Disorders of the thoracic spine
Pathology and treatment
Disorders and their treatment
Tumours of the thoracic spine
• Intraspinal tumours are sited in the spinal canal. They are further divided into intra- and extradural tumours. The former may be intra- or extramedullary.
• Extraspinal tumours involve the bony parts of the vertebrae. Benign tumours are usually located in the posterior parts (spinous and transverse processes), malignant tumours in the vertebral body.
Intraspinal tumours
Thoracic neurofibroma
Pathology
This benign tumour usually originates from the dorsal root and arises from proliferating nerve fibres, fibroblasts and Schwann cells. Sensory or motor fibres may be involved.1 Some confusion exists about the terminology: various names, such as neurofibroma, neurinoma, neurilemmoma or schwannoma have been used. Some believe that all these terms cover the same type of tumour; others distinguish some slight histological differences between them. Multiple tumours in nerve fibres and the subcutaneous tissues, often accompanied by patchy café au lait pigmentation, constitute the syndrome known as von Recklinghausen’s disease.
Neuromas are the most common primary tumours of the spine accounting for approximately one-third of the cases. They are most often seen at the lower thoracic region and the thoracolumbar junction.2 More than half of all these lesions are intradural extramedullary (Fig 1), 25% are purely extradural, 15% are both intradural and extradural, and very rarely they are seen intramedullary.3,4 They may become symptomatic in patients at any age, but the peak incidence is around the fourth and fifth decades.5 The tumours are benign, slow-growing and remain solitary,5 well circumscribed and encapsulated. Often there is a cystic degeneration within the tumour. A tumour that is within the intervertebral foramen is often shaped like an hourglass or dumb-bell, one limb of which can give rise to a paravertebral extraspinal extension, which is sometimes palpable.
History
Symptoms are usually due to the compression of dura, spinal cord and roots. Few symptoms are present until the tumour reaches a large mass. Cystic tumours have a high risk of causing progressive symptomatic worsening as a result of cyst expansion.6
In the early stage, the diagnosis is often difficult because neurofibromas usually give rise to symptoms almost identical to those of a disc lesion.7,8 Thoracic neuromas may even simulate a disc problem at the lumbar level.8–11
Involvement of the sensory fibres may result in a segmental band-shaped area of numbness. The tumour may also compress the spinal cord, affecting both motor and sensory elements (see p. 390). As a consequence, the patient may complain of stiffness of the legs, muscle spasms, extrasegmentally referred pins and needles, and disturbed sphincter function with loss of bowel or bladder control.10 Exceptionally Brown–Séquard syndrome occurs.5
Clinical examination
All signs slowly and progressively increase. Finally, neurological signs may develop but they come on much later than in malignant tumours. They may consist of a band-shaped numbness related to one dermatome. The tumour may also affect motor fibres but segmental motor deficit is very difficult to detect. Once the tumour compresses the spinal cord, any of the signs of cord compression may be encountered: depression of abdominal reflexes, hyperactive patellar and Achilles tendon reflexes and sensory loss.4
Special investigations
MRI appears to be the most sensitive investigation for identifying these lesions. Nerve sheath tumours have equal or less signal intensity on T1-weighted images and mild to marked hyperintensity on T2-weighted images as compared to the spinal cord.12 Focal areas of even greater hyperintensity on T2-weighted images often correspond to cystic portions.
Treatment and prognosis
Neurofibromas may undergo malignant change or produce cord compression. Therefore, they should be surgically removed. Small posteriorly or laterally sited tumours can usually be dealt with easily. Anterior neurofibromas present more problems and usually can be only partly excised.13
Other intraspinal masses
Clinical presentation
The primary and most universal symptom is pain, which is usually felt centrally in the back and may spread bilaterally as girdle pain.14 The pain increases progressively and is relentless, despite the patient’s attempts to limit activities. In anterior compression of the dural sac, L’hermitte’s sign is sometimes present.15
Occasionally the pain is worst at night. Although this is classically regarded as being suggestive of a tumour, it is rather rare.8,9
Straining and coughing may increase the pain as may active movement, but to a lesser degree than in mechanical disorders.9,16
The clinical pattern depends on the extent of the tumour: all tests can be completely normal or movement may be considerably limited. If the latter, anteflexion is usually involved and there is often associated muscle spasm. As a rule, in intraspinal soft tissue masses, not much is learnt from articular movements. Besides the positive articular signs, all intraspinal masses give rise sooner or later to neurological signs caused either by involvement of one or more nerve roots or by compression of the spinal cord (see p. 390).
It should be noted that a tumour is not always found where it would be expected on a clinical basis. Cases have been reported where upper thoracic tumours gave rise to pain and neurological signs in the lower limb or in the lumbar area.8,9
Further investigations
In all instances, further investigation is called for. Examination of the cerebrospinal fluid may show elevated proteins, a finding strongly suggesting a neoplasm.9
Neoplasms that are frequently associated with skeletal metastases include tumours of the breast, prostate, lung, kidney, thyroid and colon.17–20 Data from autopsy material suggest that up to 70% of patients with a primary neoplasm from one of these sources will develop pathological evidence of metastases to vertebral bodies in the thoracolumbar spine.21 Because the majority of metastases occur in the vertebral body, they may cause anterior compression of the spinal cord, either directly by tumour growth or by a pathological fracture with retropulsion of bone and disc fragments into the spinal canal.22 Finally compression of the cord can result from an intradural metastasis.23
Extraspinal tumours
Neoplasms located outside the spinal canal are called extraspinal tumours.
In general, benign tumours are located in the posterior elements of the vertebrae and are found in patients under 30 years of age, whereas malignant tumours (both primary and metastatic) are located in the anterior components of the vertebrae and are more common after the age of 50. Myelomas and metastases are the most frequent malignancies.17
Diagnosis is made on laboratory examination and radiographic evaluation.
Clinical presentation
In 95% of cases, the first symptom is local neckache or local thoracic backache, which goes on to radiate. Suspicion should arise when this occurs in patients over 50 years of age complaining for the first time of backache not preceded by trauma. The pain tends to increase in intensity progressively and to involve a larger area: expanding pain. If radicular pain is present, it is usually worse at night (Borenstein and Wiesel:1 p. 309).
Differential diagnosis (see Tables 1, 2) must be careful,8,9,16, 24–29 because the majority of the signs and symptoms also occur in ordinary thoracic disc lesions. It is based mainly on clinical examination, because up to 30% of the bone mass has to be lost before metastases may become visible on radiography.30 When there is the slightest possibility that there is a tumour, manipulation should never be done and further investigations such as a bone scan must be carried out.
Extradural haematoma
Table 2
Differential diagnosis of thoracic disc protrusion and neurofibroma
| Neurofibroma | Thoracic disc protrusion | |
| Age | Young | 20–50 years |
| Pain | Slowly increasing | Swift onset; if chronic, no increasing pain but ups and downs |
| On inspiration or cough | + | + |
| Preferred sleeping position | Sitting up | Lying down |
| Articular movements | Usually negative | Partial articular pattern |
| Side flexion away from the painful side: painful and limited? | ||
| Dural signs | ||
| Neck flexion | + | + |
| Scapular approximation | + | + |
| Neurological signs | ||
| Band-shaped area of numbness in one dermatome | + | Unusual |
| Segmental motor deficit | + | Unusual |
| Signs of cord compression | + | Unusual |
| Palpable mass | ± | _ |
Compression of the cord by a haematoma is also a well-known, although rare, complication of spinal surgery.31 The reported frequency is between 1 and 6 per 1000 operations. Traumatic bleeding in the epidural space has also been reported after epidural injections and chiropractic manipulations.32
Non-traumatic extradural spinal haematoma is an uncommon condition often associated with a poor outcome. There seems to be an increase of the incidence, probably from the increased use of thrombolytic and anticoagulant therapy.33–35 Other causes of non-traumatic extradural spinal haematoma include vasculitis such as systemic lupus erythematosus (SLE), spinal arteriovenous malformations and haemophilia.36,37
A spinal epidural haematoma may present acutely or subacutely over a number of days or weeks and with fluctuating symptoms. The patient usually suffers from increasing and expanding thoracic back pain, followed by progressive signs and symptoms of major neurological dysfunction secondary to cord compression.38 Conditions that may mimic an acute spinal haematoma include extradural abscess and extradural metastatic infiltration.
It is important to make an early diagnosis because surgery may offer the best hope of restoring neurological function. MRI is the examination of choice and provides characteristic findings that allow a prompt diagnosis.39 The technique can also provide useful information about the age of the haematoma.40
Spinal epidural haematoma has always been considered a neurosurgical emergency. The treatment of choice is decompressive surgery as soon as possible because permanent neurological disability or death may follow if neurosurgical intervention is delayed.41,42 However, during the last decade several reports have been published showing that non-operative treatment may be successful in cases with minimal neurological deficits, despite cord compression revealed by MRI.43–45
Spinal cord herniation
Spinal cord herniation is a rare, although increasingly recognized, cause of spinal cord dysfunction. It has been ascribed to a dural defect, either congenital or acquired, in the anterior surface of the dural sac through which the spinal cord herniates.46,47 The number of published cases in the English language literature markedly increased after 2000. Awareness of the clinical setting and the wider use of MRI in myelopathy are considered the pertinent factors in this increase.48
The main clinical features are thoracic pain and a Brown–Séquard syndrome. Although the dura is sensitive to pain, review of the literature shows that only 48% of the patients had thoracic pain.49,50 About 73% present with Brown–Séquard syndrome51,52 (spastic paralysis on the ipsilateral side together with numbness on the contralateral side). This is probably caused by tethering of the spinal cord at the side of the herniation which results in unilateral damage of the lateral funiculus.53 Some patients have signs of only spasticity or numbness in one leg.54
MRI is the gold standard technique for diagnosis of spinal cord herniation. On sagittal MRI, typical features are ventral displacement, sharp ventral angulation of thoracic spinal cord, and enlargement of dorsal subarachnoid space.55,56
Surgery is crucial in the management of this rare entity, and duraplasty the more widely performed method.57
Thoracic spinal canal stenosis
This may be the outcome of either congenital deformation or hypertrophy of the posterior spinal elements. Most often it occurs in association with generalized rheumatological, metabolic or orthopaedic disorders, such as achondroplasia, osteofluorosis, Scheuermann’s disease, Paget’s disease or acromegaly. It is rare in the absence of a generalized disorder.58,59 Degenerative changes in the facet joints and the intervertebral disc can diminish the volume of the spinal canal and cause cord compression.60,61 The latter is most frequently found at T11 and T12 in middle-aged people.62,63
Clinical presentation
Arterial pulses in the lower limb are normal, which largely excludes vascular problems.
Further investigations
The radiograph is often unremarkable and myelography can be misleading. CT scan and MRI are usually required.58,62
Differential diagnosis
Two differential diagnoses should be considered:
• Intermittent claudication: when pseudoclaudication is present, differential diagnosis must be made from intermittent claudication caused by vascular abnormalities. In spinal stenosis, some patients have symptoms only on standing. In intermittent claudication the pain is brought on by walking and relieved on standing still; arterial pulses are usually diminished or absent. Doppler probe and arteriography may confirm the diagnosis.
• Disc protrusion: in spinal stenosis all postures or movements that bring the spine into anteflexion usually relieve the pain, whereas in a disc protrusion the opposite is more usual. Moreover, in a simple disc protrusion, activity of the lower limbs has no influence on the symptoms.
Chest deformities
Hyperkyphosis
The normal thoracic spine is kyphotic but if the kyphosis is beyond 40°, hyperkyphosis is present.64 The condition may occur at one or more levels and may be the result of several disorders.65
Juvenile kyphosis
Juvenile kyphosis has its clinical onset in adolescence between the ages of 14 and 18 years; for this reason the condition is sometimes known as adolescent osteochondritis. There is a slight preponderance in females.66
It develops because of a disturbance in growth in the vertebral rim epiphysis akin to osteochondritis dissecans.67 Although the exact aetiology is unknown, it is generally believed to result from an anterior endplate lesion, through which herniations of the intervertebral disc protrude into the adjacent bone (Schmorl’s nodes).68 The intervertebral disc itself becomes narrowed, mainly anteriorly. The protrusion interferes with the growth of the vertebral ring epiphysis, which finally leads to about 5° of anterior wedging of the vertebral body. Should this occur over several levels, thoracic hyperkyphosis (with the apex normally around T7–T9) results. In this event, the condition is named Scheuermann’s disease.
Clinical presentation
In adolescents the disorder usually remains painless. It usually gives rise to progressive silent hyperkyphosis, with backache present only in a minority of cases.69,70 At a later age, however, there is a predisposition to thoracic pain71 (see p. e175 of this chapter).
Hyperkyphotic posture in the elderly
Age-related hyperkyphosis is an exaggerated anterior curvature in the thoracic spine that occurs commonly with advanced age. Epidemiologic studies have demonstrated that age-related hyperkyphosis commonly affects the elderly population with estimates ranging from 20% to 40%.72 The ‘dowager hump’ is well recognized, and most clinicians and patients equate this with spinal osteoporosis and vertebral compression deformity or angulation.73 Increasing thoracic kyphosis, in particular when linked to back pain, is considered a signature of possible vertebral body compression fracture. However, approximately two-thirds of those who are most hyperkyphotic don’t have vertebral fractures.74 In the absence of vertebral compression fracture, changes in the spinal support tissues (i.e. ligaments, tendons, disk annulus and nucleus) or supporting musculature could also lead to a progressive increase in curvature.75
Apart from being a cosmetic deformity, most cases of hyperkyphosis do not cause much pain or suffering. However, in some instances they may lead to an increased risk of vertebral fractures76 or may be complicated by a thoracic postural pain syndrome (see following page).
Therapy includes the treatment of the acute exacerbations (see fractures), and of a thoracic postural pain syndrome. Also pharmacological treatment of the underlying osteoporosis with calcium and vitamin D supplementation together with bisphosphonates may be indicated.77
Vertebral body fractures
The thoracolumbar spine is the most common site for vertebral fractures.78 In younger patients, thoracolumbar vertebral fractures are usually caused by high-energy accidents such as falls, or motor vehicle accidents; whereas in elderly patients, osteoporosis is the dominant aetiology.79 Vertebral body fractures may also occur spontaneously as the result of an underlying disorder, such as a vertebral tumour, infection, or ankylosing spondylitis. All are classified as pathological fractures.80
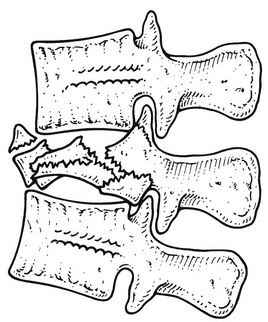
High-energy fractures usually arise from an axial load in combination with flexion or lateral bending. If a compression injury with a significant flexion component is the cause, a wedge-shaped deformity with considerable loss of anterior vertebral height is commonly present (Fig. 2), resulting in angular kyphosis. Lateral bending leads to lateral wedging. In some cases the whole vertebral body is shattered (burst fracture) which can lead to serious neurological complications (Fig 3). A uniformly flattened vertebral body is more indicative of a pure axial component. Vertebral compression fractures due to osteoporosis are usually wedge fractures and have a milder clinical appearance.
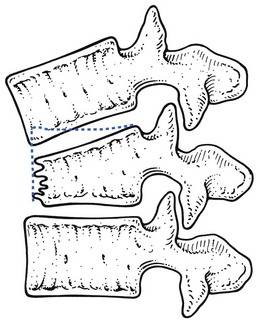
Fig 2 Wedge compression fracture: the middle column (posterior part of the vertebral body) remains intact.
Osteoporotic compression fractures
Central thoracic pain and bilateral girdle pain referred to the corresponding dermatomes. Standing or walking exacerbates the pain. Unsupported sitting is also uncomfortable. There may be twinges. Lying in the supine position generally relieves some of the discomfort.81 Spinal compression fractures can also be insidious and may then produce only modest back pain early in the course of the progressive disease. This may explain the fact that only one-third of vertebral fractures are actually diagnosed, as the patient regards his back pain as a normal part of aging.82
Plain frontal and lateral radiographs are the initial imaging study obtained for a suspected compression fracture. Compression of the anterior aspect of the vertebrae results in the classic wedge-shaped vertebral body with narrowing of the anterior portion. A decrease in vertebral height of 20% or more is considered positive for compression fracture.83 Computed tomography can be used for evaluating the posterior vertebral wall integrity and for distinguishing a wedge fracture from a burst fracture. In the latter, the middle column, consisting of the posterior half of the vertebral body, the posterior longitudinal ligament and the posterior fibres of the annulus fibrosus, is disturbed, and varying degrees of retropulsion into the neutral canal takes place, provoking neurological signs (Fig. 3).84
During the last decades vertebroplasty and kyphoplasty have been promoted for the management of severe recalcitrant pain. Initially reported by Galibert et al in 1987, vertebroplasty involved the destruction of an angioma through consolidation of the vertebral column by percutaneous injection of acrylic cement;85 however, vertebroplasty is now commonly used in treatment of painful osteoporotic vertebral compression fractures.86 Kyphoplasty involves the use of an inflatable bone tamp that when introduced into the vertebral body, restores vertebral height and forms a space for injection of acrylic cement.87
Pathological fractures
These are mainly the result of vertebral infections, ankylosing spondylitis and primary tumours or metastases.80 If the patient complains of severe pain after trivial injury, a pathological fracture should be suspected. Sometimes the event may have been so minimal that the patient does not always mention it. The clinical presentation is the same as for other fractures of abrupt onset except that the pain may not fully disappear on recumbency.
Thoracic postural pain syndrome
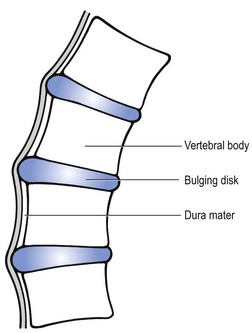
Cyriax regarded this pain syndrome, which affects mainly young adults and the middle aged, as being the result of a posterior displacement of the whole intervertebral disc content, occurring mainly in patients with a marked thoracic kyphosis (Cyriax7). Although dural compression by the entire disc is very likely, ligamentous overstretching of the supra- and interspinal ligaments may also be a contributing factor. Prolonged and repeated anteflexion stretches the posterior longitudinal ligament, which may become too elastic. At the same time, the kyphotic posture causes a posterior directed force on the disc. After some years, the whole disc content bulges posteriorly through the longitudinal ligament. At first this occurs only during prolonged anteflexion and ceases slowly on lying down – the phenomenon is self-reducing. Later the displacement may become permanent. Once the disc comes in contact with the dura mater, or when the posterior ligaments become severely overstretched, the patient complains of pain, which is initially temporary but later becomes permanent.
The whole process depends on three elements, all of which aggravate the disorder:
On clinical examination, only a few articular signs are present and they are not very pronounced. In this disorder, a deep breath usually hurts at the moment the pain is maximally present. This element draws attention to a dural involvement (Fig. 4).
Treatment of the initial stage
Early treatment is in fact prevention: all kyphotic postures should be avoided.
Anterior erosion
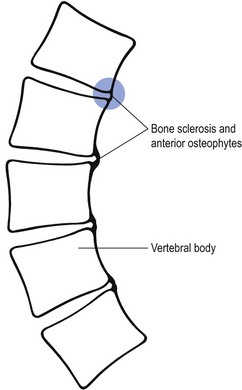
This is the final stage of the postural pain syndrome and is encountered only in the elderly. Progressive increase in hyperkyphosis finally erodes the anterior portion of the disc and the anterior bony parts of the vertebrae are in contact with each other. Bone sclerosis develops and anterior osteophytes form (Fig. 5). Pain that previously ceased at night is now constantly present and is probably due to the osseous contact between the vertebrae, with subsequent local inflammation. For this reason the pain remains local.
Treatment consists of repeated traction at intervals as the complaints demand, as for the thoracic postural pain syndrome (see earlier). Recent studies demonstrated a significant improvement in pain and posture after rehabilitation of the kyphotic spine (combination of manual mobilization, taping and exercises).88,89
Scoliosis
Scoliosis90 is defined as a lateral curvature of the spine of greater than 10°. The angle is determined by drawing lines across the upper surface of the vertebral bodies at which the curve changes direction – i.e. the vertebrae that tilt maximally into the concavity of the curve. Lines perpendicular to these are then drawn. The angle between the two perpendicular lines is Cobb’s angle (Fig. 6). If it is greater than 10°, clinical scoliosis is present.
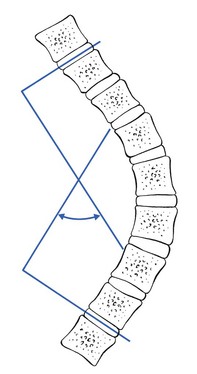
Fig 6 Cobb’s angle.
There are two types of scoliosis.64 In structural scoliosis, the deformity cannot be voluntarily corrected by the patient. It is characterized by a fixed rotation on forward bending. Some cases are congenital, others acquired. Non-structural scoliosis is frequently postural and remains under voluntary control. No fixed rotation is present on anteflexion. It is not progressive.
Structural scoliosis
Acquired scoliosis
This is either secondary or idiopathic.
In this, an underlying disorder is not present. Ninety per cent of cases are in this group, and some authors estimate the incidence as approximately 4.5% of all schoolchildren.1,91
Depending on the age of onset it is known as infantile scoliosis (0–4 years), juvenile scoliosis (4–10) and adolescent scoliosis (over 10 to skeletal maturity). Adolescent scoliosis sometimes has adult onset when it affects females between 20 and 40 years of age. Severe adolescent scoliosis affects girls four times more often than boys, whereas less pronounced deviations are seen equally in both sexes.91
One curious feature of the disorder is that in childhood, 90% of the curvatures are convex to the left, whereas in adolescence this is reversed.92 The condition worsens as bone growth increases and rarely changes after bone growth has stopped. However, if the angle of deformity between the lumbar and thoracic spines remains less than 60°, an effect on vital functions is not to be expected.
Clinical presentation
Controversy surrounds the incidence of thoracolumbar pain in scoliosis. Some authors contend that in the main the condition is asymptomatic and that patients with scoliosis do not suffer more from back pain than those with a normal back.93 Others maintain that the larger the scoliosis the more likely it is that pain will occur.94 However, some patients initially experience pain at the end of the day, which stops rapidly on lying down.94 The cause may be overstretching of ligaments, overloading of the facet joints or temporal displacement of the disc. All these possibilities are compatible with a non-articular pattern on clinical examination. The pain is usually located at the apex of the curvature. Progression of the deformity is usually associated with increased pain.
On inspection, attention must be paid to scapular asymmetry, prominence at one side of the thorax, the distance between the arms and trunk and the level of the pelvis. Because a contralateral rotation occurs on side flexion, scoliosis is associated with a unilateral thoracic prominence at the convex side of the scoliosis.64,95 Prominence of the thorax is most easily noted when the patient bends over.
Advanced scoliosis may lead to a decreased vital capacity and to cardiorespiratory dysfunction.
Further investigations
Radiography establishes the exact location of the curvature, its degree of severity (Cobb’s angle) and the stage of skeletal maturity, all of which are important for diagnosis, follow-up and treatment. Skeletal maturation is judged from the ossification and closure of the vertebral ring apophyses.64
Treatment
Different types of treatment may be indicated: observation, drugs, exercises, braces and surgery (see Box 1). It should be noted that there is an important distinction to be made between the treatment for children and that for adults.
This is undertaken in the following:
• All patients who have an immature skeleton, and with curve of less than 20°
In all such cases, observation should be made at regular intervals to detect whether the curve progresses.96 Usually it is sufficient to see the patient every 6 months. In adolescents nearing the growth spurt, a check-up every 3 months is more appropriate. Exercises are of no use in this stage.
• Brace: a young adult having a Cobb’s angle of less than 40°, in the absence of severe pain, is best treated by conservative means: non-specific anti-inflammatory agents, facet infiltrations and physical monitoring. A Milwaukee brace can also be considered. The main goal of the use of a brace is to prevent further progression, rather than to correct the curvature. A Milwaukee brace or plastic jacket can be used, which extends the spine by pushing it up cranially from the hips. However, a recent study of late-onset idiopathic scoliosis raises questions about the efficacy of spinal orthoses.97 Braces are contraindicated in skeletally mature patients or if the curvature is over 40° – the latter usually do not respond well. A brace cannot be used in thoracic lordosis. When brace treatment is given, associated exercises become important in order to keep the muscles of the trunk and the abdomen in good condition.
• Operation: if the curve is over 40° in a skeletally immature patient, or when it is progressive or over 50° in a mature skeleton, spinal fusion is indicated to straighten and stabilize the spine.96 Other indications for operative intervention are when orthotic treatment has failed, if pain becomes uncontrollable or when a thoracic lordosis is found.
Older adults are more likely to suffer from neural compression. The major aim of treatment is to maintain function. With a curve under 40° this is usually possible without surgery. If there is uncontrolled pain or a progressive curve or structural disabilities, neurological complications or cardiorespiratory problems, surgical internal fixation is essential.5,98
Lateral erosion
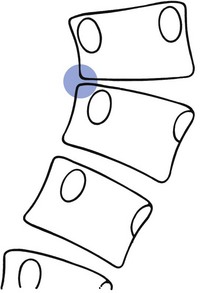
Just as hyperkyphosis may lead to thoracic postural pain syndrome, and finally to anterior erosion, moderate and severe scoliosis may result in lateral erosion. The process is the same for both, but in scoliosis the entire disc of several consecutive levels shifts laterally to one side. After some years, this may lead to bony contact between the vertebral bodies at the concave side of the scoliosis (Fig. 7).
Spinal infections
Vertebral osteomyelitis
Pyogenic osteomyelitis
Since the introduction of antibiotics this disorder has changed from a disease of childhood and adolescence to one affecting mainly adults. The lumbar spine is most commonly involved.100 The most frequent organism is a Staphylococcus sp.101 In about 40% of patients, an extraspinal primary source of infection is found, very often sited in the pelvis (urinary tract, postpartum, bowel).1 The presence of diabetes mellitus, intravenous drug abuse, underlying chronic debilitating diseases or immunosuppression, previous infections, preceding bacteraemia and recent vertebral surgery are significantly associated with pyogenic osteomyelitis.102,103
Clinical presentation
As the condition progresses, pain usually becomes more dramatic, leading to a full articular pattern with gross limitation mainly of extension, of both side flexions and rotations. Rigidity and muscle spasm are also present. Gross tenderness on local pressure over the spinous processes is found. A soft tissue mass may be palpable. Chills and weight loss complete the clinical presentation. Thecal sac neurocompression by the formation of an epidural abscess may cause severe neurological disturbances (paraplegia or paraparesis).104
Further investigations
The erythrocyte sedimentation rate (ESR) is constantly raised and is a good indicator of the activity of the disease.105,106 An increased white cell count is found in only half of the cases, and it seldom rises above 15 000.102,107
Spinal radiographs become positive at the earliest after 2 or 3 weeks of infection,107 when a narrowed disc space is the first sign found. This narrowing cannot be distinguished from narrowing that is the result of degenerative changes. Only later, after 10–12 weeks, do the adjacent endplates become more dense and eventually blurred, once destructive erosion has set in. Healing is associated with osteosclerosis and leads to bony fusion across the disc space.
Spongy bone erosion and rarefaction is apparent on CT scan before it is on plain radiography. However, gallium bone scan proves to be the most valuable technique to detect early inflammatory changes and it becomes positive earlier than does CT scan. In a recent study, gallium scanning proved to be 100% sensitive, specific and accurate.108
Tuberculosis of the spine
Further investigations
On the radiograph, involvement of one vertebra is typical.110 The most common early findings are a diminished disc space and osteolysis of the vertebral body. Later, paravertebral granulation tissue is formed, resulting in paravertebral mass.111 This is usually followed by collapse of the vertebral body with formation of an angular kyphosis.
A positive Mantoux test is not diagnostic in itself but does arouse suspicion of tuberculosis.
Intervertebral disc inflammation: discitis
The first complaint is of back or hip pain; smaller children may just refuse to walk.
Some weeks after onset, some narrowing of the intervertebral disc space and erosion of the endplates can be seen. Although these radiographic findings are usually sufficient to establish the diagnosis of discitis, MRI is the diagnostic study of choice especially in a differential diagnosis with suspected vertebral osteomyelitis.112 Immobilization of the spine and antibiotics are the main treatment required.
Lateral recess stenosis
In some older patients, the intervertebral disc becomes completely worn out and its fragments displace posteriorly because of physiological kyphosis. As a consequence, the intervertebral space decreases progressively and the posterior longitudinal ligament becomes relatively long. Under the influence of the body weight it bulges posteriorly and may come in contact with the dura mater, causing central backache radiating bilaterally around the trunk, or may compress the nerve root, causing unilateral root pain (Cyriax:7 p. 211). This condition is characterized by pain that comes on immediately on standing and, unlike lumbar lateral recess stenosis, is not altered by sitting or stooping. Only when the patient lies down for 1 or 2 minutes does the pain cease, when it stops almost immediately.
Arthritis of the costovertebral and costotransverse joints
Disorders of these joints that give rise to clinical symptoms are rare. They may be affected by osteoarthritis, ankylosing spondylitis and by rheumatoid arthritis.113,114 Degeneration is identified mainly in the costovertebral joints of the first, eleventh and twelfth ribs, all of which have only one articular facet. It is possible that these are more vulnerable to mechanical irritation by continuous rib motion, sometimes as the outcome of a mechanical injury.115 Less frequently the sixth, seventh and eighth costovertebral joints are affected, and in these it is most frequently the inferior hemifacet.116 It has been suggested that patients often have an increased thoracic kyphosis with associated scoliosis.115
Involvement of these joints gives rise to unilateral paravertebral pain radiating along the ribs. The pain is described as aching or burning, is usually worse in the morning and is aggravated by deep inspiration and a cough.115–117 Exceptionally, on breathing, a sudden twinge that arrests further inspiration is felt.
Standard radiography, CT scan and technetium bone scans may show abnormalities of these joints.
Arthritis of the thoracic facet joints
Degeneration of thoracic facet joints most frequently affects levels C7–T1, T3–T5 and T11–L1.118,119 The diagnosis should be considered in those patients whose complaint is a unilateral and local paravertebral pain. There are no dural symptoms or signs. The sole clinical finding is pain on one of the rotations, actively and/or passively performed. This can be either the rotation towards or away from the painful side, a situation resembling facet disorders in the cervical area. Other passive movements are far less painful or not painful at all and resisted rotations are painless. Extension pressure on the spinous processes elicits pain on two consecutive processes, indicating the level of the lesion.
Treatment
Cure is obtained with one or two infiltrations of 1 ml of triamcinolone over 1–2 weeks.
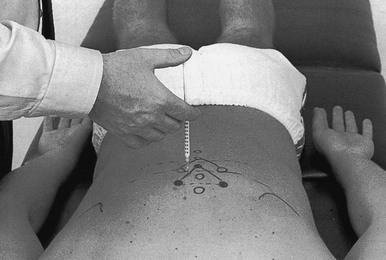
Technique: injection of a thoracic facet joint (Fig. 8)
The patient must return after 2 weeks. If there is still some pain, the injection is repeated.
If the treatment fails, a blockade with phenol solution of the medial branch of the dorsal ramus of the thoracic spinal nerve can be performed. Good results have also been reported with percutaneous radiofrequency denervation of the thoracic facet.120
Paget’s disease
This focal, non-generalized osseous disorder is characterized by thickening and deformation of the affected bone and is caused by hyperactive osteoclasts and osteoblasts, which give rise to increased bone resorption together with formation of disorganized new bone. It occurs more commonly than is generally believed and affects about 3% of the population above 40 years of age. This figure rises to about 5–10% in people aged over 80.121 There is a slight preponderance of males and the condition is most frequently encountered in Western Europe, Australia and New Zealand.
Flat bones and the ends of the long bones are mainly affected. Most frequently involved are the spine, skull, sacrum, pelvis and femurs. About 60% of patients with Paget’s disease have involvement of the lumbar spine, and 45% of the thoracic spine and sacrum. In only 15% is the cervical spine involved.122
Clinical presentation
Only about 12% of the patients affected by Paget’s disease suffer from backache from the disease as such.123 It should be recognized that other disorders, such as disc protrusions and facet joint problems, may be the basis of the pain in patients who are identified as having Paget’s disease. In two-thirds the disease remains clinically asymptomatic.124 These cases are usually detected incidentally on a radiograph taken for some other reason or by an increased serum alkaline phosphatase level.
Complications
Because of multiple arteriovenous fistulae in bone, there is lowered peripheral resistance with a rise in cardiac output, which may lead to cardiac decompensation. Enlargement of the skull, with secondary compression of peripheral nerves, may result in hearing loss, tinnitus and vertigo.1
References
1. Borenstein, D, Wiesel, S. Low Back Pain. Philadelphia: Saunders; 1989.
2. Conti, P, Pansini, G, Mouchaty, H, Capuano, C, Conti, R, Spinal neurinomas: Retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol 2004; 61:35–44. ![]()
3. Van Goethem, JW, van den Hauwe, L, Ozsarlak, O, De Schepper, AM, Parizel, PM, Spinal tumors. Eur J Radiol 2004; 50:159–176. ![]()
4. Cassidy, R, Ducker, T. Intradural neural tumours. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991:889–903.
5. Gautier-Smith, P, Clinical aspects of spinal neurofibromas. Brain 1967; 90:359–394. ![]()
6. Brügger, A. Die Erkrankungen des Bewegungsapparates und seines Nervensystems. Stuttgart: Fischer; 1980.
7. Cyriax, J. Textbook of Orthopaedic Medicine, vol I, Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
8. Love, J, Rivers, M, Spinal cord tumours, simulating protruded intervertebral discs. JAMA 1962; 179:878–881. ![]()
9. Epstein, JA, Marc, JA, Hyman, RA, Khan, A, Mardayat, M, Total myelography in the evaluation of lumbar discs. With the presentation of three cases of thoracic neoplasms simulating nerve root lesions. Spine. 1979;4(2):121–128. ![]()
10. Wiesel, S, Ignatius, P, Marvel, J, Rothman, R, Intradural neurofibroma simulating lumbar-disc disease. J Bone Joint Surg 1956; 58A:1040–1042. ![]()
11. Shah, K, Nagler, W, Dumbell neurilemoma simulating lumbar disk disease. Arch Phys Med Rehabil. 1978;59(7):340–342. ![]()
12. Colosimo, C, Cerase, A, Denaro, L, Maira, G, Greco, R, Magnetic resonance imaging of intramedullary spinal cord schwannomas. J Neurosurg (Spine) 2003; 99:114–117. ![]()
13. Borges, G, Bonilha, L, Proa, M, Jr., et al, Imaging features and treatment of an intradural lumbar cystic schwannoma. Arq Neuropsiquiatr 2005; 63:681–684. ![]()
14. Albiñana, J, Perez Grueso, FS, Lopez Barea, F, Gutierrez, F, Rib osteoblastoma: a clinical manifestation. Spine. 1988;13(2):212–214. ![]()
15. Ongerboer De Visser, B, Het teken van Lhermitte bij thoracale wervelaandoeningen. Ned Tijdschr Geneeskd. 1980;124(11):390–392. ![]()
16. Wells, F, Thomas, TL, Matthewson, MH, Holmes, AE, Neurilemmoma of the thoracic spine. A case report. Spine. 1982;7(1):66–70. ![]()
17. Onimus, O, Schraub, S, Bertin, D, Bosset, J, Guidet, M, Surgical treatment of vertebral metastasis. Spine. 1986;11(9):883–891. ![]()
18. Galasko, CSB. Skeletal Metastases. Boston: Butterworths; 1986.
19. Reith, C, Reith, HB, Baumgartner, D, Kozuschek, W, Lausberg, G, Diagnosis and interdisciplinary therapy of spinal cord compression caused by spinal metastases. Zentralbl Chir. 1991;116(15):913–916. ![]()
20. Brown, PD, Stafford, SL, Schild, SE, Martenson, JA, Schiff, D, Metastatic spinal cord compression in patients with colorectal cancer. J Neurooncol. 1999;44(2):175–180. ![]()
21. Fornasier, VL, Horne, JG, Metastases to the vertebral column. Cancer 1975; 36:590. ![]()
22. Biyani, A, Ebraheim, NA, Lu, J, Thoracic spine fractures in patients older than 50 years. Clin Orthop 1996; 328:190–193. ![]()
23. Kostuik, J, Weinstein, J. Differential diagnosis and surgical treatment of metastatic spine tumours. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991:861–888.
24. Weinstein, J. Differential diagnosis and surgical treatment of primary benign and malignant neoplasms. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991:829–860.
25. Love, G, Rivers, M, Intractable pain due to associated protruded intervertebral disc and intraspinal neoplasm. Neurology 1962; 12:60–64. ![]()
26. Scott, M, Lower extremity pain stimulating sciatica, tumours of the high thoracic and cervical cord as causes. JAMA 1956; 160:528–534. ![]()
27. Fidler, M, Bongartz, E, Laminar removal and replacement: a technique for the removal of epidural tumour. Spine. 1988;13(2):218–221. ![]()
28. Plasmans, CM, van der Eijken, JW, Laminar removal and replacement in combination with a spondylodesis in children with an intraspinal extension of a neuroblastoma: two case reports. Spine. 1988;13(2):215–217. ![]()
29. Roblot, P, Alcalay, M, Cazenave-Roblot, F, Levy, P, Bontoux, D, Osteochondroma of the thoracic spine. Report of a case and review of the literature. Spine. 1990;15(3):240–243. ![]()
30. Emsellem, H, Metastatic disease of the spine: diagnosis and management. South Med J 1986; 76:1405. ![]()
31. Cabana, F, Pointillart, V, Vital, J, Senegas, J, Postoperative compressive spinal epidural hematomas. 15 cases and a review of the literature. Rev Chir Orthop Repar Appar Mot. 2000;86(4):335–345. ![]()
32. Ruelle, A, Datti, R, Pisani, R, Thoracic epidural hematoma after spinal manipulation therapy. J Spinal Disord. 1999;12(6):534–536. ![]()
33. Cohen, JE, Ginsberg, HJ, Emery, D, Schwartz, ML, Fatal spontaneous spinal epidural hematoma following thrombolysis for myocardial infarction. Surg Neurol. 1998;49(5):520–522. ![]()
34. Sawin, PD, Traynelis, VC, Follett, KA, Spinal epidural hematoma following coronary thrombolysis with tissue plasminogen activator. Report of two cases. J Neurosurg. 1995;83(2):350–353. ![]()
35. Van Schaeybroeck, P, Van Calenbergh, F, Van De Werf, F, et al, Spontaneous spinal epidural hematoma associated with thrombolysis and anticoagulation therapy: report of three cases. Clin Neurol Neurosurg. 1998;100(4):283–287. ![]()
36. Meena, AK, Jayalakshmi, S, Prasad, VS, Murthy, JM, Spinal epidural haematoma in a patient with haemophilia-B. Spinal Cord. 1998;36(9):658–660. ![]()
37. Leach, M, Makris, M, Hampton, KK, Preston, FE, Spinal epidural haematoma in haemophilia A with inhibitors – efficacy of recombinant factor VIIa concentrate. Haemophilia. 1999;5(3):209–212. ![]()
38. Daentzer, D, Boker, DK, Spontaneous spinal hemorrhage. Outcome after surgical therapy of epidural hematomas. Nervenarzt. 2000;71(2):116–122. ![]()
39. Sklar, EM, Post, JM, Falcone, S, MRI of acute spinal epidural hematomas. J Comput Assist Tomogr. 1999;23(2):238–243. ![]()
40. Lovblad, KO, Baumgartner, RW, Zambaz, BD, et al, Nontraumatic spinal epidural hematomas. MR features. Acta Radiol. 1997;38(1):8–13. ![]()
41. Alexiadou-Rudolf, C, Ernestus, RI, Nanassis, K, Lanfermann, H, Klug, N, Acute nontraumatic spinal epidural hematomas. An important differential diagnosis in spinal emergencies. Spine. 1998;23(16):1810–1813. ![]()
42. Groen, RJ, van Alphen, HA, Operative treatment of spontaneous spinal epidural hematomas: a study of the factors determining postoperative outcome. Neurosurgery. 1996;139(3):494–508. ![]()
43. Fukui, MB, Swarnkar, AS, Williams, RL, Acute spontaneous spinal epidural hematomas. Am J Neuroradiol. 1999;20(7):1365–1372. ![]()
44. Silber, SH, Complete nonsurgical resolution of a spontaneous spinal epidural hematoma. Am J Emerg Med. 1996;14(4):391–393. ![]()
45. Serizawa, Y, Ohshiro, K, Tanaka, K, Tamaki, S, Matsuura, K, Uchihara, T, Spontaneous resolution of an acute spontaneous spinal epidural hematoma with neurological deficits. Intern Med. 1995;34(10):992–994. ![]()
46. Wortzman, G, Tasker, RR, Rewcastle, NB, Richardson, JC, Pearson, FG, Spontaneous incarcerated herniation of the spinal cord into a vertebral body: a unique cause of paraplegia. Case report. J Neurosurg 1974; 41:631–635. ![]()
47. Zairi, F, Thines, L, Bourgeois, P, Dereeper, O, Assaker, R, Spinal cord herniation: a misdiagnosed and treatable cause of thoracic myelopathy. Acta Neurochir (Wien). 2010;152(11):1991–1996. ![]()
48. Maira, G, Denaro, L, Doglietto, F, Mangiola, A, Colosimo, C, Idiopathic spinal cord herniation: diagnostic, surgical, and follow-up data obtained in five cases. J Neurosurg Spine 2006; 4:10–19. ![]()
49. Vallee, B, Mercier, P, Menei, P, et al, Ventral transdural herniation of the thoracic spinal cord. Surgical treatment in four cases and review of literature. Acta Neurochir Wien 1999; 141:907. ![]()
50. Tronnier, VM, Steinmetz, A, Albert, FK, Scharf, J, Kunze, S, Hernia of the spinal cord: case report and review of the literature. Neurosurgery 1991; 29:916–919. ![]()
51. Borges, LF, Zervas, NT, Lehrich, JR, Idiopathic spinal cord herniation: a treatable cause of the Brown-Sequard syndrome. Case report. Neurosurgery 1995; 26:1028–1032. ![]()
52. Verny, C, Mercier, P, Hayek, G, Fournier, D, Menei, P, Guy, G, Spontaneous spinal cord herniation: a little-known cause of Brown-Sequard syndrome. Report of two cases and review of the literature. Neurochirurgie 1999; 45:225–231. ![]()
53. Masuzawa, H, Nakayama, H, Shitara, N, Suzuki, T, Spinal cord herniation into a congenital extradural arachnoid cyst causing Brown-Sequard syndrome: case report. J Neurosurg 1981; 55:983–986. ![]()
54. Sasani, M, Ozer, AF, Vural, M, Sarioglu, AC, Idiopathic spinal cord herniation: case report and review of the literature. J Spinal Cord Med. 2009;32(1):86–94. ![]()
55. Karadeniz-Bilgili, MY, Castillo, M, Bernard, E, Transdural spinal cord herniation: pre- and postoperative MRI findings. Clin Imaging 2005; 29:288–290. ![]()
56. Barrenechea, IJ, Lesser, JB, Gidekel, AL, Turjanski, L, Perin, NI, Diagnosis and treatment of spinal cord herniation: a combined experience. J Neurosurg Spine 2006; 4:294–302. ![]()
57. Saito, A, Takahashi, T, Sato, S, Kumabe, T, Tominaga, T, Modified surgical technique for the treatment of idiopathic spinal cord herniation. Minim Invasive Neurosurg 2006; 49:120–123. ![]()
58. Barnett, G, Hardy, R, Little, J, Bay, J, Sypert, G, Thoracic spinal canal stenosis. J Neurosurg 1987; 66:338–344. ![]()
59. Govoni, A, Developmental stenosis of a thoracic vertebra resulting in narrowing of the spinal canal. AJR 1971; 112:401–404. ![]()
60. Milhorat, TH, Kotzen, RM, Anzil, AP, Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. 1994;80(4):716–722. ![]()
61. Yamamoto, I, Matsumae, M, Ikeda, A, et al, Thoracic spinal stenosis: experience with seven cases. J Neurosurg. 1988;68(1):37–40. ![]()
62. Okada, K, Oka, S, Tohge, K, Ono, K, Yonenoby, K, Hosoya, T, Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine. 1991;16(3):280–287. ![]()
63. Love, G, Schorn, V. Thoracic-disc protrusions. JAMA. 1965; 191:627–631.
64. Goldstein, L, Waugh, T, Classification and terminology of scoliosis. Clin Orthop Rel Res 1973; 93:10–22. ![]()
65. Guille, J, Forlin, E, Bowen, R, Congenital kyphosis. Orthop Rev 1993; 235–239. ![]()
66. Bullough, P, Boachie-Adjei, O. Atlas of Spinal Disease. Philadelphia: Lippincott; 1988.
67. Scheuermann, H. Röntgenologic studies of the origin and development of juvenile kyphosis, together with some investigations concerning the vertebral epiphyses in man and animals. Acta Orthop Scan. 1934; 5:161.
68. Schmorl, G. Über die an den Wirbelbandscheiben vorkommenden Ausdehnungs- und Zerreisungsvorgänge und die dadurch an ihnen und der Wirbelspongiosa hervorgerufenen Veränderungen. Verh Dtsch Pat Ges. 1927; 22:50.
69. Bradford, D, Juvenile kyphosis. Clin Orthop Rel Res 1977; 128:45–55. ![]()
70. Segers M, Anciaux H. Pseudo-angineuze Pijnklachten. Dienst fysische geneeskunde, Gent.
71. Murray, P, Weinstein, S, Spratt, K, The natural history and long term follow up of Scheuermann kyphosis. J Bone Joint Surg 1993; 2:236–248. ![]()
72. Becker, C, Clinical evaluation for osteoporosis. Clin Geriatr Med 2003; 19:299–320. ![]()
73. De Smet, AA, Robinson, RG, Johnson, BE, Lukert, BP, Spinal compression fractures in osteoporotic women: patterns and relationship to hyperkyphosis. Radiology 1988; 166:497–500. ![]()
74. Bartynski, WS, Heller, MT, Grahovac, SZ, Rothfus, WE, Kurs-Lasky, M, Severe thoracic kyphosis in the older patient in the absence of vertebral fracture: association of extreme curve with age. AJNR Am J Neuroradiol. 2005;26(8):2077–2085. ![]()
75. Milne, JS, Williamson, J, A longitudinal study of kyphosis in older people. Age Ageing 1983; 12:225–233. ![]()
76. Huang, MH, Barrett-Connor, E, Greendale, GA, Kado, DM, Hyperkyphotic posture and risk of future osteoporotic fractures: the Rancho Bernardo study. J Bone Miner Res 2006; 21:419–423. ![]()
77. Cummings, SR, Ensrud, K, Donaldson, MG, Bauer, DC, Sellmeyer, D, Schousboe, JT. The U.S. National Osteoporosis Foundation (NOF) guideline: recommendations for pharmacologic treatment. IBMS Bone Key. 2008; 5:137–141.
78. Jansson, KA, Blomqvist, P, Svedmark, P, Granath, F, Buskens, E, Larsson, M, Adami, J, Thoracolumbar vertebral fractures in Sweden: an analysis of 13,496 patients admitted to hospital. Eur J Epidemiol. 2010;25(6):431–437. ![]()
79. Robertsson, A, Branfoot, T, Barlow, F, Giannoudis, P, Spinal injury pattern resulting from car and motorcycle accidents. Spine 2002; 24:2825–2830. ![]()
80. Kostuik, J, Huler, R, Esses, S, Stauffer, S. Thoracolumbar spine fracture. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991.
81. Patel, U, Skingle, S, Campbell, GA, Crisp, AJ, Boyle, IT, Clinical profile of acute vertebral compression fractures in osteoporosis. Br J Rheumatol 1991; 30 418–241. ![]()
82. Kado, DM, Browner, WS, Palermo, L, Nevitt, MC, Genant, HK, Cummings, SR, Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220. ![]()
83. Nevitt, MC, Ettinger, B, Black, DM, et al, The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 1998; 128:793–800. ![]()
84. Vaccaro, AR, Lehman, RA, Jr., Hurlbert, RJ, Anderson, PA, et al, A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30(20):2325–2333. ![]()
85. Galibert, P, Deramond, H, Rosat, P, Le Gars, D, Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987; 33:166–168. ![]()
86. Rao, RD, Singrakhia, MD, Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplastyand kyphoplasty in its management. J Bone Joint Surg Am 2003; 85-A:2010–2022. ![]()
87. Lieberman, IH, Dudeney, S, Reinhardt, MK, Bell, G, Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine 2001; 26:1631–1638. ![]()
88. Kado, DM, The rehabilitation of hyperkyphotic posture in the elderly. Eur J Phys Rehabil Med. 2009;45(4):583–593. ![]()
89. Bautmans, I, Van Arken, J, Van Mackelenberg, M, Mets, T, Rehabilitation using manual mobilization for thoracic kyphosis in elderly postmenopausal patients with osteoporosis. J Rehabil Med. 2010;42(2):129–135. ![]()
90. Zorab, P. Respiratory tract disease chest deformities. BMJ. 1966; 7 May:1155–1156.
91. Drummond, D, Rogola, E, Gurr, J, Spinal deformity: natural history and the role of school screening. Orth Clin North Am. 1979;10(4):751–759. ![]()
92. James, J, The etiology of scoliosis. J Bone Joint Surg. 1970;52(3):410–419. ![]()
93. Nachemson, A, Adult scoliosis and back pain. Spine. 1979;4(6):513–517. ![]()
94. Hall, J, Nachemson, A. Debate: scoliosis. Spine. 1977; 2(4):318–324.
95. Kapandji, IA. The Physiology of the Joints. Volume three. The Trunk an the Vertebral Column, 1st ed. Edinburgh: Churchill Livingstone; 1974.
96. Bunnell, W, Treatment of idiopathic scoliosis. Orthop Clin North Am. 1979;10(4):813–827. ![]()
97. Goldberg, C, Dowling, F, Hall, J, Emans, J, A statistical comparison between natural history of idiopathic scoliosis and brace treatment in skeletally immature adolescent girls. Spine. 1993;18(7):902–908. ![]()
98. Kostuik, J, Decision making in adult scoliosis. Spine. 1979;4(6):521–525. ![]()
99. Papaioannou, T, Stokes, I, Kenwright, J, Scoliosis associated with limb-length inequality. J Bone Joint Surg. 1982;64A(1):59–63. ![]()
100. Ross, P, Fleming, J, Vertebral body osteomyelitis. Clin Orthop Rel Res 1976; 118:190–198. ![]()
101. Carragee, EJ, Pyogenic vertebral osteomyelitis. J Bone Joint Surg. 1997;79A(6):874–880. ![]()
102. Macnab, I. Backache. Baltimore: Williams & Wilkins; 1983.
103. Colmenero, JD, Jimenez-Mejias, ME, Sanchez-Lora, FJ, et al, Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Am Rheum Dis. 1997;56(12):709–715. ![]()
104. Hadjipavlou, AG, Mader, JT, Necessary, JT, Muffoletto, AJ, Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25(13):1668–1679. ![]()
105. Buchelt, M, Lack, W, Kutschera, H-P, Katterschafka, T, Kiss, H, Schneider, B, Kotz, R, Comparison of tuberculous and pyogenic spondylitis. Clin Orthop Rel Res 1993; 296:192–199. ![]()
106. Carragee, EJ, Kim, D, van der Vlugt, T, Vittum, D, The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine. 1997;22(18):2089–2093. ![]()
107. Vincent, K, Benson, D. Differential diagnosis and conservative treatment of infectious diseases. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991.
108. Hadjipavlou, AG, Cesani-Vazquez, F, Villaneuva-Meyer, J, et al, The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited. Am J Orthop. 1998;27(3):179–183. ![]()
109. Arnold, PM, Baek, PN, Bernardi, RJ, Luck, EA, Larson, SJ, Surgical management of nontuberculous thoracic and lumbar vertebral osteomyelitis: report of 33 cases. Surg Neurol. 1997;47(6):551–561. ![]()
110. Fernandez, M, Carrol, CL, Baker, CJ, Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105(6):1299–1304. ![]()
111. Polley, P, Dunn, R, Noncontiguous spinal tuberculosis: incidence and management. Eur Spine J. 2009;18(8):1096–1101. ![]()
112. Cohen, MJ, Ezekiel, J, Persellin, RH, Costovertebral and costotransverse joint involvement in rheumatoid arthritis. Am Rheum. 1978;37(5):473–475. ![]()
113. Benhamou, C, Roux, C, Tourliere, D, Gervais, T, Viala, J, Amor, B, Pseudovisceral pain referred from costovertebral arthropathies. Spine. 1993;18(6):790–795. ![]()
114. Raney, F. Costovertebral–costotransverse joint complex as the source of local or referred pain. J Bone Joint Surg. 1966; 48A(7):1451–1452.
115. Nathan, H, Weinberg, H, Robin, G, Aviad, I, The costovertebral joints. Anatomical–clinical observations in arthritis. Arthritis Rheum. 1964;7(3). ![]()
116. Grant, A, Keegan, D, Rib pain – a neglected diagnosis. Irish J Med Sci (6th series) 1966; 367:162–169. ![]()
117. Shore, L. On osteo-arthritis in the dorsal intervertebral joints. Br J Surg. 1935; 57:412.
118. Skubic, J, Kostuik, J. Thoracic pain syndromes and thoracic disc herniation. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991:1443–1461.
119. Stolker, RJ, Vervest, AC, Groen, GJ, Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir (Wien). 1993;122(1–2):82–90. ![]()
120. Kingma, M. Leerboek Orthopedie, 5th ed. Utrecht/Antwerp: Bohn, Scheltema & Holkema; 1985.
121. Meunier, P, Salson, C, Mathieu, L, et al, Skeletal distribution and biochemical parameters of Paget’s disease. Clin Orthop Rel Res 1987; 217:37–44. ![]()
122. Altman, R, Brown, M, Gargano, G, Low back pain in Paget’s disease of bone. Clin Orthop Rel Res 1987; 217:152. ![]()
123. Kane, W. Osteoporosis, osteomalacia, and Paget’s disease. In: The Adult Spine: Principles and Practice. New York: Raven Press; 1991.
124. Collins, D, Paget’s disease of bone: incidence and subclinical forms. Lancet 1956; 2:51. ![]()


 (mid-thorax) interspinal levels higher than the lower tender spinous process.
(mid-thorax) interspinal levels higher than the lower tender spinous process.
