W9 Reconstruction of the Distal Radius Facet by a Free Vascularized Osteochondral Graft
Rationale and Basic Science
Intra-articular malunions of the distal radius should be ideally treated by an outside-in1–5 or inside-out osteotomy,6 in such a way as to restore the normal joint anatomy. Sometimes the degree of damage on the articular cartilage or the degree of comminution is such that an intra-articular osteotomy is no longer an alternative. Currently, the gold standards when the lunate or scaphoid facet cartilage is irreversibly damaged are partial or total arthrodeses.7,8 Partial arthrodeses limit the range of motion and may overload neighboring joints, which may degenerate in the midterm.9
Taking into account that the original problem is the loss of cartilage in the distal radius, a more logical approach was to try to replace it with a similar piece of tissue, rather than to fuse the joint. Giachino and coworkers10,11 resurfaced irreversibly damaged distal radius articular surface by transferring nonvascularized osteochondral grafts from the proximal fibula. Their experience in two cases was positive in the short-term, but the fate of nonvascularized osteochondral grafts has been shown to be unpredictable in other joints.12–15 Ishida and colleagues12 recommend limiting the graft size to 50% of the proximal interphalangeal joint to avoid massive graft resorption. Other studies have found on histology, even with small autogenous osteochondral plugs, the appearance of multiple tidemarks on the deep layer of the cartilage,16 a finding that has been associated with osteoarthritis.15,17
The problem of restoring articular cartilage loss has been widely explored in other fields of orthopaedic surgery. There are several methods for repairing cartilage defects, including microfracture, chondrocyte cultures, mosaicplasty, fresh and frozen allografts, autologous osteochondral grafts, and perichondroplasty, but none of them totally successful.18–24 The fact that the anatomy of the hyaline cartilage is extremely complex and difficult to reproduce was considered by Poole17 as being responsible in part for the failures with these methods.
Cartilage has been said to get its nutrients from the synovial fluid.25–27 Collapse or even total joint disappearance has been shown to occur, however, when a joint or a part thereof is transferred as a nonvascularized graft.28–30 Conversely, vascularized joint transfers do maintain the joint spaces open in the long-term.31,32 Several studies have shown the blood supply of the subchondral bone is important for the nutrition of the bone-cartilage interface or even the deep layers of the hyaline cartilage.17,33 It seems logical to conclude, based on current evidence, that the cartilage’s nourishment via the subchondral blood vessels is also an important issue. It seemed to us important when transplanting a piece of cartilage to include the underlying vascularized subchondral bone. Bone that has been transferred with microsurgical technique has long been shown to maintain its biological and mechanical properties.34 Even reliable growth has been shown to occur when a vascularized epiphysis is included.35,36
My colleagues and I searched for a donor site with similar shape to the distal radius facets, which contained cartilage and a reliable blood supply. This chapter presents our experience on transferring the base of the third metatarsal as a vascularized osteochondral graft.37–39
Anatomy
Twenty fresh feet were dissected under loop magnification for this study. The proximal facet of the base of the third metatarsal, which articulates with the third cuneiform, is pear-shaped, flat or slightly concave, and has a slant of about 5 degrees dorsal to plantar and fibular to tibial. We named this the “principal” facet to distinguish it from several minor ones that exist on both sides of the metatarsal base. One of the latter, located on the dorsofibular aspect, which we named the “accessory facet,” can be advantageously used for reconstructing the sigmoid fossa (Fig. W9-1).
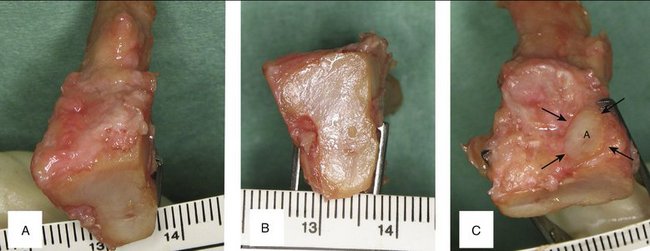
FIGURE W9-1 Anatomical features of the base of the left third metatarsal. A, Bird’s eye view to appreciate the slight inclination toward the second metatarsal. B, Proximal (principal) facet. C, Accessory (peroneal) facet.
(From del Piñal F, García-Bernal JF, Delgado J, et al: Reconstruction of the distal radius facet by a free vascularized osteochondral autograft: anatomical study and report of a case. J Hand Surg [Am]. 2005; 30:1200-1210; with permission from the American Society for Surgery of the Hand.)
The principal facet is 19 mm on its main axis with a maximal width of 12 mm dorsal and 8 mm plantar. Mekhail and associates40 studied the distal radius articular surface. They found the scaphoid facet to be a triangle measuring 18 mm dorsal × 16 mm palmar × 13 mm ulnar, and the lunate facet to be a polygon measuring 10 mm dorsal × 13 mm lateral × 14 mm palmar × 15 mm ulnar. The principal facet is approximately the right size for restoring one of the fossae. The accessory facet is about 8 × 8 mm, and slightly inclined plantarward and tibialward. This is sufficient to restore the dorsal part of the sigmoid facet in Tolat’s type I and II.41 Bearing in mind the three-dimensional shape and slanting of the different facets of the base of the third metatarsal, we can restore a die-punch defect in a single stage by harvesting the contralateral graft (Fig. W9-2).
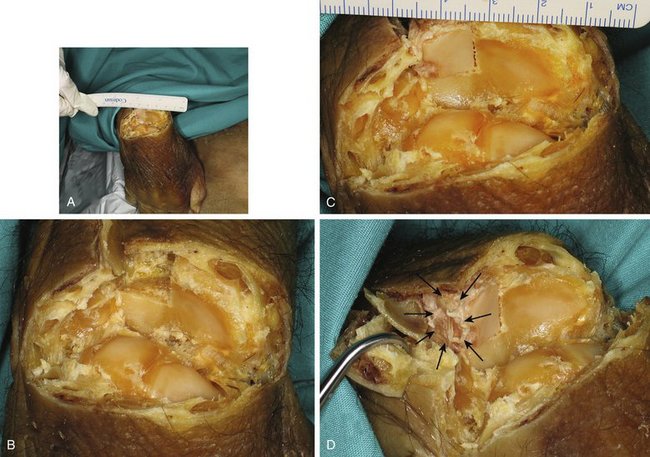
FIGURE W9-2 Simulation in a cadaver of the reconstruction of the malunited die-punch fragment. A, In this specimen, all the dorsal structures have been sharply divided at the radiocarpal level, and the wrist has been flexed to have an unimpeded view of the radius surface. B, Close-up view where the dorsal half of the right lunate facet of the distal radius has been resected in preparation for its reconstruction. C and D, The dorsal half of the left third metatarsal is replacing the dorsal half of the lunate facet of the distal radius. It matches the radius slope in both views, and the accessory facet (arrows) is located in a favorable position for reconstructing the sigmoid facet (when the contralateral metatarsal is selected).
(From del Piñal F, García-Bernal JF, Delgado J, et al: Reconstruction of the distal radius facet by a free vascularized osteochondral autograft: anatomical study and report of a case. J Hand Surg [Am]. 2005; 30:1200-1210; with permission from the American Society for Surgery of the Hand.)
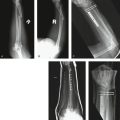
The blood supply to the dorsum of the foot has been studied by several investigators. The classic anatomical description of the dorsalis pedis artery with three lateral branches (proximal lateral tarsal artery, distal lateral tarsal artery, arcuate artery) is rare; it may represent less than 30%.42–45 We found the description of the anatomy of the dorsum of the foot by Cormack and Lamberty42 closely matches our findings. They described a plexus where all the arteries are interrelated in a competitive fashion from where the dorsal intermetatarsal arteries originate (Fig. W9-3A). In all the specimens in our anatomical study, we were able to track a periosteal artery to the dorsalis pedis. In addition, in all of the specimens, the nutrient foramina could be found in the dorsum of the proximal aspect of the third metatarsal. We found that in two thirds of the cases, the distal lateral artery was dominant, whereas in the rest the arcuate was the larger vessel (Fig. W9-3B and C). Variations in vascular anatomy must be kept in mind when harvesting this flap; as is the case in modern perforator-based flaps, deviation of anatomy is the norm. The surgeon has to be prepared to perform a “freestyle” type of harvesting,46 rather than expecting a book type of anatomical findings.
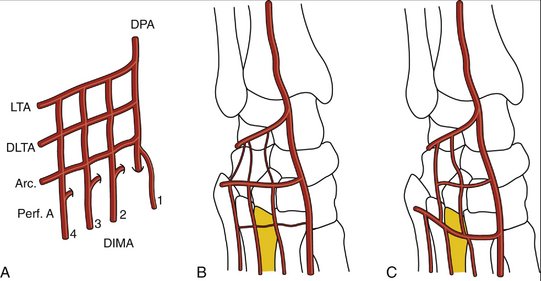
FIGURE W9-3 The blood supply to the dorsum of the foot, and the variations most commonly found (see text for details). A, Classic arterial network. B, Distal lateral tarsal artery dominance (the arcuate vessels are either hypoplastic or absent). C, Arcuate artery dominance (the distal lateral tarsal artery is hypoplastic or absent). Arc, arcuate vessels; DIMA, dorsal intermetatarsal arteries; DLTA, distal lateral tarsal artery; DPA, dorsalis pedis; LTA, lateral tarsal artery; Perf A, perforating arteries.
(From del Piñal F, García-Bernal JF, Delgado J, et al: Reconstruction of the distal radius facet by a free vascularized osteochondral autograft: anatomical study and report of a case. J Hand Surg [Am]. 2005; 30:1200-1210; with permission from the American Society for Surgery of the Hand.)
Indications
The ideal candidate for this operation is a young patient with irreversible damage to the articular cartilage of the lunate or the scaphoid facet of the distal radius. For these instances, the principal facet of the base of the third (or alternatively the second) metatarsal is used. The procedure also is indicated to reconstruct the die-punch malunion. In this instance, the dorsal aspect of the sigmoid fossa is reconstructed by the accessory facet of the base of the third metatarsal (see Fig. W9-2).
Focal cartilage loss (2 to 3 mm) and scarring on the corresponding scaphoid or the lunate’s articular surface are considered a relative contraindication. In a sedentary patient, the procedure may be unwise after the age of 50, but in an active or healthy manual worker we do not see age by itself to be a problem, provided that the patient is a nonsmoker and can tolerate a long operation.
There is no upper time limit for this procedure, provided that the joint surface of the corresponding carpal bone is preserved. This is more likely to occur when the step-off is located in the sagittal crease (the interfacetal ridge) because there is little load transfer in this area47,48 and little risk of wearing out the articular cartilage. The earlier the procedure is done, the better because there is an overload on the healthy cartilage.
Intrafacet step-offs produce not only overload on the unaffected areas,49 but also early erosion of the cartilage of the opposing carpal bone.5,50 Fernandez5 set 6 weeks as the upper limit until after which an intra-articular step-off would have caused irreversible cartilage damage on the opposing carpal bone. Rather than adhering to an exact timeline, I presently assess the joint arthroscopically and decide which operation to perform based on the quality of the cartilage on the radius and carpal surfaces. If both are preserved, we proceed with an arthroscopic-guided osteotomy; if the radius is damaged, but the carpal surface is preserved, we proceed with an osteochondral graft. If both sides of the joints are damaged, we proceed with a partial arthrodesis (Fig. W9-4).
A final indication is the central defect that is created during the correction of irregular sagittal gaps/step-offs. In those circumstances after débriding the scarred area, the surgeon creates a secondary defect (Fig. W9-5A). If this is closed directly, without interposing any tissue, the radius would be narrower in the frontal plane, the normal ligamentous restraints of the carpus would be relatively lengthened, and the carpus would translocate ulnarly. This is a painful situation for the patient and the surgeon (Fig. W9-5B). This secondary carpal translocation can be avoided by interposing a vascularized osteochondral graft (Fig. W9-5C).
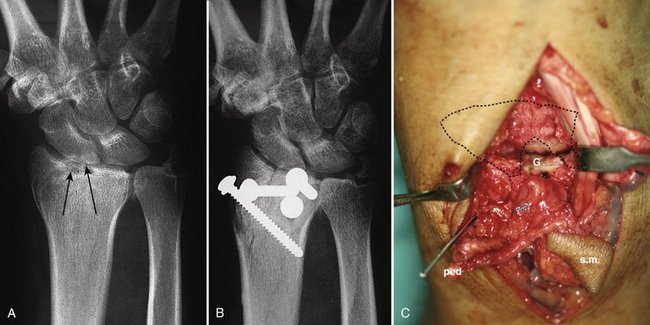
FIGURE W9-5 This patient was treated for a four-part malunion with a classic open outside-in osteotomy technique. A, The lunate fossa was anatomically reconstructed; however, the effect of resecting scarred and multifragmented tissue in the gap on the scaphoid fossa (arrows) entailed a narrower radius. B, In the early x-ray, already an ulnar carpal translocation can be seen. This became painful. (This case predated the vascularized osteochondral graft). C, To avoid this complication in case no. 4 of our series, with a similar problem in the lunate fossa, this was managed interposing a vascularized osteochondral graft (G). ped, pedicle; sm, skin monitor.
Contraindications
The procedure is absolutely contraindicated when the cartilage loss involves the corresponding carpal bone also. Under those circumstances, the metatarsal would face an eroded surface, and early osteoarthritis is to be expected.
One should not exceed the resurfacing capabilities of the graft. Anything larger than a single facet is beyond the scope of this technique and is a contraindication. In many cases, there is an area of irreversible damage (dorsally located) that is adjacent to areas of malunited, but vascularized, volar fragments. These malunited fragments are salvaged and reduced, which limits the amount of chondral resurfacing that is needed on the dorsal part of the joint (Fig. W9-6).
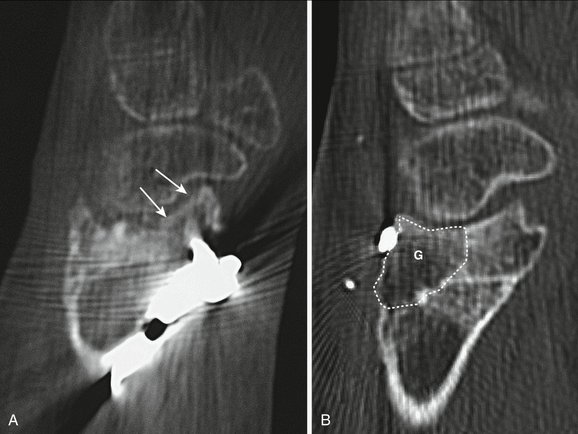
FIGURE W9-6 A and B, Reduction of the anterior malpositioned fragment (arrows in A) limited graft’s needs (G) to the dorsal half of the scaphoid fossa (B).
A final word of caution with regard to an apparently “healthy” bony fragment on the distal radius that was markedly displaced during the original trauma. The original plain films are invaluable for assessment of the degree of original displacement (Fig. W9-7). If one takes into account how the distal radius is vascularized,51,52 it is likely that some of those displaced fragments were devascularized. In this case, these fragments behave as osteochondral grafts; they look healthy on x-ray appearance, but the cartilage component of the graft is worthless in the short-term.15,28–30 If reconstruction is planned, one should take into account that this bone block also would be excised, making the actual defect much larger than that anticipated based on the x-ray appearance.
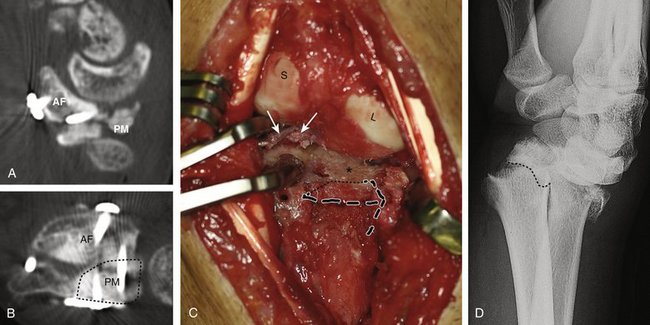
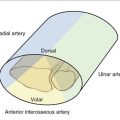
FIGURE W9-7 Detrimental effect of initial displacement of cartilage on vitality and biology after massive fragment displacement during the initial trauma. A and B, On preoperative computed tomography scan (4 months after trauma), a large “benign” anterior fragment (AF) and a posteromedial (PM) comminuted area with involvement of the distal radioulnar joint can be seen (dots). The PM fragment is to be replaced by a vascularized osteochondral graft. C, The posteromedial fragment has been removed (stippled lines) exposing into view the anterior fragment (asterisk). Massive scattered chondrolysis is evident throughout the fragment, including full-thickness chondral fragments (arrows). Despite the good condition of the cartilage of the scaphoid and lunate, the procedure was aborted in favor of a partial arthrodesis. D, The original x-ray shows that the volar fragment was the main component of a Melone type IV fracture. To the quite improbable case that this fragment had any vascular connection after such a displacement, it has to be added that it was unreduced for 4 days. The importance of the original x-rays for planning purposes cannot be overemphasized.
Surgical Technique
All patients should have preoperative plain x-rays of both wrists and a computed tomography scan of the involved joint. Axial views are invaluable to assess the size of the defect. All of our patients were operated under combined epidural and axillary block. The procedure is started by exploring the wrist under tourniquet and exsanguination. Ideally, a midline incision is used, but concessions should be given to the previous surgical approaches. This early exploration allows the surgeon to measure the actual defect and to assess the status of the articular cartilage on the adjacent carpal bones. In more recent cases, diagnostic wrist arthroscopy has been used instead. As stated previously, the operation can be aborted at any stage if conditions are not met (see Fig. W9-4).
The extensor retinaculum is opened in a zigzag manner over the fourth extensor compartment. The capsule is sharply elevated from the dorsal rim of the radius and reflected distally. Any bony excrescence, dorsal malpositioned fragment, or osteophyte that might block the view of the joint surface is excised with a small rongeur (Fig. W9-8A). Distraction with Chinese finger traps and judicious use of a lamina spreader, while protecting the blades with a silicone intravenous tubing, permits exploration of the joint (see Fig. W9-7C). Prying too strongly with the lamina spreader should be avoided because this would damage healthy cartilage areas. Sometimes scarring on the volar ligaments may limit vision, but with the help of a Freer elevator many of those adherences can be gently broken. The irreversibly damaged area (i.e., irregularly scarred tissue and bone devoid of cartilage) is then identified.
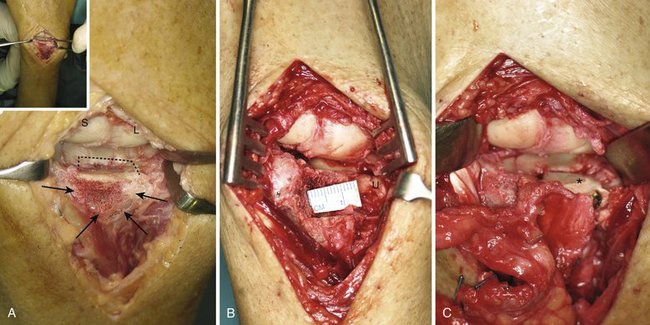
FIGURE W9-8 Intraoperative view of the same patient as in Figures 9, 10, and 11. (Inset, panoramic view). A, Area of scarring and irregularities on the dorsoulnar aspect of the radius can be seen only after the hypertrophic dorsal rim was rongeured (arrows) (see also Fig. W1-10A and W10-B. B, A three-dimensional defect has been created exposing the ulnar head (U). C, The graft has been secured by two screws (see Fig. W1-10D).
At this stage, the final decision to proceed is made. If the conditions are met (i.e., absence of carpal cartilage damage, no static dissociation, and size of defect amenable to the graft capabilities), the operation is continued; otherwise, the appropriate salvage procedure is carried out. A three-dimensional defect is created in the metaphyseal-epiphyseal area to allow room for fitting the graft, by using osteotomes, sagittal saws, and a rongeur (see Fig. W9-8B). The pins for an external fixator also are installed. During this stage, any volar fixation hardware that may block the reduction also is removed. Additionally, any intra-articular malunited fragment is osteotomized, reduced, and fixed under direct visual control. The bed is ready to fit the graft. A compressive bandage is applied, and the tourniquet is released.
The appropriate foot is exsanguinated by elevation, and the tourniquet is elevated. The foot is approached though a zigzag incision in the cleft between the extensor hallucis longus and the extensor digitorum longus. A tiny skin perforator from the dorsalis pedis is identified and traced to the dermis. A small skin island, which is dependent on this perforator, also is harvested to act as monitor. The extensor hallucis brevis is divided and retracted laterally together with the extensor digitorum longus, which exposes the blood supply to the dorsum of the foot (Fig. W9-9A). The exact location of the metatarsal to be elevated is misleading sometimes. It can be located exactly by using intramuscular needles and fluoroscopy (see Fig. W9-9A).
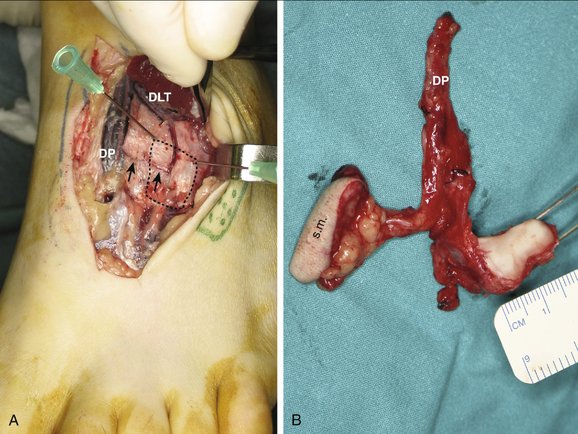
FIGURE W9-9 Intraoperative view during graft harvesting. A, The vessels going to the base of the third metatarsal (dots) are shown in this case in which the distal lateral tarsal artery is dominant. Conversely, the arcuate artery is small (arrows). Two intramuscular needles are being used to locate the graft (see text). B, The harvested flap. DP, dorsalis pedis; s.m., skin monitor.
(From del Piñal F, Innocenti M: Evolving concepts in the management of the bone gap in the upper limb: long and small defects. J Plast Reconstr Aesthet Surg. 2007; 60(7):776-792; with permission from the British Society of Plastic Surgery.)
As stated in the anatomy section, the blood supply varies, and the surgeon should have a clear picture of the blood supply of the graft before cutting any vessel. Sometimes the distal lateral tarsal artery is dominant, whereas other times the arcuate vessels are dominant, all going to the dorsalis pedis axis. In one case, the blood supply to the second metatarsal was judged to be more reliable than the supply to the third metatarsal, and the base of the former was selected as the donor graft. Under ideal circumstances, we prefer the base of the third metatarsal because the anatomy of the facet is closer to the distal radius facets. In any case, one should be prepared to alter the flap in a “freestyle” manner as necessary.46
The distal lateral artery and any other tiny supraperiosteal vessel are protected by including a cuff of periosteum because any inadvertent pulling would damage them. The base of the metatarsal is released from the soft tissues and divided with an oscillating saw about 1.5 cm distal to the cuneiform and always distal to the arcuate artery when present. The graft is dissected from its ligamentous attachments until it can be pedicled on its nutrient vessels. The intermetatarsal space is very tight, and the intermetatarsal ligaments are very deep. Rough maneuvers may put the nutrient vessels at risk. A lamina spreader is invaluable in this part of the operation to separate the metatarsals. The first dorsal metatarsal artery is ligated distal to the take-off of the arcuate artery, and the deep arterial branch to the plantar system is divided.
The graft is isolated on the dorsalis pedis vessels. Caution should be taken with the deep peroneal nerve, which travels in the neighborhood of the pedicle. If the surgeon decides that it should be divided, the nerve should be resected proximally to leave its stump proximal to the annular ligament; otherwise, a painful neuroma may develop. This complication, which we have learned through painful experience, is documented in the literature.53 The tourniquet is released, and the flap is left pedicled for 20 minutes to assess bleeding and to reduce the ischemia time from the microvascular part of the operation. In all of our clinical cases, profuse bleeding from the skin and the bone was noted in every case.
The flap’s pedicle is now divided (see Fig. W9-9B), and the arm tourniquet is elevated. The osteochondral graft is tailored to fit into the defect and rigidly fixed there (see Fig. W9-8C). Lag screws were used in all our cases; nevertheless, the load across the wrist was neutralized with an external fixator without distraction. Revascularization is done in the anatomical snuffbox. The dorsal-is pedis is anastomosed to the radial artery in an end-to-side fashion. The venae comitantes are joined in an end-to-end or end-to-side manner to local veins. In most cases, a running 9-0 nylon suture on a 150-μm needle is used. The extensor retinacular ligament is closed, taking care not to compress the pedicle or the skin island pedicle. If undue tightness is noticed at the time of closure, a small skin graft should be used; otherwise, the skin monitor, which always has a tenuous blood supply, might die.
The whole graft fitting and revascularization is best done under ischemia in less than 2 hours of tourniquet time. Just before the tourniquet is released, a bolus of 1500 U of heparin is injected intravenously. Thereafter, we presently prefer a continuous perfusion of saline and heparin (5000 U heparin in 500 mL of saline) at a rate of 25 mL/hr during the first 4 days, which is reduced to 12 mL for 1 more day. Patients were discharged on day 6 on low-molecular-weight heparin for an average of 2 weeks or less pending on ambulation. The flaps were monitored by assessing the capillary refill and color of the skin island and Doppler probe hourly for 48 hours, decreasing to 2 and then to 4 hours afterward. Doppler over the dorsum of the wrist may be unreliable because pulses may be transmitted from remote areas owing to the swelling.
The donor site is simply closed under aspiration drainage. No attempt to reconstruct the metatarsal is done. Protected ambulation with a rigid sole shoe is allowed after 3 to 5 days and with regular shoes after 3 to 4 weeks. A compressive stockinette is recommended for about 3 months, or until the foot swelling totally disappears.
At 5 weeks, the external fixator is removed in the office, and range of motion exercises are begun. A removable splint is used for an additional 2 weeks in between exercises, and the patient is instructed to perform active and assisted exercises. Thereafter patients are sent to physical therapy to improve range of motion and strengthening exercises for about 3 to 4 months. Night splints to improve wrist motion (particularly extension) are used at the discretion of the surgeon. After that time, patients are discharged. Improvement in grip and range of motion are expected during the first year (Figs. W9-10 and W9-11).
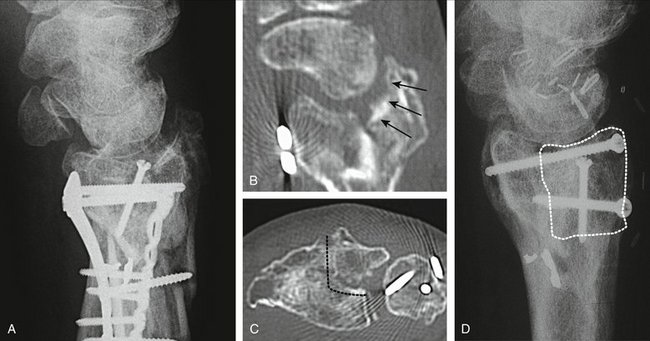
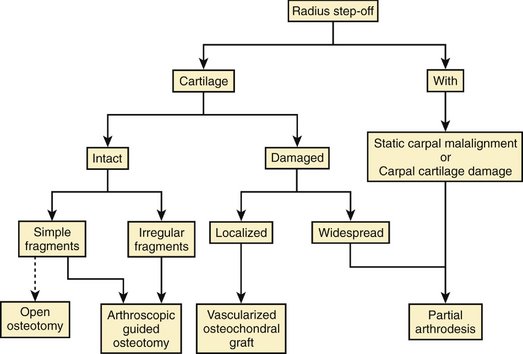
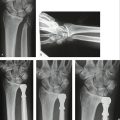
FIGURE W9-10 A, Preoperative x-rays of the same patient as in Figures W9-8 and W9-9 with a malunited lunate facet with sigmoid fossa involvement (supination −5°). B, In the sagittal computed tomography scan, damage on the malrotated fragment is highlighted by arrows. C, The area to be excised has been marked with dots in this axial view. D, X-ray 18 months after the operation. The osteochondral fragment is delimited by black dots.
(From del Piñal F, Innocenti M: Evolving concepts in the management of the bone gap in the upper limb: long and small defects. J Plast Reconstr Aesthet Surg. 2007; 60(7):776-792; with permission from the British Society of Plastic Surgery.)
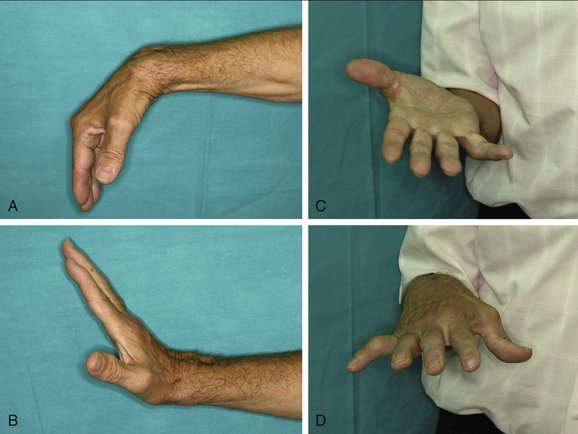
FIGURE W9-11 A to D, Functional result of the same patient as in Figures W9-8, W9-9, and W9-10.
(From del Piñal F, Innocenti M: Evolving concepts in the management of the bone gap in the upper limb: long and small defects. J Plast Reconstr Aesthet Surg. 2007; 60(7):776-792; with permission from the British Society of Plastic Surgery.)
Results
My experience with this technique is limited to four patients 33 to 56 years old, who were all manual laborers who sustained a work-related injury. In three cases, the base of the third metatarsal was transferred for reconstructing a die-punch fragment (two cases) or a scaphoid fossa (in patient no. 1). The final patient (no. 4) who had a central-dorsal lunate defect was reconstructed with the base of the second metatarsal. In every case, a skin island was used to monitor the blood supply to the transplants, which survived without complications. Patient no. 1 requested a screw removal 8 months after the operation. This removal was done under arthroscopic guidance, and allowed us to explore the joint. Bleeding points in the subchondral bone were noted after the screw was removed. In my opinion, of even more importance was the normal adherence of the cartilage to the subchondral bone (Fig. W9-12 and Video).
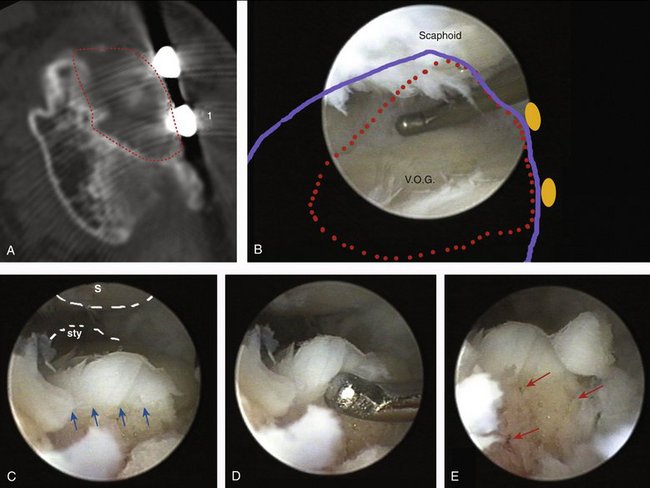
FIGURE W9-12 Arthroscopic findings at 8 months in case no. 1 (see also video). A and B, Outlines of the vascularized osteochondral graft (VOG) have been marked with red dots on the axial computed tomography scan (A) and the arthroscopic panoramic view (outline of the radius in blue; screw head positions are represented by orange circles) (B). C to E, Scope is at 6R portal looking at the dorsal edge of the osteochondral graft and focusing on the bone canal left by the screw that was fixing the graft. (This spot corresponds to 1 in A.) The scaphoid is above (S); on the very far end is the radial styloid (Sty). C, Normal cartilage and subchondral bone interface (small arrows). D, The probe is testing for delamination and is attempting to separate the cartilage from the subchondral bone by pushing distally. E, Bleeding points on the canal left after removal of the screw are highlighted with red arrows.
(From del Piñal F, García-Bernal JF, Delgado J, et al: Reconstruction of the distal radius facet by a free vascularized osteochondral autograft: anatomical study and report of a case. J Hand Surg [Am]. 2005; 30:1200-1210; with permission from the American Society for Surgery of the Hand.)
Our first three patients had a follow-up longer than 1 year (1 to 3 years). The average active range of flexion/extension was 90 degrees. Grip strength was 72% of the contralateral side. The pain as assessed using a visual analog scale (0 = no pain, 10 = unbearable) improved from 9 before the operation to 2 at the latest follow-up. There have been no problems to date of metatarsalgia on the donor foot, but in our first case a neuroma of the deep peroneal nerve required steroid injection. As noted in the surgical technique section, the deep peroneal nerve should be resected deep under the retinaculum. The eldest patient had a concomitant hip injury that forced him to retire; the others went back to their original or a similar type of work.
Conclusion
The vascularized osteochondral graft allows one to restore partial defects on the distal surface of the radius. It seems to be a logical alternative to partial arthrodeses because it avoids neighboring joint overload, with a better functional outcome in the short term. The microvascular part of the operation is easy because of the large caliber of the dorsalis pedis. The anatomy varies, however, and one has to be prepared to alter the plan pending local conditions. Rehearsal in a cadaver is strongly recommended. Radiological and clinical results seem to hold over time (Fig. W9-13).
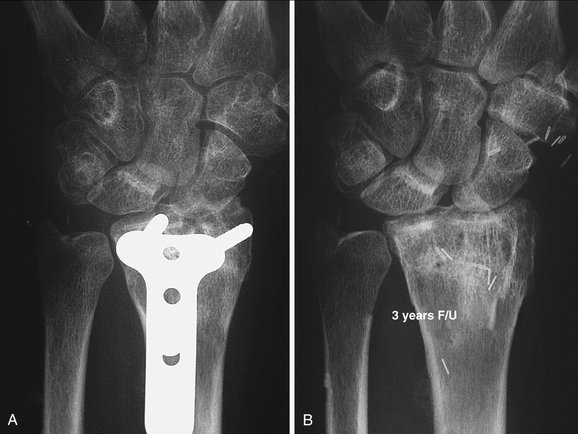
FIGURE W9-13 Preoperative and 3-year postoperative x-rays of patient no. 1. The joint spaces are well maintained. No signs of subchondral sclerosis or cysts are present on the scaphoid fossa.
1. Marx RG, Axelrod TS. Intraarticular osteotomy of distal radius malunions. Clin Orthop. 1996;327:152-157.
2. González del Pino J, Nagy L, González Hernandez E, et al. Osteotomías intraarticulares complejas del radio por fractura: indicaciones y técnica quirúrgica. Rev Ortop Traumatol. 2000;44:406-417.
3. Ring D, Prommersberger KJ, González del Pino J, et al. Corrective osteotomy for malunited articular fractures of the distal radius. J Bone Joint Surg Am. 2005;87:1503-1509.
4. Saffar P. Treatment of distal radial intra-articular mal-unions. In: Saffar P, Cooney WP III, editors. Fractures of the Distal Radius. London: Martin Dunitz, 1995.
5. Fernandez DL. Reconstructive procedures for malunion and traumatic arthritis. Orthop Clin North Am. 1993;24:341-363.
6. del Piñal F, Garcia-Bernal FJ, Delgado J, et al. Correction of malunited intra-articular distal radius fractures with an inside-out osteotomy technique. J Hand Surg [Am]. 2006;31:1029-1034.
7. Saffar P. Radio-lunate arthrodesis for distal radial intraarticular malunion. J Hand Surg [Br]. 1996;21:14-20.
8. Garcia-Elias M, Lluch A, Ferreres A, et al. Treatment of radiocarpal degenerative osteoarthritis by radioscapholunate arthrodesis and distal scaphoidectomy. J Hand Surg [Am]. 2005;30:8-15.
9. Nagy L, Buchler U. Long-term results of radioscapholunate fusion following fractures of the distal radius. J Hand Surg [Br]. 1997;22:705-710.
10. Giachino A, Mehin R, Backman D, et al. Autologous osteoarticular transfer from the proximal tibiofibular joint to the distal radial facets in the treatment of severe distal radial fractures. J Hand Surg [Br]. 2003;25(Suppl 1):36.
11. Mehin R, Giachino A, Backman D, et al. Autologous osteoarticular transfer from the proximal tibiofibular joint to the scaphoid and lunate facets in the treatment of severe distal radial fractures: a report of two cases. J Hand Surg [Am]. 2003;28:332-341.
12. Ishida O, Ikuta Y, Kuroki H. Ipsilateral osteochondral grafting for finger joint repair. J Hand Surg [Am]. 1994;19:372-377.
13. Boulas HJ. Autograft replacement of small joint defects in the hand. Clin Orthop. 1996;327:63-71.
14. Gaul JS Jr. Articular fractures of the proximal interphalangeal joint with missing elements: repair with partial toe joint osteochondral autografts. J Hand Surg [Am]. 1999;24:78-85.
15. Buckwalter JA. Articular cartilage injuries. Clin Orthop. 2002;402:21-37.
16. Huang FS, Simonian PT, Norman AG, et al. Effects of small incongruities in a sheep model of osteochondral autografting. Arthroscopy. 2004;32:1842-1848.
17. Poole AR. What type of cartilage repair are we attempting to attain? J Bone Joint Surg [Am]. 2003;85(Suppl 2):40-44.
18. Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230.
19. Brittberg M, Peterson L, Sjogren-Jansson E, et al. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85(Suppl 3):109-115.
20. Engkvist O, Johansson SH, Ohlsen L, et al. Reconstruction of articular cartilage using autologous perichondrial grafts: a preliminary report. Scand J Plast Reconstr Surg. 1975;9:203-206.
21. Foucher G, Hoang P, Citron N, et al. Joint reconstruction following trauma: comparison of microsurgical transfer and conventional methods: a report of 61 cases. J Hand Surg [Br]. 1986;11:388-393.
22. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(Suppl 2):25-32.
23. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86:455-464.
24. Shasha N, Krywulak S, Backstein D, et al. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85(Suppl 2):33-39.
25. Sledge C, Reddi AH, Walsh DA, et al. Biology of the normal joint. In Ruddy S, Harris ED, Sledge CB, editors: Kelley’s Textbook of Rheumatology, 6th ed, Philadelphia: WB Saunders, 2001.
26. Simkin PA. The musculoskeletal system. In: Klippel JH, Dieppe PA, editors. Rheumatology. St. Louis: Mosby, 1994.
27. Ulrich-Vinther M, Maloney MD, Schwarz EM, et al. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421-430.
28. Entin MA, Alger JR, Baird RM. Experimental and clinical transplantation of autogenous whole joints. J Bone Joint Surg Am. 1962;44:1518-1536.
29. Kettelkamp DB. Experimental autologous joint transplantation. Clin Orthop. 1972;87:138-145.
30. Yoshizu T, Watanabe M, Tajima T. Étude expérimentale et applications cliniques des transferts libres d’articulation d’orteil avec anastomoses vasculaires. In: Tubiana R, editor. Traité de chirurgie de la main. Paris: Masson, 1984.
31. Tsubokawa N, Yoshizu T, Maki Y. Long-term results of free vascularized second toe joint transfers to finger proximal interphalangeal joints. J Hand Surg [Am]. 2003;28:443-447.
32. Dautel G, Gouzou S, Vialaneix J, et al. PIP reconstruction with vascularized PIP joint from the second toe: minimizing the morbidity with the “dorsal approach and short-pedicle technique.”. Tech Hand Upper Extrem Surg. 2004;8:173-180.
33. Imhof H, Sulzbacher I, Grampp S, et al. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35:581-588.
34. Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533-544.
35. Taylor GI, Wilson KR, Rees MD, et al. The anterior tibial vessels and their role in epiphyseal and diaphyseal transfer of the fibula: experimental study and clinical applications. Br J Plast Surg. 1988;41:451-469.
36. Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86:1504-1511.
37. del Piñal F, García-Bernal JF, Delgado J, et al. Reconstruction of the distal radius facet by a free vascularized osteochondral autograft: anatomical study and report of a case. J Hand Surg [Am]. 2005;30:1200-1210.
38. del Piñal F, García-Bernal JF, Delgado J, et al. Injerto osteocondral vascularizado de la base del tercer metatarsiano para la malaunión irreparable de extremo distal del radio. Rev Ortop Traumatol.. 2008;52:171-178.
39. del Piñal F, Innocenti M. Evolving concepts in the management of the bone gap in the upper limb: long and small defects. J Plast Reconstr Aesthet Surg. 2007;60(7):776-792.
40. Mekhail AO, Ebraheim NA, McCreath WA, et al. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg [Am]. 1996;21:567-573.
41. Tolat AR, Stanley JK, Trail IA. A cadaveric study of the anatomy and stability of the distal radioulnar joint in the coronal and transverse planes. J Hand Surg [Br]. 1996;21:587-594.
42. Cormack GC, Lamberty BG. Lower leg. In Cormack GC, Lamberty BG, editors: The Arterial Anatomy of Skin Flaps, 2nd ed, Edinburgh: Churchill Livingstone, 1994.
43. Gilbert BJ, Horst F, Nunley JA. Potential donor rotational bone grafts using vascular territories in the foot and ankle. J Bone Joint Surg [Am]. 2004;86:1857-1873.
44. Huber JF. The arterial network supplying the dorsum of the foot. Anat Rec. 1941;80:373-391.
45. Yamada T, Gloviczki P, Bower TC, et al. Variations of the arterial anatomy of the foot. Am J Surg. 1993;166:130-135.
46. Mardini S, Tsai FC, Wei FC. The thigh as a model for free style free flaps. Clin Plast Surg. 2003;30:473-480.
47. Viegas SF, Tencer AF, Cantrell J, et al. Load transfer characteristics of the wrist, part I: the normal joint. J Hand Surg [Am]. 1987;12:971-978.
48. Craigen MA, Stanley JK. Wrist kinematics: row, column or both? J Hand Surg [Br]. 1995;20:165-170.
49. Wagner WF Jr, Tencer AF, Kiser P, et al. Effects of intra-articular distal radius depression on wrist joint contact characteristics. J Hand Surg [Am]. 1996;21:554-660.
50. Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am. 1986;68:647-659.
51. Brunelli F, Pagliei A, Smussi C. Anatomy of the distal radius. In: Saffar P, Cooney WP III, editors. Fractures of the Distal Radius. London: Martin Dunitz, 1995.
52. Sheetz KK, Bishop AT, Berger RA. The arterial blood supply of the distal radius and ulna and its potential use in vascularized pedicled bone grafts. J Hand Surg [Am]. 1995;20:902-914.
53. Rose EH, Kowalski TA. Restoration of sensibility to anesthetic scarred digits with free vascularized nerve grafts from the dorsum of the foot. J Hand Surg [Am]. 1985;10:514-521.