell carcinomas (RTOG 73-03).79 Seventy patients were randomized to definitive radiotherapy, preoperative radiotherapy followed by surgery, or surgery followed by radiotherapy. No significant difference was identified in rates of either OS or local control, although the study was grossly underpowered.
A similar study evaluating 119 patients with locally advanced head and neck cancers, including oropharyngeal cancers, demonstrated no difference in DFS or OS between surgery followed by adjuvant radiation versus adjuvant concurrent chemoradiation.80 Improved organ preservation was observed in patients randomized to chemoradiation.
Early-stage oropharyngeal carcinomas (T1 to T2, N0 to N1) can be treated with either irradiation or surgery, with the addition of adjuvant irradiation as indicated by surgical pathologic findings. Surgery and radiation therapy provide similar local and regional control rates.81 Treatment selection is consequently based on the anticipated modality-specific functional and cosmetic outcome as well as the most appropriate treatment for lymph nodes at risk. Well-lateralized tumors, particularly of the tonsillar complex, can be treated with unilateral irradiation and resultantly have a more favorable toxicity profile. Irradiation is typically used for early-stage oropharyngeal tumors based on excellent local control rates and acceptable morbidity; a higher rate of severe complications has been identified with surgery, particularly in older series. However, minimally invasive surgery with robotics or transoral laser microsurgery is being increasingly investigated at select institutions.
Surgery
Definitive surgery followed by irradiation remains a viable treatment choice in oropharyngeal cancer, particularly with smaller primary tumors that are potentially amenable to resection without significant sequela. When surgery is used, several approaches may be applicable. Traditional surgical approaches include lateral pharyngotomy, a transhyoid approach, or an anterior approach with a midline or lateral mandibulotomy with a combined lip-splitting incision. Newer surgical approaches include transoral laser microsurgery (TLM), in which the tumor is excised with either a free-beam or fiber-delivered carbon dioxide laser with visualization through an operating microscope or rod telescope.82 The carbon dioxide laser allows precision with minimal cautery artifact or oozing, and the tumor is removed as a multibloc resection. Advocates of TLM state that this maximizes oncologic excision while minimizing the potential surgical side effects seen with older approaches. A transoral robotic approach for a radical tonsillectomy has also been described.83
The Washington University experience82 of 84 patients with stage III and IV oropharyngeal cancers treated with TLM followed by irradiation as indicated described rates of disease-specific survival (DSS) and OS of 92% and 88%, respectively, at 5 years, with only one local failure and four regional failures; only 3.4% of patients remained gastrostomy-tube dependent at 3 years. Only 26% of the patients had stage T3 and T4 disease, however, and these patients had a statistically worse OS. In addition, 95% of the patients were HPV-16-positive, and this may have contributed to the high rates of locoregional control. Again, such potential benefits are weighed against the hazards of surgery, which included a 6% reoperation rate, an 11% temporary tracheotomy rate, and an 11% rate of velopharyngeal incompetence (although oral intake and speech were not interrupted).
Although the Mayo Clinic experience84,85 details a similarly high local control rate with transoral excision, others have found higher rates of local recurrence, particularly when lesions larger than stage T2 are resected. Pradier and colleagues86 described lower rates of locoregional control, DSS, and OS of 61%, 48%, and 42%, respectively, in oropharyngeal cancers treated with TLM. As a whole, this study had higher percentages with locally advanced primary tumors (stage T3 and T4) and a worse outcome in patients in which adjuvant split-course irradiation was used. The worse outcome with split-course irradiation endorses the importance of radiotherapy in this patient cohort.
Treatment Recommendations by Subsite
Tonsillar Region Carcinomas
A review of North American experiences81 published between 1970 and 2000 compiled data comparing surgery with or without adjuvant irradiation with definitive radiation with or without neck dissection. The 51 reported series represented approximately 6400 patients. Results for the tonsillar region showed strikingly similar oncologic outcomes between primary surgery and radiation therapy: local control 79% versus 76%, locoregional control 60% versus 69%, 5-year OS were 49% versus 52%, and 5-year cause-specific survival (CSS)rates were 62% versus 63%, none of which were statistically significant. However, rates of severe complications (23% vs. 6%) and fatal complications (3.2% vs. 0.8%) were significantly higher in the surgical group. The less toxic side effect profile of definitive radiation therapy led the authors to recommend this approach for most patients with SCC of the tonsillar complex.
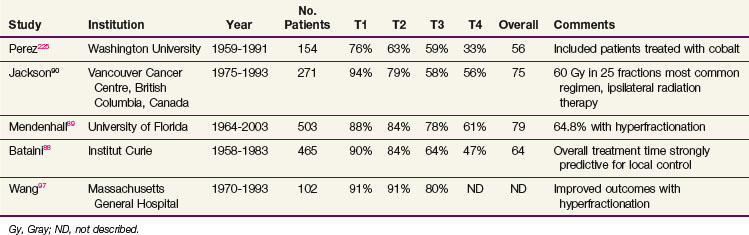
Table 31-4 summarizes institutional experiences with more than 100 patients treated with conventional irradiation for SCC of the tonsillar region (with the exception of 17 patients in the Florida series). Local control rates for T1 and T2 primary tumors are high for all series, although slightly lower control rates were seen in the Washington University series that extended back to the late 1950s. These studies confirm that the vast majority of patients with T1 and T2 disease can be cured with irradiation alone. Of tumors that recur, a significant percentage can still be cured with salvage surgery.87 Lower local control rates were seen for T3 and T4 tumors, but most of these patients would now receive concurrent chemotherapy, elevating the anticipated local control rate. Bataini and colleagues88 found that tumors originating from the glossopalatine sulcus characterized by involvement of the tongue had a worse outcome than those arising elsewhere within the tonsillar region. The overall treatment time was also found to be strongly predictive for local control. In the largest series, Mendenhall and associates89 recorded severe complications in 9% of patients. The most common severe complications were osteonecrosis requiring surgical intervention and a permanent gastrostomy tube secondary to chronic dysphagia. Most of the large series evaluating definitive radiation therapy for tonsillar region carcinomas extend well into the era before CT was available, with some series including patients from the 1950s and 1960s. Better imaging modalities, leading to stage migration, and improved planning techniques would be anticipated to improve outcomes.
TABLE 31-4 Local Control Rates Reported for Treatment of Squamous Cell Carcinoma of the Tonsillar Fossa
Unilateral Radiation
Unilateral radiation, including coverage of the ipsilateral neck but not the contralateral neck, has been given in the past using conventional techniques in patients with well-lateralized tonsillar fossa tumors. Such an approach in the era before IMRT became available offered sparing of the contralateral parotid and submandibular glands and was designed to avoid permanent xerostomia. Jackson and coauthors90 reported a risk of contralateral node recurrence in 2.5% of patients treated with ipsilateral irradiation for a tonsillar primary tumor that was clinically N0 or N1. Of the 642 patients treated for carcinoma of the tonsillar region at Princess Margaret Hospital from 1970 to 1991, 228 patients were treated with ipsilateral irradiation.91 Contralateral neck failures occurred in eight patients (3.5%), with only three patients having contralateral nodal failure alone (1.7% of patients). These three patients all had significant soft palate involvement (to within 1 cm of the midline in two cases and involving more than 1 cm of the soft palate in the other case) and ipsilateral neck disease. No contralateral neck failures were seen in patients who had clinical stage T1 disease, regardless of nodal disease extent, or N0 disease, regardless of tumor size or involvement of midline structures. The margin of coverage placed on the primary site had a significant impact on local control rates, with a 30% local control rate for a margin of less than 1 cm and an 89% local control rate for a margin of more than 1 cm. O’Sullivan and colleagues91 advocated ipsilateral irradiation after informed patient consent for all lateralized lesions with less than 1 cm of medial soft palate or tongue base extension.
Side effects appear to be reduced in patients treated to the ipsilateral neck only. The Aarhus University group found no impact on locoregional control or survival with ipsilateral radiation, but side effects were reduced for all endpoints (i.e., dysphagia, xerostomia, hoarseness, atrophy, fibrosis, and edema).92 Moderate to severe toxicity was reduced by more than 50% for all endpoints except fibrosis.
Unilateral radiation at the University of Wisconsin is confined to well-lateralized T1 to T2, N0 to N1 tonsillar cancers and has not been employed when the primary tumor extends into midline structures such as the base of the tongue or there is more than minimal extension onto the soft palate. Others have argued that the contralateral neck only needs to be covered if the tumor extends beyond the midline of the soft palate (uvula) or beyond the lateral one-third of the ipsilateral base of the tongue.93
Surgery
The use of oncologic surgery in early-stage tonsillar cancer remains controversial because of the high rates of local control that can be achieved with radiotherapy alone. Many patients present to the radiation oncology clinic having undergone tonsillectomy for either a tonsillar abnormality or as part of an evaluation for an unknown primary tumor in the setting of cervical nodal disease. Yildirim and associates94 evaluated 120 patients treated with radiotherapy at the M.D. Anderson Cancer Center following gross removal of their disease by tonsillectomy. Most of these tonsillectomies were performed for diagnostic purposes, including 29% that had no preoperative tonsillar abnormality. The population included 36 patients with stage III disease and 64 patients with stage IV disease. Only 12 patients received systemic chemotherapy. Five-year locoregional control, recurrence-free survival, and OS were very high at 97%, 92%, and 86%, respectively, with only a single patient experiencing disease recurrence at the primary site. Fifty-one percent of tumors had negative surgical margins, 35% had positive surgical margins, 10% had close margins (<5 mm), and 3% had an unknown margin status. The tonsillar bed received 66 Gy of irradiation in 82% of patients. The authors concluded that the high local control rate present, particularly with T2 disease, may be a result of patient selection rather than the potential benefit of tonsillectomy because there was not invasion into adjacent mucosal sites such as the soft palate or base of the tongue in this patient group. Margin status and nodal disease dictated that adjuvant irradiation be delivered, making the benefit from surgery with its associated toxicities, including potential velopharyngeal insufficiency, unclear. Surgeries may be useful in small, stage T1 or T2 primary tumors without nodal disease in which adjuvant irradiation may not be required postoperatively, but this is a small subset of patients with early disease.95
Base of Tongue Tumors
Squamous cell carcinomas of the base of the tongue tend to be more locally, regionally, and systemically aggressive with worse outcomes when compared with tonsillar primary tumors. Parsons and coauthors81 also reviewed the radiation oncology and surgical literature from 1970 to 2000 and compared reported outcomes between surgery with and without irradiation and definitive irradiation with or without a lymph node dissection for base of tongue primaries. For surgical-based versus radiation-based treatments, similar outcomes were identified for rates of local control (79% vs. 76%), 5-year OS (49% vs. 52%), and 5-year CSS (62% vs. 63%).81 Locoregional control rates were slightly better with irradiation as the primary modality (60% vs. 69%). Again, the rate of severe complications was higher if primary surgery was performed (32% vs. 3.8%), as was the rate of fatal complications (3.5% vs. 0.4%). The authors concluded that radiation therapy with or without lymph node dissection was preferable for the majority of squamous cell carcinomas of the base of the tongue based on the discrepant toxicity profiles.
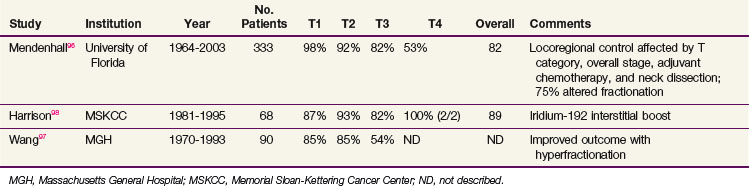
Reported local control rates of large series published in the last 20 years are listed in Table 31-5. The largest series from the University of Florida describes a local control rate of 93% for T1 to T2 tumors.96 Multivariate analysis demonstrated that the T category, histologic differentiation, adjuvant chemotherapy (if T4 disease), and race all affected local control rates. More poorly differentiated cancers had better local control rates when compared with the more differentiated neoplasms. Locoregional control rates were affected by the same factors and were also statistically associated with postradiotherapy neck dissections and overall stage. Seventy-five percent of patients in this series were treated with hyperfractionated regimens (standard hyperfractionation or concomitant boost), although this was not correlated with local or locoregional control. Wang and colleagues97 described improved local control rates with accelerated split-course therapy, although this represented an inter-era comparison. Compared with tonsillar fossa tumors, base of tongue tumors had lower rates of local control, particularly clinically T3.
TABLE 31-5 Local Control Rates Reported for Treatment of Squamous Cell Carcinoma of the Base of Tongue
Sixteen percent of patients (52 patients) developed one or more severe complications from treatment, including the need for a permanent gastrostomy tube (21 patients) and mandibular osteoradionecrosis (8 patients). Eleven patients had surgery-related complications following either a post-irradiation neck dissection or salvage surgery.96
Brachytherapy
High local and regional control rates have been achieved using a combination of external beam radiation therapy (EBRT) and a brachytherapy boost. Harrison and associates98 treated 68 patients with this approach, with a high overall local control rate of 89%. EBRT was delivered to the primary site and upper neck (54 Gy) and low neck (50 Gy), followed by a boost of 20 to 30 Gy of iridium-192 (192Ir) brachytherapy approximately 3 weeks later. Contraindications for a brachytherapy implant included disease extending below the hyoid bone, into the pre-epiglottic space, mandibular invasion, or posterior pharyngeal wall extension, with 15% to 20% of cases being unsuitable for brachytherapy. Similar high rates of local control have been identified at other institutions,99,100 although the University of Florida discontinued interstitial implant because of inferior results compared with conventional EBRT.96
Surgery
Foote and coauthors101 evaluated 55 patients treated with surgery alone for tongue base SCC from January 1971 to December 1986 at the Mayo Clinic. A partial glossectomy was performed in 54 patients; additionally, 5 patients had an associated total laryngectomy, 2 patients a supraglottic laryngectomy, and 11 patients a partial mandibulectomy. The median hospital stay postoperatively was 16 days. The local control rate at 5 years was 74%, but locoregional control at 5 years was only 48%, with OS of 55%. By clinical T category, local control was T1, 77%; T2, 81%; and T3, 77%. Complications included fistula or abscess formation in eight patients, osteomyelitis with bone necrosis in six patients, pectoralis flap failure in two patients, and hemorrhage in two patients, and the surgical mortality rate was 4% (two patients). Based on this experience, locoregional control appears to be inferior with surgery alone and the complication rate is substantial. Caveats from this study are the facts that patients were included who were treated before CT scanning became available, only nine patients underwent bilateral neck dissection, and adjuvant irradiation was absent in the presence of high-risk neck disease.
The morbidity of primary surgery was confirmed by Sessions and colleagues,102 who compared outcomes for patients with base of tongue SCC at Washington University who underwent local or composite resection with or without radiation therapy compared with radiation therapy alone. No difference in DSS was identified between modalities. Major complications occurred in 45% of patients; rates were significantly higher for combined surgery and irradiation (53%) versus surgery alone (25%) or irradiation alone (40%). The accompanying quality-of-life data found that patients treated with radiation therapy alone had a statistically higher ability to swallow, speak, and work compared with patients treated with primary surgery. The toxicity profile of surgery-based interventions drives the use of radiation therapy as the dominant treatment modality for early- to intermediate-stage base of tongue cancers, given the apparent equivalence in oncologic outcome.
Soft Palate Carcinomas
Carcinomas originating from the soft palate are a small minority of oropharyngeal cancers, occurring less frequently than cancer arising from the base of the tongue or tonsillar regions, and they typically account for 10% to 20% of the total cases.103 A higher prevalence is seen in studies evaluating early-stage (stage I and II) carcinomas of the oropharynx because soft palate carcinomas are less likely to be locally advanced or regionally metastatic and resultantly represent a higher percentage of the early-stage tumors.87 Because of the lower frequency of soft palate carcinomas, accumulation of large numbers of patients for studies is challenging. Generally, in patients who have not been previously irradiated, definitive irradiation is preferred to surgery. Surgical defects can lead to velopharyngeal incompetence, affecting both speech and swallowing. The drainage pattern from the soft palate can also include the retropharyngeal nodes, which are unaddressed in a standard neck dissection.
Chera and associates104 reviewed the University of Florida experience of 145 patients treated curatively with definitive irradiation for soft palate carcinomas between 1963 and 2004. Hyperfractionation was used in 42% of patients, an orthovoltage intraoral cone in 27%, and an interstitial implant in 6%. Thirteen percent had synchronous squamous cell carcinomas elsewhere in the upper aerodigestive tract. At 5 years, local control rates were T1, 90%; T2, 91%; T3, 67%; and T4, 57%. The OS at 5 years was 44%, with many patients dying of co-morbid disease and second primary tumors (CSS at 5 years was 73%). An overall treatment time of more than 47 days and a higher overall stage both adversely affected locoregional control rates, whereas nodal category affected OS. In an inter-era comparison, patients treated after 1985 had improved control rates (87% vs. 73%). Similar high rates of local control were identified in older single-institution studies. Keus and coauthors105 reported on 146 patients treated at Institut Curie for soft palate carcinoma, 71% with EBRT alone and 29% with an intraoral applicator. Patients receiving irradiation through the intraoral applicator had fewer normal tissue complications, although most patients receiving this had smaller primary tumors (stage T1 or T2). Local control rates at 3 years for T1 and T2 tumors were 92% and 70%, respectively. Neck disease recurred in 19 patients, of whom 9 had no component of local recurrence; reexamination of the treatment portals suggested a marginal miss in 7 patients. No dose response was ascertained in locally advanced tumors receiving between 60 and 75 Gy, leading the authors to recommend limiting the EBRT dose to 70 Gy to minimize complications.
Several French groups have described high locoregional control rates with a combination of interstitial brachytherapy and EBRT. Mazeron and colleagues106 described 5-year local control and nodal control of 91% and 95%, respectively, in patients treated with EBRT followed by a 192Ir implant for T1 and T2 soft palate carcinomas. The 5-year necrosis rate in this group was just under 20%. When compared with rates for patients treated with EBRT alone, both local control and OS were improved.
Lateral and Posterior Pharyngeal Wall Tumors
Both surgery and radiation therapy have been employed to treat pharyngeal wall cancers, and comparison of the two modalities is problematic because of the low incidence and inherent staging variability of these lesions as well as the limited amount of literature addressing them. Hull and associates107 reported on 148 patients with squamous cell carcinomas of the pharyngeal wall treated with definitive irradiation between 1964 and 2000; 93 lesions (63%) arose from the hypopharynx and 55 (37%) arose from the oropharynx. Local control rates at 5 years were T1, 93%; T2, 82%; T3, 59%; and T4, 50%. With surgical salvage, the local control rate increased to 87% for T2 tumors. Earlier-stage disease (stage I and II), an oropharyngeal primary site, and twice-daily fractionation were all associated with improved locoregional control. Treatment complications resulted in the deaths of 5% of the patients, and 16% of patients experienced severe complications, the most frequent of which was the need for a permanent gastrostomy tube.
Julieron and coauthors108 reviewed 77 patients treated surgically for posterior pharyngeal wall tumors, of which 22 required a total laryngectomy. For previously untreated patients, local failure occurred in 11% of individuals treated with surgery and adjuvant irradiation and in 18% with tumors larger than 4 cm. The total failure rate, including local, regional, and distant relapses and second primary tumors, was 50% in the primary surgery group. Nineteen percent continued to rely on a gastrostomy tube at 1 year. Local failure was seen in 52% of patients who had received prior radiation therapy, and 5-year OS was inferior to that of the nonrecurrent disease group; the authors concluded that conservative surgery should not be performed in the setting of postradiation failure.
Locally Advanced Disease and Palliation
Chemoradiation
Concurrent Chemoradiation
The benefit of concurrent chemotherapy added to locoregional treatment of locally advanced squamous cell carcinoma of the head and neck has been established by the Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC), a meta-analysis based on individual patient data, first published in 2000 and most recently updated in 2007 and 2009.109,110,111 The updated meta-analysis included 87 randomized trials that enrolled 16,485 patients comparing locoregional treatment versus the same locoregional treatment plus chemotherapy. Among these, 50 were concomitant trials that enrolled 9615 patients, with a median follow-up of 5.6 years. The hazard ratio of death was 0.88 (95% CI, 0.85 to 0.92) for the entire group, with the following hazard ratios of death for concomitant, induction, and adjuvant chemotherapy, respectively: 0.81 (95% CI, 0.78 to 0.86), 0.96 (95% CI, 0.90 to 1.02), and 1.06 (95% CI, 0.95 to 1.18). For the 50 concomitant trials, the absolute benefit for chemotherapy was 6.5% at 5 years. The proportion of deaths not resulting from head and neck cancer increased progressively with age from 15% in patients younger than 50 years to 39% in those aged 71 years and over. There is a concomitant diminishing benefit of chemotherapy on survival such that patients aged 71 years or older essentially derived no improvement at 5 years.111
Table 31-6 summarizes some of the recently published randomized phase III trials of concurrent chemoradiation using optimal EBRT schedules.112,113–116 In general, the addition of concurrent chemotherapy confers a survival benefit when it is combined with standard fractionation EBRT or with altered fractionation EBRT. Rates of acute toxicities such as mucosal, skin, and hematologic adverse events were either similar or slightly increased by the addition of concurrent chemotherapy, but no statistically significant differences in late toxicities have been reported in any of these studies.
TABLE 31-6 Recently Published Large, Randomized Phase III Trials of Concurrent Chemoradiation Containing Optimal Radiation Schedules
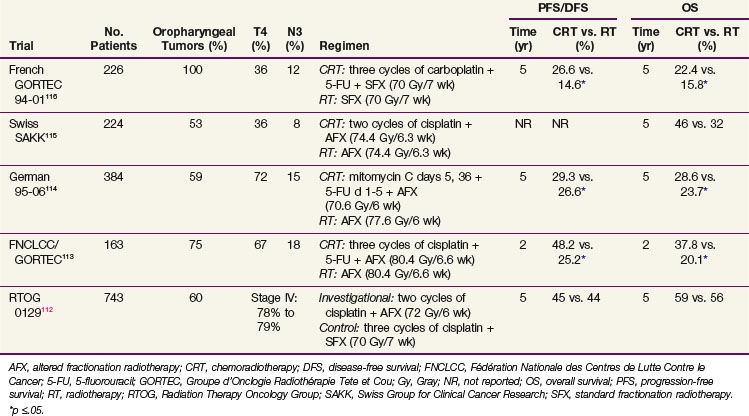
The RTOG 0129 trial is a randomized phase III trial that compared cisplatin 100 mg/m2 administered every 3 weeks for three cycles with standard fractionation EBRT, with cisplatin 100 mg/m2 administered every 3 weeks for two cycles with accelerated concomitant boost radiotherapy in 743 patients with stage III to IV SCC of the head and neck112 (see Table 31-6). The first analysis of this trial after a median time of 4.8 years has demonstrated no difference in outcomes or in rates of acute or late toxicities between the two arms. An interesting subgroup analysis of HPV status by in situ hybridization among 317 of 433 oropharyngeal cancers revealed that 61% of these cases were HPV-16-positive.117 Patients with HPV-positive oropharyngeal cancers had better OS than those with HPV-negative oropharyngeal cancers (p <.0001; 2-year OS of 87.5% [range, 82.8% to 92.2%] vs. 67.2% [range, 58.9% to 75.4%]) and better PFS (p <.0001; 2-year PFS of 71.9% [range, 65.5% to 78.2%] vs. 51.2% [range, 42.4% to 59.9%]). The hazard ratios of death for HPV-negative cases with 20 pack-years or more of smoking history, HPV-negative cases with less than 20 pack-years, and HPV-positive cases with 20 pack-years or more, were 4.33, 2.41, and 1.79, respectively, relative to HPV-positive cases with less than 20 pack-years (HR, 1). These results confirm that tumor HPV status is strongly associated with clinical outcome among oropharyngeal cancer patients receiving standard chemoradiation and that it should be incorporated as a stratification factor for future clinical trials, including oropharyngeal cases. In addition, the design of separate clinical trials based on tumor HPV status should be considered.
Sequential Chemoradiation
In the updated MACH-NC meta-analysis, individual patient data from 31 trials (5311 patients) of induction chemotherapy were analyzed, and they reported a nonsignificant 5-year absolute benefit of 2.4% in survival (HR, 0.96; 95% CI, 0.90 to 1.02).111 However, among trials that used induction regimens that contained a platinum and fluorouracil, the hazard ratio was more favorable at 0.90 (95% CI, 0.82 to 0.99). Induction chemotherapy appears to have some benefits in reducing distant metastases, but it has minimal effects on locoregional control rates.
Two large randomized, phase III trials comparing triplet (i.e., taxane plus platin plus 5-FU) versus doublet (platin plus 5-FU) induction chemotherapy, followed by concurrent chemoradiation, have been completed.118,119 Both the Madrid trial (Hitt and colleagues119) and the TAX 324 trial (Posner and associates118) demonstrated that the triplet therapy was associated with higher rates of complete response, improvements in time to treatment failure, PFS, and OS compared with the doublet therapy. Triplet therapy was associated with a higher rate of neutropenia with or without fever but with less mucositis than doublet therapy. The control arms in these two trials both used a sequential strategy, as opposed to the present standard of concurrent chemoradiation. Hence, until the results of ongoing randomized trials comparing sequential versus concurrent strategies are available, sequential therapy remains an investigational approach in locally advanced squamous cell carcinoma of the head and neck. Although the sequential strategy appears promising, comparisons of its therapeutic index and late toxic effects against current standards are needed.
The European Organization for Research and Treatment of Cancer (EORTC) 24971/TAX 323 trial compared triplet induction chemotherapy with docetaxel, cisplatin, and fluorouracil with doublet induction chemotherapy with cisplatin and fluorouracil, followed by radical irradiation (conventional, accelerated, or hyperfractionated) without concurrent chemotherapy, in patients with unresectable stage III and IV head and neck squamous cell carcinoma.120 Similar improvements in clinical outcome, and similar toxicity data, were reported by the EORTC 24971/TAX 323 and the Madrid and TAX 324 trials. Taken together, these trials suggest that as an induction regimen, triplet taxane-based therapy is superior to a doublet platinum-fluorouracil regimen, both in terms of efficacy and rates of most toxicities. However, as mentioned above, given the lack of comparative data against standard chemoradiation, sequential approaches should be used in the context of clinical trials.
Biologic Therapy
The Bonner study is a landmark study that compared EBRT alone (once-daily dose of 70 Gy in 35 fractions; twice-daily dose of 72 to 76.8 Gy in 60 to 64 fractions; concomitant boost of 72 Gy in 42 fractions) with EBRT plus the anti-EGFR monoclonal antibody cetuximab.121 This study proved a paradigm that the use of a biologic agent in combination with EBRT can improve survival rates for locoregionally advanced squamous cell carcinoma of the head and neck, and led to the approval of cetuximab by the U.S. Food and Drug Administration (FDA) in 2006 for this indication. The primary endpoint of the study was the duration of locoregional control, defined as the absence of progression of locoregional disease at the scheduled follow-up visits. The median duration of locoregional control was 24.4 months in the combined-therapy arm and 14.9 months in the radiotherapy alone arm (HR for locoregional progression or death, 0.68; 95% CI, 0.52 to 0.89; p = .005). The 2-year PFS, 5-year OS, and median survival durations were 46% versus 37%, 45.6% versus 36.4%, and 49 months versus 29.3 months (HR, 0.73; p = .018), respectively, favoring the combined-therapy arm.121,122 With the exception of acneiform rash and infusion reactions, the incidence of grade III or greater toxic effects, including mucositis, did not differ significantly between the two groups. Rates of grade III or IV late toxicities were similar between the two groups.
Management of the Neck
Adjuvant Radiation or Chemoradiation
Two large, multicenter, randomized phase III trials, EORTC 22931 and RTOG 9501, have evaluated the role of adjuvant high-dose cisplatin chemotherapy administered concomitantly on days 1, 22, and 43 with EBRT (60 to 66 Gy in 30 to 33 fractions) in the postoperative setting for patients with locally advanced squamous cell carcinoma of the head and neck123,124 (Table 31-7). Both trials have demonstrated statistically significant improvements in rates of locoregional control and DFS/PFS. The EORTC 22931 trial also demonstrated a significant benefit of postoperative chemoradiation for OS, whereas the RTOG 9501 trial only showed a trend in the same direction. The eligibility criteria of the two trials differed, and the overall patient population appeared to have somewhat worse baseline characteristics in the EORTC 22931 trial. For instance, there were fewer patients with oropharyngeal cancer (30% vs. 42%) and more with hypopharyngeal cancer (20% vs. 10%) in the EORTC 22931 trial than the RTOG 9501 trial. A meta-analysis of these two trials has since been published, identifying microscopically involved resection margins and extracapsular spread of tumor from neck nodes as the two most important prognostic factors for poor outcome.125 Patients with these risk factors appear to gain the most benefit from concurrent chemoradiation. The percentage of patients having one or both of these factors accounted for 70% and 59% of the EORTC 22931 and RTOG 9501 trials, respectively. Based on the comparative analysis, patients with one or both of the high-risk factors who are medically fit should be treated with postoperative chemoradiation, whereas radiation therapy alone should remain as the standard of care for those without these high-risk factors.
TABLE 31-7 Comparison of Two Concurrent Postoperative Radiation Plus Chemotherapy Trials (RTOG 9501 and EORTC 22931)
| Trial Characteristic | RTOG 9501 | EORTC 22931 |
|---|---|---|
| Sample Size | 459 randomized; 416 eligible | 334 |
| Primary Site | ||
| Oropharynx | 43% | 30% |
| Hypopharynx | 10% | 20% |
| Pathologic Risk Factors for inclusion | Positive resection margin; ≥2 nodes involved; extracapsular extension | Positive resection margin; extracapsular extension; perineural involvement; vascular tumor embolism; oral cavity or oropharyngeal tumors with involvement of level IV or V lymph nodes |
| High-risk Groups | ||
| Positive resection margin | 6% | 13% |
| Extracapsular extension | 49% | 41% |
| Both | 4% | 16% |
| Total | 59% | 70% |
| Outcome Endpoint, Chemoradiation vs. Radiation | 3-yr estimates | 5-yr estimates |
| Locoregional recurrence | 22% vs. 33%* | 18% vs. 31%* |
| Progression (disease)-free survival | 47% vs. 36%* | 47% vs. 36%* |
| Overall survival | 56% vs. 47% | 53% vs. 40%* |
| Median Follow-up | 45.9 mo | 60 mo |
EORTC, European Organization for Research and Treatment of Cancer; RTOG, Radiation Therapy Oncology Group.
The RTOG 0234 trial is a randomized phase III trial of surgery followed by chemoradiation plus cetuximab with either cisplatin or docetaxel in patients with stage III or IV squamous cell carcinoma of the head and neck with high-risk pathologic features, including either microscopically positive resection margins, two or more metastatic neck nodes, or extranodal capsular spread.126 Accrual of 238 patients to this trial, which evaluated two chemoadditive strategies, has been completed, and efficacy results are awaited.
In 2009, the RTOG activated a randomized phase III trial (RTOG 0920) comparing irradiation alone versus irradiation plus the anti-EGFR monoclonal antibody cetuximab in patients with locally advanced squamous cell carcinoma of the oral cavity, oropharynx, or larynx whose tumors exhibited one or more of the following intermediate-risk factors: perineural invasion; lymphovascular invasion; a single lymph node greater than 3 cm or two or more lymph nodes (all <6 cm) and no extracapsular extension; close margin(s) of resection (close margins defined as cancer extending to within 5 mm of a surgical margin); a T3 or T4a primary tumor; and T2 oral cavity cancer with more than 5 mm depth of invasion.127 The primary endpoint of the RTOG 0920 trial is the OS, with a target sample size of 700 patients, to detect the ability of cetuximab to produce a 26% reduction in the death rate when delivered in conjunction with postoperative radiotherapy.
Post-Radiation Neck Dissection
Current issues in the management of the neck in oropharyngeal squamous cell carcinomas revolve around the dose and volume of irradiation delivered to nodal regions and the role of posttreatment neck dissections. Before the IMRT era, a common approach was to deliver 50 Gy to the draining regional lymphatics followed by a boost to the nodal disease using photon or electron beam therapy to 60 Gy in patients presenting with palpable cervical lymph node metastases. Irradiation was subsequently followed by a post-radiation planned neck dissection. Reported neck control rates with this approach at the Memorial Sloan-Kettering Cancer Center were 96% overall and 100% in patients who went on to have neck dissection.128 Pathologic complete response was observed in 70% of patients. In the patients with residual disease, over half had a complete clinical response and 83% had multiple residual pathologically positive nodes. The downside of this approach is potential overtreatment of the neck; the patients with a pathologic complete response could have avoided neck dissection and the associated potential for complications and heightened late effects. The late side effects of neck dissection are not negligible; a meta-analysis of multiple RTOG studies showed that neck dissection after chemoradiation was one of the risk factors for severe late toxicity.129
Despite concern for related side effects, caution is warranted in choosing to forego a planned neck dissection in patients with N2 or higher nodal disease. Early, planned neck dissections have several distinct advantages. Most important are improvements in oncologic outcomes such as DSS and regional control.130 Most studies have found residual nodal disease in at least 20% of patients, with the caveat that pathologic presence following treatment does not establish cancer viability. Neck dissections performed during the 6 to 12 weeks of irradiation also take advantage of a window of time between the acute and late effects of radiation treatment. Although technically demanding in the post-radiation setting, complications requiring additional surgery are typically around 5%, although they may be increased by high doses and concurrent chemotherapy.131,132 Subcutaneous fibrosis is limited before the 12-week mark, allowing selective neck dissections to occur. In oropharyngeal cancer, a selective neck dissection involving levels II to IV is usually sufficient, although decision making regarding the neck dissection should be driven by pretreatment clinical and radiographic findings. In a primarily oropharyngeal cohort, residual nodal disease outside of levels II to IV was seen in less than 5% of patients following irradiation.133 Although salvage surgery can be performed in the undissected neck with recurrent disease, it carries a greater risk of complications, often requires a more extensive resection, and may lead to inferior regional control rates.
Evaluation for residual disease occurs 8 to 12 weeks following irradiation with a PET/CT scan, diagnostic neck CT scan, and clinical neck examination. No isolated nodal failures were identified in a prospective trial in which neck dissection was foregone in patients with N2 and N3 disease and clinical and radiographic complete responses.134 A radiographic complete response required that any previous lymphadenopathy measure less than 1 cm in maximum dimension and have no radiographically suspicious features. Of note, only 59% of patients with N2 and N3 disease had a clinical and radiologic complete response, and this dropped to 40% when considering N3 patients alone. Liauw and coauthors130 reported the University of Florida experience, which similarly found a negative predictive value of 94% for a radiographic complete response and no regional relapses in 34 patients with a radiographic complete response who did not undergo a post-radiation neck dissection.
Salvage Surgery
A meta-analysis of 1080 patients treated with salvage surgery from 28 institutions showed a 5-year OS of 39%, with this better than expected outcome buoyed by the large number of recurrent early-stage laryngeal cancers.135 Pharyngeal primaries in this meta-analysis had a 2-year DFS of 25% and a 5-year OS of 26%. The operative mortality rate was 5.2%, with a complication rate of 39% and a major complication rate of 27%. Surgical complication rates appear to be significantly increased following primary chemoradiation compared with irradiation alone.136 A prospective trial that accompanied the meta-analysis found that OS declines progressively with a more advanced recurrent stage (24.3 months for stage I disease vs. 9.3 months for stage IV disease) and the prior use of chemotherapy (26.9 months vs. 8.8 months with prior chemotherapy).135 The author concluded that stage I and II recurrent cancer patients had a 70% chance of being disease-free at 2 years and a 60% to 85% probability of a successful outcome relative to quality of life and a low surgical complication rate. On the other hand, recurrent cancer patients that initially had stage IV lesions had a less than 25% chance of being disease-free at 2 years, with disease recurring in half of these patients within 5 months. Significant complications were present in 30% of stage IV salvage surgeries, and 30% of patients had a good quality-of-life outcome.
Bachar and colleagues137 reviewed the largest single-institution oropharynx salvage experience, in which 239 out of 640 patients treated definitively at the Princess Margaret Hospital with radiation for tonsillar carcinomas had subsequent recurrence. Of the 239, 175 (73.5%) were surgical salvage candidates. The median time to death was 1.3 years, and 5-year CSS and OS were 40% and 23%, respectively. The majority of patients died with disease. Both the T and N categories were predictors of time to death. Although the prognosis was poor overall for recurrent disease, the survivorship of 23% of patients at 5 years defines a finite group of patients cured by salvage surgery and argues in favor of a salvage surgical procedure when feasible.
Reirradiation
In attempting to determine which patients might benefit from repeating full-dose irradiation to the head and neck, some have warned that “experience has shown that indiscriminate use of high-dose reirradiation often makes a bad matter worse.”138 Reirradiation has been pursued at some centers in the situation of a patient with good performance status, locoregional recurrence, and no other potentially curative treatment options. Appropriate patient selection is key, and patients should be fully informed of the increased complication rate incurred by cumulative radiation doses frequently topping 100 Gy.
Investigations at several academic centers have demonstrated that between 10% and 20% of selected patients who are reirradiated can be long-term survivors. The University of Chicago reported on 115 patients who underwent reirradiation with a median lifetime dose of 131 Gy, including 30 patients with oropharyngeal carcinoma.139 Radiation was delivered with concurrent chemotherapy as part of seven consecutive phase I and II protocols in which radiation was given for five fractions every other week. Median OS and DFS were limited at 11 and 7 months, respectively. A subset of patients had long-term benefits from the repeat treatment, however, as 3-year OS, PFS, and locoregional control were 22%, 33%, and 51%, respectively. Improved outcomes were seen with higher doses of radiation (>58 Gy), multiagent chemotherapy, and surgery before repeat radiation. Conversely, only 11% of patients treated with repeat radiation alone were alive at 3 years. Side effects were substantial, with 19 patients (16.5%) dying of treatment-related complications and another 13 (11%) requiring surgery for osteoradionecrosis of the mandible.
The inability to undergo surgery as a negative prognostic indicator was reinforced by the RTOG 9610 clinical trial, which delivered 60 Gy at 1.5 Gy twice daily with concurrent chemotherapy. Patients were ineligible for surgical resection, and 2-year and 5-year OS were 15.2% and 3.8%, respectively. A follow-up study (RTOG 9911) with concurrent cisplatin and paclitaxel found 2-year OS of 25.9%, which exceeded the results generally seen with chemotherapy alone, although 8% of patients still died of treatment-related complications.140
The side effects of reirradiation may be partially tempered by employing highly conformal treatment techniques. An M.D. Anderson Cancer Center 5-year experience of 74 patients reirradiated with IMRT reported a 20% rate of major toxic events, defined as hospitalization or corrective surgery, and one patient death.141 The median lifetime radiation dose was 116.1 Gy, and 41% of the lesions were oropharyngeal in origin. Two-year OS and locoregional control were encouraging at 58% and 64%, respectively. Salvage surgical resection had been previously performed in 27% of patients, and 81% of patients were treated for gross disease with margins only. Locoregional failure rates decreased with IMRT in the Memorial Sloan-Kettering Cancer Center experience, which described 2-year OS of 37% and a severe late complication rate of 11.4%.142
Brachytherapy143 and intraoperative irradiation (IORT)144 can also be used in the setting of focal reirradiation with reasonable local control. IORT as a treatment option is discussed further in the Head and Neck Overview section of the textbook.
Janot and associates145 reported on a randomized trial evaluating reirradiation in patients who had recurrent disease or a second primary tumor in a previously irradiated region, no major sequelae from the prior treatment, a good performance status, no distant metastases, and salvage surgery with macroscopic complete resection. The radiation dose was 60 Gy over 11 weeks (five fractions, followed by the next week off) with concurrent 5-FU and hydroxyurea. Repeat irradiation improved the DFS (HR, 1.68) but did not affect OS. Grade 3 or 4 late toxicities at 2 years were 39% in the radiation therapy arm and 10% in the observation arm (p = .06).
Recurrent or Metastatic Disease
Cytotoxic Chemotherapy
Large randomized clinical trials that spanned the last two decades evaluating cytotoxic chemotherapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck have demonstrated little progress; the median survival time remains only 6 to 8 months. Single agents such as methotrexate typically yield lower objective response rates (≈10%) compared with cytotoxic combinations (≈25% to 30%), but the latter have not demonstrated survival benefits in this patient population.146,147 Cytotoxic chemotherapy, especially when given as doublets or triplets, can cause significant toxicity, and its effects on the quality of life in recurrent and/or metastatic disease have not been well studied. Intensification of chemotherapeutic regimens, such as the use of high-dose paclitaxel in combination with cisplatin and growth factor support in the ECOG study E1393, has led to excessive toxicity and failed to improve survival outcomes.148 Such observations have motivated oncologists to continue to seek novel treatments to improve disease control and palliation for these patients.
Molecularly Targeted Agents
The biologic relevance of EGFR in squamous cell carcinoma of the head and neck is validated not only through clinicopathologic correlations149 but also by the results of the Bonner trial, which demonstrated improvements in both locoregional control and overall survival when cetuximab was added to definitive EBRT in locally advanced disease.121 Web-only Table 31-1![]() summarizes the results of published phase II and III clinical trials of anti-EGFR molecularly targeted therapies in recurrent or metastatic squamous cell carcinoma of the head and neck. Overall, large molecules such as monoclonal antibodies appear to have a slight advantage in terms of higher objective response rates (10% to 13%) than small molecule tyrosine kinase inhibitors (≈2% to 10.6%) in this patient population.
summarizes the results of published phase II and III clinical trials of anti-EGFR molecularly targeted therapies in recurrent or metastatic squamous cell carcinoma of the head and neck. Overall, large molecules such as monoclonal antibodies appear to have a slight advantage in terms of higher objective response rates (10% to 13%) than small molecule tyrosine kinase inhibitors (≈2% to 10.6%) in this patient population.
WEB-ONLY TABLE 31-1 Clinical Trials of Anti-EGFR Molecularly Targeted Therapies in Recurrent or Metastatic SCCHN
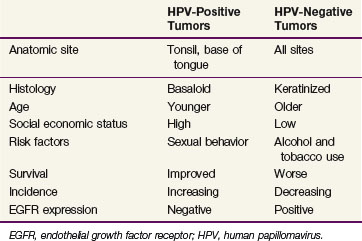
Vermorken and coauthors120 evaluated single-agent cetuximab in a multicenter phase II trial enrolling 103 recurrent or metastatic SCC patients that had progressed with platinum-based chemotherapy. The objective response rate for treatment with cetuximab alone was 13% (95% CI, 7% to 21%) and the stable disease rate was 33%. The median time to progression and overall survival time were 2.3 and 6 months, respectively. This trial formed the basis for regulatory approval of cetuximab as monotherapy by the FDA for this indication.120
The recently published EXTREME trial, which compared a 5-FU–plus-platinum combination with or without cetuximab in previously untreated patients with recurrent or metastatic squamous cell carcinoma of the head and neck, demonstrated benefits in rates of overall objective response, PFS, and OS with the addition of cetuximab. However, increased incidences of grade 3 or 4 sepsis, skin reactions, anorexia, and hypomagnesemia were encountered in the cetuximab arm.150 Other molecular targets besides EGFR, such as the antiangiogenic, PI3-kinase-Akt-mTOR, cancer stem cell pathways, amongst others, are also being interrogated in the attempt to continue to identify promising therapeutic targets in head and neck squamous cell carcinoma.