I-gel® spraglottic device (with permission from Intersurgical Ltd, Wokingham, Berkshire, United Kingdom).
The 2010 American Heart Association (AHA) guidelines on cardiopulmonary resuscitation suggest that continuous monitoring of expired carbon dioxide is mandatory if the patient is being ventilated via an endotracheal tube or supraglottic airway (Figure 9-2).[2]
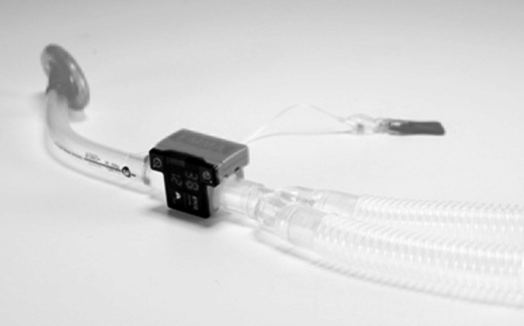
CO2 detector.
In addition to capnography, monitoring should include pulse oximetry, ECG, and noninvasive blood pressure measurement.
Staff must be trained and regularly updated in the management of emergencies and the use of drugs, equipment and monitoring required. The use of protocol based drills enables health-care providers to become proficient in the skills required. Teamwork practice in the actual work environment is important. The evidence that higher fidelity simulation training in the management of emergencies improves patient outcomes does not yet exist. As emergencies are rare, easily accessible checklists and algorithms of care are recommended and have been shown to impact patient outcome.[3]
Finally it is crucial that there is a validated transfer protocol in place. The likely time for transfer, the means by which it occurs, the destination and the staff who will be involved should be determined in advance. Protocols, documentation, and equipment must be agreed upon by transferring and receiving parties. The unexpected emergency department arrival of a patient suffering a complication from an office based procedure, without prior communication and planning, should be viewed as a failure of care.
Cardiac arrest
Introduction
Ischemic heart disease is the leading cause of death among women in the United States and is associated with increasing levels of obesity, smoking, hypertension, diabetes, and hypercholesterolemia;[4] 60% of deaths from ischemic heart disease are due to sudden cardiac arrest. Many of the complications discussed in this chapter may lead to cardiac arrest. The more rapidly a return of spontaneous circulation occurs, the more likely the outcome will be good; up to 30%–40% survival is thought to be possible with prompt cardiopulmonary resuscitation (CPR).
Survival after out-of-hospital cardiac arrest is poor with only 50% victims receiving bystander CPR. Survival in the office setting should approach that of hospital in-patients. The American Society of Anesthesiologists guidelines already require that personnel trained in advanced life support are present whenever patients receive sedation/anesthesia.[5]
The components that lead to a successful outcome have been termed the “chain of survival” and include early recognition and summoning help, early CPR with effective chest compressions, early defibrillation if indicated, effective advanced life support and effective post-resuscitation care.
Presentation
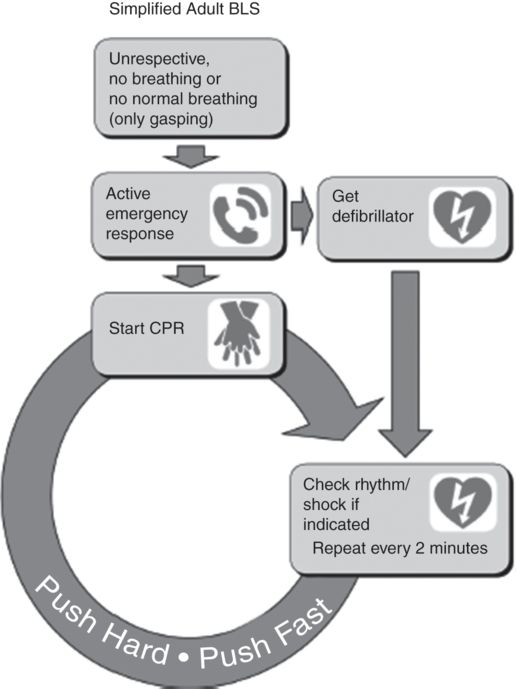
Prompt recognition is vital. The patient is unresponsive since cardiac arrest leads to unconsciousness within 15–30 seconds. Pulses are absent and abnormal gasping respiration or apnea is present. The pupils will usually be dilated. Seizures may occur. The 2010 AHA guidelines recommend summoning help and beginning CPR on an unresponsive patient who is breathing abnormally or apneic, as time can be wasted trying to palpate for a pulse. The advice to “look, listen, feel” has been withdrawn.
Initial management
Initial management follows the AHA basic life support algorithm (Figure 9-3).[6] Chest compressions should start before airway maneuvers or rescue breaths. The emphasis is on effective compressions with minimal interruptions. The ratio of compressions to breaths is 30:2 with a rate of 100 compressions per minute. Compression only resuscitation is an option for adults not suffering drowning. As long as the airway is patent, air will be drawn into the lung and the oxygen (21%) will be sufficient to address hypoxemia.
Securing the airway allows the patient to be ventilated without having to halt compressions. Attempts to secure the airway, however, must not lead to compression interruption. As soon as possible, an AED should be attached. If a shockable rhythm is detected the 2010 guidelines suggest a single shock immediately followed by compressions. Compressions should cease only once the pads have been attached to the chest and the machine charged to further minimize interruption to compressions.
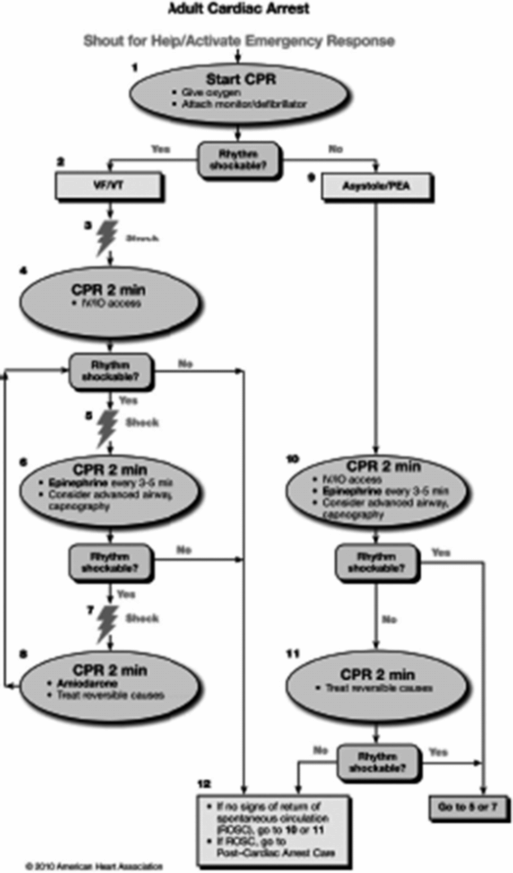
Intravenous access should be secured as early as possible in order to administer epinephrine 1 mg every 3–5 minutes as per the advanced life support (ALS) algorithm (Figure 9-4). If ventricular fibrillation persists after three shocks amiodarone 300 mg should be administered. Atropine is no longer recommended. If intravenous access is impossible the interosseous route can be used. The tibial and humeral sites are usually accessible and fluids as well as drugs can be administered by this route when the equipment is available (Figure 9-5). Reversible causes of cardiac arrest that may arise and can be treated in the office setting include hypoxia, hypovolemia (hemorrhage), and toxins (e.g., local anesthetic toxicity).
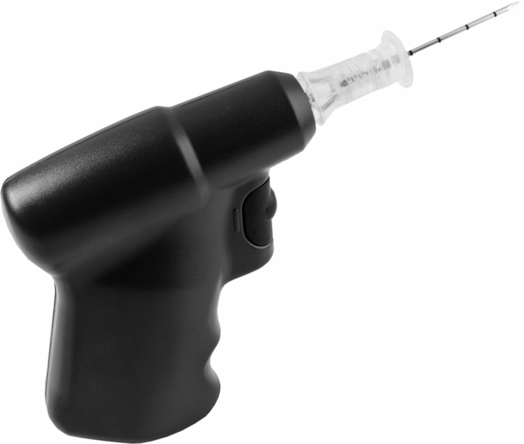
Ezio interosseous drill.
Compressions and defibrillation should be continued during transfer and compressions should not cease until there are clear signs of return of spontaneous circulation.
Investigations
As mentioned previously, continuous waveform capnography is recommended in ventilated patients. Return of spontaneous circulation will be indicated by an increase in expired CO2. Blood pressure should be measure and oxygen saturations measured. A 12-lead ECG should be performed, and blood taken for biochemistry and full blood count.
Acute coronary syndrome
Introduction
Acute coronary syndrome (ACS) refers to a diagnosis of myocardial infarction or unstable angina. Myocardial infarction (MI) may be further subdivided into ST elevation MI (STEMI) and non-ST elevation MI (NSTEMI). NSTEMI and unstable angina occur as a result of the development of thrombus on an atheromatous plaque. In contrast to STEMI, the thrombus is nonocclusive, but distal vasospasm or embolization may lead to subsequent myocardial necrosis.[7]
It is estimated that MI goes unnoticed or unreported in women in approximately one third of cases as symptoms may be more subtle than in men.[8] Furthermore, there is now a rising population of young women in their third and fourth decades of life who have undetected but significant coronary artery atheroma. Consequently, clinicians working in the office environment need to be alert to the possibility of ACS occurring in women of any age group, particularly when underlying cardiac disease may be exacerbated by anxiety and stress in the perioperative period.
Presentation
Acute coronary syndrome presents classically with severe central chest pain which is crushing or squeezing in character and may be referred to the left arm or shoulder. There may be associated symptoms of sweating, dyspnea, nausea, or vomiting. In women with ACS, these symptoms may be more prominent than the pain itself. Pain secondary to MI will often last several hours with no respite. In unstable angina, pain usually occurs at rest due to critical myocardial perfusion. This pain will often resolve after 20–30 minutes, either due to spontaneous clot lysis and restoration of a patent coronary artery lumen, or due to relaxation of coronary artery spasm.[7]
Initial management
In the acute setting, it may be difficult for the clinician to differentiate between MI and unstable angina since their clinical presentation may be almost identical. Initial treatment, however, remains the same for both conditions.
Pain in ACS is due to inadequate perfusion and a lack of oxygen delivery to the cardiac muscle. The initial management should therefore aim to maximize the availability of oxygen to the heart and to restore coronary artery patency. High-flow oxygen should be administered via a face mask at 10 L–15 L/minute. The patient should be asked to chew aspirin 300 mg, or to take clopidogrel 300 mg if there is a hypersensitivity to aspirin or major gastrointestinal intolerance.[9, 10]
Sublingual administration of glyceryl trinitrate spray (400 mcg) or administration of an oral nitrate will also help to promote vasodilatation and increase coronary artery perfusion. Nitrates should be avoided if the patient is already hypotensive. The pain of ACS is severe, and intravenous morphine should be titrated to help with this in addition to the administration of an antiemetic.
Investigations
A 12-lead ECG should be performed as early as possible to look for ST segment elevation, T wave inversion or new left bundle branch block. It should be noted, however, that a normal ECG does not rule out ACS since NSTEMI is more common than STEMI. Cardiac-specific troponins (Troponin I or T) are gold standard diagnostic tests. Troponin level may be normal initially, but usually rises within 2–12 hours and peaks at 12–24 hours post-MI. In an office setting and with a normal ECG, the diagnosis of ACS remains a clinical one, and immediate transfer to a center with percutaneous coronary intervention (PCI) capabilities is indicated, regardless of ECG findings.
Acute asthma
Introduction
Acute asthma remains a major cause of death among young adults. In the United States there are 5,000–6,000 deaths per year, a good proportion being pre-hospital.
Acute attacks account for 2 million visits to emergency departments every year, with one in 4 leading to admission. Up to one in five inpatients need intensive care with a third of these requiring mechanical ventilation.
Death is most commonly due to asphyxia as a result of a combination of bronchospasm, airway inflammation, and mucous plugging. Other potentially fatal complications include tension pneumothorax, pulmonary edema, pneumonia, and atelectasis.[11]
Presentation
The patient may give a history of a respiratory tract infection or worsening of previously well-controlled asthma. She may not be able to complete sentences. Although wheeze is common, the severity is not related to the degree of airway compromise: a silent chest indicates almost complete obstruction. The respiratory rate is raised and greater than 25 per minute indicates acute severe asthma, as does a pulse rate greater than 110 bpm. The condition is life threatening when the patient becomes exhausted, has altered level of consciousness, or becomes cyanosed with a silent chest. Arrhythmia and hypotension may intervene.
Initial management
Treatment should be started immediately even if transfer time is short, as patients can become critically ill very rapidly. Initial treatment includes oxygen, inhaled β2 agonists, and steroids. Intravenous access should be obtained. All patients should be given high-flow oxygen, regardless of whether their oxygenation is within the normal range as it may decrease with treatment (see later in the chapter).
Inhaled β2 agonists such as albuteral are potent bronchodilators with a dose-dependent effect and minimal side effects. There is no evidence that nebulizers are more effective than metered dose inhalers; if one method produces no improvement it is sensible to try the alternative. Bronchodilation may result in a decrease in oxygenation due to an increase in ventilation perfusion mismatch. The addition of an anticholinergic agent such as ipratropium bromide has been shown to produce a modest improvement over Inhaled β2 agonists alone.
Because steroids take up to 12 hours to exert their anti-inflammatory effect they should be given early. Dexamethasone 10 mg or methylprednisolone 125 mg should both be given intravenously.
Magnesium sulfate relaxes bronchial smooth muscle and has been shown to be effective in severe acute asthma. The dose is 2 g given over 20 minutes, intravenously.
Investigations/monitoring
Any asthmatic patient suffering an attack severe enough to prevent her from finishing sentences, who is tachypnoeic, tachycardic, or showing signs of exhaustion should have continuous pulse oximetry and not be left alone. Baseline spirometry for FEV1 (forced expiratory volume in one second) or peak expiratory flow should be measured and repeated at 30 minute intervals.
Seizures
Introduction
Seizures occurring in the office environment are most likely to be secondary to known seizure disorder or a first presentation of epilepsy in a patient. In the United States alone, nearly 2.2 million people suffer from epilepsy; approximately 150,000 new cases of the illness are diagnosed per year.[12]
Although many seizures occur unprovoked, some of the commonest causes of seizures in the absence of primary head trauma include electrolyte disturbance (hypoglycemia is the most common precipitant, particularly in diabetic patients), an intracranial lesion or spontaneous hemorrhage, noncompliance with anti-epileptic medication or sub-therapeutic dosage, stress or anxiety, and use of drugs and/or alcohol.
Iatrogenic causes of seizures which may be directly associated with the performance of certain office gynecological procedures include local anesthetic toxicity and endoscopic fluid resorption leading to water intoxication syndrome.
Initial management
Most seizures are self-terminating, but in some cases a seizure may persist or there may be seizure recurrence. Status epilepticus is defined as a seizure that lasts longer than five minutes or recurrence of seizures without an intervening return of consciousness.[13]
The incidence of permanent brain damage increases with seizure duration.[14] Consequently, in status epilepticus, drug treatment to terminate the seizure state is required as a matter of urgency.
Regardless of the cause of the seizure, the primary goals of treatment in all cases are the same: the protection of the patient’s airway, the prevention of physical injury secondary to convulsions and the rapid termination of the seizure itself. If possible, a patient who is suffering from a seizure should be turned into the left lateral or recovery position to help prevent the aspiration of any regurgitated gastric contents. If the patient is on the operating table, a degree of head-down tilt may also be used to facilitate the drainage of gastric fluid away from the lungs.
High-flow oxygen at 10 L–15 L/minute should be administered via a face mask, vital signs monitored and a patent airway maintained. This can be achieved by simple airway opening maneuvres such as a chin lift or jaw thrust that will prevent the patient’s tongue from falling backward and obstructing the oropharynx. Suction of the oropharynx may be necessary if gastric regurgitation occurs, but this should be performed carefully and under direct vision to avoid causing trauma to friable tissues or a vagal response. In the post-ictal phase, the patient will still need to be monitored closely due to the risk of repeat seizures. Airway opening maneuvres may also still be required at this stage, since airway obstruction can occur secondary to a reduced level of consciousness in the post-ictal state.
If the seizure has not resolved after five minutes, then pharmacologic therapy will be required. First-line drug treatment that can be undertaken in the office environment should be the administration of a benzodiazepine such as lorazepam, diazepam, or midazolam. Intravenous lorazepam (0.1 mg/kg adult dose) is the drug of choice; however, buccal, intramuscular, or rectal benzodiazepines may be quicker to administer in cases where an intravenous cannula is not already in place or where cannulation is difficult. One further dose of the same or a different benzodiazepine should be administered five minutes after the first dose if the seizure is still ongoing.
If no seizure resolution occurs following two doses of benzodiazepine, then the patient will need to be transferred to a definitive care facility for ongoing treatment. The patient is likely to require endotracheal intubation for definitive airway protection prior to transfer, and so urgent and early anesthetic assistance should be sought.
Investigations
As part of the initial office management of the patient with a seizure, a finger-stick blood glucose should be checked urgently. If this is found to be low (<3 mmol/L or 50 mg/dL), an intravenous infusion of dextrose should be commenced immediately. If there is on site access to a blood gas analyzer, then a venous or arterial blood gas sample may be taken to allow detection and correction of any other abnormal electrolyte levels.
Hemorrhage
Introduction
Most procedures undertaken in an office setting are not associated with a high risk of hemorrhage. Occasionally large loop excision of transition zone is associated with significant blood loss. Should there be an undetected coagulopathy (pathological or iatrogenic and undeclared), however, the risk of significant hemorrhage from office procedures is significant.
Presentation
The patient may complain of an unusual amount of pain for the procedure. Early signs of acute hypovolemia include increased respiratory rate and pulse rate. The patient on beta blockers, however, will not mount a tachycardia. Occasionally there is a paradoxical bradycardia. The patient may complain of feeling faint, dizzy, feeling generally unwell, and/or become agitated or confused. Her skin may become cold and clammy. Capillary refill will be delayed.* Pulse pressure (the difference between systolic and diastolic) may be narrowed: The diastolic is increased. Especially in the pregnant patient, a falling systolic blood pressure is a late sign; it would be a mistake to wait for this sign before initiating treatment.[15]
In pregnancy, the signs of hypovolemia are hidden until a much greater amount of blood is lost from the circulation (see Figure 9-6). Small women have smaller blood volumes and will decompensate earlier.
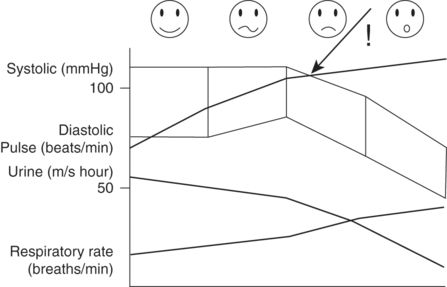
Clinical parameters following increasing blood loss in the pregnant woman.
Initial management
Intravenous access with as wide bore cannula (two are preferable) should be achieved as early as possible. High-flow supplementary oxygen should be given. If the bleeding point is accessible compression should be applied. Crystalloid or colloid, warmed if possible, should be rapidly infused to achieve a rise in blood pressure/fall in pulse rate/improvement in mental state. There is little advantage to colloid over crystalloid although a slightly smaller volume of the former may initially produce a response. Given the choice, a balanced salt solution is preferable to normal saline as large quantities of the latter produce a hyperchloremic acidosis. From mid trimester it is vital to minimize aortocaval compression that will further compromise tissue perfusion in the pregnant patient. This is most effectively achieved by placing the patient in the left lateral position. As a temporizing measure if the blood pressure is significantly low and there are signs of vital organ underperfusion (e.g., deteriorating conscious level), the legs can be raised. Small boluses of a vasoconstrictor may also be used (consider ephedrine 5 mg/metaraminol 0.5 mg–1 mg/phenylephrine 25 mcg–50 mcg). Monitoring the patient suspected of hemorrhage should include frequently repeated pulse rate and blood pressure assessment. Oxygen saturation monitoring is desirable. If possible the patient should be catheterized so that urine output – a sensitive indicator of hypovolemia – can be measured.
Investigations and subsequent management
Early transfer to a unit with imaging to detect site of blood loss, and appropriate facilities should surgery be required, should not be delayed. The patient will require close monitoring during transfer and the receiving facility will need to be given up to date clinical details. The severity of hemorrhage is frequently underestimated and delayed resuscitation and surgical control of bleeding were sited as contributory factors leading to a fatal outcome in a recent report on maternal mortality in the UK.[16]
Anaphylaxis
Introduction
Anaphylaxis is defined as a severe, life-threatening, generalized or systemic hypersensitivity reactions.[17]
It may be subdivided into “allergic” anaphylaxis, which is usually an IgE-mediated reaction, and “nonallergic anaphylaxis,” which is not immunologically mediated. In the office setting, anaphylaxis is most likely to be triggered by exposure to drugs (especially β-lactam-based antibiotics such as penicillins and cephalosporins,[18] latex, antiseptic cleaning solutions, or intravenous colloid.
Presentation
Signs and symptoms of anaphylaxis may be evident immediately after exposure to the allergenic agent, but they often develop between 30 minutes and one hour post exposure. The most common manifestation of an anaphylactic reaction is severe hypotension that may lead rapidly to cardiac arrest. Wheeze secondary to bronchoconstriction may also be an early presenting feature. It is a common misconception that anaphylaxis is always associated with an urticarial rash. This may be the initial presenting feature in as few as 4% of cases or may be a late sign.[19] Consequently, clinicians must retain a high index of suspicion for anaphylaxis in all situations of cardiovascular collapse, even in the absence of a cutaneous reaction.
Initial management
The immediate management of a suspected anaphylactic reaction is focussed on the removal of any suspected triggers, restoration of cardiac output, and provision of adequate oxygenation. If the patient is in cardiac arrest, cardiopulmonary resuscitation must be commenced immediately according to standard life support algorithms.
High-flow oxygen at 10 L–15 L/minute should be administered immediately via a face mask in a conscious patient. Airway edema may develop rapidly and may be heralded by worsening cough, wheeze or voice changes. In such cases it is vital for the airway to be secured rapidly via endotracheal intubation and an anesthetist should be called immediately. In the unconscious patient, administer oxygen via a bag-valve-mask device while waiting for anesthetic assistance.
The key treatment step in the management of anaphylaxis is the administration of intramuscular epinephrine (0.5 mg/kg; 0.5 mL of 1:1,000 solution for an adult). Epinephrine is used in preference to other drugs as it has both alpha and beta adrenergic effects. The alpha adrenergic effects of the drug help to restore the blood pressure by reversing the widespread vasodilatory response to the allergen. The beta adrenergic effects of epinephrine promote relaxation of smooth muscle spasm thus reducing bronchoconstriction. They also attenuate the leakage of capillary fluid that helps to reduce edema, and counteract the negative inotropic effects on the heart which are induced by circulating inflammatory mediators.[20]
Since the effects of epinephrine are short-lived, repeated doses of the drug may be required at five-minute intervals if symptoms are severe.
A liter of crystalloid solution (e.g., Ringer’s lactate) should be given through a large bore cannula and the patient’s legs elevated above the level of the heart. These measures will assist in the restoration of blood pressure and cardiac output by optimizing preload. If colloid is suspected to have been the trigger for the anaphylactic reaction then ensure that the entire giving set and cannula are changed before the administration of crystalloid solution.
Secondary management of an anaphylactic reaction includes the administration of chlorpheniramine (10 mg IV adult dose) and hydrocortisone (200 mg IV adult dose). Chlorpheniramine will help to prevent further mast cell degranulation, whereas the administration of hydrocortisone helps to dampen down the inflammatory response over the following hours. All patients who have suffered an anaphylactic reaction should be transferred to the nearest emergency department as soon as possible. Even those patients who appear to have recovered well following initial treatment have a propensity to develop recurrent symptoms up to six hours after the initial reaction (biphasic response).
Investigations
Mast cell tryptase levels are elevated in most but not all patients who have had an anaphylactic reaction. Levels should be taken at one hour, six hours, and 24 hours post reaction provided that this does not interfere with treatment and stabilization of the patient. Since patients who suffer an anaphylactic reaction in the office will be transferred elsewhere for ongoing management, it is unlikely that the office clinician will have to perform these blood tests on site.
Embolic complications
Introduction
Pregnancy and gynecologic cancers are associated with an increased risk of thromboembolic complications. Additionally, thrombosis and thromboembolism is now the leading obstetric cause of maternal mortality in the latest UK report.[21] In nononcology gynecological patients the risks are increased with rising BMI, increasing age, and certain comorbidities. Young patient who have recently undertaken long-haul flights are also at increased risk.
Gas emboli have classically been linked to laser ablation procedures but can occur during any hysteroscopic or laparoscopic procedures.[22] Carbon dioxide as a distending medium has the advantage of being highly soluble, allowing relatively large amounts to dissolve in the circulation without the formation of bubbles. This, however, leads to acidosis and hypercarbia and subsequent cardiac arrhythmias. Eventually bubbles will form. Air emboli are potentially much more dangerous as air is highly insoluble, so even very small amounts in the circulation can be fatal. Air embolization occurs if air is allowed to enter the distension tubing, usually when bags of irrigation fluid are being changed.
The venous side of the circulation can accommodate relatively large volumes of gas. Conversely even tiny bubbles can cause havoc on the arterial side. The pulmonary capillaries should protect the latter by trapping small bubbles. However this is bypassed in the 20%–35% of the healthy population who have a patent foramen ovale allowing a direct right to left shunt.
Initial presentation
Although pulmonary embolism can present as a major life-threatening event, the presentation is often insidious and easily missed. There may be a combination of symptoms including cough, hemptysis, dyspnea, pleuritic chest pain and low-grade fever. The patient may be tachypneic and/or tachycardic. only a couple of these may be present initially. Signs of right heart failure, such as raised internal jugular pressure, liver distension, and ECG changes are unlikely unless the patient is in extremis. Massive pulmonary embolism may present with marked dyspnea and cyanosis. There may be chest pain due to right ventricular myocardial ischemia, hypotension and circulatory collapse and pulseless electrical activity cardiac arrest.
Gas embolism may present with tachypnea and tachyarthymias. The most sensitive indicator of gas in the pulmonary circulation is a sudden fall in expired CO2 associated with a fall in oxygen saturation indicating interrupted gas exchange. Gas in the cerebral circulation will cause convulsions, paralysis, and sudden death. Intracardiac gas can mechanically obstruct the circulation and cause myocardial ischemia through coronary artery occlusion. The typical “mill wheel” cardiac murmur is not always heard. Pulmonary edema is common.
Initial management
The procedure should be terminated and if gas embolism is suspected the patient should be placed head down in the left lateral position to minimize gas transit to the brain. Regardless of the etiology, high-flow (preferably 100%) oxygen should be administered and intravenous access secured. Cardiopulmonary resuscitation should be initiated without delay if required, and the patient rapidly transferred for investigation and further management.
Monitoring should include ECG, blood pressure, oxygen saturations, and if feasible end-expired CO2 concentrations.
Investigations
Once transferred, arterial blood gas analysis, complete blood count, electrolyte analysis and liver functions tests should be sent. Other investigations such as chest x-ray, ventilation/perfusion lung scan, or computed tomography pulmonary angiogram and echocardiology may be indicated. Doppler ultrasound is the most sensitive detector of gas in the precordium.
Local anesthetic toxicity
Introduction
Local anesthetics are routinely used to provide analgesia and anesthesia for a variety of office gynecological procedures. Toxicity may occur following excessive dosage and absorption or secondary to inadvertent intravascular injection. Clinicians should be alert to the possibility of local anesthetic toxicity in their patients and take steps to reduce the risk of development. These include the use of the smallest effective clinical dose of local anesthetic, careful aspiration, and use of a test dose to help ascertain correct needle placement, slow and incremental injection, and adequate monitoring of patient vital signs and mental state. Early detection of this complication is important.
Presentation
The signs and symptoms of local anesthetic toxicity may be neurological or cardiovascular. Neurological effects tend to occur first; the conscious patient may develop peri-oral numbness or tingling, a metallic taste in the mouth, tinnitus, lightheadedness, agitation, and/or disorientation. Seizures may occur in severe cases. Cardiovascular effects tend to occur with higher blood levels of local anesthetic. These signs include the development of arrhythmias, chest pain, hypotension, and cardiac arrest. Of the local anesthetics in common use, bupivacaine in particular is very cardiotoxic. It adheres strongly to sodium channels in the myocardium and inhibits oxidative phosphorylation.
Initial management
The American Society of Regional Anesthesia and Pain Medicine (ASRA) has published an algorithm for the management of local anesthetic toxicity which is available on its website.[23]
It is recommended that this algorithm is prominently displayed in all office environments where local anesthetics are in common use.
Since hypoxia and acidosis can exacerbate the toxic effects of local anesthetic, the initial management of local anesthetic toxicity involves the administration of high-flow oxygen via face mask at 10 L–15 L/minute. If seizures are present, a small dose of a benzodiazepine should be given to try to terminate the seizures in addition to taking measures to protect the patient from aspiration and to ensure a patent airway is maintained.
The use of a 20% lipid emulsion, such as Intralipid®, is now recommended as first-line treatment of local anesthetic toxicity. The lipid acts as a “sink” for circulating local anesthetic and prevents it from binding to cardiac muscle. It can also reverse neurological signs and symptoms including seizures and altered mental state, and thus is useful even when cardiovascular symptoms are absent.[24]
The initial intravenous bolus dose for an adult is 1.5 mL/kg lean body mass administered over one minute (~100 mL), and this should be followed immediately by a continuous infusion of 0.25 mL/kg/minute (~ 18 mL/minute).
Persistent cardiovascular collapse may require repeated bolus doses of lipid emulsion every five minutes (maximum of two repeated doses). The lipid infusion rate may be doubled to 0.5 mL/kg/minute at any point after five minutes if cardiovascular instability or hypotension persists. Treatment with lipid emulsion should be in conjunction with standard cardiac advanced life support measures if cardiorespiratory arrest occurs. Good quality cardiac compressions are vital in ensuring that lipid reaches the coronary circulation. Recovery from cardiac arrest secondary to local anesthetic toxicity may require prolonged cardiopulmonary resuscitation (>1 hour).
Any patient who develops severe local anesthetic toxicity should be transferred to a site where she can be fully monitored for further complications. A potential exists for a recurrence of cardiovascular depression up to 12 hours after the initial injection of local anesthetic.[13] Use of lipid emulsion should be reported to the international registry at www.lipidregistry.org and cases of local anesthetic toxicity recorded at www.lipidrescue.org.
Investigations
To date, there have been no serious side effects reported in relation to the use of lipid emulsion for resuscitation in local anesthetic toxicity. It is recommended, however, that the total dose of lipid emulsion be limited to approximately 10 mL–12 mL/kg over 30 minutes (~1,000 mL).[24] In addition, amylase levels should be monitored in a place of definitive care, since there may be the potential for hyperlipidemic pancreatitis to develop when large lipid volumes are used.
Vasovagal reactions
Introduction
The vagus forms the major part of the parasympathetic nervous system. Vagal reflexes can cause bronchospasm, laryngospasm, and bradycardia. The latter can be associated with complete heart block and even asystole. Stimulation through dilatation of the cervix is a particularly potent vagal stimulant associated with both bradycardia and bronchospasm (the Brewer-Lickhardt reflex). Stimulation of mesentery, uterus, bladder, urethra can also initiate such reflexes.
Presentation
The patient may give a history of inability to tolerate gynecologic procedures such cervical screening. The conscious patient will complain of feeling dizzy, faint, and unwell and become cool and clammy with a slow pulse and hypotension. She may lose consciousness, suffer a seizure, or even decrease cardiac output. In the anesthetized patient, bradycardia is a fairly common response to cervical manipulation as is distension of the abdomen during laparascopy. Brochospasm or laryngospasm will cause a rise in inflation pressures and problems with ventilation.
Immediate management
Frequently all that is required is for the stimulus to be stopped. To prevent recurrence an anticholinergic such as atropine or glycopyrronium should be given. Glycopyrronium has the advantage that it does not cross the blood brain barrier. A dose of 200 mcg–600 mcg intravenously should be sufficient to bock further vagal reflexes.
Water intoxication syndrome
Introduction
Endometrial ablation, transcervical resection of the endometrium (TCRE), and other hysteroscopic procedures performed in the office setting often require the use of fluid for irrigation, distention of the uterine cavity, and optimal visualization of the surgical field. The most commonly used fluids for these purposes are saline or glycine 1.5% since they are relatively inexpensive, have good optical properties, and lack significant allergic side effects. Alternative fluids that are sometimes used include mannitol, dextran, and sorbitol solutions. Fluid overload complicates 0.1%–0.2% of hysteroscopic gynecologic procedures, although some procedures such as resection of uterine septa and myomectomy are associated with a greater risk.[25]
Fluid overload can result in dilutional coagulopathy, pulmonary and cerebral edema, and heart failure; these can be fatal. Hyponatremia and hyposmolarity result from fluid overload with electrolyte free, low viscosity fluids. Premenopausal women are 25 times more likely to die or suffer permanent neurological damage from cerebral edema caused by hyponatremia than the postmenopausal or male population due to inhibition of the NA-K adenosine pump by estrogens. Fluid overload with metabolic complications can, however, also occur with the use of isotonic solutions. Normal saline overload has been associated with dilutional coagulopathy and hyperchloremic acidosis.[26]
The driving force for fluid absorption into the circulation is the pressure of the fluid itself. When the fluid pressure exceeds the venous pressure of ~1.5 kPa, fluid is forced into open veins which have been severed during surgery. The volume absorbed increases significantly with the length of time at which the fluid pressure exceeds the venous pressure.[27]
Presentation
The syndrome may manifest itself either intraoperatively or postoperatively (often 30–45 minutes post procedure) with a mixture of circulatory and neurological signs and symptoms. These will be dependent on both the volume of fluid absorbed and the type of fluid used. Symptoms may occur with as little as 500 mL of fluid absorption and are due to a combination of intravascular volume overload, intracellular edema and hyponatremia, with or without complications directly related to the choice of irrigation fluid.
The most consistent signs of water intoxication syndrome are bradycardia and arterial hypotension.[28]
Chest pain, pulmonary edema, and oliguria may also occur. Conscious patients may become agitated or confused and experience nausea and vomiting or headache. Glycine is an inhibitory neurotransmitter that has particularly marked effects on the transmission of retinal impulses to the central nervous system; it is metabolized to ammonia by the liver. Transient visual disturbances occur in approximately 10% of patients who absorb an excess of 500 mL of glycine. Cerebral edema has been reported after the absorption of >1 L of glycine during TCRE. In the most severe cases, water intoxication syndrome may be associated with seizures (serum sodium < 120 mmol/L), loss of consciousness, and cardiorespiratory arrest.
Initial management
The prevention of water intoxication syndrome should be the main aim. There is growing evidence to suggest that preoperative treatment with gonadotropin-releasing hormone (GnRH) agonists both reduces the amount of fluid absorbed and minimizes the effects of hyponatremic hypo-osmolar encephalopathy by reversing the estrogen inhibition of the Na-K adenosine triphosphatase pump. Simple preventative measures include ensuring that the patient has a normal serum sodium concentration and is euvolemic prior to undertaking surgery. There should also be careful monitoring of the amount of fluid being used and absorbed intraoperatively in all cases.
If water intoxication syndrome does occur or is suspected, then the treatment is mainly supportive. There should be a predetermined volume of absorption; after this level, electrolytes should be measured and diuretics given. Furosemide should not be given when hypotonic fluid is implicated as it may worsen the hyponatremia. The maximum fluid deficit tolerated without terminating the procedure will depend on the fluid used and presence of comorbidities. A maximum deficit of 1,000 mL has been recommended when hypotonic solutions are used in the healthy patient, (although complications of hyponatremia have been associated with as little as 200 mL deficit). Up to 2,500 mL is suggested when isotonic solutions are used.[29] Obviously the procedure should be halted earlier should there be clinical signs of overload.
Investigations
Serum sodium and serum osmolality should be measured. The administration of hypertonic saline may be indicated after transfer to higher level care if there is evidence of cerebral edema or the serum sodium level falls below 120 mmol/L. Correction of serum sodium should not occur at a rate exceeding 1 mmol/L/hour due to the risk of cerebral pontine myelinolysis.[28]
Concluding remarks
In order to minimize the likelihood of an office emergency occurring, it is important that careful patient selection occurs prior to surgery, particularly with reference to underlying comorbidities and general patient health. When dealing with an office emergency, staff must follow relevant and current guidelines and these must be readily available for review. Frequent multidisciplinary training and simulation in emergency scenarios should be undertaken to ensure that staff are fully prepared, and emergency equipment and drugs should be regularly checked and kept up to date. Clear protocols must be in place to allow the rapid transfer of a patient to a definitive care facility once initial emergency treatment has been instigated.
References
* To test capillary refill the nail bed should be pressed for five seconds. Capillary refill time (CRT) is normal if color returns within two seconds of the compression being released.