CHAPTER 27 Olfactory Groove Meningiomas 
INTRODUCTION
Olfactory groove meningiomas, which account for 10% of all intracranial meningiomas, arise from the cribriform plate or the frontosphenoid suture. Their microscopic appearance, pathologic classification, and female preponderance reflect the characteristics of meningiomas found elsewhere.1 An olfactory groove meningioma was successfully resected by Durante in 1885, and this constitutes the first reported successful removal of a meningioma.2
Olfactory groove meningiomas are usually midline, but as their size increases they may become asymmetric. The frontal lobes are always displaced superiorly and posteriorly, and in larger tumors, inferior and lateral displacement of the optic nerves and chiasm is observed. Their growth can also occur inferiorly through the cribriform plate into the ethmoid sinus, through the planum sphenoidale into the sphenoid sinus, or laterally through the orbit.3,4
Olfactory groove meningiomas can be highly vascularized tumors, as observed in meningiomas in other locations. Their main blood supply is derived from contributions of the external carotid artery. Olfactory groove meningiomas typically receive their blood supply from the anterior and posterior ethmoidal arteries as well as branches of the middle meningeal artery and meningeal branches of the ophthalmic artery.5,6 As they enlarge, variable contributions from the anterior cerebral arteries are observed.
CLINICAL PRESENTATION
Olfactory groove meningiomas are commonly diagnosed when their size is significant and causes local mass effect.7,8 The initial complaints of many patients are nonspecific, leading to a high rate of misdiagnosis. The slow progression of symptoms in many cases may be accompanied by apathy in these patients, which decreases the likelihood they will seek medical care. The time frame noted from the development of initial symptoms to the time of diagnosis varies substantially between different series and has been reported to be up to 14 years.9
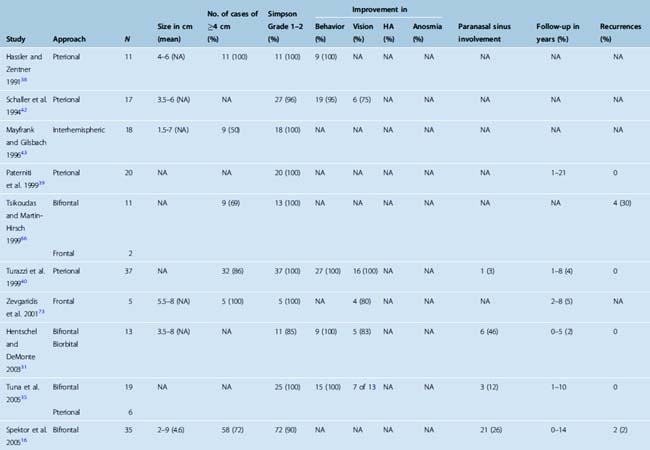
Hyposmia or anosmia is one of the earliest presenting symptoms, but few patients seek medical care for this isolated symptom. At the time of diagnosis, anosmia is noted in more than 50% of cases (Table 27-1). In a rare case, cacosmia has been reported secondary to an olfactory groove meningioma.10 Later aspecific symptoms consist of headaches and personality changes, such as apathy and akinesia, which are commonly misdiagnosed as being associated with depression or aging. Occasionally, an early stage of aggressiveness has been reported.11,12 Exceptional cases of euphoria have also been described.13 Patients then develop a flat affect as well as some short-term memory problems from subtle frontal lobe dysfunction. Later progression can lead to dementia and urinary incontinence. The symptoms usually progress over several months to years, and the impact on the social and professional lives of patients can be dramatic. Mental disturbance is the most commonly reported presenting symptom in published series and occurs in more than 50% of cases (see Table 27-1).
TABLE 27-1 Clinical characteristics of patients with olfactory groove meningiomas reported since 1990
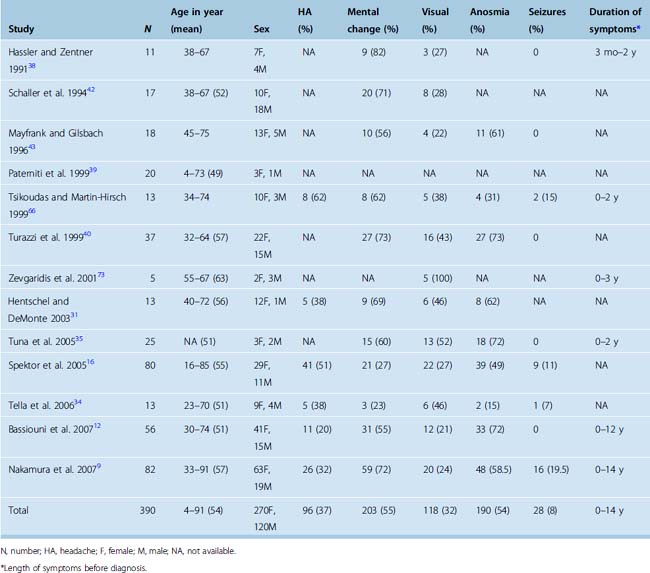
Late findings may include visual symptoms from compression of the optic nerve or optic chiasm. Less frequently, visual symptoms may be due to an intraorbital invasion of the tumor. Varying degrees of visual field cuts as well as loss of visual acuity have been reported in about one third of patients at the time of diagnosis (see Table 27-1).14,15 The presence and severity of a visual defect seems to correlate to the size of the tumor. The triad of anosmia and unilateral optic atrophy with contralateral papilledema first described in the Foster Kennedy syndrome is rare and can be observed in other conditions.16–19 In addition to the Foster Kennedy syndrome, optociliary shunting may be found on funduscopic examination.20
PREOPERATIVE EVALUATION
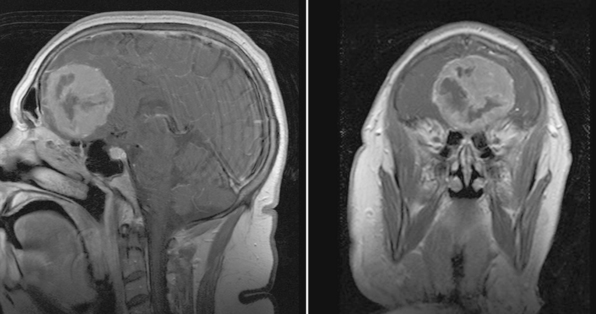
Magnetic resonance imaging (MRI) with and without gadolinium enhancement is the study of choice to confirm the diagnosis of an olfactory groove meningioma. The appearance of these tumors on MRI is similar to that of meningiomas found in other regions of the intracranial cavity or spinal canal. Olfactory groove meningiomas classically appear as a homogeneously enhancing lesion with a dural attachment centered on the cribriform plate (Fig. 27-1). They are usually isointense to gray matter on T1-weighted sequences and iso- to hyperintense on T2-weighted sequences. Ethmoidal or intranasal extension of the tumor can be ascertained as well, and the relationship of the optic nerve and chiasm is visible. The visual apparatus is displaced posteriorly and inferiorly in olfactory groove meningiomas. The anterior cerebral arteries are usually displaced posteriorly and superiorly, and their relationship to the capsule is noted. Encasement of the anterior cerebral arteries within the tumor is rare. Frontal lobe edema is observed to varying degrees. Cerebral edema is present preoperatively in up to 58% of case, prompting the use of steroids.9
A computed tomography (CT) scan with fine cuts through the anterior skull base is useful to evaluate the extent of the paranasal sinus or orbital involvement. The location and degree of hyperostosis may influence the surgical aggressiveness used. Paranasal sinus involvement seems to occur in about 19% of cases (Table 27-2). Intraorbital involvement through erosion of the medial orbital wall is uncommon but has been reported in up to 5% of cases.9
TABLE 27-2 Clinical outcomes and tumor characteristics of patients with olfactory groove meningiomas reported since 1990
A cerebral angiogram is seldom required for diagnosis or definition of the relation to normal vasculature because the anterior cerebral arteries are easily visible on the MRI study. A cerebral angiogram demonstrates a vascular blush at the anterior skull base with posterior and superior displacement of the anterior cerebral arteries. A flow-related saccular aneurysm of the anterior ethmoidal artery has been reported in association with an olfactory groove meningioma, with resolution of the aneurysm on resection of the tumor.21
The size of the tumor at time of diagnosis is highly variable. Tumor sizes of up to 10 cm have been reported at the time of diagnosis.9 At diagnosis, most olfactory groove meningiomas are large and have a diameter greater than 4 cm (see Table 27-2). They can also present as incidental findings on imaging in up to 10% of cases.9
DIFFERENTIAL DIAGNOSIS
The differential diagnosis is usually limited, and the MRI findings are usually very characteristic of these tumors. An olfactory nerve schwannoma or subfrontal schwannoma not associated with any cranial nerves is a rare tumor that can arise in that location.22–25 Key preoperative imaging findings include the presence of bone scalloping as well as the absence of bone sclerosis and a dural tail.26 In one case, a nasal schwannoma has been found in conjunction with an olfactory groove meningioma.27 Occasionally, olfactory groove meningiomas can be difficult to distinguish from esthesioneuroblastomas. A young patient can alert to the possibility of these tumors. An olfactory groove meningioma can also develop after radiation for therapy for the treatment of an esthesioneuroblastoma.28 Dural-based metastases must also be considered in the differential diagnosis. In this regard, rare cases of subdural metastatic disease, notably breast carcinoma, have been reported, sometimes metastasizing an associated meningioma.29 With improved screening and local tumor control for breast carcinoma, this situation has become exceptional.
SURGICAL APPROACHES FOR THE RESECTION OF OLFACTORY GROOVE MENINGIOMAS
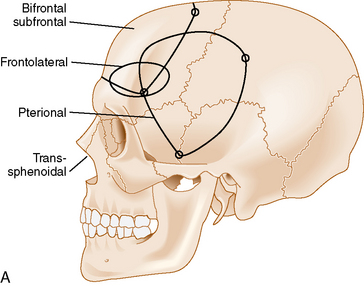
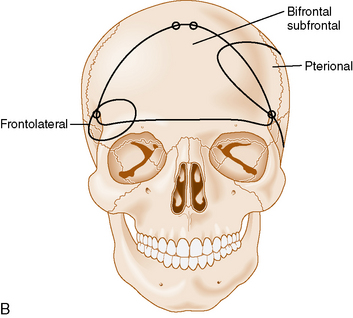
Olfactory groove meningiomas usually require surgical treatment at time of diagnosis because of their size and associated mass effect. Several surgical approaches are commonly used when treating olfactory groove meningiomas: the subfrontal,6,30–36 pterional,33,35,37–42 or interhemispheric approaches.43 Cases with extensive paranasal sinus involvement require a craniofacial approach.16,44,45 The transsphenoidal approaches are also an option in selected cases.46,47
Subfrontal Approaches
The bifrontal subfrontal approach was the first approach described for the treatment of olfactory groove meningiomas. It remains the most widely used approach and different surgeons have developed additions to the approach (see Table 27-2).
The standard bilateral subfrontal approach is performed through a bicoronal skin incision with the patient’s head placed in slight extension in rigid head fixation. The head is optimally positioned to permit unobstructed venous return and to provide maximal separation of the frontal lobes from the tumor. A pericranial flap is harvested and reflected anteriorly, with preservation of the supraorbital nerves and arteries. An interfascial dissection is performed anteriorly over the temporalis muscle to protect the frontalis branch of the facial nerve. The standard bilateral subfrontal approach is made through two burr holes on either side of the superior sagittal sinus, anterior to the coronal suture, as well as burr holes at the anatomic keyhole (Fig. 27-2). The temporalis muscle is cut at the level of the keyhole to the cranium along the direction of the planned craniotomy, permitting muscle closure over the keyhole burr holes for better cosmesis. The craniotomy is completed when adequate lateral exposure is ensured. The lower limit of the craniotomy is approximately a centimeter above the orbital rims. Resection of the frontal sinus mucosa is performed early, and the remainder of the frontal sinus is cranialized, increasing the frontal exposure.
The dura can be opened horizontally in the low frontal area. Bilateral dural opening with ligation of the superior sagittal sinus can be performed anteriorly. The tumor is easily accessible, and its capsule is coagulated anteriorly and inferiorly to achieve maximal devascularization.36 Internal debulking of the tumor is then accomplished. The superior and posterior aspects of the tumor are addressed last. The capsule is dissected off the optic nerves, chiasm, and anterior cerebral arteries using microsurgical techniques. A reasonable arachnoid plane is usually found posteriorly and should be followed for dissection. It is important to dissect the feeding arteries before coagulating them so as to clearly visualize and preserve the anterior cerebral arteries and the perforating branches to the hypothalamus. Frontal lobe retraction is usually necessary at this stage of the operation for good visualization.
Complete resection of the tumor should be possible. Dura mater that is involved with the tumor should be resected when feasible and balanced against the risk of developing a postoperative CSF leak. The anterior skull base should then be inspected, and the crista galli or any areas of hyperostotic bone should be drilled with a high-speed diamond burr drill. Dura should be closed in a watertight fashion to prevent the formation of a postoperative CSF leak. Dural patching may be necessary. The pericranium is used to reconstruct the floor of the anterior skull base and is sutured in place to the dura posterior and inferior to the posterior edge of the frontal sinus. The bone flap is secure to the skull by leaving a sufficient ridge at its inferior surface to prevent necrosis or edema of the pericranial flap.48 Sufficient inferior fixation is also necessary to prevent the bone flap from sinking in and providing an unsatisfactory cosmetic result.
The subfrontal approach can be complemented by the addition of orbital osteotomies as a means of improving access to the tumor and limiting the need for any brain retraction. The keyhole burr hole is extended anteriorly to permit entry into the orbit. A subperiosteal orbital dissection is performed, and control of the anterior and posterior ethmoidal arteries can be accomplished before any tumor dissection.49 The orbital osteotomies are completed with a cut at the frontozygomatic and frontonasal sutures (see Fig. 27-2). The orbital osteotomies can be performed in a unilateral as well as bilateral approach.50
Other variations to the subfrontal approach limit the craniotomy to the size of the patient’s frontal sinus.51 The anterior table of the frontal sinus is resected at the limit of the sinus with an oscillating saw. Cranialization of the frontal sinus is then performed. The dura is opened in a transverse fashion in the low frontal area with sacrifice of the superior sagittal sinus, and resection of the tumor is completed in a similar fashion. The theoretical advantages to this approach consist of exposing less of the superior sagittal sinus. Some debate has arisen as to the safety of surgically dividing the superior sagittal sinus, even in its anterior portion.52 Variations to these approaches have been developed that preserve the superior sagittal sinus while maximizing exposure by incising the inferior part of the falx.53
A less invasive subfrontal approach can be performed through a unilateral craniotomy in the frontolateral approach. Entry into the frontal sinus is not always mandatory and the craniotomy size is smaller (see Fig. 27-2). The approach has a more lateral angle, enabling early CSF drainage by splitting the sylvian fissure proximally. The need for frontal lobe retraction is minimized, and the superior sagittal sinus is preserved. The major limitation to the approach is the limited view offered into the paranasal sinuses or toward the orbits. Reconstruction of the anterior skull base is possible with a free pericranial graft but may be more challenging. The vascular structures are encountered toward the final stages of the operation as in the bifrontal approach. A unilateral osteotomy can also be included in this approach.30,54
The Pterional Approach
The pterional approach has been used extensively in neurosurgery. Yasargil55 advocated and popularized using the approach for the treatment of cerebral aneurysms. Its main advantage consists of an early visualization of the optic nerve, chiasm, and anterior cerebral arteries. The frontal sinus is also left intact, which makes the approach less time-consuming with a decreased risk of infection and postoperative CSF leak. As in the subfrontal approaches, some head extension facilitates the separation of the frontal lobes from the tumor. It is important to balance the degree of head rotation that can be achieved with the need for visualization of the anterior skull base and the extent of contralateral visualization. The reach to the contralateral side is longer than with the subfrontal approach. Early release of CSF facilitates brain relaxation. The tumor may be difficult to access on the contralateral side, especially posteriorly. Smaller tumors are more difficult to access through this approach and may require the use of brain retraction. Access to the tumor’s vascular supply can be performed early in the operation but access to the tumor within the ethmoid sinus is more difficult. Closure of dural defects at the anterior skull base is more difficult in cases requiring significant anterior skull base reconstruction than through a subfrontal approach. The superior sagittal sinus is not exposed and risk of injury is minimal (see Fig. 27-2). The pterional approach is probably optimal in large tumors that are lateralized with minimal disruption of the anterior skull base.
An orbital osteotomy is also used by some authors in conjunction with a pterional approach to improve the subfrontal access to the tumor, maximizing the benefits of the subfrontal approaches as well as the pterional approaches.16,56
The Interhemispheric Approach
The interhemispheric approach has been described as another alternative when resecting olfactory groove meningiomas. The patient’s head is gently flexed in these cases to allow for good visualization of the anterior skull base. A unilateral craniotomy is performed on the side with the most tumor or on the right side in cases in which the tumor is midline. The craniotomy is extended anteriorly until the superior limit of the frontal sinus (see Fig. 27-2). Dissection is performed along the falx cerebri, and the superior pole of the tumor is first encountered with this approach. The working corridor is usually narrow (about 1 cm) between the falx and the frontal lobes. Early devascularization of the tumor is possible in cases in which tumor volume is limited. In cases of large meningiomas, a transtumoral route is necessary to reach the area of the crista galli and the ethmoids to adequately devascularize the tumor. Internal debulking followed by capsular dissection is performed under good direct visualization. The anterior cerebral arteries, optic nerves, optic chiasm, and pituitary stalk are well visualized in this approach. Removal of tumor in the ethmoids is also facilitated by the trajectory of the approach. Good inspection of the anterior skull base can be performed with removal of the dura and hyperostotic bone when feasible.
The Transsphenoidal and Transethmoidal Approaches
The use of transsphenoidal approaches has increased when treating anterior skull base lesions because of improvements in endoscopic optics and instrumentation. Olfactory groove meningiomas have been resected through an endonasal transcribriform route57 or through a glabellar transethmoidal route (see video).58,59 The devascularization of the tumor is performed early, with control of the ethmoidal arteries. Although early devascularization by removing the anterior skull base attachment is an advantage, the major limitations to the approach remain removal of lateral extensions of the tumor over the orbits and the risk of CSF leak postoperatively. The increased use of the endoscopy has facilitated more recent interest in this approach to anterior skull base meningiomas. Reported cases have been limited to small case reports, and their long-term outcomes have not been well defined.
SURGICAL RESULTS AND OUTCOMES
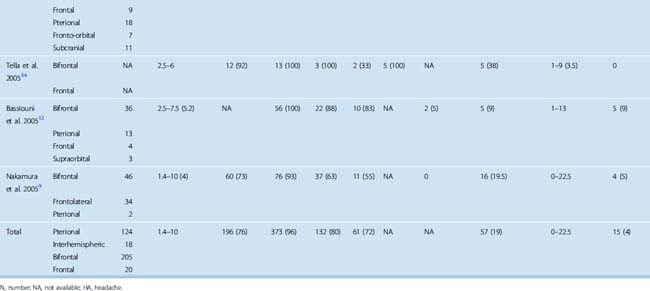
The goals of surgery consist of a gross total removal of the tumor as well as its dura, combined with the drilling of any hyperostotic areas of bone. A Simpson grade 1 or 2 resection should be accomplished in the vast majority of cases. Rates of complete resection (Simpson grade 1 or 2) have varied greatly in published series and have been reported as low as 50%,9,11,13,37,40,60–66 although recent surgical series have all reported high complete resection rates (see Table 27-2). When all studies published since 1990 were reviewed, a combined complete resection rate of 96% was observed. The surgical resection rates accomplished as a Simpson grade 1 versus grade 2 have not been studied in detail.
The choice of surgical approach does not seem to influence the extent of the surgical resections. Simpson grade 1 and 2 resection rates were similar in a single-institution series in which the use of a bifrontal approach was compared with a frontolateral approach.9 When comparing all published series since 1990, similar resection rates have been reported with the interhemispheric, subfrontal, and pterional approaches (see Table 27-2).
A significant heterogeneity also exists between different series as to the extent of removal of hyperostotic bony areas. Hyperostosis is the result of tumor invasion rather than a reactive process.3,67 Some authors remove the superficial area of hyperostosis with coagulation of the dura to limit the risk of a postoperative CSF leak.9,11,32,37 Other authors have suggested removal of all abnormal bone to prevent recurrence.3,13,56,64,68 In anatomic studies, the normal cribriform plates thickness varies between 1 and 16 mm.69 The benefit of aggressive versus more limited bony removal is difficult to assess, although an increase in postoperative CSF leaks has been observed in a more aggressive approach.16,37,40
Patients commonly improve their functional status postoperatively, with mental status generally improving the most, followed by visual disturbance.70 Improvement in mental status occurs in 80% of cases, and full recovery has been reported. Some correlation has been observed between the mental status improvements and the regression of frontal lobe edema.12 Improvement of visual symptoms is reported in 72% of patients who presented with an objective preoperative finding. The degree of visual improvement is linked to the severity and length of the preoperative deficit.71–73 Younger patients also have a better chance at having a better visual outcome.72,73 Postoperative visual deficits are most commonly due to ischemia of the optic apparatus. Some cases may be due to vasospasm, and these respond to blood pressure elevation combined with treatment with calcium channel blockers.31 Most patients who presented with seizures preoperatively are seizure free and off anticonvulsant therapy postoperatively.9 Anosmia is almost universally permanent except in a few exceptions.12,74 Olfactory function may be lost even when anatomic preservation of at least one olfactory nerve was accomplished.75
MORBIDITY
Surgery for olfactory groove meningiomas is now accomplished with good results. The use of the microscope combined with microsurgical techniques has decreased the risk of stroke and visual deterioration. The most common reported complication is the presence of a postoperative CSF leak, which occurs in 7% of patients. The vast majority of CSF leaks respond to the use of a lumbar drain, and further surgery is needed in fewer than 2% of patients (Table 27-3). Infections, in the form of meningitis, are more frequent after craniotomy to resect olfactory groove meningiomas than after craniotomies for other indications. The rate of postoperative wound infections or infections of the bone flap is less than 1% (see Table 27-3). Visual deterioration remains exceptional and is thought to occur secondary to ischemic damage to the optic nerve. Ischemic strokes are rare and are related to the dissection of the tumor capsule along neurovascular structures. The authors of recent surgical series have agreed on a preference for completing a subtotal resection rather than an aggressive resection in cases in which the tumor capsule is adherent to the neurovascular structures. Some authors have suggested that lateral-based approaches (pterional, fronto-orbital, frontolateral) may be associated with a lower incidence of ischemic strokes than subfrontal approaches and their variants.9
TABLE 27-3 Morbidity and mortality associated with the surgical resection of olfactory groove meningiomas in reported series since 1990
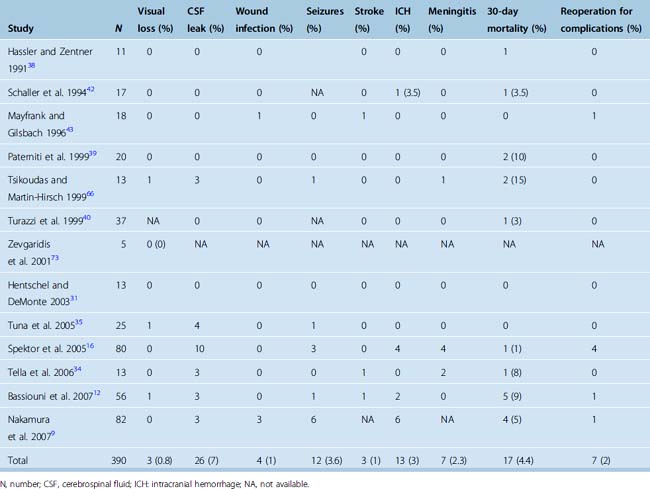
Postoperative intracranial hemorrhages occur in 3% of cases and are explained by the large size of most tumors as well as the risk of injury to the superior sagittal sinus or its draining veins. Occasional reoperation may be necessary. The surgical treatment of larger tumors has been associated with a higher risk of a postoperative hemorrhage.9 Postoperative intracranial hemorrhages are most commonly located in the surgical resection bed, but epidural hematomas have been described in remote locations from the initial craniotomy.16
Reported morbidity rates vary significantly between series, but this variation is probably a reflection of the limited number of cases in each series (see Table 27-3). Most series also cover significant time spans, and a decrease in morbidity over time has been demonstrated in some single-surgeon series.9
The approach used in the different series does not seem to directly affect the incidence of postoperative morbidity. Some authors have been reluctant to use skull-base approaches because of a theoretical increased risk of a postoperative CSF leak or infection.37,40 Authors who have reported their results from large series of skull-base approaches with a breach in the frontal sinus have not observed increased infections when an adequate reconstruction technique was used.9,30,64
The incidence and significance of postoperative edema is poorly reported in the different series. Frontal lobe contusions or edema can occur from the use of frontal lobe retraction, which has led some authors to avoid bifrontal exposures.56 Other authors suggest that the sacrifice of the superior sagittal sinus may induce severe postoperative edema in some cases and approaches should be used to avoid any manipulation of the sinus.9,41,52 The different impact of each approach on frontal lobe function is not known at this time.
MORTALITY
Mortality rates after surgery for olfactory groove meningiomas also differ greatly among series. Mortality is most commonly secondary to supratentorial herniation in cases in which severe postoperative edema, strokes, or intracranial hemorrhages are encountered. Other causes of reported mortality include myocardial infarction, pulmonary embolism, or sepsis. Overall postoperative mortality has historically varied between 0 and 33%.11,13,40,60,61,64,66,76 When all clinical series published since 1990 are included, an overall 30-day mortality rate of 4.4% is observed (see Table 27-3).
CHOICE OF SURGICAL APPROACH
Most published series have exclusively used one approach to the removal of olfactory groove meningiomas, based on the senior author’s preference. The size and location of the tumor did not affect the trajectory used. A few series have tailored the approach to the tumor’s size and laterality and degree of involvement of the paranasal sinuses. The bifrontal subfrontal approach, first described by Tönnis,77 has been advocated as the best approach when dealing with large tumors.64 It gives the best direct access to most sides of the tumor and necessitates the least amount of traction on the frontal lobes. A unilateral subfrontal approach has been advocated in cases of smaller tumors.13,64 The pterional approach is still favored by some authors because it offers better early visualization of the vascular and optic structures.37,38,40 Orbital osteotomies have been used in addition to the pterional or subfrontal approaches to shorten the working distance and decrease the need for frontal lobe retraction.16 Orbital osteotomies have been proposed in addition to a bifrontal cranitotomy,50 frontotemporal craniotomy,16,56 or unilateral frontal craniotomy.30,54 The use of orbital osteotomies increases the risk of a postoperative CSF leak and makes the approach more time consuming. Some authors have preferred the use of unilateral approaches to avoid further cognitive dysfunction from bifrontal lobe manipulation or retraction.30,50 Cases in which there is extensive paranasal sinus involvement require the use of a subcranial approach or combined craniofacial approach.
Some single-institution series performed by one surgeon have reported decreased morbidity and mortality rates when substituting the bilateral subfrontal approach for a more lateral approach.9,52 Such findings have not been reproduced in other series and may reflect the surgeon’s improved experience.
RECURRENCE OF OLFACTORY GROOVE MENINGIOMAS
The overall recurrence rate after resection of olfactory groove meningiomas is difficult to assess. Recurrence rates of 0 to 41% have been reported after surgical treatment.13,62–64,66,68,76 The ranges observed in the recurrence rates are influenced by the degree of initial resection, length of follow-up, and degree of orbital or paranasal sinus involvement. Any surgical series with a least 10 years follow-up have reported recurrence ranges from 5 to 40%.13,62,63,66,68,76 Recurrence rates for olfactory groove meningiomas are higher than those of meningiomas affecting the cerebral convexities.63,78 When all series published since 1990 were reviewed, a recurrence rate of 4% was observed. Follow-up ranged from a few months to 22 years, but the mean follow-up of most studies is less than 10 years (see Table 27-2).
The extent of surgical resection at the initial operation influences the risk of recurrence.1,62,65,76,79–81 Recurrence rates are also higher in cases in which tumor-involved bone was not completely resected at the previous operation.3,63,64,68,82 Recurrences most commonly occur at the cranial base or in the paranasal sinuses.64,83,84 Recurrences up to 15 years after the initial resection have been described.83
The MIB-1 labeling indexes may also affect the incidence of recurrence. A MIB-1 labeling index greater than 10 has been shown to greatly increase the risk of recurrence in large series of meningiomas from various locations.85 Specific data concerning olfactory groove meningiomas specifically are lacking.
RADIOSURGERY FOR OLFACTORY GROOVE MENINGIOMAS
Radiosurgery is rarely a first-line treatment option in cases of olfactory groove meningiomas because of the size of the tumors at time of diagnosis. Radiosurgery could be considered in elderly patients or in poor surgical candidates in cases of small tumors. Radiosurgery may be necessary in cases of tumor recurrence after resection for tumor control in patients who do not wish to undergo further surgery. Radiosurgery is also a useful adjunct in cases of residual tumor after microsurgical debulking in cases of adherence of the meningioma to important structures. Specific data regarding the use of radiosurgery for management of olfactory groove meningiomas in comparison with its use for meningiomas in other locations is lacking. Overall tumor control rates of 93% and tumor volume reductions of more than 10% were observed in 74% of patients with World Health Organization (WHO) grade I and II meningiomas.86
ATYPICAL MENINGIOMAS
Rare cases of atypical or anaplastic olfactory groove meningiomas have been reported.16 Their aggressive behavior and previous history of radiation mimics atypical and anaplastic meningiomas in other locations. Their low prevalence has limited their study to a few case reports. Aggressive surgery with a Simpson grade 1 resection offers the best chance of long-term tumor control.87 The use of stereotactic radiosurgery is recommended in all cases except in atypical meningiomas without brain invasion with a low proliferation index.87 The use of chemotherapy is anecdotal and limited to cases of recurrence. The prognosis of atypical and anaplastic olfactory groove meningiomas is probably better than in cases in other locations because of a higher likelihood of an aggressive resection.
[1] Black P.M. Meningiomas. Neurosurgery. 1993;32:643-657.
[2] Durante F. Estirpazione di un tumore endocranio. Arch Soc Ital Chir. 1885;2:252-255.
[3] Derome P.J., Guiot G. Bone problems in meningiomas invading the base of the skull. Clin Neurosurg. 1978;25:435-451.
[4] Bonfils P., Brasnu D., Roux F.X., Laccourreye H. [Olfactory meningioma with ethmoido-orbital extension. Diagnostic and therapeutic problems. Apropos of a case]. Ann Otolaryngol Chir Cervicofac. 1988;105:179-181.
[5] Hullay J., Gombi R., Velok G., Rozsa L., Borus F. Planum sphenoidale meningioma. Attachment and blood supply. Acta Neurochir (Wien). 1980;52:9-12.
[6] DeMonte F. Surgical treatment of anterior basal meningiomas. J Neurooncol. 1996;29:239-248.
[7] Bakay L. Olfactory meningiomas. The missed diagnosis. JAMA. 1984;251:53-55.
[8] Carvi M.N. Volume assessment of intracranial large meningiomas and considerations about their microsurgical and clinical management. Neurol Res. 2007;29:787-797.
[9] Nakamura M., Struck M., Roser F., Vorkapic P., Samii M. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60:844-852.
[10] Ayad T., Khoueir P., Saliba I., Moumdjian R. Cacosmia secondary to an olfactory groove meningioma. J Otolaryngol. 2007;36:E21-E23.
[11] Bakay L., Cares H.L. Olfactory meningiomas. Report on a series of twenty-five cases. Acta Neurochir (Wien). 1972;26:1-12.
[12] Bassiouni H., Asgari S., Stolke D. Olfactory groove meningiomas: functional outcome in a series treated microsurgically. Acta Neurochir (Wien). 2007;149:109-121. discussion 121
[13] Solero C.L., Giombini S., Morello G. Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir (Wien). 1983;67:181-194.
[14] Slavin M.L. Acute, severe, symmetric visual loss with cecocentral scotomas due to olfactory groove meningioma. J Clin Neuroophthalmol. 1986;6:224-227.
[15] Rohmer F., Philippides D., Buchheit F., Ben-Amor M. [Neuro-ophthalmologic signs of olfactory meningiomas (apropos of 21 cases)]. Rev Otoneuroophtalmol. 1970;42:335-338.
[16] Spektor S., Valarezo J., Fliss D.M., et al. Olfactory groove meningiomas from neurosurgical and ear, nose, and throat perspectives: approaches, techniques, and outcomes. Neurosurgery. 2005;57:268-280.
[17] Kennedy F. Retrobulbar neuritis as an exact diagnostic sign of certain tumors and absecesses in the frontal lobes. Am J Sci. 1911;142:355-368.
[18] Yildizhan A. A case of Foster Kennedy syndrome without frontal lobe or anterior cranial fossa involvement. Neurosurg Rev. 1992;15:139-142.
[19] Markand O.N., Chandrakar K.L. Foster-Kennedy syndrome in a case of olfactory-groove meningioma. J All India Ophthalmol Soc. 1965;13:75-78.
[20] Fukuyama J., Hayasaka S., Setogawa T., et al. Foster Kennedy syndrome and optociliary shunt vessels in a patient with an olfactory groove meningioma. Ophthalmologica. 1991;202:125-131.
[21] Tachikawa T., Adachi J., Nishikawa R., Matsutani M. An anterior ethmoidal artery aneurysm associated with an olfactory groove meningioma. Case illustration. J Neurosurg. 2002;97:1479.
[22] Tan T.C., Ho L.C., Chiu H.M., Leung S.C. Subfrontal schwannoma masquerading as meningioma. Singapore Med J. 2001;42:275-277.
[23] de Souza H.L., Ramos A.M., Ramos C.C., et al. [Olfactory groove schwannoma: case report]. Arq Neuropsiquiatr. 2003;61:125-128.
[24] Yako K., Morita A., Ueki K., Kirino T. Subfrontal schwannoma. Acta Neurochir (Wien). 2005;147:655-657. discussion 657–8
[25] Timothy J., Chakrabarty A., Rice A., Marks P. Olfactory groove schwannoma revisited. Acta Neurochir (Wien). 1999;141:671-672.
[26] Amador A.R., Santonja C., Del Pozo J.M., Ortiz L. Olfactory schwannoma. Eur Radiol. 2002;12:742-744.
[27] O’Brien D.F., Farrell M., Pidgeon C.N. Combined nasal and skull base pathology: adjacent nasal schwannoma and olfactory groove meningioma. Br J Neurosurg. 2005;19:446-448.
[28] Zirkin H.J., Puterman M., Tovi F., Tiberin P. Olfactory groove meningioma following radiation therapy for esthesioneuroblastoma. J Laryngol Otol. 1985;99:1025-1028.
[29] Sampaio P., Telles C., Parise M. [Meningioma of the olfactory groove and breast neoplasms: report of 2 cases]. Arq Neuropsiquiatr. 1992;50:212-215.
[30] Babu R., Barton A., Kasoff S.S. Resection of olfactory groove meningiomas: technical note revisited. Surg Neurol. 1995;44:567-572.
[31] Hentschel S.J., DeMonte F. Olfactory groove meningiomas. Neurosurg Focus. 2003;14(6):e4.
[32] El Gindi S. Olfactory groove meningioma: surgical techniques and pitfalls. Surg Neurol. 2000;54:415-417.
[33] Rubin G., Ben David U., Gornish M., Rappaport Z.H. Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien). 1994;129:26-30.
[34] Tella Jr O.I., Paiva Neto M.A., Herculano M.A., et al. [Olfactory groove meningioma]. Arq Neuropsiquiatr. 2006;64:83-87.
[35] Tuna H., Bozkurt M., Ayten M., et al. Olfactory groove meningiomas. J Clin Neurosci. 2005;12:664-668.
[36] Wei C.P., Wang A.D., Tsai M.D. Resection of giant olfactory groove meningioma with extradural devascularization. Skull Base. 2002;12:27-31.
[37] Hassler W., Zentner J. Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery. 1989;25:942-945. discussion 945–7
[38] Hassler W., Zentner J. Surgical treatment of olfactory groove meningiomas using the pterional approach. Acta Neurochir Suppl (Wien). 1991;53:14-18.
[39] Paterniti S., Fiore P., Levita A., et al. Venous saving in olfactory meningioma’s surgery. Clin Neurol Neurosurg. 1999;101:235-237.
[40] Turazzi S., Cristofori L., Gambin R., Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999;45:821-825. discussion 825–6
[41] Paterniti S., Fiore P., Levita A., et al. Basal meningiomas. A retrospective study of 139 surgical cases. J Neurosurg Sci. 1999;43:107-113. discussion 113–4
[42] Schaller C., Rohde V., Hassler W. Microsurgical removal of olfactory groove meningiomas via the pterional approach. Skull Base Surg. 1994;4:189-192.
[43] Mayfrank L., Gilsbach J.M. Interhemispheric approach for microsurgical removal of olfactory groove meningiomas. Br J Neurosurg. 1996;10:541-545.
[44] Boyle J.O., Shah K.C., Shah J.P. Craniofacial resection for malignant neoplasms of the skull base: an overview. J Surg Oncol. 1998;69:275-284.
[45] Shah J.P., Sundaresan N., Galicich J., Strong E.W. Craniofacial resections for tumors involving the base of the skull. Am J Surg. 1987;154:352-358.
[46] Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3.
[47] Couldwell W.T., Weiss M.H., Rabb C., et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-547. discussion 547–50
[48] Jensen R., McCutcheon I.E., DeMonte F. Postoperative swelling of pericranial pedicle graft producing intracranial mass effect. Report of two cases. J Neurosurg. 1999;91:124-127.
[49] McDermott M.W., Rootman J., Durity F.A. Subperiosteal, subperiorbital dissection and division of the anterior and posterior ethmoid arteries for meningiomas of the cribriform plate and planum sphenoidale: technical note. Neurosurgery. 1995;36:1215-1218. discussion 1218–9
[50] Al Mefty O. Tuberculum Sella and Olfactory Groove Meningiomas. New York: Raven Press, 1993.
[51] Hallacq P., Moreau J.J., Fischer G., Beziat J.L. [Frontal sinus approach to olfactory groove meningiomas]. Neurochirurgie. 1999;45:329-337.
[52] Auque J., Civit T. Les dangers du sacrifice du sinus longitudinal superieur dans son tiers anterieur lors de la chirurgie des meningiomes olfactifs. Neurochirurgie. 1996;42:84-87.
[53] Seeger W. Microsurgery of the Cranial Base. New York: Springer, 1983.
[54] Delashaw Jr J.B., Jane J.A., Kassell N.F., Luce C. Supraorbital craniotomy by fracture of the anterior orbital roof. Technical note. J Neurosurg. 1993;79:615-618.
[55] Yasargil M. Microneurosurgery I. Stuttgart: Thieme, 1984.
[56] Sekhar L.N., Nanda A., Sen C.N., et al. The extended frontal approach to tumors of the anterior, middle, and posterior skull base. J Neurosurg. 1992;76:198-206.
[57] Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
[58] Jho H.D., Alfieri A. Endoscopic glabellar approach to the anterior skull base: a technical note. Minim Invasive Neurosurg. 2002;45:185-188.
[59] Jho H.D., Ko Y. Glabellar approach: simplified midline anterior skull base approach. Minim Invasive Neurosurg. 1997;40:62-67.
[60] Chan R.C., Thompson G.B. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52-60.
[61] Holub K. Intrakranielle Meningeome. Acta Neurochir (Wien). 1956;4:355-401.
[62] Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
[63] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[64] Obeid F., Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53:534-542. discussion 542–3.
[65] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[66] Tsikoudas A., Martin-Hirsch D.P. Olfactory groove meningiomas. Clin Otolaryngol Allied Sci. 1999;24:507-509.
[67] Pieper D.R., Al-Mefty O., Hanada Y., Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-746. discussion 746–7
[68] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7. discussion 8–9
[69] Keros P. [On the practical value of differences in the level of the lamina cribrosa of the ethmoid.]. Z Laryngol Rhinol Otol. 1962;41:809-813.
[70] Chee C.P., David A., Galbraith S., Gillham R. Dementia due to meningioma: outcome after surgical removal. Surg Neurol. 1985;23:414-416.
[71] Andrews B.T., Wilson C.B. Suprasellar meningiomas: the effect of tumor location on postoperative visual outcome. J Neurosurg. 1988;69:523-528.
[72] Rosenstein J., Symon L. Surgical management of suprasellar meningioma. Part 2: Prognosis for visual function following craniotomy. J Neurosurg. 1984;61:642-648.
[73] Zevgaridis D., Medele R.J., Muller A., et al. Meningiomas of the sellar region presenting with visual impairment: impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir (Wien). 2001;143:471-476.
[74] Gerber M., Vishteh A.G., Spetzler R.F. Return of olfaction after gross total resection of an olfactory groove meningioma: case report. Skull Base Surg. 1998;8:229-231.
[75] Welge-Luessen A., Temmel A., Quint C., et al. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry. 2001;70:218-221.
[76] Melamed S., Sahar A., Beller A.J. The recurrence of intracranial meningiomas. Neurochirurgia (Stuttg). 1979;22:47-51.
[77] Tönnis W. Zur Operation der Meningiome der Siebbeinplatte. Zentralbl fur Neurochir. 1938;3:1-6.
[78] Yao Y.T. Clinicopathologic analysis of 615 cases of meningioma with special reference to recurrence. J Formos Med Assoc. 1994;93:145-152.
[79] Adegbite A.B., Khan M.I., Paine K.W., Tan L.K. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51-56.
[80] Al-Mefty O., Holoubi A., Rifai A., Fox J.L. Microsurgical removal of suprasellar meningiomas. Neurosurgery. 1985;16:364-372.
[81] Crompton M.R., Gautier-Smith P.C. The prediction of recurrence in meningiomas. J Neurol Neurosurg Psychiatry. 1970;33:80-87.
[82] Roser F., Nakamura M., Jacobs C., et al. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005;64:37-43. discussion 43
[83] Maiuri F., Salzano F.A., Motta S., et al. Olfactory groove meningioma with paranasal sinus and nasal cavity extension: removal by combined subfrontal and nasal approach. J Craniomaxillofac Surg. 1998;26:314-317.
[84] Snyder W.E., Shah M.V., Weisberger E.C., Campbell R.L. Presentation and patterns of late recurrence of olfactory groove meningiomas. Skull Base Surg. 2000;10:131-139.
[85] Ho D.M., Hsu C.Y., Ting L.T., Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94:1538-1547.
[86] Feigl G.C., Samii M., Horstmann G.A. Volumetric follow-up of meningiomas: a quantitative method to evaluate treatment outcome of gamma knife radiosurgery. Neurosurgery. 2007;61:281-286. discussion 286–7
[87] Modha A., Gutin P.H. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57:538-550.