2 Optimal Processing of Diagnostic Lung Specimens
Optimal specimen handling is essential for the accurate interpretation of biopsies and cytologic preparations obtained in the course of evaluating the patient with lung disease.1–9 The limited number of sampling techniques available can be divided into three general categories: bronchoscopy, transthoracic needle core biopsy or aspiration, and surgical wedge biopsy of peripheral lung through a transthoracic approach.8,10–13
The focus of this chapter is on these techniques and the specimens thereby obtained, with emphasis on how they should be prepared and handled in the laboratory. Once an appropriate sample of adequate quality has been obtained, the addition of pertinent clinical data and radiologic information greatly increases the likelihood of a meaningful and accurate diagnosis.13–15 Even when the diagnostic goal is simply to rule out malignancy, the effect of other information may be substantial, especially when the sample is of marginal quality or size. In the case of diffuse non-neoplastic lung diseases (often referred to as “interstitial lung diseases”), a reasonable amount of clinical and radiologic information is essential for accurate interpretation. Without such information, even the experienced lung pathologist may need to resort to a purely descriptive diagnosis.15
In this chapter, specimen characteristics and processing steps are presented for each of the common lung samples taken in the course of clinical evaluation for pulmonary disease. Also, for each type of sample, the benefits and limitations are reviewed. Such a working knowledge of specimen handling for each procedure ensures the greatest likelihood of success in establishing a specific diagnosis and, in the end, a rational treatment plan. An overview of biopsy procedures and the specimens generated is presented in Table 2-1.
Table 2-1 Diagnostic Sampling Techniques, Specimens Obtained, and Common Analyses Performed
| Sampling Technique | Specimens/Common Analyses |
|---|---|
| Sputum expectoration | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Bronchoscopy with: Washings |
Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Brushings | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Endobronchial biopsy | Forceps tissue biopsy specimen, 2–3 mm in size Fixed and processed for histopathologic examination Microbiologic cultures and other testing performed as indicated |
| Transbronchial biopsy | Forceps tissue biopsy specimen, 2–3 mm in size Processed for histopathologic examination Microbiologic cultures and other testing performed as indicated |
| Bronchoalveolar lavage (BAL) | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination and biochemical analysis Microbiologic cultures and other testing performed as indicated |
| Transbronchial fine needle aspiration | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures and other testing performed as indicated |
| Surgical “wedge” lung biopsy (either video-assisted or open) | 3- to 5-cm peripheral lung tissue sample including pleura and alveolar parenchyma Fixed and processed for histopathologic examination Microbiologic cultures and specialized testing performed as indicated |
| Transthoracic needle core biopsy and aspiration | Core biopsy fragment(s), cytologic smears and centrifuge preparations Smears and cellular preparations: fixed or air-dried, then stained for cytopathologic examination, with special stains for organisms and other specialized techniques as indicated Core tissue specimens: fixed and processed for histopathologic examination Microbiologic cultures and specialized assays performed as indicated |
| Thoracentesis | Cytologic centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures, biochemical analysis, and specialized assays performed as indicated |
Specimens Obtained through the Flexible Bronchoscope
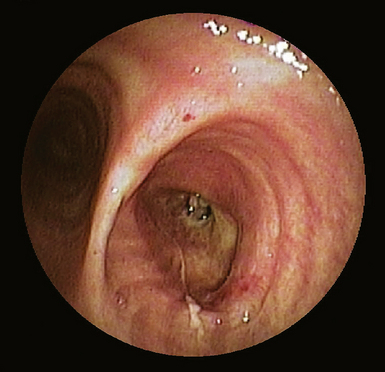
The flexible bronchoscope was introduced in the United States in the late 1960s, after successful use in Japan.8 Despite several decades of experience with the rigid bronchoscope, the advent of the flexible bronchoscope (Fig. 2-1) allowed evaluation of the major conducting airways without use of general anesthesia and with less morbidity.8,16 Furthermore, the flexible instrument has the advantage of providing better access to more distal and obliquely branched airways. The rigid bronchoscope still has major uses in certain settings, mainly those in which the device’s larger bore is an advantage, but today pulmonary endoscopy is dominated by the flexible bronchoscope.
Endobronchial Biopsy
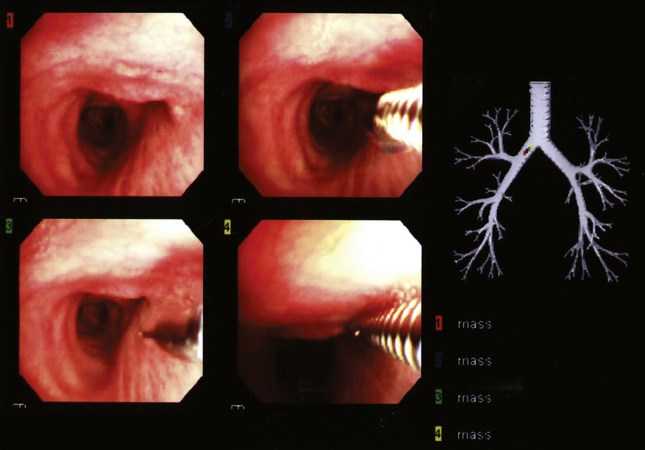
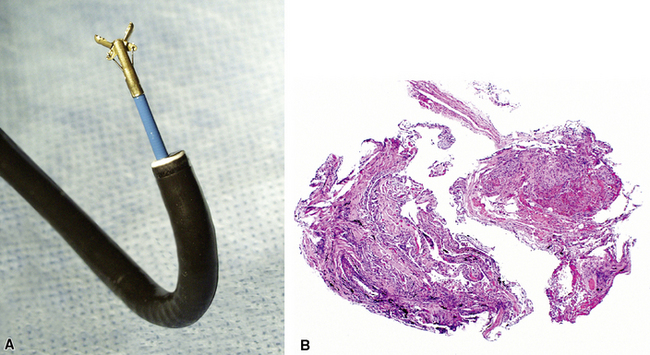
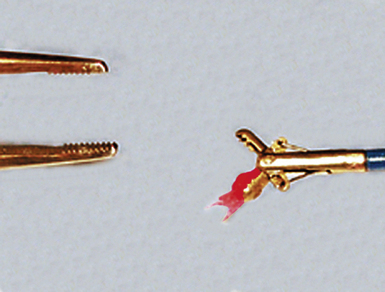
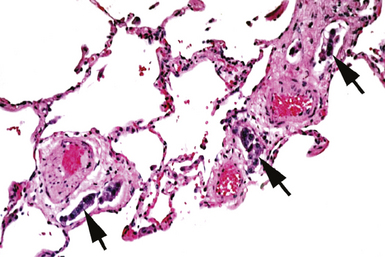
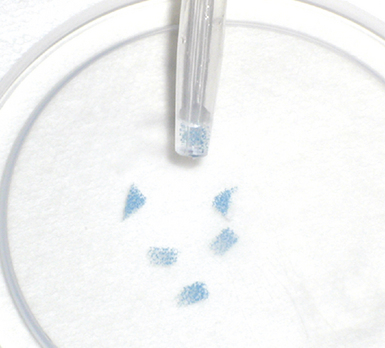
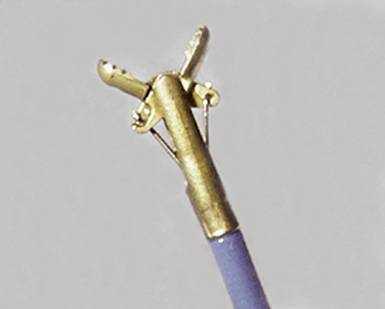
Modern flexible bronchoscopes allow the operator to accurately visualize the structural integrity of the bronchial tree and its mucosal surfaces, commonly as far distal as the sixth order bronchi17,18 (Fig. 2-2). Biopsy of visualized mucosal lesions most commonly is performed using cupped forceps (Fig. 2-3A) introduced through the flexible shaft of the bronchoscope.8 With this technique, the airway mucosa, lamina propria, and musculature are sampled with or without fragments of cartilage (see Fig. 2-3B). The closed forceps is extracted from the bronchoscope and the biopsy is dislodged from the cupped ends of the device and placed in fixative or other solution (see later discussion). A sterile needle or fine-tipped forceps (Fig. 2-4) is useful for removing the delicate tissue specimen. The tissue specimens obtained in this way average 2 to 3 mm in greatest dimension. The lymphovascular network of the peribronchial sheath often is included in these samples, making it possible to identify metastatic disease when present in lymphatic or vascular channels (Fig. 2-5).
For transferring bronchoscopic biopsy specimens from carrier or fixative solutions into cassettes for paraffin embedment, a useful device is a polystyrene pipette with the tip cut off with scissors (Fig. 2-6). This pipetting device allows the operator to transfer delicate specimens without tearing or crushing. Transferring of these specimens using forceps is to be avoided.
When infection is a consideration, bronchoscopic biopsy specimens can be transported directly to the microbiology laboratory for processing.4,19,20 In most scenarios, biopsy samples are sent for histopathologic evaluation and microbiologic studies directly from the bronchoscopy suite or bedside. For endobronchial and transbronchial samples, the optimal number of biopsy specimens varies depending on the radiologic distribution of disease,15,21–23 bronchoscopy findings,8,11,16 and the specific diagnostic entities under consideration.8,24,25 As a general guideline, if the patient is tolerating the procedure well, the greater the number of biopsy specimens, the greater the likelihood of establishing a definitive diagnosis.8,23,25
Transbronchial Biopsy
In contrast with endobronchial biopsy, the transbronchial biopsy technique is intended to sample alveolar lung parenchyma beyond the cartilaginous bronchi.8,16,17,26 This technique uses either crocodile-style (Machida) forceps or cupped forceps manipulated by the operator (Fig. 2-7). To obtain the biopsy specimen, the forceps is advanced with the jaws closed into a distal airway until resistance is met. The forceps is retracted slightly and then advanced slightly, with the jaws open. The jaws are then closed and the forceps is pulled out through the bronchoscope. Advancing the forceps at end-expiration can be helpful in forcing the bronchiolar wall and peribronchiolar lung parenchyma into the mouth of the device. The successful parenchymal biopsy specimen appears finely ragged (Fig. 2-8) and usually measures between 2 and 3 mm in diameter.26–28
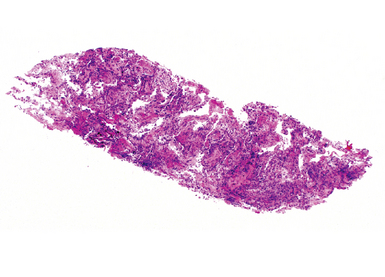
Figure 2-8 Transbronchial biopsy. Low-magnification image of a transbronchial biopsy specimen of generous size.
As with endobronchial samples, the transbronchial sample is teased from the forceps with a sterile needle, and the same precautions are advised to avoid damage during handling and transfer to fixative or other solution. The truncated pipette technique also is useful in this setting. Once processed, both types of biopsy specimens should be serially sectioned for thorough microscopic evaluation.2
Bronchial Brushings
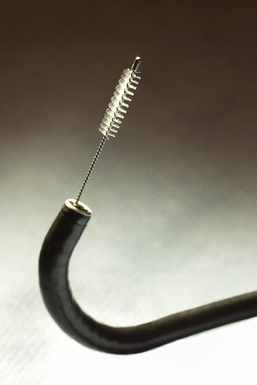
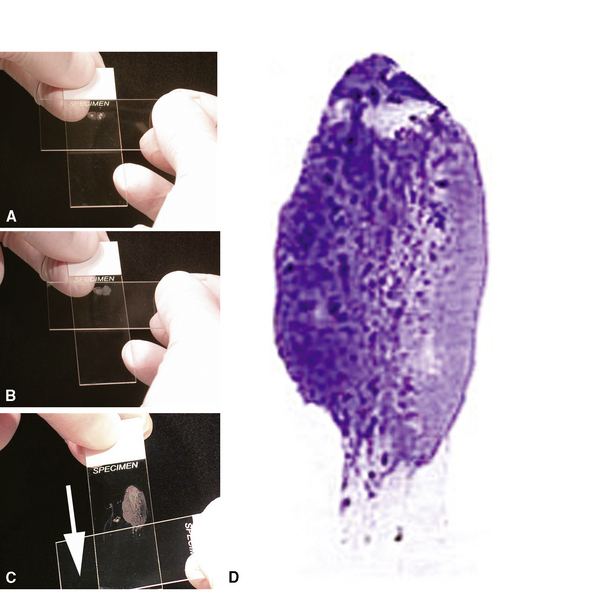
Visualized lesions of the airway epithelium can be sampled for cytologic evaluation.8,11,16 The technique involves the use of a conical bristle brush (Fig. 2-9). Under direct visualization, the brush is agitated against the mucosal surface of the airway, forcing cells into the interstices of the bristles (Fig. 2-10). The brush is removed from the bronchoscope and can be applied directly to glass slides. Cells and secretions smeared on slides can be immediately fixed for cytologic evaluation using the Papanicolaou staining method or air-dried for use with the Wright-Giemsa staining technique (this choice is best made in consultation with the pathologist). Immediate fixation of slides is best accomplished by direct immersion in 95% alcohol immediately after smearing of the sample on the slide. Each fixation and staining technique produces characteristic artifacts, and the use of one over the other depends on operator training and preference.
If slides are not available for smear preparations, the brush can be cut off and placed directly into a small vial of sterile saline, which is then shaken vigorously to dislodge cells into the fluid. This fluid sample of suspended cells is sent to the laboratory for millipore filtration or cytocentrifuge-type application onto slides,8,11,16,29,30 analogous to the handling of washings and lavage specimens, discussed next. With ever-increasing demands for molecular genetic information for use in patient management (typically in the setting of malignant tumor), aliquoting this liquid suspension of cells and saving a portion of it in collaboration with the local pathologist is advisable and may avoid the need for resampling of tumor in cases in which surgical tumor removal is not an option.
Bronchial Washings and Bronchoalveolar Lavage
Bronchial washings and bronchoalveolar lavage (BAL) specimens are less “lesion-directed” sampling techniques and rely on the presence of shed cells within the airways and more peripheral lung.11,31–35 Bronchial washings are obtained by aspiration of sterile saline solution applied near the tip of the bronchoscope (Fig. 2-11). The washings consist of a rather concentrated cellular preparation of bronchial epithelial cells and macrophages with variable amounts of inflammatory cells and mucus. Because of the relatively small sample volume (as with the bronchial brush sample when this is placed into solution), a limited number of assays can be performed on the bronchial washing specimen. Cytologic examination and cultures are the most commonly ordered tests on these samples. If a diagnosis of tumor is likely, saving an aliquot for special studies is prudent. If initial aliquots are judged to be nondiagnostic, the saved sample can be recruited.
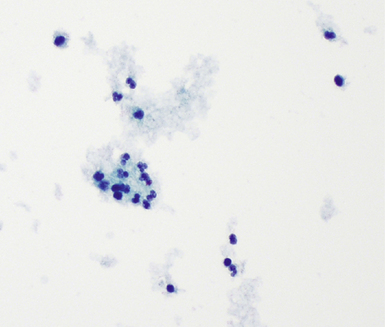
The lavage procedure is performed by instilling multiple aliquots of sterile saline (20–50 mL), followed, after a variable dwell time, by subsequent aspiration of this fluid into a flask or syringe at the bedside. BAL fluid from normal lungs consists primarily of macrophages with a few inflammatory cells34 (Fig. 2-12). A potential advantage of BAL over the bronchial washing technique is the capability to analyze noncellular elements included, such as surfactant content, serum proteins (e.g., albumin, immunoglobulins, enzymes), and mucus.36,37 In current practice, the BAL technique is used primarily in the clinical setting for the diagnosis of infection in the immunocompromised host.36,38–41 As a research tool in the study of interstitial lung diseases, however, BAL has been used extensively for quantitation of cellular components.19,28,36,42–44
Transbronchial Fine-Needle Aspiration
Transbronchial fine-needle aspiration (TBNA) initially was introduced by Wang and Terry in 198345 as a staging tool in the evaluation of patients with lung cancer. The use of TBNA has expanded considerably since that time46–48 for the diagnosis of both central and peripheral lung lesions, even in the absence of endobronchial abnormalities.48 Endoscopic ultrasound imaging has increased the accuracy of TBNA in recent years and is becoming a standard part of the TBNA procedure in many settings.49–51 The specimens generated by this technique are very small, sometimes no more than a drop or two, and when the target is solid tissue, these samples consist of thick cellular material. To prepare direct smear preparations for cytopathologic evaluation and rapid stains for organisms, the needle is removed from the syringe (or other aspiration device) and then reattached after air has been aspirated into the syringe. The air is then rapidly forced out through the needle tip, forcing the sample out of the needle hub and onto a slide. Alternatively, a drop can be expressed directly onto a slide (close to the label end) and smeared using a feathering technique (Fig. 2-13). The slide smear is then either air-dried or immediately fixed before staining. For microbiology cultures or fluid-based cytocentrifuge preparations, the needle is rinsed directly into culture or cytopathology fixative medium, respectively.
Rigid Bronchoscopy
Rigid bronchoscopy is a procedure that has been used for more than 125 years.52,53 With the introduction of the flexible bronchoscope, use of rigid bronchoscopy has declined, but some lesions are more readily biopsied using this device, especially when larger quantities of tissue are required53,54 (Fig. 2-14). The procedure requires use of general anesthesia and expertise in airway management. Large fragments of tumor (or foreign bodies) can be removed and cautery can be applied to control any bleeding encountered. For large, highly vascular, or mostly necrotic tumors, this may be the most prudent and useful procedure for obtaining diagnostic specimens.
Specimens Obtained by Transthoracic Needle Biopsy and Aspiration
Thoracentesis
Thoracentesis derives its greatest practical application in the evaluation of pleural effusion samples for cells and noncellular elements.55–57 As with BAL fluid examination, a number of specific analyses typically are performed. If collected after hours, the sterile thoracentesis fluid can be stored unfixed at 4°C for processing the next day. The aliquoted thoracentesis fluid specimens are distributed to the appropriate laboratory for analysis (e.g., microbiology, chemistry, hematology). Chemical determinations of glucose, amylase, lactate dehydrogenase, and other analytes are compared with cellular composition determined by cytopathology evaluation. The cytocentrifuge or millipore filter also can be evaluated cytopathologically for the presence of malignant neoplasm. Rapid stains for microorganisms can be performed as indicated.
Closed Pleural Biopsy
Available pleural needle biopsy devices include the Cope, Abrams, and Tru-Cut needles that produce a very small biopsy sample (Fig. 2-15). Inflammatory, infectious, and neoplastic diseases of the pleura can be diagnosed using these devices, despite the limitations of biopsy size and somewhat randomness of sampling.58 These small specimens should be handled in a fashion analogous to those obtained from bronchoscopic biopsy techniques (as described previously).
Transthoracic Fine-Needle Core Aspiration and Biopsy of the Lung
In current practice, the use of transthoracic needle aspiration biopsy has become commonplace.10,59–64 This procedure typically is performed in the radiology department, because biopsy by this method is always performed under radiologic guidance. Samples are similar to those obtained by transbronchial needle aspiration and should be handled accordingly (as discussed previously). Assistance from a cytotechnologist during the procedure is a cost-effective benefit to ensure adequacy of the specimen before termination of the procedure.65 Recent advances in needle biopsy devices have allowed for better tissue samples and greater likelihood of accurate diagnosis.66–69 If the technique generates a semiliquid sample, this should be handled as described for transbronchial needle aspiration specimens. If a core of tissue is generated (typically 1 mm in diameter), this can be processed like other needle core samples received in surgical pathology. In addition to routine hematoxylin and eosin–stained sections and any number of sectioning levels that typically are obtained for routine evaluation, the histology laboratory also should make four to six unstained sections (mounted on slides designed for immunohistochemical stains) at the time of initial sectioning in histology. Having these extra sections available saves time and avoids having to return to the tissue block later when special stains may be necessary, which can be a problem because block resurfacing between sectioning wastes a certain amount of tissue.
Specimens Obtained by Thoracoscopy
Surgical biopsy of lung parenchyma is indicated in several specific situations:
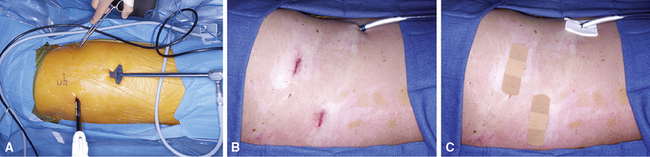
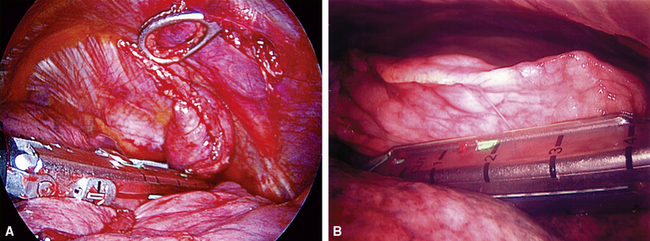
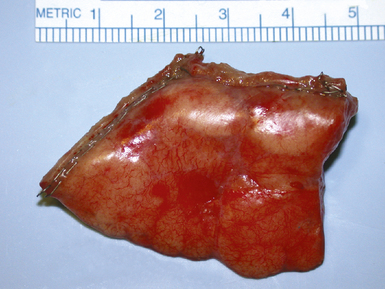
The introduction of a high-resolution video endoscopic system has changed the practice of elective thoracic surgery. With this procedure, smaller incisions are made and a thoracoscope with a video camera is introduced along with the instruments (Fig. 2-16). Video-assisted thoracic surgery (VATS) has become the standard of care for obtaining most surgical biopsy specimens. It has been used in the diagnosis and treatment of pulmonary diseases since the early 1990s.72–75 The mortality rate is low, duration of hospital stay is decreased, and patient recovery is hastened in comparison with standard thoracotomy.73 Specimens measuring 2 to 3 cm across and larger (Fig. 2-17) are easily obtained, and patients often can be discharged from the hospital less than 24 hours. With VATS, the same thoracic access ports also provide access for sampling ipsilateral lymph nodes that may contain neoplastic disease or other abnormalities.
Although it confers undeniable benefits, VATS usually requires the collapse of one lung, so all conditions that contradict this maneuver may prevent use of this approach. Tumor deposition and dissemination can be prevented by adherence to well-established oncologic surgical principles.76,77 Meticulous technique is required to avoid laceration of tumor during excision and contamination of the distant pleura or lung by the instruments. The use of an impermeable “endocatch” bag for specimen retrieval is mandatory (Fig. 2-18). A sterile lavage of all port sites also is done at the conclusion of surgery.
Specimen Processing
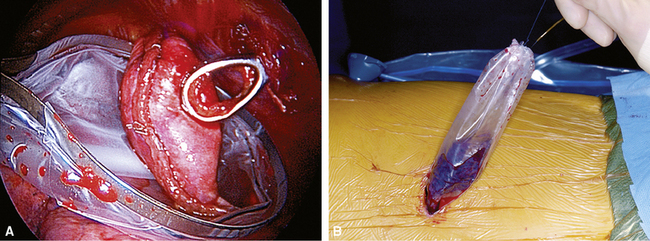
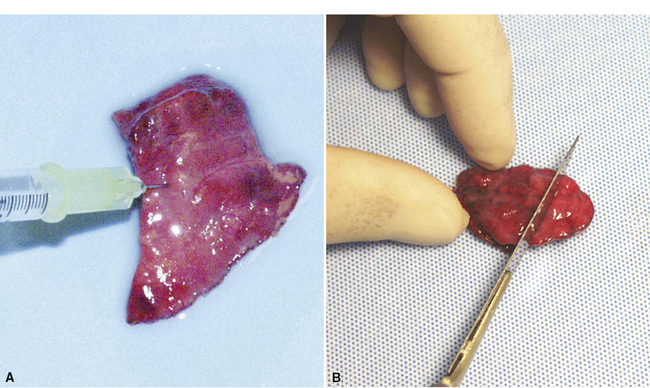
Processing of the wedge lung biopsy specimen requires techniques different from those used in handling specimens from other organs. A typical surgical lung biopsy specimen as it is received from surgery is shown in Figure 2-19. If the wedge tissue sample is to be divided for different types of analysis (e.g., microbiological cultures, electron microscopy, molecular diagnostic studies), these portions can be separated before routine processing for morphologic assessment. Preparing frozen sections from air-filled lung tissue poses some special problems. Unfixed lung tissue is easily compressed, especially when attempts are made to slice it into thin (typically less than 5 mm) sections unfixed. Severe compression may result in artifactual atelectasis, thereby compounding the difficulty of histopathologic assessment. The simplest and most reliable technique for preparing frozen sections is to cut a 5- to 6-mm slab from the biopsy specimen using a fresh scalpel and freezing it without further preparation. For most lung diseases, the frozen sections generated this way are reasonably interpretable. Alternatively, some authors have recommended injecting the actual slab section (not the whole biopsy) with a stabilizing solution before freezing. To accomplish this, a dilute solution of embedding compound can be gently infused into the cut surface of the slab using a 21- to 23-gauge needle attached to a 5-mL syringe.
After any intraoperative consultation has been completed, the remainder of the specimen can be prepared for processing using a number of techniques, all designed to restore the normal inflated state of the tissue (VATS specimens are received deflated and stapled closed). We prefer a simple technique that is as good as either of the two more elaborate methods also described here and requires no special equipment or needles: After all staples have been removed from the sample, the specimen is vigorously shaken for 2 minutes in a container half-filled with fixative solution (appropriately sealed with paraffin film). This maneuver forces the sample repeatedly against the inner walls of the container and nicely distributes fixative within the elastic lung parenchyma. In most instances, atelectatic areas are fully restored. A second technique uses a small volume of carbonated water added to the fixative solution to assist in reexpansion of alveoli (no agitation required). Finally, some authors have proposed using a small-gauge needle and syringe to gently reinflate the sample with fixative. This procedure should be performed only after the staples have been removed from the sample to avoid overinflation. Injection into the cut lung surface is preferable to injecting through the pleural surface (Fig. 2-20A). After fixation of 1 to 2 hours, the specimen can be safely and easily sliced into 3- to 5-mm sections for final processing (see Fig. 2-20B).
1 Mikel U., editor. Advanced Laboratory Methods in Histology and Pathology. Washington, DC: American Registry of Pathology, 1994.
2 Nagata N., Hirano H., Takayama K., et al. Step section preparation of transbronchial lung biopsy. Significance in the diagnosis of diffuse lung disease. Chest.. 1991;100:959-962.
3 Bonetti F., Chiodera P.L., Pea M., et al. Transbronchial biopsy in lymphangiomyomatosis of the lung. HMB45 for diagnosis. Am J Surg Pathol.. 1993;17:1092-1102.
4 Chastre J., Fagon J.Y., Soler P., et al. Diagnosis of nosocomial bacterial pneumonia in intubated patients undergoing ventilation: comparison of the usefulness of bronchoalveolar lavage and the protected specimen brush. Am J Med.. 1988;85:499-506.
5 Miller R., Nelems B., Müller N.L., et al. Lingular and right middle lobe biopsy in the assessment of diffuse lung disease. Ann Thorac Surg.. 1987;44:269-273.
6 Rosen P. Frozen section management of a lung biopsy for suspected Pneumocystis pneumonia. Am J Surg Pathol.. 1977;1:79-82.
7 Travis W., Borok Z., Roum J.H., et al. Pulmonary Langerhans cell granulomatosis (histiocytosis X). A clinicopathologic study of 48 cases. Am J Surg Pathol.. 1993;17:971-986.
8 Zavala D. Diagnostic fiberoptic bronchoscopy: techniques and results of biopsy in 600 patients. Chest. 1975;68:12-19.
9 Churg A. An inflation procedure for open-lung biopsies. Am J Surg Pathol.. 1983;7:69-71.
10 Yang P., Lee Y.C., Yu C.J., et al. Ultrasonographically guided biopsy of thoracic tumors. A comparison of large-bore cutting biopsy with fine-needle aspiration. Cancer. 1992;69:2553-2560.
11 Popp W., Rauscher H., Ritschka L., et al. Diagnostic sensitivity of different techniques in the diagnosis of lung tumors with the flexible fiberoptic bronchoscope. Comparison of brush biopsy, imprint cytology of forceps biopsy, and histology of forceps biopsy. Cancer. 1991;67:72-75.
12 Burt M.E., Flye M.W., Webber B.L., Wesley R.A. Prospective evaluation of aspiration needle, cutting needle, transbronchial and open lung biopsy in patients with pulmonary infiltrates. Ann Thorac Surg.. 1981;32:146-153.
13 Leslie K.O., Lanza L.A., Helmers R.A., Colby T.V. Diagnostic sampling of lung tissues and cells. In: Davis G., Marcey T., Seward E., editors. Medical Management of Pulmonary Diseases. New York: Marcel Dekker; 1999:213-220.
14 Leslie K., Colby T. Classification and pathology of diffuse interstitial lung disease. Semin Clin Immunol.. 1999;1:7-15.
15 Leslie K., Colby T., Swensen S. Anatomic distribution and histopathologic patterns in interstitial lung disease. In: Schwarz M., King T.J., editors. Interstitial lung disease. Hamilton: BC Decker; 2002:31-50.
16 Mitchell D., Emerson C.J., Collins J.V., Stableforth D.E. Transbronchial lung biopsy with the fibreoptic bronchoscope: analysis of results in 433 patients. Br J Dis Chest.. 1981;75:258-262.
17 Kovnat D.M., Rath G.S., Anderson W.M., Snider G. Maximal extent of visualization of bronchial tree by flexible fiberoptic bronchoscopy. Am Rev Respir Dis.. 1974;110(1):88-90.
18 Joos L., Patuto N., Chhajed P.N., Tamm M. Diagnostic yield of flexible bronchoscopy in current clinical practice. Swiss Med Wkly. 2006;136(9–10):155-159.
19 Haslam P., Turton C.W., Heard B., et al. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980;35:9-18.
20 Delvenne P., Arrese J.E., Thiry A., et al. Detection of cytomegalovirus, Pneumocystis carinii, and Aspergillus species in bronchoalveolar lavage fluid. A comparison of techniques. Am J Clin Pathol. 1993;100:414-418.
21 Guinee D.G.Jr, Feuerstein I., Koss M.N., Travis W.D. Pulmonary lymphangioleiomyomatosis. Diagnosis based on results of transbronchial biopsy and immunohistochemical studies and correlation with high-resolution computed tomography findings. Arch Pathol Lab Med. 1994;118:846-849.
22 Colby T.V., Swensen S.J. Anatomic distribution and histopathologic patterns in diffuse lung disease: correlation with HRCT. J Thorac Imaging. 1996;11(1):1-26.
23 Popovich J.Jr, Kvale P.A., Eichenhorn M.S., et al. Diagnostic accuracy of multiple biopsies from flexible fiberoptic bronchoscopy. A comparison of central versus peripheral carcinoma. Am Rev Respir Dis.. 1982;125:521-523.
24 Gilman M., Wang K. Transbronchial lung biopsy in sarcoidosis: an approach to determine the optimal number of biopsies. Am Rev Respir Dis.. 1980;122:721-724.
25 Flint A., Martinez F.J., Young M.L., et al. Influence of sample number and biopsy site on the histologic diagnosis of diffuse lung disease. Ann Thorac Surg.. 1995;60:1605-1607.
26 Andersen H. Transbronchoscopic lung biopsy for diffuse pulmonary diseases. Results in 939 patients. Chest. 1978;73(suppl 5):734-736.
27 Cazzadori A., Di Perri G., Todeschini G., et al. Transbronchial biopsy in the diagnosis of pulmonary infiltrates in immunocompromised patients. Chest. 1995;107(1):101-106.
28 Guilinger R., Paradis I.L., Dauber J.H., et al. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med. 1995;152:2037-2043.
29 Willcox M., Kervitsky A., Watters L.C., King T.E.Jr, et al. Quantification of cells recovered by bronchoalveolar lavage. Comparison of cytocentrifuge preparations with the filter method. Am Rev Respir Dis. 1988;138(1):74-80.
30 Robb J., Melello C., Odom C. Comparison of Cyto-Shuttle and cytocentrifuge as processing methods for nongynecologic cytology specimens. Diagn Cytopathol. 1996;14(4):305-309.
31 Poletti V., Romagna M., Allen K.A., et al. Bronchoalveolar lavage in the diagnosis of disseminated lung tumors. Acta Cytol. 1995;39:472-477.
32 Winterbauer R., Lammert J., Selland M., et al. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352-361.
33 The BAL Cooperative Steering Committee. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S169-S202.
34 Merchant R., Schwartz D.A., Helmers R.A., et al. Bronchoalveolar lavage cellularity. The distribution in normal volunteers. Am Rev Respir Dis. 1992;146:448-453.
35 Cobben N., Jacobs J.A., van Dieijen-Visser M.P., et al. Diagnostic value of BAL fluid cellular profile and enzymes in infectious pulmonary disorders. Eur Respir J.. 1999;14:496-502.
36 American Thoracic Society Statement. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 2001;142:481-486.
37 Reynolds H.Y., Fulmer J.D., Kazmierowski J.A., et al. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest.. 1977;59(1):165-175.
38 Abramson M.J., Stone C.A., Holmes P.W., Tai E.H. The role of bronchoalveolar lavage in the diagnosis of suspected opportunistic pneumonia. Aust N Z J Med.. 1987;17(4):407-412.
39 Bye M., Bernstein L., Shah K., et al. Diagnostic bronchoalveolar lavage in children with AIDS. Pediatr Pulmonol. 1987;3:425-428.
40 Kahn F.W., Jones J.M. Diagnosing bacterial respiratory infection by bronchoalveolar lavage. J Infect Dis. 1987;155:862-869.
41 Martin W.J.II, Smith T.F., Sanderson D.R., et al. Role of bronchoalveolar lavage in the assessment of opportunistic pulmonary infections: utility and complications. Mayo Clin Proc. 1987;62:549-557.
42 Goldstein R.A., Rohatgi P.K., Bergofsky E.H., et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis.. 1990;142:481-486.
43 Hunninghake G.W., et al. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol.. 1979;97:149-206.
44 Kvale P.A. Bronchoscopic biopsies and bronchoalveolar lavage. Chest Surg Clin N Am.. 1996;6(2):205-222.
45 Wang K., Terry P. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis.. 1983;127(3):344-347.
46 Rosenthal D., Wallace J. Fine-needle aspiration of pulmonary lesions via fiberoptic bronchoscopy. Acta Cytol. 1984;28:203-210.
47 Wagner E.D., Ramzy I., Greenberg S.D., Gonzalez J.M. Transbronchial fine-needle aspiration. Reliability and limitations. Am J Clin Pathol.. 1989;92:36-41.
48 Mehta A.C., Kavuru M.S., Meeker D.P., et al. Transbronchial needle aspiration for histology specimens. Chest. 1989;96:1228-1232.
49 Eloubeidi M.A. Endoscopic ultrasound-guided fine-needle aspiration in the staging and diagnosis of patients with lung cancer. Semin Thorac Cardiovasc Surg.. 2007;19(3):206-211.
50 Vilmann P., Puri R. The complete “medical” mediastinoscopy (EUS-FNA + EBUS-TBNA). Min Med.. 2007;98(4):331-338.
51 Zias N., Chroneou A., Gonzalez A.V., et al. Changing patterns in interventional bronchoscopy. Respirology. 2009;14(4):595-600.
52 Helmers R.A., Sanderson D.R. Rigid bronchoscopy. The forgotten art. Clin Chest Med. 1995;16(3):393-399.
53 Melo N., Salero S., Fernandes G., et al. Rigid bronchoscopy—a 2.5 year experience. Rev Port Pneumol.. 2006;6(suppl 1):30-31.
54 Chao Y.K., Liu Y.H., Hsieh M.J., et al. Controlling difficult airway by rigid bronchoscope—an old but effective method. Interact Cardiovasc Thorac Surg.. 2005;4(3):175-179.
55 Berquist T.H., Bailey P.B., Cortese D.A., Miller W.E. Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc.. 1980;55(8):475-481.
56 Crosby J.H., Hager B., Hoeg K. Transthoracic fine-needle aspiration. Experience in a cancer center. Cancer. 1985;56(10):2504-2507.
57 Larscheid R.C., Thorpe P.E., Scott W.J. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest. 1998;114(3):704-709.
58 Von Hoff D.D., LiVolsi V. Diagnostic reliability of needle biopsy of the parietal pleura. A review of 272 biopsies. Am J Clin Pathol.. 1975;64(2):200-203.
59 Sanders C. Transthoracic needle aspiration. Clin Chest Med.. 1992;13(1):11-16.
60 Böcking A., Klose K.C., Kyll H.J., Hauptmann S. Cytologic versus histologic evaluation of needle biopsy of the lung, hilum and mediastinum. Sensitivity, specificity and typing accuracy. Acta Cytol. 1995;39:463-471.
61 Milman N. Percutaneous lung biopsy with semi-automatic, spring-driven fine needle—preliminary results in 13 patients. Respiration. 1993;60:289-291.
62 Smyth R., Carty H., Thomas H., et al. Diagnosis of interstitial lung disease by a percutaneous lung biospy sample. Arch Dis Child.. 1994;70:143-144.
63 Lohela P., Tikkakoski T., Ammälä K., et al. Diagnosis of diffuse lung disease by cutting needle biopsy. Acta Radiol. 1994;35:251-254.
64 Williams A., Santiago S., Lehrman S., Popper R. Transcutaneous needle aspiration of solitary pulmonary masses: how many passes? Am Rev Respir Dis.. 1987;136:452-454.
65 Nasuti J., Gupta P., Baloch Z. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol. 2002;27(1):1-4.
66 Zavala D.C., Bedell G.N. Percutaneous lung biopsy with a cutting needle. An analysis of 40 cases and comparison with other biopsy techniques. Am Rev Respir Dis.. 1972;106(2):186-193.
67 Zavala D.C. The diagnosis of pulmonary disease by nonthoracotomy techniques. Chest. 1973;64(1):100-102.
68 Zavala D.C., Rossi N.P. Nonthoracotomy diagnostic techniques for pulmonary disease. Arch Surg. 1973;107(2):152-154.
69 Zavala D.C. Pulmonary biopsy. Adv Intern Med.. 1976;21:21-45.
70 Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery. The Thorax Group. Ann Thorac Surg.. 1996;1:202-204. discussion 204–205
71 Chang A.C., Yee J., Orringer M.B., Iannettoni M.D. Diagnostic thoracoscopic lung biopsy: an outpatient experience. Ann Thorac Surg.. 2002;74(6):1942-1946. discussion 46–47
72 Allen M.S., Deschamps C., Jones D.M., et al. Video-assisted thoracic surgical procedures: the Mayo experience. Mayo Clin Proc.. 1996;71(4):351-359.
73 Jaklitsch M.T., DeCamp M.M.Jr, Liptay M.J., et al. Video-assisted thoracic surgery in the elderly. A review of 307 cases. Chest. 1996;110(3):751-758.
74 Rubin J.W., Finney N.R., Borders B.M., Chauvin E.J. Intrathoracic biopsies, pulmonary wedge excision, and management of pleural disease: is video-assisted closed chest surgery the approach of choice? Am Surg.. 1994;60(11):860-863.
75 Solaini L., Prusciano F., Bagioni P., et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc. 2008;22(2):298-310.
76 Ang K.L., Tan C., Hsin M., Goldstraw P. Intrapleural tumor dissemination after video-assisted thoracoscopic surgery metastasectomy. Ann Thorac Surg.. 2003;75(5):1643-1645.
77 Anraku M., Nakahara R., Matsuguma H., Yokoi K. Port site recurrence after video-assisted thoracoscopic resection of chest wall schwannoma. Interact Cardiovasc Thorac Surg.. 2003;2(4):483-485.