Chapter 23 Organ Protection during Cardiopulmonary Bypass
CENTRAL NERVOUS SYSTEM INJURY
Incidence and Significance of Injury
Although the incidence of this dysfunction varies greatly, the significance of these injuries cannot be overemphasized. Cerebral injury is a most disturbing outcome of cardiac surgery. To have a patient’s heart successfully treated by the planned operation but discover that the patient no longer functions as well cognitively or is immobilized from a stroke can be devastating. There are enormous personal, family, and financial consequences of extending a patient’s life with surgery, only to have the quality of the life significantly diminished. Mortality after CABG, although having reached relatively low levels in the past decade (approximately 1% overall), is increasingly attributable to cerebral injury.1
Risk Factors for Central Nervous System Injury
Stroke is better characterized with respect to risk factors. Although studies differ somewhat as to all the risk factors, certain patient characteristics consistently correlate with an increased risk for cardiac surgery–associated neurologic injury. In a study of 2108 patients from 24 centers in a study conducted by the Multicenter Study of Perioperative Ischemia, incidence of adverse cerebral outcome after CABG surgery was determined and the risk factors analyzed.2 Two types of adverse cerebral outcomes were defined. Type I included nonfatal stroke, transient ischemic attack (TIA), stupor or coma at time of discharge, and death caused by stroke or hypoxic encephalopathy. Type II included new deterioration in intellectual function, confusion, agitation, disorientation, and memory deficit without evidence of focal injury. A total of 129 (6.1%) of the 2108 patients had an adverse cerebral outcome in the perioperative period. Type I outcomes occurred in 66 (3.1%) of 2108 patients, with type II outcomes occurring in 63 (3.0%) of 2108 patients. Stepwise logistic regression analysis identified eight independent predictors of type I outcomes and seven independent predictors of type II outcomes (Table 23-1).
Table 23-1 Risk Factors for Adverse Cerebral Outcomes after Cardiac Surgery
| Risk Factor | Type I Outcomes | Type II Outcomes |
|---|---|---|
| Proximal aortic atherosclerosis | 4.52 [2.52 to 8.09]* | |
| History of neurologic disease | 3.19 [1.65 to 6.15] | |
| Use of IABP | 2.60 [1.21 to 5.58] | |
| Diabetes mellitus | 2.59 [1.46 to 4.60] | |
| History of hypertension | 2.31 [1.20 to 4.47] | |
| History of pulmonary disease | 2.09 [1.14 to 3.85] | 2.37 [1.34 to 4.18] |
| History of unstable angina | 1.83 [1.03 to 3.27] | |
| Age (per additional decade) | 1.75 [1.27 to 2.43] | 2.20 [1.60 to 3.02] |
| Admission systolic BP > 180 mm Hg | 3.47 [1.41 to 8.55] | |
| History of excessive alcohol intake | 2.64 [1.27 to 5.47] | |
| History of CABG | 2.18 [1.14 to 4.17] | |
| Arrhythmia on day of surgery | 1.97 [1.12 to 3.46] | |
| Antihypertensive therapy | 1.78 [1.02 to 3.10] |
BP = blood pressure; CABG = coronary artery bypass graft surgery; IABP = intra-aortic balloon pump.
* Adjusted odds ratio [95% confidence intervals] for type I and type II cerebral outcomes associated with selected risk factors from the Multicenter Study of Perioperative Ischemia.
From Arrowsmith JE, Grocott HP, Reves JG, et al: Central nervous system complications of cardiac surgery. Br J Anaesth 84:378, 2000.
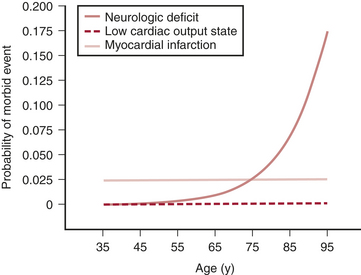
Of all the factors in the Multicenter Study of Perioperative Ischemia analysis, age appears to be the most overwhelmingly robust predictor of stroke and of neurocognitive dysfunction after cardiac surgery. Age has a greater impact on neurologic outcome than it does on perioperative myocardial infarction or low cardiac output states after cardiac surgery (Fig. 23-1).
Atheromatous disease of the ascending, arch, and descending thoracic aorta has been consistently implicated as a risk factor for stroke in cardiac surgical patients. The increased use of transesophageal echocardiography (TEE) and epiaortic ultrasonography has added new dimensions to the detection of aortic atheromatous disease and the understanding of its relation to stroke risk. These imaging modalities have allowed the diagnosis of atheromatous disease to be made in a more sensitive and detailed manner, contributing greatly to the information regarding potential stroke risk. Studies have consistently reported higher stroke rates for patients with increasing atheromatous aortic involvement (particularly the ascending and arch segments). This relationship is outlined in Figure 23-2.
Cause of Perioperative Central Nervous System Injury
Because central nervous system dysfunction represents a wide range of injuries, differentiating the individual causes of these different types of injuries becomes somewhat difficult (Box 23-1). They are frequently grouped together and superficially discussed as representing different severities on a continuum of similar injury. This likely misrepresents the different causes of these injuries. The following section addresses stroke and cognitive injury (Table 23-2).
Table 23-2 Causes of Cognitive Dysfunction after Cardiac Surgery
| Cause | Possible Settings |
|---|---|
| Cerebral microemboli | Generated during cardiopulmonary bypass (CPB); mobilization of atheromatous material or entrainment of air from the operative field; gas injections into the venous reservoir of the CPB apparatus |
| Global cerebral hypoperfusion | Hypotension, occlusion by an atheromatous embolus leading to stroke |
| Inflammation (systemic and cerebral) | Injurious effects of CPB, such as blood interacting with the foreign surfaces of pump-oxygenator; upregulation of proinflammatory cyclooxygenase mRNA |
| Cerebral hyperthermia | Hypothermia during CPB; hyperthermia during and after cardiac surgery, such as aggressive rewarming |
| Cerebral edema | Edema from global cerebral hypoperfusion or from hyponatremia; increased cerebral venous pressure from cannula misplacement |
| Blood-brain barrier dysfunction | Diffuse cerebral inflammation; ischemia from cerebral microembolization or increased intracranial pressure |
| Pharmacologic influences | Anesthetic-related cognitive damage; necrosis of neonatal brains; proteomic changes |
| Genetic influences | Effects of single nucleotide polymorphisms on risk for Alzheimer’s disease or for acute coronary syndromes and other thrombotic disorders |
Cerebral Embolization
Macroemboli (e.g., atheromatous plaque) and microemboli (i.e., gaseous and particulate) are generated during CPB, and many emboli find their way to the cerebral vasculature. Macroemboli are responsible for stroke, and microemboli are fundamental to the development of neurocognitive dysfunction. Sources for the microemboli are numerous and include those generated de novo from the interactions of blood within the CPB apparatus (e.g., platelet-fibrin aggregates) and those generated within the body by the production and mobilization of atheromatous material or entrainment of air from the operative field.3
Neuroprotective Strategies
Management of Aortic Atherosclerosis
A combination of epiaortic scanning and atheroma avoidance techniques (with respect to cannulation, clamping, and vein graft anastomosis placement) have been used to attempt to reduce neurocognitive deficits.4 The incidence of cognitive decline may be lower in patients who had an avoidance technique guided by epiaortic scanning compared with no epiaortic scanning. It is an area that requires more investigation. One of the difficulties in interpreting studies that have evaluated atheroma avoidance strategies is the absence of any form of blinding of the investigators. For the most part, a strategy is chosen based on the presence of known atheroma, and the results of these patients are compared with historical controls. Multiple techniques can be used to minimize atheromatous material liberated from the aortic wall from getting into the cerebral circulation. These range from optimizing placement of the aortic cannula in the aorta to an area relatively devoid of plaque to the use of specialized cannulas that reduce the sandblasting of the aortic wall. Alternative aortic cannulas and using different locations possess the ability to decrease embolization of atheromatous plaque. The avoidance of partial occlusion clamping for proximal vein graft anastomosis using a single-step automated anastomotic device and the use of alternatives to cross-clamping all possess the ability to mitigate injury due to embolization.
Acid-Base Management: α-Stat versus pH-Stat
Optimal acid-base management during CPB has long been debated. Theoretically, α-stat management maintains normal CBF autoregulation with the coupling of cerebral metabolism (CMRO2) to CBF, allowing adequate oxygen delivery while minimizing the potential for emboli. Although early studies were unable to document a difference in neurologic or neuropsychologic outcome between the two techniques, later studies showed reductions in cognitive performance when pH-stat management was used, particularly in cases with prolonged CPB times. pH-stat management (i.e., CO2 is added to the fresh oxygenator gas flow) results in a higher CBF than is needed for the brain’s metabolic requirements. This luxury perfusion risks excessive delivery of emboli to the brain. Except for congenital heart surgery, for which most outcome data support the use of pH-stat management due to its improvement in homogenous brain cooling before circulatory arrest, adult outcome data support the use of α-stat management.5
Glucose Management
The appropriate type of perioperative serum glucose management and whether it adversely affects neurologic outcome in patients undergoing CPB remain unclear. The major difficulty in hyperglycemia treatment is the relative ineffectiveness of insulin therapy. Using excessive amounts of insulin during hypothermic periods may lead to rebound hypoglycemia after CPB. Chaney and associates6 attempted to maintain normoglycemia during cardiac surgery with the use of an insulin protocol and came to the conclusion that even with aggressive insulin treatment, hyperglycemia is often resistant and may actually predispose to postoperative hypoglycemia. Attempting to mediate injury may actually predispose to additional injury.
ACUTE RENAL INJURY
Despite the recognition, made more than 40 years ago, of acute renal injury as a serious complication of cardiac surgery, postoperative renal dysfunction persists today as an independent predictor of postoperative mortality. Even during procedures where there is no evidence of acute renal injury as reflected by a rise in serum creatinine, more subtle markers have demonstrated renal tubular injury. Patients with increasing degrees of acute renal injury after cardiac surgery require disproportionately more short- and long-term resources and have increasingly poorer outcome and greater costs.7 The association of acute renal dysfunction with mortality and major morbidity may reflect its role as a marker of, rather than as a contributor to, the insult of cardiac surgery; however, there is compelling evidence that acute renal injury itself also contributes directly to adverse outcome. Accumulation of “uremic toxins” and release of inflammatory mediators from the injured kidney also appear to contribute significantly to remote organ dysfunction.
Incidence and Significance
Definitions and the incidence of renal injury vary, with approximately 8% to 15% of cardiac surgery patients sustaining a moderate renal injury (defined by a peak rise in the serum creatinine level of more than 1.0 mg/dL) and 1% to 5% requiring dialysis. Each type of cardiac surgical procedure has its own specific pattern of renal injury. As a result, surgical procedure type is an important variable when examining the incidence and severity of postoperative acute renal injury. For example, elevation of the serum creatinine level, a common marker of acute renal injury, typically peaks on the second day after CABG with CPB and, in most cases, returns to baseline by postoperative day 4 or 5.8
Risk Factors for Surgery-Related Acute Renal Injury
Numerous studies have characterized risk factors for nephropathy after cardiac surgery (Table 23-3). Preoperative variables are most useful for renal risk stratification with intraoperative and postoperative issues contributing to overall clinical management decisions. Equivalent renal injury for a patient with preoperative renal dysfunction does result in a greater rise in serum creatinine due to the nonlinear relationship between reductions in glomerular filtration rate (GFR) and creatinine rise. Even small amounts of additional renal impairment may lead to dialysis for a surgical patient with severe renal disease at baseline.
| Important Factors | Probably Not Important |
|---|---|
| Preoperative Factors | |
| Advanced age | Gender |
| Obesity | Renal artery stenosis |
| Preexisting renal insufficiency | |
| Diabetes | |
| Hyperglycemia | |
| Hypertension | |
| Ascending aortic atherosclerosis | |
| Peripheral vascular disease | |
| IABP | |
| COPD | |
| Loop diuretic therapy | |
| Corticosteroid therapy | |
| Nephrotoxins (radiocontrast dye, cyclosporin, aminoglycosides, cephalosporins, NSAIDs) | |
| Genetic variants | |
| Intraoperative Factors | |
| Aprotinin | Lysine analogs |
| Loop diuretic (increased risk) | Low-dose dopamine |
| Transfusion | Mannitol |
| Hyperglycemia | |
| Procedure type (CABG < valve < HCA) | |
| Emergency procedure | |
| Reoperation | |
| IABP | |
| CPB management | CPB management |
| Extended CPB duration | Hypothermia |
| Hemodilution | Blood pressure management |
| Postoperative Factors | |
| Low cardiac output | |
| Excessive inotrope use | |
| Prolonged ventilation | |
| Transfusion | |
| Sepsis | |
| CMV infection | |
CABG = coronary artery bypass graft surgery; CPB = cardiopulmonary bypass; CMV = controlled mechanical ventilation; COPD = chronic obstructive pulmonary disease; HCA = hypothermic circulatory arrest; IABP = intra-aortic balloon pump; NSAIDs = nonsteroidal anti-inflammatory drugs.
Causes and Pathophysiology of Renal Injury
Ischemia-Reperfusion Injury
A long-standing assumption regarding the causes and pathophysiology of renal dysfunction after cardiac surgery is that hypoperfusion with resulting renal ischemia plays a central role (Box 23-2). This assumption is supported by an understanding of the precarious oxygen supply and demand physiology of the renal medulla, particularly during CPB, as well as the responsiveness of experimental acute renal injury to improved renal perfusion. However, clinically applied techniques directed at improving perfusion (e.g., including low-dose dopamine) and reducing oxygen consumption (e.g., loop diuretics) have been uniformly unsuccessful in cardiac surgical patients and in most other settings. Other risk factors supporting the ischemia-reperfusion hypothesis of acute renal injury and nephropathy after cardiac surgery include episodes of circulatory arrest and perioperative low cardiac output states and prolonged bypass duration.9
Drug-Related Nephropathy
Concern continues regarding the use of antifibrinolytic agents during cardiac surgery. Benign proximal tubule effects of lysine analog agents cause “tubular proteinuria” in cardiac surgery patients; however, no change in the incidence of acute renal injury has been documented in longitudinal studies examining institutional rates of renal injury before and after periods of ε-aminocaproic acid use.10 Aprotinin also interacts with the proximal renal tubule and is thought to saturate tubular transport mechanisms, resulting in tubular proteinuria. Aprotinin has been implicated in postoperative renal dysfunction after cardiac surgery.11
Strategies for Renal Protection
Pharmacologic Intervention
dopamine
Mesenteric dopamine-1 (D1) receptor agonists increase renal blood flow, decrease renal vascular resistance, and enhance natriuresis and diuresis. Despite the absence of clinical evidence of renoprotection, this rationale has been used to justify the use of low-dose (“renal-dose”) dopamine (<5 μg/kg/min) for decades. Despite the lack of benefit and accumulating concerns regarding the use of low-dose dopamine, continued use of this agent for renoprotection is prevalent.12
fenoldopam
Fenoldopam mesylate, a benzazepine derivative, is a selective D1-receptor agonist. Although first approved as an antihypertensive agent, fenoldopam has shown promise in the prevention of contrast-induced nephropathy. A recent meta-analysis by Landoni et al provides evidence that fenoldopam may confer significant benefits in preventing renal replacement therapy and reducing mortality in patients undergoing cardiovascular surgery.13
MYOCARDIAL INJURY
Incidence and Significance of Myocardial Dysfunction after Cardiopulmonary Bypass
Unlike other organs at risk of damage during cardiac surgery, it is assumed, owing to the very nature of the target of the operation being performed, that all patients having cardiac surgery suffer some degree of myocardial injury. Although the injury can be subclinical, represented only by otherwise asymptomatic elevations in cardiac enzymes, it frequently manifests more overtly. The degree to which these enzymes are released by injured myocardium, frequently to levels sufficiently high to satisfy criteria for myocardial infarction, has been related to perioperative outcome after cardiac surgery. CK-MB values more than 10 times the upper limit of normal during the initial 48 hours after CABG were significantly associated with 6-month mortality (P < .001).14
Risk Factors for Myocardial Injury
With an increasingly sicker cohort of patients presenting for cardiac surgery,15 many with acute ischemic syndromes (e.g., often with evolving myocardial infarction) or significant left ventricular dysfunction, the need has never been greater for optimizing myocardial protection to minimize the myocardial dysfunction consequent to aortic cross-clamping and cardioplegia. The continued increase in cardiac transplantation and other complex surgeries in the heart failure patient has served to fuel the search for better myocardial protection strategies.
Myocardial Protection during Cardiac Surgery: Cardioplegia
Optimizing the metabolic state of the myocardium is fundamental to preserving its integrity. The major effects of temperature and functional activity (i.e., contractile and electrical work) on the metabolic rate of myocardium have been extensively described.16 With the institution of CPB, the emptying of the heart significantly reduces contractile work and myocardial oxygen consumption 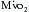 . Nullifying this cardiac work reduces the
. Nullifying this cardiac work reduces the 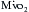 by 30% to 60%. With subsequent reductions in temperature, the
by 30% to 60%. With subsequent reductions in temperature, the 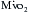 further decreases, and with induction of cardiac arrest and hypothermia, 90% of the metabolic requirements of the heart can be reduced. Temperature reductions diminish metabolic rate for all electromechanical states (i.e., beating or fibrillating) of the myocardium.
further decreases, and with induction of cardiac arrest and hypothermia, 90% of the metabolic requirements of the heart can be reduced. Temperature reductions diminish metabolic rate for all electromechanical states (i.e., beating or fibrillating) of the myocardium.
Composition of Cardioplegia Solutions
The composition of the various cardioplegia solutions used during cardiac surgery varies as much between institutions as it does between individual surgeons. In very general terms, cardioplegia can be classified into blood-containing and non–blood-containing (i.e., crystalloid) solutions. Table 23-4 outlines the various additives to cardioplegia solutions along with their corresponding rationale for use. Although all cardioplegia solutions contain higher than physiologic levels of potassium, solutions used for the induction of diastolic arrest contain the highest concentrations of potassium as opposed to solutions used for the maintenance of cardioplegia. In addition to adjustment of electrolytes, manipulation of buffers (e.g., bicarbonate, tromethamine), osmotic agents (e.g., glucose, mannitol, potassium), and metabolic substrates (e.g., glucose, glutamate, and aspartate) constitutes the most common variations in cardioplegia content.
Table 23-4 Strategies for the Reduction of Ischemic Injury with Cardioplegia
| Principle | Mechanism | Component |
|---|---|---|
| Reduce O2 demand | Hypothermia | Blood, crystalloid, ice slush, lavage |
| Perfusion | ||
| Topical/lavage | ||
| Asystole | KCl, adenosine(?), hyperpolarizing agents | |
| Substrate supply and use | Oxygen | Blood, perfluorocarbons, crystalloid(?) |
| Glucose | Blood, glucose, citrate-phosphate-dextrose | |
| Amino acids | Glutamate, aspartate | |
| Buffer acidosis | Hypothermia (Rosenthal factor), intermittent infusions | |
| Buffers | Blood, tromethamine, histidine, bicarbonate, phosphate | |
| Optimize metabolism | Warm induction (37°C), warm reperfusion | |
| Reduce Ca2+ overload | Hypocalcemia | Citrate, Ca2+ channel blockers, K channel openers(?) |
| Reduce edema | Hyperosmolarity | Glucose, KCl, mannitol |
| Moderate infusion pressure | 50 mmHg |
From Vinten-Johansen J, Thourani VH: Myocardial protection: An overview. J Extra Corpor Technol 32:38, 2000.
Cardioplegia Delivery Routes
Retrograde cardioplegia, where a cardioplegia catheter is introduced into the coronary sinus, allows for almost continuous cardioplegia administration. Retrograde delivery is useful in settings where antegrade cardioplegia is problematic, such as with severe aortic insufficiency or during aortic root or aortic valve surgery (Box 23-3). It also allows the distribution of cardioplegia to areas of myocardium supplied by significantly stenosed coronary vessels. Retrograde cardioplegia has proved safe and effective for cardioplegia in patients with coronary artery disease and in those undergoing valve surgery. With the administration of retrograde cardioplegia, certain provisos should be considered. The acceptable perfusion pressure to limit perivascular edema and hemorrhage needs to be limited to less than 40 mmHg.17
GASTROINTESTINAL COMPLICATIONS
Incidence and Significance
Gastrointestinal complications after cardiac surgery, although occurring relatively infrequently (0.5% to 5.5%), portend a significantly increased incidence of overall adverse patient outcome. The variability in the reported incidence of gastrointestinal complications is partly a reflection of how they are defined and the variable patient and operative risk factors in the studied cohorts. Although the most commonly considered gastrointestinal complications include pancreatitis, gastrointestinal bleeding, cholecystitis, and bowel perforation or infarction, hyperbilirubinemia (total bilirubinemia > 3.0 mL/dL) has also been described as an important complication after cardiac surgery. In one of the largest prospective studies examining these complications after CPB, McSweeney and associates studied 2417 patients undergoing CABG (with or without concurrent intracardiac procedures) in a multicenter study in the United States. The overall incidence of gastrointestinal complications in this study was 5.5%, ranging from 3.7% for hyperbilirubinemia to 0.1% for major bowel perforation or infarction.18
Risk Factors
A long list of preoperative, intraoperative, and postoperative risk factors for gastrointestinal complications have been identified in a number of studies.19 As many factors are associated with one another, it is only when these risk factors are examined in multivariable analyses that a more accurate understanding of what the most significant risk factors for visceral complications after cardiac surgery are. Preoperatively, age (>75 years), history of congestive heart failure, presence of hyperbilirubinemia (>1.2 mg/dL), combined cardiac procedures (e.g., CABG plus valve), repeat cardiac operation, preoperative ejection fraction less than 40%, preoperative elevations in partial thromboplastin time, emergency operations; intraoperatively, prolonged CPB, use of TEE, and blood transfusion; and postoperatively, requirements for prolonged inotropic vasopressor support, IABP use for the treatment of low cardiac output; and prolonged ventilatory support are all risk factors. These factors identify patients at high risk, and they lend some credence to the overall pathophysiology and suspected causes of these adverse events. If there is a common link between all these risks, it is that many of these factors would be associated with impairment in oxygen delivery to the splanchnic bed.
Protecting the Gastrointestinal Tract during Cardiac Surgery
As with other aspects of organ protection, critical etiologic factors need to be addressed with specific targeted therapies (Box 23-4). Unfortunately, as with most other organ-protective strategies, the major limitation in making definitive recommendations is an overall lack of large, well-controlled, prospectively randomized studies to provide supportive data for any one particular technique.
Off-Pump Cardiac Surgery
There is little evidence that the use of off-pump cardiac surgery is in any way beneficial to the gastrointestinal tract. Three retrospective studies20 have shown no differences in gastrointestinal complications. One reason for this lack of apparent difference between on-pump and off-pump cardiac surgery may again be related to the common denominator of splanchnic perfusion. OPCAB is fraught with hemodynamic compromise that may lead to prolonged periods of splanchnic hypoperfusion by itself or as a result of the concurrent administration of vasopressors to maintain normal hemodynamics during the frequent manipulations of the heart.
LUNG INJURY DURING CARDIAC SURGERY
Incidence and Significance
Pulmonary dysfunction was one of the earliest recognized complications of cardiac surgery employing CPB.21 However, as improvements in operative technique and CPB perfusion technologies occurred, the overall frequency and severity of this complication decreased. Juxtaposed to the improvements in cardiac surgery, which led to an overall reduction in complications, is an evolving patient population that now comprises a higher-risk group with a higher degree of pulmonary comorbidities, increasing their risks of postoperative pulmonary dysfunction. With the advent of fast-track techniques, even minor degrees of pulmonary dysfunction have reemerged as significant contributors to patient morbidity and the potential need for extended postoperative ventilation. As with most postoperative organ dysfunction, there is a range of dysfunction severity. Arguably, some degree of pulmonary dysfunction occurs in most patients after cardiac surgery; however, it manifests clinically only when the degree of dysfunction is particularly severe or the pulmonary reserve is significantly impaired. As a result, even minor CPB-related pulmonary dysfunction can cause significant problems in some patients.
Pulmonary Protection
Ventilatory Strategies
The inspired oxygen content of the gases that the lungs see during the period of apnea during CPB may have an effect on the A-a DO2 gradient, probably because of the enhanced effect of higher FIO2 on the ability of atelectasis (so-called absorption atelectasis) on these gradients. With these findings in mind, it would be prudent to reduce the FIO2 to room air levels during CPB. Several simple therapies can be introduced before separation from CPB, including adequate tracheobronchial toilet and the delivery of several vital capacity breaths that may reduce the amount of atelectasis that has occurred during bypass (Box 23-5).
SUMMARY
1. Newman M.F., Grocott H.P., Mathew J.P., et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874.
2. Arrowsmith J.E., Grocott H.P., Reves J.G., et al. Central nervous system complications of cardiac surgery. Br J Anaesth. 2000;84:378.
3. Djaiani G., Fedorko L., Borger M., et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356.
4. Bar-Yosef S., Anders M., Mackensen G.B., et al. Aortic atheroma burden and cognitive dysfunction after coronary artery bypass graft surgery. Ann Thorac Surg. 2004;78:1556.
5. Duebener L.F., Hagino I., Sakamoto T., et al. Effects of pH management during deep hypothermic bypass on cerebral microcirculation: Alpha-stat versus pH-stat. Circulation. 2002;106(suppl 1):I103.
6. Chaney M.A., Nikolov M.P., Blakeman B.P., et al. Attempting to maintain normoglycemia during cardiopulmonary bypass with insulin may initiate postoperative hypoglycemia. Anesth Analg. 1999;89:1091.
7. Stafford-Smith M., Phillips-Bute B., Reddan D.N., et al. The association of postoperative peak and fractional change in serum creatinine with mortality after coronary bypass surgery. Anesthesiology. 2000;93:A240.
8. Ostermann M.E., Taube D., Morgan C.J., et al. Acute renal failure following cardiopulmonary bypass: A changing picture. Intensive Care Med. 2000;26:565.
9. Sreeram G.M., Grocott H.P., White W.D., et al. Transcranial Doppler emboli count predicts rise in creatinine after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2004;18:548.
10. Stafford-Smith M., Phillips-Bute B., Reddan D.N., et al. The association of epsilon-aminocaproic acid with postoperative decrease in creatinine clearance in 1502 coronary bypass patients. Anesth Analg. 2000;91:1085.
11. Mangano D.T., Tudor J.C., Dietzel C., et al. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353.
12. Bellomo R., Chapman M., Finfer S., et al. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australia and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139.
13. Landoni G., Biondi-Zoccai G., Marino G., et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: A meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27.
14. Chaitman B.R. A review of the GUARDIAN trial results: Clinical implications and the significance of elevated perioperative CK-MB on 6-month survival. J Card Surg. 2003;18(suppl 1):13.
15. Ferguson T.B.Jr., Hammill B.G., Peterson E.D., et al. A decade of change-risk profiles and outcomes for isolated coronary artery bypass grafting procedures. 1990-1999: A report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480.
16. Vinten-Johansen J., Thourani V.H. Myocardial protection: An overview. J Extra Corpor Technol. 2000;32:38.
17. Flack J.E.3rd, Cook J.R., May S.J., et al. Does cardioplegia type affect outcome and survival in patients with advanced left ventricular dysfunction? Results from the CABG Patch Trial. Circulation. 2000;102(suppl 3):III-84.
18. McSweeney M.E., Garwood S., Levin J., et al. Adverse gastrointestinal complications after cardiopulmonary bypass: Can outcome be predicted from preoperative risk factors? Anesth Analg. 2004;98:1610.
19. Hessel E.A.2nd. Abdominal organ injury after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:243.
20. Sanisoglu I., Guden M., Bayramoglu Z., et al. Does off-pump CABG reduce gastrointestinal complications? Ann Thorac Surg. 2004;77:619.
21. Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:185.