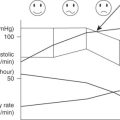
Cardiac evaluation and care algorithm for noncardiac surgery based on active clinical conditions, known cardiovascular disease, or cardiac risk factors for patients 50 years of age or greater.
* Active clinical conditions include unstable coronary syndromes, decompensated heart failure – HF (NYHA functional class IV worsening or new-onset HF), significant arrhythmias, and severe valvular disease.
† Class III recommendations for noninvasive stress testing.
‡ Estimated MET level equivalent.
§ Noninvasive testing may be considered before surgery in specific patients with risk factors if it will change management.
// Clinical risk factors include ischemic heart disease, compensated or prior heart failure, diabetes mellitus, renal insufficiency, and cerebrovascular disease.
¶ Consider perioperative beta blockade for populations in which this has been shown to reduce cardiac morbidity/mortality.
Abbreviations: ACC/AHA – American College of Cardiology/American Heart Association; HR eheart rate; LOE alevel of evidence; MET Tmetabolic equivalent.
Although most patients undergoing elective gynecologic surgery would likely be classified in ASA 1 through ASA 3, it is important to consider the high-risk patient with multiple comorbidities including cardiac disease. As the female population ages, the prevalence of stable cardiovascular disease is bound to increase with the concomitant increase in number of elective gynecologic procedures.[30] In this context, the American College of Cardiology (ACC) and the American Heart Association (AHA) have developed joint guidelines for preoperative cardiovascular evaluation for noncardiac surgery, incorporating clinical predictors and functional status into the preoperative risk-assessment algorithm.[30]
Patients are stratified according to major, intermediate, or minor “clinical predictors” of increased cardiac risk. Those with no risk factors may proceed to surgery without further cardiac evaluation. Patients with one or two risk factors planning for at least intermediate surgery are able to proceed with heart rate control. Noninvasive testing can be considered if results would potentially change management.[30]
To summarize, the obstetrician-gynecologist should choose preoperative testing wisely, with consideration of patient’s functional status as assessed during the office visit, risk profile, and risk level of the proposed procedure. Evidence suggests no value of preoperative testing prior to low-risk surgery.[32] For intermediate risk surgery, preoperative testing should be obtained only if the functional status of the patient is questionable. No invasive testing is required for patients with moderate functional capacity (four to seven METs) and intermediate clinical predictors (mild stable angina, prior myocardial infarction, diabetes mellitus, compensated or prior congestive heart failure) with asymptomatic stable disease.[33] Few experts would recommend performing pulmonary function testing in the preoperative assessment of the gynecologic patient. Data also suggests low value from routine preoperative chest x-rays.[5]
Optimization
In a generally low-risk patient population undergoing low to intermediate-risk procedures, surgical risk should be low. In this context, quality improvement processes are imperative for identifying characteristics or comorbidities that predispose women to increased perioperative complications.[2] Outcomes data exists to help the obstetrician-gynecologist with risk stratification and surgical planning.
Modifiable risk
The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), which was started in the Veterans Health Administration in 1991, performs central analysis including additional centers to generate observed/expected ratios for 30-day mortality and morbidity for each of eight major surgical specialties including gynecology. Notably, since the initiation of this program, the 30-day morbidity after major surgery has decreased by 45% and the 30-day mortality has decreased by 31%.[2] This decrease suggests that outcomes data can be used to create a clinical prediction tool to differentiate women at risk. ACS NSQIP data demonstrates complications in gynecologic surgery associated with preoperative anemia, ASA 3 category, smoking, and morbid obesity with BMI greater than 40.[3] The obstetrician-gynecologist largely performs elective surgery in the context of longitudinal and comprehensive care and, thus, is in a unique position to work closely and diligently with patients to improve health and reduce risks in these targeted areas.
In addition, in the perioperative setting, the obstetrician-gynecologist can impact the incidence of known complications of surgical site infection, deep venous thrombosis, and blood transfusion with established evidence-based guidelines.
Surgical site infection prophylaxis
Surgical site infection is the most common surgical complication, occurring in up to 5% of patients undergoing operative procedures. Aseptic technique has been associated with a dramatic decrease in surgical site infection. However, contamination of the operative site with endogenous bacterial flora is inevitable; this includes aerobic gram-positive cocci from the skin, anaerobic bacteria and gram-negative aerobes from the perineum, and mixed aerobes and anaerobes in the vagina. The goal of antimicrobial prophylaxis is to augment natural immune-defense mechanisms, helping to kill bacteria that are inoculated into the wound. Timing of the prophylaxis is essential. To achieve adequate concentrations of the antibiotic given at the beginning and during the procedure, the recommendation is for administration to occur within an hour before incision.[6] For most minor procedures (elective suction curettage abortion is an exception) antibiotic prophylaxis is not indicated. For major procedures where there is contamination of the peritoneal cavity from the vagina (i.e., hysterectomy), one dose is usually sufficient but an additional dose may be recommended either with blood loss greater than 1,500 mL or at intervals of one or two times the half-life of the drug (for cefazolin when the duration of surgery approaches three hours). Morbidly obese patients (BMI over 35) should receive a higher antibiotic dose to achieve adequate tissue concentrations. Guidelines for the prevention of surgical site infection have been outlined by ACOG and by the US Centers for Disease Control and Prevention (CDC).[34, 35] The adequate and appropriate use of prophylactic antibiotics has been shown to reduce the postoperative infection burden as well as morbidity and associated costs.[6]
Bacterial vaginosis is a known risk factor for surgical site infection after hysterectomy. Although ACOG does not offer specific screening guidelines in the preoperative setting, ACOG does recommend that patients found to have preoperative bacterial vaginosis be treated before hysterectomy.[34] In a 2011 cost-comparison study, McElligott et al. suggest that the optimal strategy clinically and economically would be a “treat all” strategy to add metronidazole to standard surgical site prophylaxis before hysterectomy.[36]
Venous thromboprophylaxis
Postoperative venous thromboembolism (VTE) ranges from 15% to 40% in women undergoing major gynecologic surgery without thromboprophylaxis, and about 40% of all deaths after gynecologic surgery are directly associated with pulmonary embolus (PE).[6] The components of Virchow’s triad – stasis, endothelial injury, and hypercoagulability – are easily present in gynecologic surgery. Pertinent risk factors for postoperative VTE include age, prior deep venous thrombosis (DVT), varicose veins, infection, malignancy, smoking, obesity, immobility, and trauma associated with extent or prolonged surgery (duration over 300 minutes and blood loss of more than 600 mL).[6]
The strategic plan to avoid VTE in gynecologic surgery should begin in the preoperative period. Patients should be classified into one of four risk categories – (1) low, (2) medium, (3) high, and (4) highest risk – to determine the appropriate thromboprophylaxis regimen.[39] Risk is determined based on procedure type and duration, age, and presence of other risk factors. For patients with history of extensive or recurrent thromobosis, or family history of thrombosis, testing for inherited and acquired thrombophilias should be considered.
Only the patient under 40 years of age with no risk factors, undergoing a short procedure less than 30 minutes in duration, will require no prophylaxis. All others should receive one or a combination of pharmacological and mechanical prophylaxis. Mechanical methods such as graded compression stockings or intermittent pneumatic compression devices reduce venous stasis and promote endogenous fibrinolysis. Low-dose unfractionated heparin and low molecular weight heparin (LMWH) prevent clot formation by disrupting the clotting cascade.[37] All methods have each been shown to effectively reduce development of VTE. Gynecologic surgeons depend on data from the general surgery literature, which suggests that a combined regimen of medical and mechanical prophylaxis may improve efficacy, especially in patients at highest risk of VTE.
Preoperative discontinuation of hormonal contraceptives and hormone replacement is not a routine recommendation in gynecologic surgery. Although the Women’s Health Initiative demonstrated an increased risk of VTE with estrogen and progesterone use, no existing trials demonstrate that discontinuation of hormones in the preoperative setting leads to a reduction in postsurgical VTE.[37] General surgery literature often recommends discontinuation of hormones four to six weeks prior to surgery.[5] However, gynecologic surgeons must consider the risks of unintended pregnancy in addition to the therapeutic benefits of hormonal treatments in the perioperative period. Hormonal medications should be considered in risk stratification for VTE prophylaxis.
Blood loss preparation
Patients frequently undergo gynecologic surgery for indications commonly associated with anemia. Importantly, perioperative anemia is associated with a fivefold increased risk of blood transfusion in patients undergoing major gynecologic surgery and has been associated with poor clinical outcome in noncardiac surgery patients.[38] Gynecologic surgeons thus should recognize preoperative anemia as an important and modifiable risk factor for surgery.[39] The preoperative evaluation provides the opportunity to detect and correct anemia prior to elective surgery. Iron deficiency anemia can be corrected with oral or intravenous iron supplementation. Oral iron, inexpensive and highly effective, is considered first line treatment for iron deficiency anemia.[40] Hormonal medications and pharmacologic therapies such as ibuprofen or tranexamic acid to decrease menstrual blood loss in the preoperative period can also be useful either alone or in combination with iron therapy. A Cochrane review on preoperative gonadotropin-releasing hormone (GnRH) analogue therapy before hysterectomy or myomectomy for uterine fibroids concludes that the use for three to four months prior to fibroid surgery is beneficial in the correction of preoperative iron deficiency anemia, and does reduce intraoperative blood loss.[41]
Concluding remarks
Preoperative risk assessment and optimization performed in the office setting can facilitate a smooth and safe intraoperative and postoperative course and improve overall outcomes. A thorough preoperative office evaluation for gynecologic surgery includes a complete history including a review of systems and assessment of functional status, a comprehensive physical examination, and the procurement of more information through laboratory and radiographic studies as indicated, for diagnostic and/or cardiopulmonary risk assessment. In general, the female patient undergoing an elective gynecologic low to intermediate-risk procedure is healthy. However, as the population ages and becomes more complex, it is important for the obstetrician-gynecologist to understand and use the algorithms for cardiac risk stratification for surgery. Preoperative testing should be chosen wisely. Evidence-based practices surrounding the use of thromboprophylaxis and antibiotics and for the treatment of perioperative anemia are followed to minimize surgical morbidity. Finally, the office preoperative evaluation is an opportunity to anticipate postoperative needs and set expectations for healing and recovery. The obstetrician-gynecologist plays a unique role in the longitudinal and comprehensive care of the female patient. Well equipped with knowledge of evidence-based guidelines and outcomes data to choose tests and interventions wisely, the obstetrician-gynecologist can guide the patient safely through the surgical experience.