Chapter 14 Oncology
Long Case
Oncology
The oncology long case provides an opportunity for a general paediatrician to display competence in assessing and managing a child with cancer, and his or her family, both within multiple contexts: the domestic situation, schooling requirements, wider social relationships and within the medical framework. The medical needs reflect the multi-layered levels of care required for the patient and delivered by the general practitioner, the general paediatrician, the paediatric oncologist and other consultants whose help may be required from time to time.
Of great importance is the role of medical practitioners to provide long-term sensitive surveillance and follow-up. A web-based comprehensive set of guidelines for management of such survivors, the Children’s Oncology Group (COG) Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, can be found at www.survivorshipguidelines.org. Both these initiatives have expanded the literature significantly and are very useful to many candidates.
In Australia and New Zealand, all major paediatric centres are members of the US-based Children’s Oncology Group (COG), a consortium of childhood cancer centres that promotes clinical and laboratory research trials in paediatric oncology.
An approach to any oncology long case could potentially fit into the following scheme.
History: an overview
Before diagnosis
1. Initial symptoms (with particular reference to the usual non-specific nature of oncology symptoms). A notable difference from adult presentation is the paucity of these symptoms. Unlike adults with cancer, it is very unusual for a child to present with weight loss, haemoptysis or haematemesis and melaena. Symptoms that may present in children include a mass, and the eight ‘Ps’: Pyrexia, Pain, Pallor, Purpura, Persistent squint, Personality change, Posterior fossa symptoms, and do not forget the Pretenders (i.e. ALL or neuroblastoma impersonating arthritis, or mediastinal lymph nodes impersonating asthma).
2. Symptom duration (may give a guide to disease ‘tempo’). For example, patients with B-cell lymphoma may present as extremely ill, with a history of illness spanning a few days, compared to a patient with a brain tumour or other solid tumour, who may have experienced symptoms for many months.
3. Parental guilt about acts of commission or omission may have contributed to their child developing cancer.
4. An enquiry about the parent’s feeling towards the diagnosis is often revealing. Most parents are initially very angry, an anger that may be self-directed and related to guilt, or directed at doctors or others who they perceive as having failed in their duty to provide an early diagnosis—although, at the time, the non-specific symptoms may not have suggested malignancy as a likely possibility.
Post-diagnosis phase
Important aspects in the history include an appreciation of the parents’ understanding of the details and significance of the diagnosis, or even whether the family is aware of the precise diagnosis. The candidate should enquire about the level of knowledge of treatment-related details that the family has acquired in the interval from diagnosis. The examinee should determine the family’s understanding of the prognosis, which falls into two broad categories: what they have been told by their doctor, and what they believe. The level of parental awareness and understanding of the condition is important, as this affects the parents’ level of care for the child and compliance with medication requirements and appointments. It also influences whether they strive to maintain a near-normal lifestyle for the child, or withdraw him or her from social interaction in anticipation of early death, perceived needs of additional protection and suchlike. The candidate should enquire about the impact of the diagnosis on siblings, the marital relationship, the financial situation for the family and job stresses. Parents may respond inappropriately in the post-diagnosis phase, by selling their house to relocate closer to the hospital, resigning from stable employment, or moving away from familiar and supportive communities, because they believe they are acting in the best interests of the child.
Management plan
Discussion points
Growth and development
If there has been a bone marrow transplant, the eyes should be checked by a paediatric ophthalmologist regularly (say, 12–24 monthly) for cataracts from total body irradiation (or from steroids). Hearing may be impaired by drugs such as cisplatin or aminoglycosides, and requires regular review and audiological assessment. Ideally, a formal psychological assessment should be carried out before any cranial irradiation. After treatment, psychological assessment may be repeated on a regular basis, in conjunction with neurological examination, MRI scanning and assessment of school performance, to monitor neuropsychological outcome and provide early rehabilitative intervention if needed. The spectrum of central nervous system damage varies from decreased performance at school, to frank leukoencephalopathy, spasticity and significant intellectual impairment. Intravenous methotrexate may result in leuko-encephalopathy in children with ALL, especially after irradiation. Pubertal development may be early (rarely precocious); however, delayed puberty is more common, with high gonadotropin levels from end-organ gonadal damage.
Infection
The child on chemotherapy
If the neutrophil count is low (below 0.5 × 109/L), the child should be admitted to hospital. The management of febrile neutropenia should include broad-spectrum parenteral antibiotic therapy (e.g. ceftriaxone, tobramycin, teicoplanin). Antibiotics ideally should be commenced within 1–2 hours of the child reaching the emergency room. It is inappropriate to wait for the results of the blood count before starting therapy with antibiotics, especially if there is any delay in processing the sample in the laboratory. In such circumstances, antibiotics should be started. If the count is normal, there is less cause for concern. Remember, however, that many patients have central venous access devices in place, which can be the cause of serious infections despite normal neutrophil numbers. Always remind the family that fevers occurring within 6–8 hours of flushing a central line may be due to the introduction of bacteria into the patient from an infected CVL. For more details, see below.
Immunisation
Some authorities avoid administering live measles and mumps vaccine to siblings, as there is a small risk of transmission. Others consider this risk minimal and the vaccine safe. Influenza vaccine is safe and may have a place in patients with chronic chest symptoms. Hepatitis B recombinant vaccine is safe, but as these patients are immunosuppressed, adding a further booster dose is recommended. Titres may be checked 12 months later to assess efficacy. There are now recommendations regarding immunisation in two distinct groups: those who receive standard therapy and those who require stem cell therapy.
Issues related to ongoing chemotherapy
1. Check that relatives are well before visits.
2. Patients can swim in swimming pools (even with central lines, but they require prompt cleaning after the swim). Swimming policy varies between units—generally advise against swimming if there is an external central line.
3. Children can go shopping in supermarkets and attend group gatherings (e.g. church, birthday parties).
Crisis intervention
The candidate should be familiar with medical practices in his or her own teaching hospital’s oncology unit. For example, prophylactic platelet transfusions for severe thrombocytopenia (platelet count below 10 × 109/L) is not standard practice in all units.
Supportive care
Mouth care
Mouth care is important in children with mouth ulceration or mucositis, or in those at risk of these if they are neutropenic (<1.0 × 109/L). Chlorhexidine mouthwash is useful, as it is bactericidal. In very young patients, chlorhexidine gel is appropriate, and before meals xylocaine viscous is useful.
Allogeneic stem cell transplantation: selected haematological malignancies
Acute lymphoblastic leukaemia: Certain patients who relapse after initial treatment, plus subgroups of patients with high-risk features of relapse, will proceed to an allogeneic SCT; in the latter, this can occur while in the first remission. SCT may be the only curative therapy for the former. Relapsed ALL is the fourth-commonest malignant disease in childhood (with a higher incidence than many newly diagnosed paediatric tumours). Outcomes for SCT are best when performed during first or second complete remission.
Acute myeloid leukaemia: The best therapy during first remission is controversial; SCT is preferred for patients with less favourable prognoses, such as those with complex karyotypes (e.g. -5, del(5q), -7, 3q abnormalities) or hyperleucocytosis. Allogeneic SCT during the second remission is associated with better outcome after relapse.
High-grade myelodysplastic syndrome: Allogeneic SCT is the treatment of choice.
Therapy-related myelodysplastic (MDS) syndrome and AML are clonal malignant disorders occurring after exposure to cytotoxic agents; these tend to be more aggressive than the de novo disorders, and SCT may be the only curative therapy available.
Chronic myeloid leukaemia (CML): Allogeneic transplantation from a matched related family donor is the only curative approach.
Alternative transplantations: umbilical cord blood transplantation (UCBT) and haploidentical transplantation
Umbilical cord blood (UCB) is an alternative source for transplantable stem and progenitor cells. CB can be collected from the placenta via the umbilical vein after the baby is delivered. GVHD occurs at lower rates than matched-sibling BMTx. Cord blood is available at short notice. Disadvantages include the potential for unknowing transmission of genetic diseases (e.g. immune deficiency and storage diseases), and the limitation of volume and cell content of what is collected (i.e. the finite number of stem cells in the CB unit). CBT may be very useful for those without a matched donor for BMTx. Predictors of success after UCBT include the number of cells in the UCB graft, and the degree of HLA disparity; a cell dose of 3 × 107 per kg have a much higher survival rate.
Therapeutic modifications: risk-adapted therapy
The dying child
There are many issues that need to be addressed in a child for whom no further beneficial therapy is possible. Most children nowadays die at home. Palliative treatment involves issues of control of pain, nutrition and appreciation of the psychosocial dynamics of the family. These children may show an inability to cope by features such as denial of their illness, withdrawal or unwarranted anger.
Short Case
Late effects of oncology treatment
Later effects of oncology treatment plus common signs of disease relapse (in particular, acute leukaemia) are outlined in Table 14.1. This is incomplete, of course, due to the nature of the wide range of tumours and their individual modes of relapse. Some of the findings mentioned will be relevant only to children still on chemotherapy. This approach may be found useful in assessing children in follow-up clinics. Several of the findings can only be ascertained fully by involving other specialised areas, such as neuropsychology and audiology. Neurocognitive dysfunction is the area that worries survivors of cancer and their parents the most. Survivors of (especially) ALL and central nervous system (CNS) tumours, head and neck tumours requiring radiation therapy and patients treated with HSCT are most at risk. Cranial radiotherapy is the main risk factor for adverse neurocognitive outcome. In ALL patients, exposure to chemotherapy alone will still be associated with neurocognitive decline in two thirds of patients. These children tend to have problems with executive function, memory, attention and concentration, processing speed and visual perceptual skills, which translates to inattention in class, inconsistent academic performance, not finishing homework, incomplete assignments, careless errors, and trouble with planning and organisation, with specific problems in the areas of mathematics, reading and spelling. It is usually in the later years of school that these sorts of problems become apparent, when rote learning is replaced with reasoning, and organisational skills and time management skills become of paramount importance. Simple educational accommodations may help (sitting at the front of the class, being allowed extra time to complete exams). These features cannot be assessed in a short-case setting, but the examiners will appreciate the candidate mentioning the importance of these areas, after the physical examination is complete.
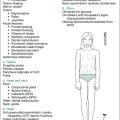
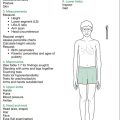
| General observations |
| Introduce yourself: ask name, age, school; assess intelligence (is child alert, conversant?) (leukoencephalopathy secondary to treatment [XRT,MTX]; subnormal IQ with intracranial tumours); note hearing aids, glasses, glass eye, deformities, amputations, prostheses |
| Parameters |
|
• Height (short stature: growth hormone deficiency, panhypopituitarism [cranial XRT, MTX], hypothyroidism [thyroid XRT or MIBG], steroid therapy, skeletal changes from spinal XRT) • Upper segment:lower segment (US:LS) ratio (skeletal changes from radiation of spine) • Percentile charts, noting trend since treatment, look at growth velocity |
• Pallor: anaemia from marrow suppression (most agents), or bone marrow relapse of leukaemia (or development of secondary leukaemia)
• Bruises: thrombocytopenia from marrow suppression (most agents) or (rare) coagulopathy from L-asparaginase, or bone marrow relapse of leukaemia
• Dermatitis (MTX, 6-MP, 6-TG)
• Hyperpigmentation (chronic GVHD from BMT)
• Aniridia (association with Wilms’ tumour, not a late effect)
• Conjunctival pallor (marrow suppression, various agents)
• Scleral icterus (bleomycin, MTX, 6-MP, 6-TG, radiation to liver)
• External ocular movements for evidence of cranial nerve palsy (CNS relapse of leukaemia, VCR neuropathy)
ADR = adriamycin; BMT = bone marrow transplantation; CNS = central nervous system; CPA = cyclophosphamide; GVHD = graft-versus-host disease; MIBG = radioactive iodine metaidobenzoguanidine; MTX = methotrexate; 6-MP = 6-mercaptopurine; 6-TG = 6-thioguanine; VCR = vincristine; XRT = radiotherapy.