Chapter 123
Occupational Vascular Problems
Mark K. Eskandari, Hari R. Kumar
Upper extremity work-related injuries are a major societal problem in regard to disability, cost, and loss of workdays. Occupational injuries affecting the shoulders, arms, and hands have been recognized for nearly 300 years and are generally categorized into injuries caused by accidents or injuries resulting from long-standing repetitive tasks.1 Injuries in the latter category are due to small but additive amounts of tissue damage sustained from repetitive motions; they are known collectively as cumulative trauma disorders. According to 2011 data released by the U.S. Bureau of Labor Statistics, injuries sustained from repetitive motion often result in longer duration of time away from work compared with other classes of injury.2 Although most of these injuries affect the musculoskeletal system, injuries to arteries and veins are known to occur.3 Work-related vascular injuries develop from excessive or exaggerated activity involving the shoulders, arms, or hands. Box 123-1 lists arterial disorders associated with occupational trauma.
Manual Labor Injuries
Hand-Arm Vibration Syndrome
The first cases of this injury were reported by Loriga in 1911 among Italian miners presenting with “dead finger.”4 Hamilton in 1918 made the connection between cold-induced blanching and numbness of the hands and use of pneumatic drills by stonecutters in Indiana.5 Subsequent work by Taylor and Pelmear6 and Ashe et al7 firmly established hand-arm vibration syndrome (HAVS) as a discrete clinical entity.
The disease has been referenced by many names, from the original dead finger to Raynaud’s of occupational origin, traumatic vasospastic disease, and vibration-induced white finger. The current designation of HAVS was introduced to reflect that the extent of involvement is more of the upper extremity than just the digit. Regardless of the name, the common initial symptoms are those of Raynaud’s phenomenon secondary to prolonged use of vibrating mechanical tools.
Clinical Findings and Risk Factors
The Taylor and Pelmear staging system is presented in Table 123-1.6 In the early stages, vibration injury may be manifested as slight tingling and numbness. Later, the tips of one or more fingers experience attacks of blanching, usually precipitated by cold. With continued progression, the affected area increases in size, and the blanching extends to the entire finger. Attacks of white finger typically last about 1 hour and terminate with reactive hyperemia (red flush) and often considerable pain. Prolonged exposure may induce bluish black cyanosis in the affected fingers. Only about 1% of cases progress to ulceration or gangrene.8
Table 123-1
Stages of Hand Arm Vibration Syndrome
| Stage | Condition of Digits | Work and Social Interference |
| 0 | Vibration exposure but no signs or symptoms | No complaints |
| 0T | Intermittent tingling | No interference with activities |
| 0N | Intermittent numbness | No interference with activities |
| 1 | Blanching of one or more fingertips, with or without tingling and numbness | No interference with activities |
| 2 | Blanching of one or more fingers with numbness, usually in winter | Slight interference with home and social activities; no interference with work |
| 3 | Extensive blanching; frequent episodes in summer and winter | Definite interference at work, at home, and with social activities; restriction of hobbies |
| 4 | Same as stage 3: extensive blanching, most fingers involved, frequent episodes in summer and winter | Same as stage 3, but occupation changed to avoid further vibration exposure because of the severity of signs and symptoms |
Updated from Taylor W, et al, editors: Vibration white finger in industry, New York, 1975, Academic Press.
Vibrating handheld machines (e.g., pneumatic hammers and drills, grinders, chain saws) have been implicated in HAVS. Such injury potential is not restricted to a few types of tools but applies to a variety of situations in which workers’ hands are subjected to transmission of vibration energy.9 There appears to be a linear relationship between the acceleration exposure dose (amount of acceleration and years of exposure) and the onset and severity of HAVS.10
The exact mechanism of injury is unknown. The frequency and intensity of vibration affect the extent of damage to the endothelium.11 Local platelet adhesion seems to be an important factor in arterial occlusion. It has been shown that sympathetic hyperactivity, in combination with local factors such as vibration-induced hyperresponsiveness of the digital vessels to cold, may be responsible for the finger-blanching attacks.12 In those with exposure to vibration injury, appearance of symptoms has also been correlated to smoking status.13
Diagnosis
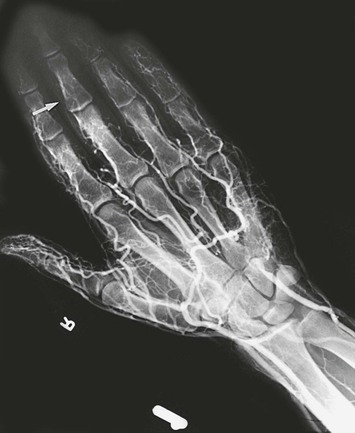
Key features in the history include use of vibrating tools and symptoms of Raynaud’s phenomenon. For a vasospastic condition, the most promising single objective test is cold provocation and recording of the time until digital temperature recovers (see Chapter 15). Digital artery occlusion is best detected by recording the systolic pressure of the affected fingers with transcutaneous Doppler ultrasound14–16 or duplex scanning.17 In advanced disease, arteriography is helpful. Barker et al18 documented arterial occlusion by brachial arteriography in workers who complained of hand blanching and attacks of numbness. Other authors have reported on the use of arteriography in this injury.19–21
Arteriographic changes in HAVS can include multiple segmental occlusions of the digits and a corkscrew configuration of vessels in the hands.21 The extent of digital artery occlusion appears dependent on the frequency and duration of exposure.22
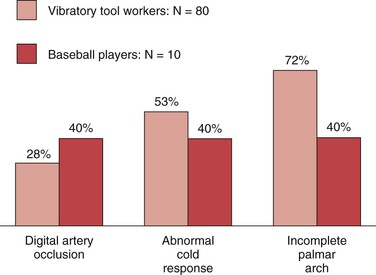
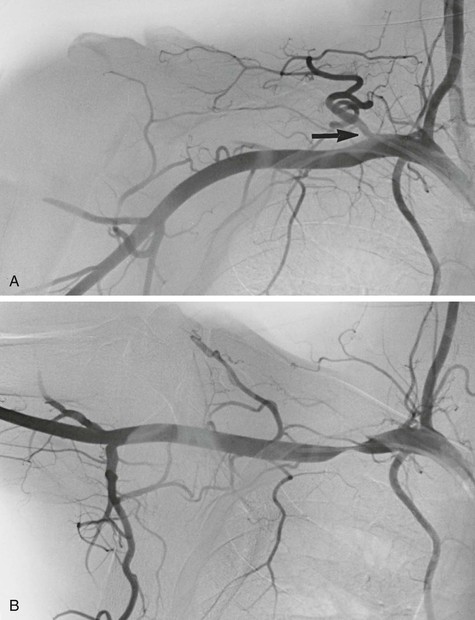
Of 80 workers (chippers) with HAVS investigated at the Blood Flow Laboratory at Northwestern University, 25 (31%) exhibited a significant reduction in systolic pressure in one or more digits.23 In 6 of the 25 workers, arteriography showed digital artery occlusion (Fig. 123-1). Incompleteness of the palmar arch was seen not only in the symptomatic hand but also in the contralateral, asymptomatic one. Raynaud’s phenomenon was present in 73 of 80 workers (91%). Abnormal cold response was observed in 53% of these workers (Fig. 123-2).
Treatment and Prevention
With onset of neurovascular symptoms that interfere with social or work activities, the initial treatment is discontinuation of use of vibratory tools.10 In advanced cases, a calcium channel blocker such as nifedipine (30 to 80 mg daily) may be useful. Calcium antagonists inhibit the response of arterial smooth muscle to norepinephrine and have been reported to be effective.24 Intravenous infusion of a prostanoid (prostaglandin E1, prostacyclin, or iloprost) is usually reserved for patients with digital gangrene.25 Surgical treatment, such as cervical sympathectomy or digital sympathectomy, is rarely needed.
Prevention is likely to be more effective than treatment. Personal protective equipment, such as gloves that limit exposure to cold and dampen transmission of vibration, should be used. Standards that limit the dose and duration of exposure to vibration have been set in place for factories and workplaces in the United States and internationally.10
Hypothenar Hammer Syndrome
Etiology and Incidence
The anatomy of the ulnar artery at the hypothenar eminence makes it vulnerable to injury with repetitive use of the palm of the hand in activities that involve pushing, pounding, or twisting. The ulnar artery and nerve travel in a tunnel known as Guyon’s canal that is bound by the pisiform and hamate bones. In this region, the ulnar artery is superficial and covered only by skin, subcutaneous tissue, and the palmaris brevis muscle (Fig. 123-3). Use of the palm as a hammer compresses the artery against the hook of the hamate bone, which acts as an anvil.
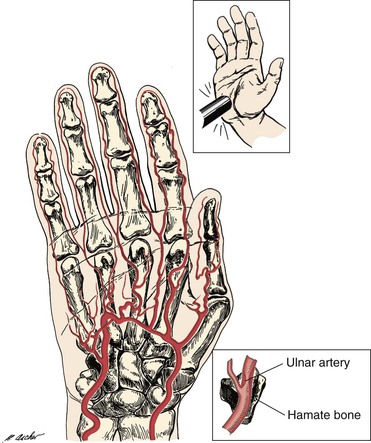
Figure 123-3 Mechanism of ulnar artery injury (upper inset) in a patient with hypothenar hammer syndrome. The terminal branch of the ulnar artery is vulnerable to injury because of its proximity to the hamate bone (lower inset).
Von Rosen (1934)26 and Guttani (1772)27 published the original reports of the disease, but it was Conn et al28 who first recognized the anatomic mechanism of injury and coined the term hypothenar hammer syndrome. Mechanics, factory workers, carpenters, masons, and any laborers who habitually use their hands as a hammer are at risk for the disease.29–31
The incidence of this rare entity is not precisely known as it is likely underrecognized. In Little and Ferguson’s study of 79 mechanics, 14% had evidence of ulnar artery occlusion but none had symptoms severe enough to seek medical attention.29 Studies focusing on cases of hypothenar hammer syndrome among referrals to tertiary centers for hand ischemia have found an incidence of 1.1% to 1.6%.31,32
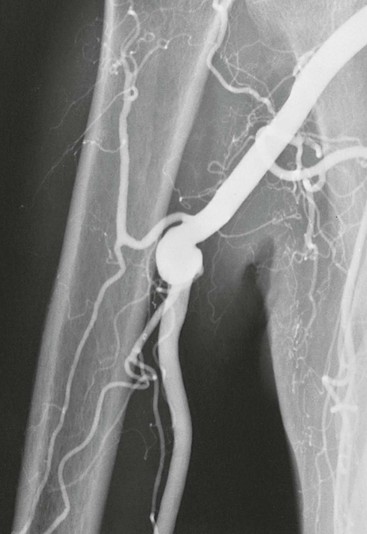
The type of arterial abnormality observed often depends on the nature of the vessel injury but includes thrombotic occlusion, aneurysm formation, or both. Vasospasm and damage to the intima can result in platelet aggregation and thrombus formation.33 Damage to the media can lead to aneurysmal degeneration of the artery (Fig. 123-4), although this occurs less frequently. Thrombus formation can also occur within the aneurysm itself and lead to distal embolization.
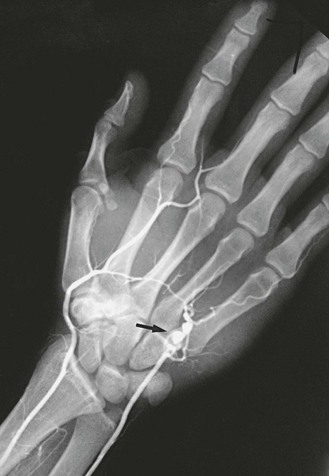
Figure 123-4 Arteriogram of the hand of a carpenter. Note the aneurysm of the ulnar artery (arrow) caused by repetitive trauma from using the hand as a hammer.
Research by Ferris et al32 demonstrated that, in addition to vascular damage from repetitive trauma, underlying vessel abnormalities may also be responsible. Histologic examination of 19 resected ulnar arteries showed hyperplastic proliferation of the intima or media and disruption of the internal elastic lamina suggestive of fibromuscular dysplasia. In 13 patients for whom bilateral angiograms were available, 12 of 13 (92%) showed an abnormality in the contralateral asymptomatic ulnar artery. On the basis of this evidence, they proposed that the etiology of hypothenar hammer syndrome depends on the presence of underlying ulnar artery fibromuscular dysplasia with superimposed repetitive palmar trauma.
Clinical Findings and Diagnosis
Patients typically present with Raynaud’s phenomenon. However, several key features distinguish hypothenar hammer syndrome from similar syndromes.33 There is a preponderance of male smokers with a concomitant history of repetitive trauma to the hand.31 The distribution is asymmetrical, often involving only the dominant upper extremity. The cyanosis and pallor phases can occur, but the hyperemic redness is usually lacking.30 The digits affected tend to be the ulnar three fingers, with a distinct lack of involvement of the thumb.30,32
Physical examination may reveal cool or mottled digits; severe cases present with ischemic ulcers. A callus may be present over the hypothenar eminence. The result of Allen’s test is often positive, indicating ulnar artery occlusion. On occasion, an aneurysm is observed as a pulsatile mass in the hypothenar eminence.
Noninvasive imaging studies such as duplex ultrasound and contrast-enhanced computed tomography or magnetic resonance imaging have been used to make the diagnosis of hypothenar hammer syndrome.34–36 However, the “gold standard” imaging modality in hypothenar hammer syndrome remains invasive arteriography. Arteriography is extremely useful in diagnosis and planning of surgical treatment as it defines the type of vascular lesion (spasm, aneurysm, occlusion), shows the site and extent, and identifies the anatomy of the palmar arch and significant collateral vessels. A corkscrew pattern is typically observed in affected vessels.32 Regardless of which imaging modality is used, proximal arterial segments should be included in the examination to rule out upstream sources of embolization.
Treatment
The majority of patients with hypothenar hammer syndrome will improve with nonsurgical interventions.31 Conservative measures include smoking cessation and hand protection involving avoidance of exposure to cold environments and avoidance of further trauma. Medical therapy consists of calcium channel blockers and antiplatelet drugs. Anticoagulation has been used in cases of digital necrosis.
Surgical therapy is reserved for severe cases of digital ischemia or the presence of an aneurysm. Simple ligation of an aneurysm can be performed if there is adequate collateral circulation. More often, surgical intervention requires resection with primary reconstruction or vein graft interposition. Several large case series report that a majority of patients have improvement in symptoms of hand ischemia after reconstruction and that long-term results are satisfactory.32,37–39
Exposure Injuries
Occupational Acro-osteolysis
Wilson et al40 first described this disease in workers exposed to polyvinyl chloride. Many of these workers experienced ischemic symptoms in the hand with resorption of the distal phalangeal tufts, similar to that seen with scleroderma. The dominant initial symptoms are those of Raynaud’s syndrome. A few reports of angiography in this syndrome document damage to the digital arteries.41–43 Findings include multiple stenoses and occlusions of the digital arteries along with nonspecific hypervascularity adjacent to the areas of bone resorption. The reason for the hypervascularity is not clear, but it may be related to stasis of contrast medium in digital pulp arteries secondary to shortening and retraction of the fingers. Some of these digits were clubbed, a finding that has also been associated with hypervascularity in the fingertips. Treatment is supportive.
Electrical Burns
Electrical energy inflicts tissue destruction in relation to the voltage applied. Currents less than 1000 V cause injuries limited to the immediate underlying skin and soft tissue. High voltage (>1000 V) usually causes extensive damage as the current travels from the point of entry to the point of exit. No tissue is immune to the devastating effects of high-voltage injury, and arterial injury may occur. The upper extremity, especially the hand, is involved more often than are other parts of the body because of its grasping function. The arterial injury is often manifested by arterial necrosis with thrombus or bleeding, and gangrene of the digits occasionally develops.
Bookstein41 described the angiographic changes in the upper extremity after electrical injury, including extensive occlusion of the ulnar and digital arteries and thrombosis of the radial artery. Arterial spasm may also be present. Later, damage to the media may cause aneurysm formation. Figure 123-5 shows a brachial artery aneurysm in a patient who had sustained electrical burns 9 months earlier. Treatment and salvage of the upper extremity depend on the associated soft tissue and bone injuries.44 Occlusion of a major artery documented by arteriography requires bypass grafting, and good results have been reported.45
Extreme Thermal Injuries
Vasomotor disturbances in the hands of individuals exposed to extreme chronic thermal trauma are typically manifested as Raynaud’s syndrome. Workers at highest risk for thermal injuries are those in a profession in which their hands are subjected to chronic exposure to cold, such as slaughterhouses, canning factories, and fisheries.46,47 Epidemiologic studies examining this dilemma are limited. The action of alternating ice-cold and hot exposure, use of plastic gloves in cold exposure, and long-term exposure to cold seem to be identifiable risk factors. Treatment is supportive.
Athletic Injuries
Athletes, particularly professionals who engage in strenuous or exaggerated hand or shoulder activity, may be susceptible to upper extremity ischemia as a result of arterial injury. Hand ischemia is often manifested as Raynaud’s syndrome, symptoms of sudden arterial occlusion, or embolization to the digits. Injury can occur anywhere along the course of the upper extremity from the subclavian artery to the digital vessels. Recognized syndromes occur anatomically at the thoracic outlet, the quadrilateral space, and the humeral head and in the hand. The exact incidences of these syndromes are unknown. The majority of reported arterial injuries in the elite athlete involve baseball players; however, vascular injury has been reported in such sports as karate, volleyball, handball, Frisbee, lacrosse, golf, weightlifting, and butterfly swimming.48,49
Hand Ischemia
Clinical Findings and Risk Factors
The mechanisms of hand ischemia fall into two main categories: direct digital artery injury and embolization from a proximal source in the upper extremity. Hand activity in any sport can cause blunt-force injury to the arteries50; however, direct digital arterial trauma is encountered more often in handball players, baseball catchers, and practitioners of karate. Figure 123-6 illustrates occlusion of the palmar arch in a Frisbee player; ischemia of all fingers occurred suddenly after he caught the Frisbee. It has been suggested that handball players with more than 200 hours of accumulated playing time are at greater risk for symptomatic alterations in perfusion.51,52
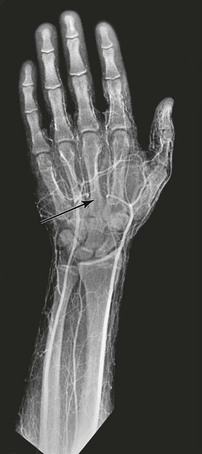
Figure 123-6 Occlusion of the palmar arch (arrow) in a Frisbee player. Because of this injury, there is poor filling of contrast medium in the second, third, fourth, and fifth fingers.
Baseball players, particularly catchers because of the tremendous and frequent impact of the baseball, are predisposed to the development of chronic hand ischemia. Many catchers exhibit Raynaud’s symptoms, especially in the off-season when they engage in outdoor activity in cool autumn or winter weather. Lowrey53 reported decreased digital perfusion to the index finger of the glove hand in 13 of 22 baseball catchers examined by Doppler flow and the Allen test. Of 10 professional catchers studied in that author’s laboratory, 40% had evidence of digital artery occlusion.53
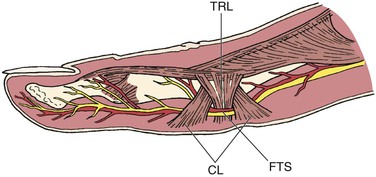
Another form of hand ischemia occasionally observed in baseball pitchers is compression of the digital artery by Cleland’s ligament. These ligamentous structures are found on the palmar surface of the digits and span from the phalanx to the subcutaneous tissue (Fig. 123-7). The proposed mechanism is compression of the digital vessels with hyperextension of the proximal interphalangeal joints.
Treatment
Treatment of hand ischemia depends on the clinical findings. With acute injury, a conservative approach involving intravenous infusion of dextran and pain control is in order. Surgical intervention is rarely needed; however, attempts at digital sympathectomy or release of Cleland’s ligaments have met with some short-term success.54 Preventing injury is important and can be accomplished by the use of gloves with padding and other protective devices.53
Quadrilateral Space Syndrome
The quadrilateral space is defined as the area bordered by the teres minor superiorly, the humeral shaft laterally, the teres major inferiorly, and the long head of the triceps muscle medially (Fig 123-8).55 Found within this space are the posterior humeral circumflex artery and the axillary nerve.
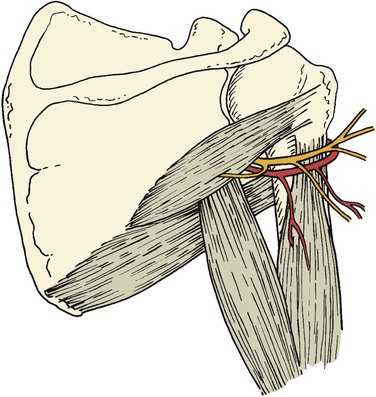
Figure 123-8 The posterior humeral circumflex artery and axillary nerve traverse the interval of the quadrilateral space.
Cahill et al56 first reported in 1983 the diagnosis and surgical treatment of 18 patients with this entity. Compression of the posterior humeral circumflex artery within this space occurs with the arm in the “cocked” position (abduction and external rotation). Chronic compression and trauma to this artery in overhand motion athletes, particularly pitchers and volleyball players, can lead to aneurysmal dilatation or occlusion (Fig. 123-9). Aneurysms in this location are prone to distal embolization in the hand.57,58
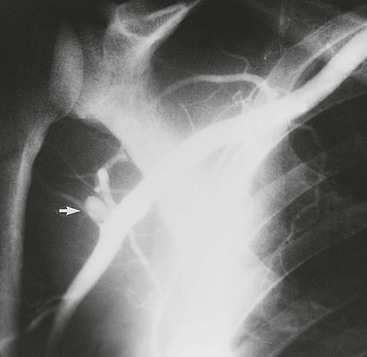
Figure 123-9 Artery of a major league baseball pitcher. Note the aneurysm of the posterior humeral circumflex artery (arrow).
Thrombolytic therapies may be used initially to dissolve the distal emboli, but surgery to treat the aneurysm should then be addressed. Surgical treatment of aneurysms in this location involves ligation of the posterior humeral circumflex artery.59 The anterior and posterior humeral circumflex arteries provide blood supply to the humeral head, and at least one of the two vessels must be preserved or repaired to prevent avascular necrosis.
Humeral Head Compression of the Axillary Artery
Lord et al60 first reported compression of the third portion of the axillary artery by the head of the humerus. With overhead throwing or striking motions in which the arm is abducted and externally rotated, there is downward compression of the humeral head against the axillary artery.57,61 Sonographic studies have demonstrated the impedance of flow through the axillary artery by the humeral head with these overhand motions.62
Symptoms typically include arm fatigue and loss of pitch velocity after several innings. Finger numbness, Raynaud’s syndrome, and cutaneous embolization can be seen as well. Evaluation includes duplex studies and arteriography with the arm at rest and in the provocative position.
Treatment is based on extent of injury. For those with compression of the artery alone, modification of throwing motion has resulted in improvement of symptoms. Saphenous vein patch angioplasty has been used for those whose symptoms do not improve.63,64 In patients with structural injury to the vessel, resection with saphenous vein bypass has been performed either anatomically tunneled or extra-anatomically above the pectoralis minor. Long-term results are good, and many of the athletes treated at major centers are able to return to competition after rehabilitation.64–66
Thoracic Outlet Syndrome
Athletes who engage in overextended shoulder motion, such as baseball pitchers, butterfly swimmers, weightlifters, and oarsmen, are potential candidates for thoracic outlet compression. Although not as common as neurogenic and venous thoracic outlet syndrome (TOS), injuries to the subclavian artery have been reported in these athletes. Because a comprehensive review of TOS is provided elsewhere (see Chapters 125 to 128), only a brief discussion as it relates to athletic activities is given here.
Clinical Findings and Risk Factors
Compression of the subclavian or axillary artery can occur at the scalene muscles, costoclavicular space, or pectoralis minor muscle. A significant portion of cases of arterial TOS are due to underlying bone abnormalities, such as a cervical rib or anomalous first rib; but in athletes, hypertrophy of the scalene muscles or pectoralis minor muscles can lead to symptoms as well.64 With chronic compression and vessel wall damage, a spectrum of disease can develop, including thromboembolism, occlusive disease, and aneurysm formation.
Whereas arterial TOS has been recognized for more than 2 centuries, the association with elite athletes has only been recent. Tullos et al67 in 1972 reported an axillary artery thrombus secondary to compression by the pectoralis minor in a major league pitcher. In 1978, Strukel et al68 reported on three competitive baseball pitchers with thoracic outlet compression. However, until the 1986 report of Fields et al69 on athletic injury in the thoracic outlet, the injury had received little attention. In their fascinating report, they describe a major league pitcher who suffered a catastrophic stroke resulting from subclavian artery thrombosis with proximal clot propagation.
Symptoms are similar to those previously described in humeral head compression of the axillary artery and include arm fatigue, loss of pitch velocity, finger numbness, Raynaud’s syndrome, and cutaneous embolization. It is often difficult to make the diagnosis, and evaluation by an orthopedic surgeon to rule out musculoskeletal abnormalities is useful. Duplex scanning and transcutaneous Doppler studies with the athlete in the pitching position help detect compression of the subclavian or axillary artery. A definitive diagnosis is established by arteriography with positional exposure (Fig. 123-10).
Treatment
Treatment depends on the extent of injury. Non-bone compression alone can be treated by division of the offending muscle or tendon.63 If an anomalous first rib or cervical rib is the culprit, a transaxillary approach can be used for decompression. If the subclavian artery requires reconstruction because of severe occlusive disease or aneurysmal disease, combined supraclavicular and infraclavicular incisions provide better exposure. Saphenous vein is the preferred conduit for bypass, although prosthetic grafts or the femoral vein can also be used.64,70 Long-term patency rates are good, approaching 90% to 100%. The ability to return to competition after repair is not well documented.
Selected Key References
Ferris BL, Taylor LM Jr, Oyama K, McLafferty RB, Edwards JM, Moneta GL, Porter JM. Hypothenar hammer syndrome: proposed etiology. J Vasc Surg. 2000;31:104.
McLelland D, Paxinos A. The anatomy of the quadrilateral space with reference to quadrilateral space syndrome. J Shoulder Elbow Surg. 2008;17:162.
A great reference and illustration of the quadrilateral space and the associated syndrome..
Nasu Y, Kurozawa Y, Fujiwara Y, Honma H, Yanai T, Kido K, Ikeda T. Multicenter study on finger systolic blood pressure test for diagnosis of vibration-induced white finger. Int Arch Occup Environ Health. 2008;81:639.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Buckle PW. Fortnightly review: work factors and upper limb disorders. BMJ. 1997;315:1360.
2. Nonfatal occupational injuries and illnesses requiring days away from work, 2011. United States Department of Labor Bureau of Labor Statistics. [Released November 8, 2012. Available at] http://www.bls.gov/news.release/osh2.nr0.htm.
3. Rempel DM, et al. Work-related cumulative trauma disorders of the upper extremity. JAMA. 1992;267:838.
4. Loriga G. Il lavoro con i martelli pneumatici. Boll Ispett Lavoro. 1911;2:35.
5. Hamilton A. A study of spastic anemia in the hands of stonecutters: an effect of the airhammer on the hands of stonecutters. [Bulletin 236, Industrial Accidents and Hygiene Series No. 19] United States Bureau of Labor Statistics: Washington, DC; 1919.
6. Taylor W, et al. Vibration white finger in industry. Academic Press: New York; 1975.
7. Ashe WF, et al. Raynaud’s phenomenon of occupational origin. Arch Environ Health. 1962;5:63.
8. Griffin MJ. Vibration injuries of the hand and arm: their occurrence and the evolution of standards and limits. Her Majesty’s Stationery Office: London; 1980.
9. Yodaiken RE, et al. The Raynaud phenomenon of occupational origin. Advances in microcirculation. Karger: Basel, Switzerland; 1985:6. Altura BM, Davis E. Advances in microcirculation. vol 12.
10. National Institute for Occupational Safety and Health (NIOSH) document 89-106. Criteria for a recommended standard: occupational exposure to hand-arm vibration. [Available at] http://www.cdc.gov/niosh/docs/89-106; 1989.
11. Newem RM. Vibration-induced arterial shear stress: the relationship to Raynaud’s phenomenon of occupational origin. Arch Environ Health. 1973;26:105.
12. Bovenzi M. Some pathophysiological aspects of vibration-induced white finger. Eur J Appl Physiol. 1986;55:381.
13. Letz R, et al. A cross sectional epidemiological survey of shipyard workers exposed to hand-arm vibration. Br J Ind Med. 1992;49:53.
14. Pearce WH, et al. Noninvasive vascular diagnostic testing. Current problems in surgery. Year Book Medical Publishers: Chicago, Ill; 1983. Ravitch MM. Current problems in surgery. vol 20.
15. Sumner DS. Vascular laboratory diagnosis and assessment of upper extremity vascular disorders. Machleder HI. Vascular disorders of the upper extremity. Futura: Mt. Kisco, NY; 1983:1.
16. Nasu Y, et al. Multicenter study on finger systolic blood pressure test for diagnosis of vibration-induced white finger. Int Arch Occup Environ Health. 2008;81:639.
17. Payne KM, et al. B-mode imaging of the arteries of the hand and upper extremity. Bruit. 1986;10:168.
18. Barker NW, et al. Arterial occlusion in the hands and fingers associated with repeated occupational trauma. Mayo Clin Proc. 1944;19:345.
19. Shatz IJ. Occlusive arterial disease in the hand due to occupational trauma. N Engl J Med. 1963;268:281.
20. Ashe WF, et al. Occupational Raynaud’s: II. Further studies of this disorder in uranium mine workers. Arch Environ Health. 1964;9:425.
21. Wegelius U. Angiography of the hand: clinical and postmortem investigations. Acta Radiol Suppl (Stockh). 1972;315:1.
22. Griffin MJ, et al. Dose-response patterns for vibration-induced white finger. Occup Environ Med. 2003;60:16.
23. Bartel P, et al. The value of noninvasive tests in occupational trauma of the hands and fingers. Bruit. 1984;8:15.
24. Kahan A, et al. Nifedipine and allied substances in the treatment of Raynaud’s phenomenon. Advances in microcirculation. Karger: Basel, Switzerland; 1985:95. Altura BM, Davis E. Advances in microcirculation. vol 12.
25. Chetter IC, et al. The hand arm vibration syndrome: a review. Cardiovasc Surg. 1998;6:1.
26. Von Rosen S. Ein Fall von Thrombose in der Arteria ulnaris nach Einwirkung von stumpfer Gewalt. Acta Chir Scand. 1934;73:500.
27. Guttani C. De externis aneursmatibus manu chirurgica methodice pertractandis. Erichsen JE. Observations on aneurysm. Snydeham Society: London; 1884:316–318.
28. Conn J, et al. Hypothenar hammer syndrome: post-traumatic digital ischemia. Surgery. 1970;68:1122.
29. Little JM, et al. The incidence of the hypothenar hammer syndrome. Arch Surg. 1972;105:684.
30. Pineda CJ, et al. Hypothenar hammer syndrome: form of reversible Raynaud’s phenomenon. Am J Med. 1985;79:561.
31. Marie I, et al. Long-term follow up of hypothenar hammer syndrome: a series of 47 patients. Medicine (Baltimore). 2007;86:334.
32. Ferris BL, et al. Hypothenar hammer syndrome: proposed etiology. J Vasc Surg. 2000;31:104.
33. Swanson KE, et al. Hypothenar hammer syndrome: a case and brief review. Vasc Med. 2012;17:108.
34. Taute BM, et al. Ultrasound image of the hypothenar hammer syndrome. Ultraschall Med. 1998;19:220.
35. Winterer JT, et al. Diagnosis of the hypothenar hammer syndrome by high-resolution contrast-enhanced MR angiography. Eur Radiol. 2002;12:2457.
36. Blum AG, et al. Pathologic conditions of the hypothenar eminence: evaluation with multidetector CT and MR imaging. Radiographics. 2006;26:1021.
37. Kleinert HE, et al. Aneurysms of the hand. Arch Surg. 1973;106:554.
38. Lifchez SD, et al. Long-tem results of surgical treatment for hypothenar hammer syndrome. Plast Reconstr Surg. 2009;124:210.
39. Dethmers RS, et al. Surgical management of hypothenar and thenar hammer syndromes: a retrospective study of 31 instances in 28 patients. J Hand Surg Br. 2005;30:419.
40. Wilson R, et al. Occupational acro-osteolysis. JAMA. 1967;201:577.
41. Bookstein JJ. Arteriography. The hand in radiologic diagnosis with gamuts and pattern profiles. ed 2. WB Saunders: Philadelphia; 1984:97. Poznanski AK. The hand in radiologic diagnosis with gamuts and pattern profiles. vol 1.
42. Veltman G. Raynaud’s syndrome in vinyl chloride disease. Heidrich H. Raynaud’s phenomenon. TM-Verlag: Berlin; 1979:11.
43. Falappa P, et al. Angiographic study of digital arteries in workers exposed to vinyl chloride. Br J Ind Med. 1982;39:169.
44. d’Amato TA, et al. High-voltage electrical injury: a role for mandatory exploration of deep muscle compartments. J Natl Med Assoc. 1994;86:535.
45. Wang X, et al. Early vascular grafting to prevent upper extremity necrosis after electrical burns: commentary on indications for surgery. Burns. 1985;11:359.
46. Kaminski M, et al. Risk factors for Raynaud’s phenomenon among workers in poultry slaughterhouses and canning factories. Int J Epidemiol. 1997;26:371.
47. Mackiewicz Z, et al. Raynaud’s phenomenon following long-term repeated action or great differences of temperature. J Cardiovasc Surg (Torino). 1977;18:151.
48. Jackson MR. Upper extremity arterial injuries in athletes. Semin Vasc Surg. 2003;16:232.
49. Arko FR, et al. Vascular complications in high-performance athletes. J Vasc Surg. 2001;33:935.
50. Noel B, et al. A tennis player with hand claudication. Vasa. 2000;29:151.
51. Buckhout BC, et al. Digital perfusion of handball players: effects of repeated ball impact on structures of the hand. Am J Sports Med. 1980;8:206.
52. McCue FC III, et al. Soft-tissue injuries to the hand. Pettrone FA. Upper extremity injuries in athletes. CV Mosby: St. Louis; 1986:85.
53. Lowrey CW. Digital vessel trauma from repetitive impacts in baseball catchers. J Hand Surg. 1976;1:236.
54. Itoh Y, et al. Circulatory disturbances in the throwing hand of baseball pitchers. Am J Sports Med. 1987;15:264.
55. McLelland D, et al. The anatomy of the quadrilateral space with reference to quadrilateral space syndrome. J Shoulder Elbow Surg. 2008;17:162.
56. Cahill B, et al. Quadrilateral space syndrome. J Hand Surg. 1983;8:65.
58. Nijhuis HHAM, et al. Occlusion of the brachial artery by thrombus dislodged from a traumatic aneurysm of the anterior humeral circumflex artery. J Vasc Surg. 1991;13:408.
59. McAdams TR, et al. Surgical decompression of the quadrilateral space in overhead athletes. Am J Sports Med. 2008;36:528.
60. Lord JW, et al. Neurovascular compression syndromes of the upper extremity. Clin Symp. 1958;10:35.
61. Fleisig GS, et al. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21:421.
62. Rohrer MJ, et al. Axillary artery compression and thrombosis in throwing athletes. J Vasc Surg. 1990;11:761.
63. McCarthy WJ, et al. Upper extremity arterial injury in athletes. J Vasc Surg. 1989;9:317.
64. Durham JR, et al. Arterial injuries in the thoracic outlet syndrome. J Vasc Surg. 1995;21:57.
65. Duwayri YM, et al. Positional compression of the axillary artery causing upper extremity thrombosis and embolism in the elite overhead throwing athlete. J Vasc Surg. 2011;53:1329.
66. Ishitobi K, et al. Extra-anatomic bypass graft for management of axillary artery occlusion in pitchers. J Vasc Surg. 2001;33:797.
67. Tullos HS, et al. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169.
68. Strukel RJ, et al. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6:35.
69. Fields WS, et al. Thoracic outlet syndrome: review and reference to stroke in a major league pitcher. AJR Am J Roentgenol. 1986;146:809.
70. Cormier JM, et al. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9:778.