Obstructive Pulmonary Disease and General Management Principles
A The acronym for chronic obstructive pulmonary disease (COPD) is applied to patients with long-term chronic obstructive pulmonary disease, who show persistent airway obstruction, normally manifested by decreased expiratory flow rates. The airflow obstruction may be associated with airway hyperactivity and may be partially reversible.
1. COPD is the fourth leading cause of death in the United States, preceded by cerebrovascular disease, cancer, and heart disease.
2. In 2000, the World Health Organization estimated that 2.74 million deaths worldwide were attributed to COPD.
3. Between 1985 and 1995, the number of physician visits for COPD in the United States increased from 9.3 million to 16 million.
4. Approximately 14 million people in the United States have been diagnosed with COPD, and this number has increased by 42% since 1982.
5. There seems to be a greater incidence of COPD in men than in women; however, the percentage of women with COPD is steadily increasing.
6. On autopsy, some degree of emphysema appears in a large percentage of the population.
7. Emphysema is the second leading cause of disability, arteriosclerotic heart disease being first.
1. Smoking: Of all risk factors, smoking shows the highest correlation with COPD; however, individual response to short-term and long-term exposure varies considerably from individual to individual. The reasons for this variation in response are not well understood. Smoking:
2. Pollution: Particulate and gaseous.
3. Passive smoking: Evidence indicates the passive inspiration of smoke from the environment increases the risk of COPD. Passive smoking exposes the individual to the same toxic substance, although in lower concentration than the active smoker.
4. Occupational exposure to dusts and fumes.
5. Infection, which may cause decreased pulmonary clearance, resulting in an increased incidence of recurrent infection.
a. β1-Antitrypsin deficiency, which results in emphysematous changes measurable in the third and fourth decades (see Section II, Emphysema).
7. Allergies (e.g., chronic asthma), which can lead to permanent pulmonary changes.
8. Socioeconomic status: Higher incidence has been demonstrated in low socioeconomic groups.
9. Alcohol ingestion, although no direct link has been demonstrated. Alcohol ingestion:
a. Decreases ciliary function.
b. Decreases alveolar macrophage function.
10. Aging, which causes natural degenerative changes in the respiratory tract resembling emphysematous changes.
D Physical appearance of patient
1. Barrel-chested: A result of increased air trapping
a. Increase in anteroposterior diameter
b. Increase proportional to increase in functional residual capacity (FRC)
2. Clubbing (pulmonary hypertrophic osteopathy): Bulbous enlargement of terminal portion of the digits, altering the cuticular angle, may be present if there have been frequent pulmonary infections.
3. Cyanosis: A result of hypoxemia coupled with secondary polycythemia
4. Decreased and adventitious breath sounds
5. Often a hyperresonant chest
7. Malnourished, secondary to loss of appetite (anorexia)
10. May be edematous with jugular vein distention if congestive heart failure (CHF) present
E General pulmonary function changes (Table 20-1)
TABLE 20-1
Changes in Pulmonary Function Associated with Obstructive and Restrictive Lung Disease
| Pulmonary Function Study | Obstructive Disease | Restrictive Disease |
| TLC | Normal or increased | Decreased |
| VC | Normal or decreased | Decreased |
| FRC | Increased | Normal or decreased |
| RV | Increased | Normal or decreased |
| RV/TLC ratio | Increased | Normal |
| FEV1% | Decreased | Normal |
| MMEFR25%-75% | Decreased | Normal or decreased |
1. Pulmonary compliance is frequently increased.
2. Airway resistance increases as a result of mucosal edema and bronchiolar wall weakening.
3. Prolonged expiratory times when numbers 1 and 2 are present.
5. Increased residual volume (RV).
6. Increased RV/total lung capacity (TLC) ratio.
8. Decreased or normal vital capacity (VC).
9. Decreased or normal inspiratory capacity (IC) and inspiratory reserve volume (IRV) secondary to increased FRC.
10. Increased expiratory reserve volume (ERV).
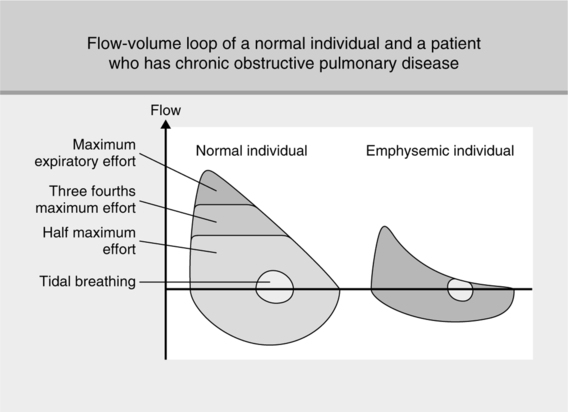
11. Decreased expiratory flow studies: FEV1%, FEV3%, maximum midexpiratory flow rate between 25% and 75% (MMEFR25%-75%), forced expiratory flow determined between the first 200 ml and 1200 ml of exhaled volume (FEF200-1200), and maximum voluntary ventilation (MVV). The level of reduction is associated with severity of disease (Figure 20-1).
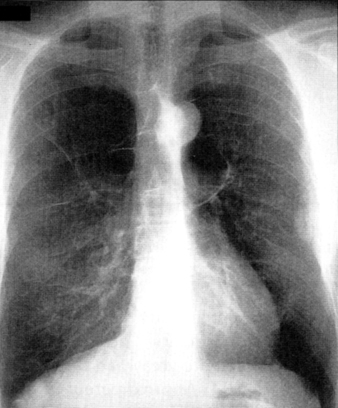
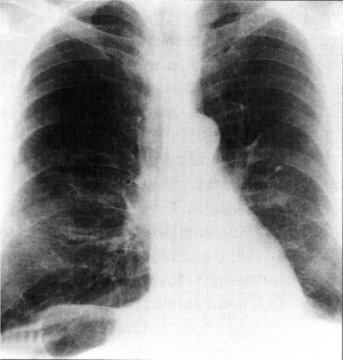
F General radiographic findings (Figure 20-2)
1. Increased anteroposterior diameter
4. Pulmonary vascular engorgement with increased vascular markings
5. Increased retrosternal airspace
6. Normal or increased heart shadow
7. Normal or thin elongated mediastinum
9. Possible peripheral bullae or blebs
10. In severe cases or end-stage disease, frequently right ventricular hypertrophy and CHF
1. In all patients dyspnea on exertion is one of the first noticeable symptoms.
2. As the disease process progresses, dyspnea becomes apparent even at rest.
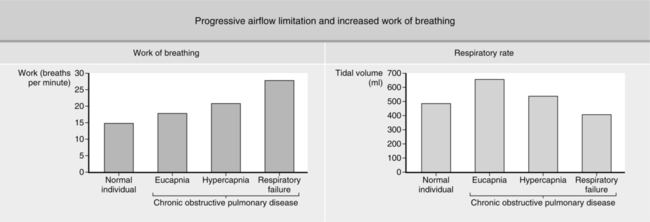
3. Dyspnea normally increases as the work of breathing progressively increases.
4. The percentage of the oxygen consumed to ventilate is increased, severely limiting the patient’s level of physical exertion as the disease progresses.
1. The ventilatory drive of COPD patients may vary considerably.
2. Some continue to increase their ventilatory efforts as the disease progresses, despite increases in work of breathing.
a. These patients possess normal or increased ventilatory drives. In the past these patients were referred to as “pink puffers.”
b. This group usually does not become carbon dioxide retainers, despite continual disease progression.
c. As a result administration of high FIO2 does not depress ventilation. Normal carbon dioxide responsiveness continues until there is complete failure of ventilatory muscles.
d. These patients present in acute distress, with normal or decreased Pco2. levels.
e. The level of Pco2. rapidly increases when failure overwhelms ventilatory capabilities (Figure 20-3).
3. Conversely, many COPD patients have marked alterations in ventilatory drive.
a. Their sensitivity to oxygen frequently is increased, and carbon dioxide is reduced.
b. This group in the past was referred to as “blue bloaters.”
c. They experience progressive increases to baseline Pco2 levels as their disease progresses.
d. At presentation with an acute exacerbation of the disease, markedly elevated Pco2 levels may be noted. Blood gas interpretation is usually acute respiratory acidosis superimposed on chronic respiratory acidosis with severe hypoxemia.
e. These patients are classified as individuals who will not breathe during failure, whereas those with normal drives simply cannot breathe at the time of failure.
I General pattern of arterial blood gas changes demonstrated by carbon dioxide retainers as their disease progresses from mild to severe:
1. Because of the pathophysiology of COPD, ventilation/perfusion inequalities develop.
2. Mismatching of ventilation and blood flow results in hypoxemia. It should be noted that hypoxemia normally is the first measured blood gas abnormality.
3. Hypoxemia becomes increasingly worse as the disease process progresses, resulting in stimulation of peripheral chemoreceptors.
4. Stimulation of peripheral chemoreceptors may result in hyperventilation, the body’s attempt to correct hypoxemia.
5. If hyperventilation persists, the kidneys compensate for the acid-base imbalance. Blood gas analysis reveals compensated respiratory alkalosis (chronic alveolar hyperventilation) with hypoxemia.
6. Hyperventilation continues until oxygen consumption by the patient’s respiratory musculature exceeds the benefits received by hyperventilation.
7. The percentage of total oxygen consumption used for ventilation becomes greatly increased because the efficiency of the respiratory system is greatly reduced by disease and increased accessory muscle use.
8. The body can no longer maintain the level of alveolar ventilation necessary to maintain adequate oxygen tensions without severely compromising oxygen delivery to other organs.
9. Because of the depressed ventilatory drive of the individual and the high cost of breathing, carbon dioxide is allowed to increase in an attempt to conserve energy.
10. This results in further progression of the hypoxemia.
11. The total oxygen reservoir may be decreased even further.
12. This is counterbalanced to a degree by a reduction in oxygen consumption by the respiratory muscles, a decrease in the patient’s overall level of activity, and secondary polycythemia.
13. Alveolar ventilation continues to decrease. This is evidenced by increasing carbon dioxide levels and further development of hypoxemia.
14. With time the patient begins to retain carbon dioxide. Blood gases at this time reveal compensated respiratory acidosis with moderate to severe hypoxemia.
15. It is at this point when carbon dioxide starts to be retained that the patient’s primary stimulus to breathe may become oxygen.
16. If oxygen were administered in sufficient amounts, the hypoxic stimulus to breathe could be reduced, potentially to the point of apnea in a few select patients.
a. This is an unusual occurrence.
b. Most patients who are CO2 retainers do not function on a hypoxic drive.
c. However, care should always be exercised when administering oxygen, but oxygen should not be withheld from a hypoxemic patient because of fear of further hypoventilation.
17. The disease continues to progress with increasing levels of carbon dioxide retention and more severe hypoxemia.
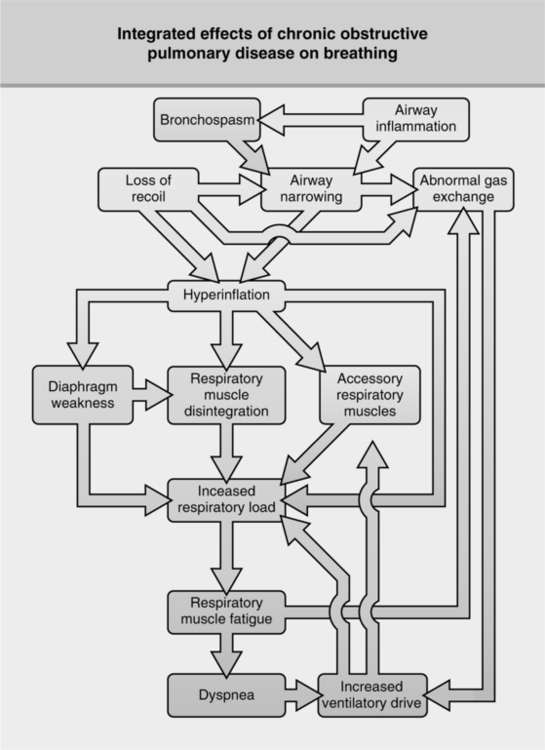
18. The disease process becomes end stage and terminal. The patient’s level of physical activity is severely limited, and he or she is reduced to a pulmonary cripple (Figure 20-4).
1. Cor pulmonale denotes right ventricular hypertrophy secondary to abnormalities of lung structure and function. CHF may or may not be present.
2. It is a frequent sequel to chronic bronchitis and cystic fibrosis.
a. Developing pulmonary disease results in increasing hypoxemia, which causes constriction of the pulmonary arterioles.
b. Constriction causes pulmonary hypertension. The decreased size of the capillary bed seen with advancing pulmonary disease also contributes to development of pulmonary hypertension.
c. Pulmonary hypertension causes the right side of the heart to work harder. With time, right ventricular hypertrophy develops.
d. Pulmonary hypertension, if not controlled, precipitates the development of right ventricular failure.
e. This results in peripheral edema because of increased resistance to venous return and decreased right ventricular function.
f. Failure of the right side of the heart is more frequently seen in association with pulmonary disease than is left-sided heart failure.
g. However, over time the patient with right-sided heart failure can also develop left-sided heart failure.
A Emphysema is characterized by enlargement of air spaces distal to terminal bronchioles, with loss of elastic tissue and destruction of alveolar septal walls.
1. Smoking (high correlation with emphysema)
2. High correlation with long-term air pollution.
a. Destructive changes occur primarily in the respiratory bronchioles.
b. Incidence is much higher in men than women.
c. Primary lesions appear in the upper lobes.
d. There is a high correlation with centrilobular emphysema and smoking; frequently it is a sequel to chronic bronchitis.
a. Changes at alveolar level where destruction of septa predominates.
b. Effects seemingly generalized in distribution.
c. Seen with α1-antitrypsin deficiency and the natural aging process.
1. Shortness of breath, developing gradually
3. Frequent respiratory infections
E Chest radiography findings (Figure 20-5)
F Pulmonary function studies (as outlined in Section I, General Comments)
G Management (as outlined in Section V, General Management Principles in COPD)
1. Acute inflammation of tracheobronchial tree with production of excessive mucus.
4. Treatment: Usually by administration of antibiotics, expectorants, aerosol therapy, and occasionally antitussives.
5. Normally a self-limiting process without serious complications or residual effects.
1. Chronic cough with excessive sputum production of unknown specific etiology for 3 months per year for 2 or more successive years
2. Caused by frequent acute episodes of bronchitis, which may result from:
a. Onset normally insidious, with patient rarely aware of its development
(1) Size increases in relation to wall thickness; normally gland size is approximately one third of the height of the bronchial walls.
(2) In chronic bronchitis, gland size is approximately two thirds of the height of the bronchial walls.
b. Submucosal gland hypertrophy
c. Increased population of goblet cells replacing ciliated columnar cells (epithelial metaplasia)
a. Early in the disease, radiographic changes are not significant, especially if the disease is associated only with larger airways.
b. If the disease has moved to the periphery, hyperinflation with flattened hemidiaphragms may be noticed.
c. Peripheral pulmonary vasculature may be prominent.
d. Cardiac shadow is enlarged.
e. There is pulmonary vascular engorgement.
f. Chest radiography is usually of little use in establishing diagnosis.
g. A positive patient history usually is the best diagnostic tool.
a. In early stages all pulmonary function studies may be normal, except for slight decreases in expiratory flow rates.
b. As the disease process progresses, pulmonary function results are consistent with those presented in Section I, General Comments.
a. Most important: Removal of patient from irritants.
b. Aerosol and bronchodilator therapy if there is a reversible component to airflow obstruction.
c. Antibiotics if indicated for bacterial infection.
d. Treatment regimen fairly consistent with that outlined in Section V, General Management Principles in COPD.
A Permanent abnormal dilation and distortion of bronchi and/or bronchioles
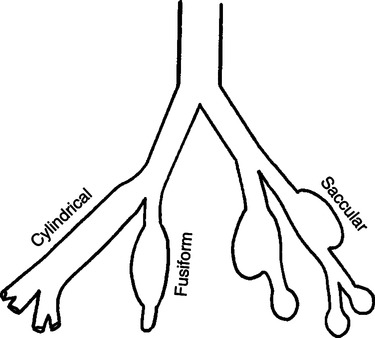
B Classification: There are three types of bronchiectasis (Figure 20-6).
a. Bronchial walls are dilated, with regular outlines.
b. It is the least severe type because bronchiectatic areas drain fairly well.
a. Bronchial walls have large, irregularly shaped distortions with bulbous ends.
b. Evidence of bronchitis or bronchiolitis often is present.
a. There is complete destruction of bronchial walls.
1. Despite controversy, probable contributing factors are as follows:
3. Sloughing of mucosa with ulceration and possible abscess formation
4. Adjacent and distal lung tissue generally has reduced volume with patchy scarring and consolidation, all believed to be secondary to the obstruction of the bronchi.
1. The bronchogram is the only absolute diagnostic tool.
2. Bronchoscopy may afford direct visualization of bronchiectatic lesions.
3. Chest radiography findings (see Section IV-G, Chest Radiographic Findings)
4. Sputum examination (see Section IV-F, Clinical Manifestations)
1. Chronic loose cough, often exacerbated by change of position
4. Increased sputum production of a characteristic three-layer nature on standing
b. Middle layer: Turbid, mucopurulent
c. Bottom layer: Opaque, mucopurulent to purulent with mucous plugs (Dittrich’s plugs), sometimes foul smelling
1. Usually normal unless disease is advanced and associated with other types of COPD
V General Management Principles in COPD
a. Regardless of level of disability, cessation of smoking is beneficial.
b. Progress of disease is reduced if smoking stopped.
c. However, no treatment can reverse or stop disease progression.
a. Response to bronchodilator therapy with pulmonary function studies generally should be demonstrated (≥15% increase in baseline FEV1 is considered positive).
b. However, many believe bronchodilator therapy should be administered regardless of demonstrated response.
c. Long-acting β agonists taken twice daily may be preferable.
d. Some patients respond to anticholinergics (atropine-like drugs) better than β agonists because of altered autonomic receptor distribution with age (see Chapter 17).
e. A combination of β agonist and anticholinergic therapy may be helpful and is available in metered dose inhaler (MDI) form (Combivent).
a. Corticosteroids are used primarily to reduce mucosal edema.
b. Aerosolized approaches are generally desired over systemic because of side effects of the systemic route; however, neither has been shown to have a significant effect.
a. Some recommend low-dose long-term antibiotic therapy for those with persistent and frequently reoccurring pulmonary infections.
6. Oxygen therapy (see Chapter 34)
7. Mechanical ventilation (see Chapter 21)
8. Nocturnal nasal continuous positive airway pressure (CPAP) therapy (see Chapter 32)
9. Improvement of patient’s exercise tolerance by general graded body toning and stamina-developing exercises
10. Maintenance of cardiovascular status by management of CHF
11. Avoidance of exposure to all types of airway irritants
12. Proper education and psychological and sociologic support
1. Acute exacerbations may be associated with a number of specific concomitant problems (Table 20-2).
TABLE 20-2
Comorbid Conditions That Present as Exacerbations of COPD
| Condition | Common Clinical Symptoms | Diagnostic Laboratory Tests | Treatment |
| Acute bronchitis | Productive cough, increased dyspnea, substernal discomfort, purulent sputum | Leukocytosis, sputum, Wright stain, and Gram stain | Antibiotics, systemic and airway hydration |
| Pneumonia | Fever, productive cough, pleuritic chest pain | As above, plus chest radiograph and blood cultures | As above; if toxic or in impending respiratory failure, hospitalization |
| Asthmatic bronchoconstriction | Increased cough, dyspnea, wheeze | Increased blood and sputum eosinophil count, elevated IgE in bronchial asthma | Corticosteroids, avoidance of causative agent if possible, desensitization when indicated sodium cromolyn |
| Medication errors and noncompliance, tobacco smoke exposure, industrial smoke and fume exposure, failure to comply with exercise conditioning regimen | Progressive dyspnea “worsening” | Persistent eosinophilia, nontherapeutic serum theophylline levels, presence of carboxyhemoglobin, work history | Patient and family education, use of intelligent caregivers, job modification, closer work with pulmonary rehabilitation team |
| Malnutrition or weight gain | Weakness, weight loss or gain | Weight, characteristic blood chemistry and hematologic abnormalities | Nutrition, counseling dietary supplementation as indicated |
| Pneumothorax | Acute dyspnea, chest pain, syncope | Chest radiograph | Hospitalization for thoracotomy tube to suction |
| Acute myocardial infarction and/or CHF | Increasing dyspnea, may not have typical anginal chest pain | ECG, chest radiograph (may not be typical), cardiac enzymes | Hospitalization for cardiovascular monitoring |
| Pulmonary embolism and infarction | Acute dyspnea, hemoptysis (in infarction) | Chest radiograph, ventilation/perfusion lung scan (may be difficult to interpret), pulmonary angiogram | Anticoagulation or thrombolysis |
| Bronchogenic carcinoma | Weight loss recurrent pneumonia, hemoptysis, chest pain | Chest radiograph, computed tomography scan, cytology, bronchoscopy | Thoracotomy and resection (if possible), radiation, chemotherapy |
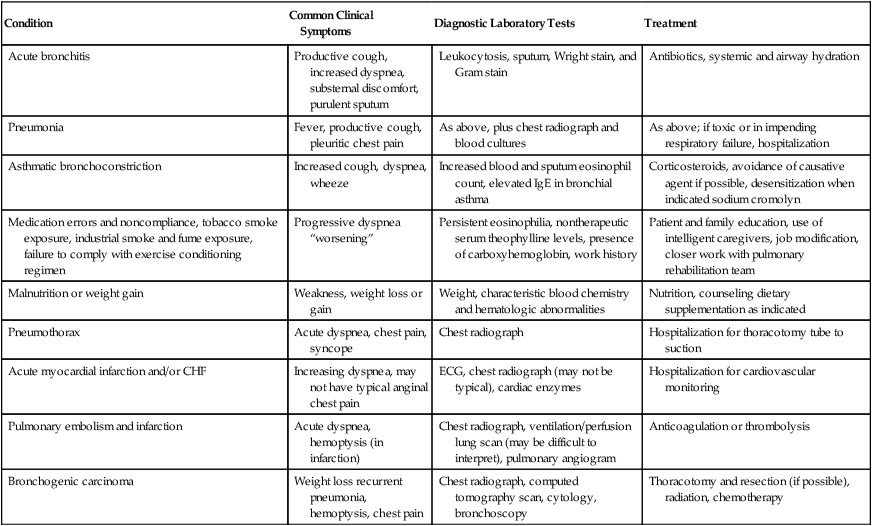
From Burton GG: Exacerbations of chronic obstructive pulmonary disease: pharmacologic management. In Kacmarek RM, Stoller JK (eds). Current Respiratory Care. Toronto, BC Decker, 1988. BC Decker
2. Oxygen therapy: FIO2 generally is titrated to maintain Po2 in the 60 mm Hg range. No need exists to increase Po2 to the 90 mm Hg range.
3. Antibiotic therapy: Acute exacerbations are commonly associated with pneumonia. Appropriate therapy should begin immediately.
4. Bronchodilator therapy should be started immediately.
a. Aminophylline: Use of aminophylline has declined over the past several years. The exact mechanism of action is not clear. It had previously been thought to increase cellular levels of cyclic adenosine monophosphate; however, this is not the case. Possible mechanisms of action likely include increased intracellular calcium transport, adenosine antagonism, and inhibition of prostaglandin E2.
a. With some, appropriate systemic hydration markedly increases the mobility of secretions because many patients are dehydrated, increasing viscosity of pulmonary secretions.
a. Use of aerosolized steroids at home is common.
b. During acute exacerbation systemic steroids may be helpful for some patients. Therapy consists of 0.5 mg/kg of methylprednisone given intravenously every 6 hours and may be abruptly withdrawn after 72 hours of treatment.
a. Many COPD patients also have CHF.
b. Appropriate diuretic therapy to normalize fluid balance is indicated.
a. Many COPD patients demonstrate chronic nutritional deficiencies.
b. Preceding admission, nutritional maintenance decreases as respiratory symptoms progress.
c. See Chapter 24 for details.
9. Bronchial hygiene techniques, although controversial, are often used (see Chapter 36).
D Ventilatory management in acute exacerbations of COPD
1. Indication for ventilatory support varies among experts but generally should be associated with reversibility of the event responsible for failure. Unless the state before the acute exacerbation can be reestablished, the likelihood of chronic ventilatory support is highly probable.
2. General principles: The goal of ventilator management for these patients generally is to provide rest for fatigued ventilatory muscles while minimizing the potential for ventilator-induced lung injury (see Chapter 21).
3. Before intubation and mechanical ventilation a trial of noninvasive ventilation should be attempted. Patients experiencing acute exacerbation of COPD are the patients most likely to have a favorable response to this intervention.
A Asthma, according to the American Thoracic Society, is “characterized by an increased responsiveness of the trachea and bronchi to various stimuli and is manifested by widespread narrowing of the airways that changes in severity either spontaneously or as the result of treatment.”
1. Allergic: Implies that asthma is a result of an antigen-antibody reaction on mast cells of the respiratory tract. This reaction causes release of histamine, bradykinins, eosinophilic chemotactic factor of anaphylaxis, and slow-reacting substance of anaphylaxis. These substances then elicit the clinical responses associated with an asthmatic attack and cause high serum immunoglobulin (Ig) E levels along with sputum and serum eosinophilia.
2. Idiopathic: Implies that asthma is a result of an imbalance of the autonomic nervous system (i.e., the response of β-and α-adrenergic sites and cholinergic sites of the autonomic nervous system is not properly coordinated).
3. Nonspecific: Implies that the origin of asthmatic reactions is unknown. The asthmatic attack may follow viral infection, emotional changes, or exercise.
1. The complete causes generally are unknown, but heredity plays a significant role. Allergies and environmental factors also are frequently implicated.
2. If the disease develops between ages 5 through 15 years, it usually has an allergic basis.
3. If onset is after age 30 years, the disease normally is considered nonspecific.
4. Incidence: The actual incidence of asthma is difficult to determine. It is based on diagnosis, which varies because of several factors, including the definition used and access to health care.
1. Diagnosis depends on skin testing for antibodies and on the patient’s and the patient’s family history.
2. In allergic asthma, antibody IgE serum levels are approximately six times that of normal.
3. In idiopathic asthma, patients show an abnormal response to drug therapy: decreased β-sympathetic response and increased α-sympathetic response.
4. In nonspecific asthma, frequent presenting symptoms are nasal polyps and aspirin intolerance.
1. Thickening of subepithelial membranes
2. Hypertrophy of mucous glands
3. Eosinophilic infiltrates common in sputum and serum
4. Decrease in number of pulmonary mast cells
1. Severe respiratory distress
2. Rapid, shallow respiratory pattern
3. Wheezing that is often audible without a stethoscope
5. Tachycardia and hypertension
8. Barrel-chested appearance with hyperresonance
10. Intercostal, substernal, and subcostal retractions
1. During an attack a classic hyperinflation pattern is seen.
2. Between attacks the chest radiographic findings may be normal.
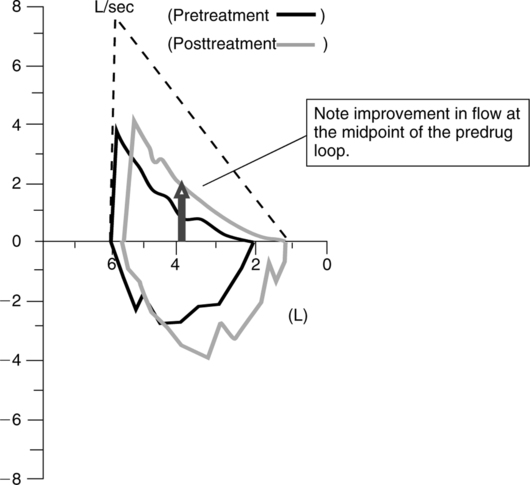
1. During an attack expiratory flow rates and VC are reduced. Bronchodilator therapy during pulmonary function testing often results in a significant improvement in test results (Figure 20-7).
2. Between attacks pulmonary function studies may be normal or show decreased expiratory flow rates.
I Exacerbating factors: One of the hallmarks of asthma is that it varies in severity and resolves either spontaneously or with treatment. Between attacks the patient may have little or no evidence of disease. Exacerbating factors or “triggers” result in acute onset and may include the following:
2. Allergens (e.g., grass, tree pollen, pets, house dust, and mites)
5. Occupation (e.g., environment and sensitizing agents)
1. Status asthmaticus is a sustained asthmatic attack that does not respond to conventional therapy.
K Management of stable asthma: The National Institutes of Health, Heart, Lung, and Blood recommend a stepwise approach based on classification of the severity of the patient’s disease to maintain control of asthma with the least amount of medication and hence minimal risk of side effects.
1. Classification of asthma severity (Table 20-3)
TABLE 20-3
Goals of Treatment and Classification of Asthma Severity
| CLASSIFY SEVERITY OF ASTHMA | |||
| Clinical Features Before Treatment* | |||
| Symptoms† | Nighttime Symptoms | Lung Function | |
| Step 4 (severe persistent) | Frequent | ||
| Step 3 (moderate persistent) | >1 time a week | ||
| Step 2 (mild persistent) | >2 times a month | ||
| Step 1 (mild intermittent) | ≤2 times a month | ||
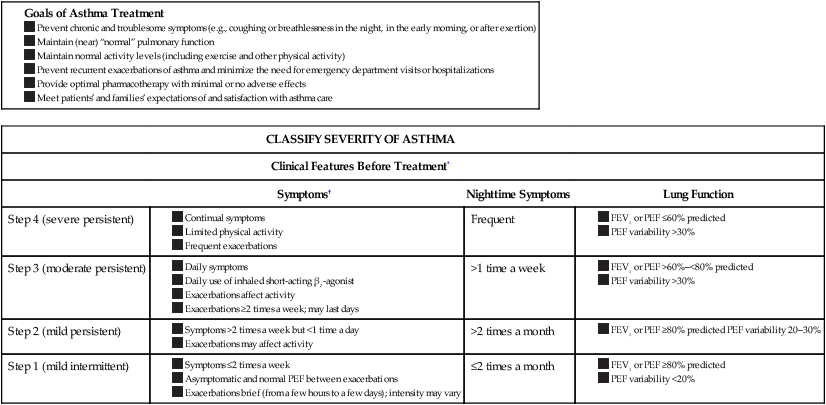
*The presence of one of the features of severity is sufficient to place a patient in that category. An individual should be assigned to the most severe grade in which any feature occurs. The characteristics noted in this figure are general and may overlap because asthma is highly variable. Furthermore, an individual’s classification may change over time.
†Patients at any level of severity can have mild, moderate, or severe exacerbations. Some patients with intermittent asthma experience severe and life-threatening exacerbations separated by long periods of normal lung function and no symptoms.
From National Institutes for Health: Guidelines for the Diagnosis and Management of Asthma Publication Number 97–4051, 1997).
2. Stepwise approach to managing asthma includes long-term control, quick relief, and education and is based on the following goals (Table 20-4):
TABLE 20-4
Stepwise Approach for Managing Asthma in Adults and Children Over 5 Years of Age*
• The stepwise approach presents general guidelines to assist clinical decision-making; it is not intended to be a specific prescription. Asthma is highly variable; clinicians should tailor specific medication plans to the needs and circumstances of individual patients.
• Gain control as quickly as possible; then decrease treatment to the least medication necessary to maintain control. Gaining control may be accomplished by either starting treatment at the step most appropriate to the initial severity of the condition or starting at a higher level of therapy (e.g., a course of systemic corticosteroids or higher dose of inhaled corticosteroids).
• A rescue course of systemic corticosteroids may be needed at any time and at any step.
• Some patients with intermittent asthma experience severe and life-threatening exacerbations separated by long periods of normal lung function and no symptoms. This may be especially common with exacerbations provoked by respiratory infections. A short course of systemic corticosteroids is recommended.
• At each step, patients should control their environment to avoid or control factors that make their asthma worse (e.g., allergens, irritants); this requires specific diagnosis and education.
• Referral to an asthma specialist for consultation or co-management is recommended if there are difficulties achieving or maintaining control of asthma or if the patient requires step 4 care. Referral may be considered if the patient requires step 3 care.
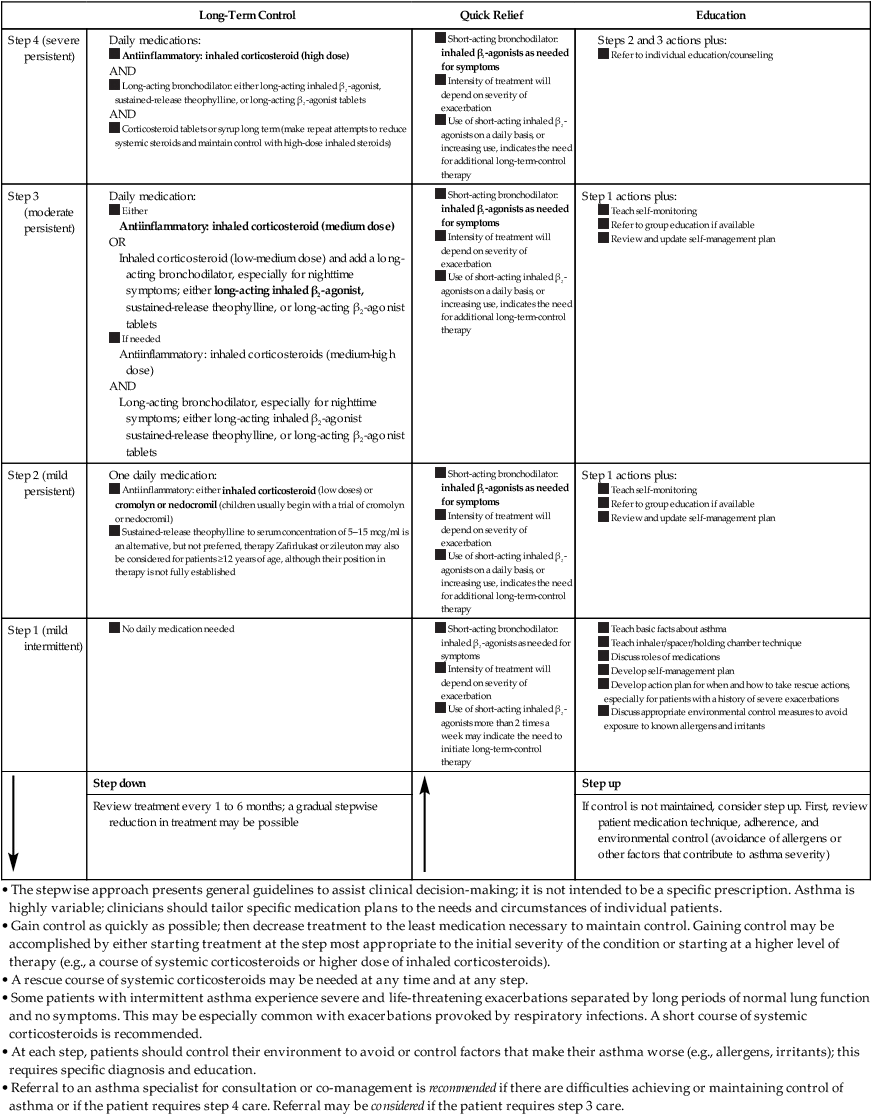
• The stepwise approach presents general guidelines to assist clinical decision-making; it is not intended to be a specific prescription. Asthma is highly variable; clinicians should tailor specific medication plans to the needs and circumstances of individual patients.
• Gain control as quickly as possible; then decrease treatment to the least medication necessary to maintain control. Gaining control may be accomplished by either starting treatment at the step most appropriate to the initial severity of the condition or starting at a higher level of therapy (e.g., a course of systemic corticosteroids or higher dose of inhaled corticosteroids).
• A rescue course of systemic corticosteroids may be needed at any time and at any step.
• Some patients with intermittent asthma experience severe and life-threatening exacerbations separated by long periods of normal lung function and no symptoms. This may be especially common with exacerbations provoked by respiratory infections. A short course of systemic corticosteroids is recommended.
• At each step, patients should control their environment to avoid or control factors that make their asthma worse (e.g., allergens, irritants); this requires specific diagnosis and education.
• Referral to an asthma specialist for consultation or co-management is recommended if there are difficulties achieving or maintaining control of asthma or if the patient requires step 4 care. Referral may be considered if the patient requires step 3 care.
*Preferred treatments are in bold print.
From National Institutes for Health: Guidelines for the Diagnosis and Management of Asthma Publication Number 97-4051, 1997.
a. Preventing chronic and troublesome symptoms (e.g., coughing or breathlessness in the night, in the early morning, or after exertion).
b. Maintaining (near) “normal” pulmonary function.
c. Maintaining normal activity levels (including exercise and physical activity).
d. Preventing recurrent exacerbations of asthma and minimizing the need for emergency department visits or hospitalizations.
e. Providing optimal pharmacotherapy with minimal or no adverse effects.
f. Meeting patients’ and families’ expectations of and satisfaction with asthma care.
3. Key recommendations for long-term asthma management:
a. Persistent asthma is most effectively controlled with daily long-term control medication, specifically, antiinflammatory therapy.
b. A stepwise approach to pharmacologic therapy is recommended to gain and maintain control of asthma.
(1) The amount and frequency of medication are dictated by asthma severity and directed toward suppression of airway inflammation.
(2) Therapy should be initiated at a higher level than the patient’s step of severity at the onset to establish prompt control and then stepped down.
(3) Continual monitoring is essential to ensure that asthma control is achieved.
(4) Step-down therapy is essential to identify the minimal medication necessary to maintain control.
c. Regular follow-up visits (at 1- to 6-month intervals) are essential to ensure that control is maintained and the appropriate step down in therapy is considered.
d. Therapeutic strategies should be considered in concert with clinical-patient partnership strategies; education of patients is essential to achieve optimal pharmacologic therapy.
e. At each step patients should be advised to avoid or control allergens, irritants, or other factors that make the patient’s asthma worse.
f. Referral to an asthma specialist for consultation or co-treatment of the patient is recommended if there are difficulties achieving or maintaining control of asthma or if the patient requires step 4 care. Referral may be considered if the patient requires step 3 care. For infants and young children referral is recommended if the patient requires step 3 or step 4 care and should be considered if the patient requires step 2care.
4. Other steps to be taken by the patient for the management of asthma:
a. Avoid irritants and allergens.
b. Avoid occupational and environmental triggers.
c. Perform daily monitoring of peak flow to serve as an early warning sign of an impending onset.
5. Pharmacologic treatment: Pharmacologic treatment is divided into two categories. Drugs that are used to manage asthma are called relievers, whereas those that are used to prevent asthma attacks are referred to as controllers (see Chapter 17).
6. Immunotherapy is used against identified antigens. Immunotherapy can be expected to:
a. Increase serum and/or secretory IgG and IgA levels that block IgE capacity to bind to mast cells.
L Management of acute exacerbation and status asthmaticus
1. Pharmacologic management centers on relief of airway obstruction.
a. Inhaled β2-adrenergic agents
d. Corticosteroids (normally do not demonstrate efficacy until 6 to 8 hours after administration)
e. Management generally is similar to stable asthma, except dosages and frequencies are increased, and more invasive routes of administration are chosen.
2. Systemic hydration demonstrates variable results depending on the length of time the asthmatic attack has been in progress before admission and the level of dehydration.
3. The use of airway clearance techniques, expectorants, and mucolytic agents during the acute phase is questionable.
4. Oxygen therapy should be administered liberally by cannula or simple mask if hypoxemia is present. A cannula is normally much better tolerated than a mask.
5. Heliox: The administration of a helium oxygen mixture is a useful adjunct to reducing the work of breathing and oxygen consumption. Patients who fail to respond to the conventional approach outlined previously may have a favorable response to inhaled heliox and thus be spared intubation and mechanical ventilation.
M Ventilatory management of status asthmaticus: Intubation and mechanical ventilation generally are difficult for patients with asthma and if possible should be avoided through the aggressive use of more conservative therapies (see Chapter 21).
1. Indications for mechanical ventilation
a. Acute ventilatory failure with a Pco2 >50 mm Hg is an indication for ventilation.
b. Normally persons with asthma are relatively healthy before an acute attack; thus they can be expected to hyperventilate in the presence of hypoxemia.
c. If the severity of the attack persists or increases despite treatment, that is, if
d. Once Pco2 returns to normal (40 to 45 mm Hg) with persistent symptomatology failure is imminent in the presence of a continued acute asthma.
e. Intubation and mechanical ventilation should be immediately instituted to prevent further clinical deterioration.
(1) Positive end-expiratory pressure (PEEP) generally is contraindicated in asthma because it may increase air trapping.
(2) However, in mechanically ventilated patients demonstrating auto-PEEP, low levels of PEEP (≤5 cm H2O) may reduce air trapping and improve ventilation (see Chapter 40).
2. If bronchospasm persists, some recommend the use of gaseous anesthetic agents that are potent bronchodilators. However, intravenous anesthetics also have potent bronchodilator effects.
3. Once the acute attack is resolved, ventilator discontinuance should progress rapidly.
4. Many persons with asthma respond adversely (i.e., increase in bronchospasm) to an artificial airway when sedation has been reversed. Once it has been established that spontaneous ventilation is feasible, rapidly extubate.