Oncologic Emergencies (Case 36: A Problem Set of Three Common Cases)
Minal Dhamankar MD and Zonera Ali MD
Case 1: A 77-year-old man with history of CLL presents with severe fatigue, nausea, and mild abdominal discomfort. He is found to have an elevated white count, splenomegaly, and bulky lymphadenopathy. He is admitted and started on chemotherapy. His basic metabolic panel is as follows: potassium 6.8 mEq/L, calcium 8.1 mg/dL, phosphate 7.0 mg/dL, LDH 28,900 U/L, uric acid 14.3 mg/dL, and creatinine 2.6 mg/dL (baseline creatinine before treatment was 1.0 mg/dL).
Differential Diagnosis
|
Renal Failure in Cancer Patients |
||
|
Tumor lysis syndrome (TLS) |
Infiltration of kidneys by the underlying neoplastic process |
Renal failure secondary to nephrotoxic chemotherapeutic agents |
|
Prerenal azotemia from volume depletion |
Ureteral obstruction due to adenopathy |
|
Differential Diagnosis
|
Low Back Pain and Leg Weakness in a Cancer Patient |
|
|
Brain metastasis |
Asthenia |
|
Lambert-Eaton myasthenic syndrome |
Spinal cord compression (SCC) |
Case 3: A 55-year-old man with a history of acute myelogenous leukemia (AML) presents for a scheduled routine red blood cell (RBC) transfusion and reports fatigue. He is also receiving outpatient chemotherapy via a peripherally inserted central venous catheter (PICC). His temperature is 101°F, and blood pressure is 82/58 mm Hg with orthostatic changes. He is given 1 L of IV fluids and has routine laboratory samples drawn as he is transferred to the hospital. Upon admission, he is having rigors. His lab work shows a white blood cell count of 200 cells/µL and an absolute neutrophil count of 60 cells/µL.
Differential Diagnosis
|
Fever in a Cancer Patient |
||
|
Tumor fever |
Neutropenic fever |
Transfusion reaction |
|
Catheter-related sepsis |
Drug fever |
|
Speaking Intelligently
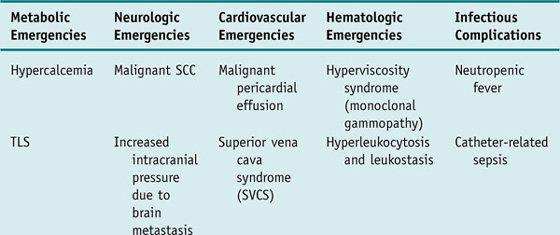
Patients with cancer are subject to developing a unique set of complications that require emergent evaluation and treatment. These oncologic emergencies can be broadly classified as those resulting from the disease itself and those resulting from therapy directed against the cancer; however, they can also be classified according to organ systems to facilitate recognition and management as follows (selected emergencies are discussed in more detail in the Clinical Entities section).
PATIENT CARE
Clinical Thinking
History
• New back pain that is not responding to routine pain medication, worsens when the patient lies down, or is associated with the development of leg weakness, urinary incontinence, and loss of sensory function warrants consideration of epidural SCC.
Physical Examination
Tests for Consideration
Laboratory studies include:
|
$11 |
|
|
• Basic metabolic profile: Tumor lysis syndrome leads to a large number of metabolic derangements. |
$12 |
|
$6 |
|
|
$45 |
| Clinical Entities | Medical Knowledge |
|
Tumor Lysis Syndrome |
|
|
Pφ |
Initiation of cytotoxic chemotherapy in malignancies with high proliferative rate, large tumor burden, and/or a high sensitivity to treatment can result in the rapid lysis of tumor cells. This releases massive quantities of intracellular contents into the systemic circulation, leading to hyperkalemia, hyperphosphatemia, secondary hypocalcemia, hyperuricemia, and acute renal failure. Hyperuricemia is a consequence of the catabolism of purine nucleic acids to hypoxanthine and xanthine, and then to uric acid via the enzyme xanthine oxidase. Overproduction and overexcretion of uric acid in TLS lead to crystal precipitation and deposition in the renal tubules, with resultant acute renal failure. Rapid tumor breakdown can lead to hyperphosphatemia, as phosphorus concentration in malignant cells is higher than in normal cells, which can cause secondary hypocalcemia. Allopurinol blocks the catabolism of xanthine; this can result in xanthine stone formation and resultant acute renal failure despite adequate hydration. |
|
Patients generally have a history of recently started chemotherapy. The tumors most frequently associated with TLS are high-grade non-Hodgkin lymphomas and acute lymphoblastic leukemia (ALL). Underlying hypovolemia or renal failure predisposes to TLS. The symptoms largely reflect the associated metabolic abnormalities. These include nausea, vomiting, diarrhea, anorexia, lethargy, hematuria, heart failure, cardiac dysrhythmias, seizures, muscle cramps, tetany, syncope, and possible sudden death. |
|
|
Dx |
The Cairo-Bishop definition, proposed in 2004, provides specific laboratory criteria for the diagnosis of TLS both at presentation and within 7 days of treatment. Laboratory TLS is defined as any two or more serum values revealing the following abnormalities: • Serum uric acid ≥ 8 mg/dL or 25% increase from baseline • Serum potassium ≥ 6.0 mmol/L or 25% increase from baseline • Serum calcium ≤ 7 mg/dL (1.75 mmol/L) or 25% decrease from baseline These abnormalities must be present within 3 days before and 7 days after instituting chemotherapy in the setting of adequate hydration (with or without alkalinization) and use of a hypouricemic agent. Clinical TLS is defined as laboratory TLS plus one or more of the following that was not directly attributable to a therapeutic agent: increased serum creatinine concentration (≥1.5 times the upper limit of normal [ULN]), cardiac arrhythmia/sudden death, or a seizure. |
|
Tx |
The risk of TLS can be reduced by maintaining adequate hydration status and administering allopurinol for 2 to 3 days before planned chemotherapy. Patients at high risk, such as those with tumors of high proliferative rate, high baseline uric acid, large tumor burden, and chemosensitive disease, may benefit from IV recombinant urate oxidase (rasburicase). Patients with established TLS need hospital admission and may need cardiac monitoring. IV fluids should be given to maintain urine output of ≥100 mL/m2 per hour. Aggressive treatment of hyperkalemia is indicated. Calcium gluconate and sodium bicarbonate should be used in addition to insulin, dextrose, and sodium polystyrene sulfonate (Kayexalate), as severe hyperkalemia is associated with cardiac conduction disturbance. |
|
Hyperphosphatemia can be treated by restricting phosphate intake and with phosphate binders such as aluminum hydroxide. Dialysis is indicated in severe cases, including patients with oliguric renal failure, congestive heart failure, or severe hyperkalemia, or in patients who do not respond to medical therapy. Hypocalcemia should not be treated unless symptomatic. See Cecil Essentials 32, 48, 51, 74, 87. |
|
|
Spinal Cord Compression |
|
|
Pφ |
SCC develops when tumors metastasize to the vertebral bodies and subsequently erode into and encroach on the spinal cord. The thoracic spine is the most common location. Some lung cancers, lymphomas, and sarcomas, which may not cause bony destruction, can lead to spinal cord damage; these tumors occupy the paraspinous space and may enter the spinal canal through the intervertebral foramen, leading to cord compression. The mechanism of injury to the spinal cord from an epidural tumor is due to direct compression of the neural elements interrupting axonal flow or via a vascular mechanism. Venous plexus obstruction can cause marked cord edema, whereas tumor occlusion of the arterial blood supply to the spinal cord creates an acute infarction, leading to abrupt and irreversible cord ischemia. Multiple inflammatory mediators and cytokines can increase the edema and the ischemia, resulting in irreversible neuronal injury. |
|
The most common presenting symptom is back pain. Pain is often worse with recumbency, secondary to distension of the epidural venous plexus. Other symptoms include radicular pain, motor weakness that is usually symmetrical, gait disturbance, and dysfunction of bladder and bowel function. Sensory involvement is less common. Because neurologic deficits may not improve with treatment, it is imperative to consider the possibility of SCC before neurologic dysfunction develops. |
|
|
Dx |
The diagnosis depends upon the demonstration of a neoplastic mass that extrinsically compresses the thecal sac. MRI of the entire spine is the preferred modality for the initial evaluation of a patient with a suspected SCC. It can provide an accurate evaluation of the extent of disease within the thecal sac and of involvement of adjacent soft tissues and bone. CT myelography can be used if MRI is contraindicated or not available. |
|
Tx |
Therapy should be initiated as soon as possible. Glucocorticoids should be given immediately if there is a delay in performing the imaging studies. Dexamethasone is typically given as an initial IV dose of 10 to 16 mg followed by 4 mg every 6 hours. Higher doses (up to 100 mg) may be associated with slightly better outcome but have a higher incidence of adverse effects. Patients without motor deficits or massive invasion of the spine on imaging studies may do well without corticosteroids. Radiation therapy has been the mainstay of the treatment, but recent studies have challenged that belief. Surgery is generally considered for patients with a good performance status and the ability to withstand an extensive operation, gross instability of the spine, rapidly progressive symptoms, progressive symptoms during radiation therapy, or when tissue for diagnosis is needed. See Cecil Essentials 58. |
|
Neutropenic Fever |
|
|
Pφ |
Most episodes of febrile neutropenia occur in patients receiving chemotherapy. Less commonly, patients with acute leukemias, myelodysplastic syndromes, or other diseases that create leukopenias may present de novo with febrile neutropenia. The risk of developing febrile neutropenia depends on both the depth and the duration of the neutrophil nadir, as well as comorbid conditions or complications such as mucositis. The neutrophil nadir typically occurs 5–10 days after the last dose. Usually, white blood cell recovery occurs within 5 days of this nadir. |
|
TP |
Fever is commonly the only symptom, but patients may also have localizing symptoms and physical findings. Common infections may present atypically as a result of the lack of neutrophils. Skin infections may manifest as a subtle rash or erythema. Patients with meningitis may not have the typical physical findings such as nuchal rigidity; furthermore, urinary tract infections may be asymptomatic, and there may be no pyuria. Moreover, because of profound neutropenia, patients can have lung infections without pulmonary infiltrates. |
|
Dx |
A complete workup to identify the source of infection should be undertaken as noted previously. |
|
Tx |
Once febrile neutropenia is diagnosed, antibiotics should be administered immediately once the necessary cultures have been obtained. Initial antibiotic selection should be guided by the patient’s history, allergies, symptoms, signs, recent antibiotic use, culture results, and institutional nosocomial infection patterns. Ideally, antibiotics should be bactericidal. There is no clear optimal choice for empirical antibiotic therapy; combination therapy and monotherapy have led to similar outcomes. Monotherapy: Cefepime or ceftazidime, or a carbapenem (meropenem or imipenem). Dual therapy: Aminoglycoside plus anti-pseudomonal β-lactam (piperacillin or piperacillin/tazobactam), cephalosporin (cefepime or ceftazidime), or a carbapenem (meropenem or imipenem). |
|
Antibiotic therapy should be altered if there is evidence of progressive disease or a new complication. Routine use of gram-positive antibiotic coverage (e.g., vancomycin or linezolid) is not recommended except in the following circumstances: presence of hypotension, mucositis, skin or catheter site infection; history of methicillin-resistant Staphylococcus aureus (MRSA) colonization; recent quinolone prophylaxis; or overall clinical deterioration. Antifungal or antiviral drugs are usually not needed as a part of initial therapy. The echinocandins (e.g., caspofungin) are used as a first-line antifungal therapy in neutropenic patients with no obvious source of infection who are persistently febrile for 5 days despite broad-spectrum antibacterial therapy. Routine use of prophylactic antibiotics or colony-stimulating factors is not recommended. The latter have not been shown to decrease mortality and beneficial effects are quite modest, but they may be used in critically ill patients such as those with pneumonia, hypotension, or organ dysfunction, and in patients whose bone marrow recovery is expected to be especially prolonged. See Cecil Essentials 58, 95, 109. |
|

a. Hypercalcemia: This is discussed in Chapter 45, Paraneoplastic Syndromes.
Practice-Based Learning and Improvement: Evidence-Based Medicine
Title
Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial
Authors
Patchell RA, Tibbs PA, Regine WF, et al.
Institution
Department of Surgery (Neurosurgery), University of Kentucky Medical Center, Lexington, Kentucky
Reference
Lancet 2005; 366:643–648
Problem
Radiation therapy has been the mainstay of the treatment, but recent studies have challenged that belief. This study showed that direct decompressive surgery followed by radiation therapy is superior to radiation therapy alone.
Intervention
In this randomized, multi-institutional, nonblinded trial, patients with SCC caused by metastatic cancer were randomly assigned to either surgery followed by radiation therapy (n = 50) or radiation therapy alone (n = 51). Radiation therapy for both treatment groups was given in 10 fractions of 3 Gy each. The primary end point was the ability to walk. Secondary end points were urinary continence, muscle strength and functional status, the need for corticosteroids and opioid analgesics, and survival time.
Comparison/control (quality of evidence)
The group receiving surgery followed by radiation therapy was compared to the group receiving radiation therapy alone.
Outcome/effect
Significantly more patients in the surgery group (42/50, 84%) than in the radiation therapy group (29/51, 57%) were able to walk after treatment (odds ratio 6.2; 95% confidence interval 2.0–19.8; P = 0.001). Patients treated with surgery also retained the ability to walk significantly longer than did those with radiation therapy alone (median 122 days vs. 13 days, P = 0.003). Thirty-two patients entered the study unable to walk; significantly more patients in the surgery group regained the ability to walk than patients in the radiation group: 10/16 (62%) vs. 3/16 (19%); P = 0.01. The need for corticosteroids and opioid analgesics was significantly reduced in the surgical group.
Historical significance/comments
Direct decompressive surgery plus postoperative radiation therapy is superior to treatment with radiation therapy alone for patients with SCC caused by metastatic cancer.
Interpersonal and Communication Skills
Alert Patients to Potential Complications of Treatment
Educating patients proactively about potential oncologic emergencies in a language that they understand will encourage them to seek timely help and prevent catastrophes. For example, educating patients about neutropenia related to chemotherapy and instructing them to notify the health-care provider of any fever while being treated can lead to earlier intervention, reducing the likelihood of an adverse outcome. A reminder to patients about adequate hydration before and during chemotherapy is also helpful in preventing metabolic derangements.
Professionalism
Inform Patients and Families When Requesting Consultation
In cases of oncologic emergencies, the approach to definitive therapy is multidisciplinary, involving surgeons, radiation oncologists, medical oncologists, and other specialists. Appropriate guidance should be sought from consultants in a timely manner and often on an emergent basis. It is important to inform the patient and family about whom you have consulted for help and why. When things happen quickly in the care of a patient, this matter is often overlooked. Families and patients become anxious quickly when multiple new physicians are suddenly involved. Keep everyone informed and up to date with your plan of management.
Systems-Based Practice
Consider Patient Compliance in Outpatient Treatment
Selected patients with fever and neutropenia can be treated in the outpatient setting. Close follow-up and unrestricted access to health-care personnel are essential when patients are receiving outpatient therapy. Situations such as a history of noncompliance, inability to care for oneself, lack of caregivers, no telephone, or lack of reliable transportation are contraindications for outpatient treatment. The best-studied oral regimen used in outpatient treatment for fever/neutropenia is a combination of ciprofloxacin (500 mg every 8 hours) and amoxicillin/clavulanate (500 mg every 8 hours). When using such a regimen, patients should be assessed daily for the first 3 days to assess compliance, response to therapy, and development of any adverse effects. All patients should be given clear instructions as to when and how to seek medical attention.
Suggested Readings
Bosly A, Sonet A, Pinkerton CR, et al. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients with cancer. Cancer 2003;98:1048–1054.
Cairo MS, Bishop M. Tumor lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3–11.
Coiffier B, Mounier N, Bologna S, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Groupe d’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol 2003;21:4402–4406.
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52:e56-e93.
Halfdanarson TR, Hogan WJ, Moynihan TJ. Oncologic emergencies: diagnosis and treatment (abstract). Mayo Clin Proc 2006;81:835–848.
Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol 2005;23:2028–2037.
Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643–648.
Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer 1998;83:1593–1601.

