Chapter 18 Nutritional Antioxidants
Dermatologists today have the capability of preventing damage to normal skin and even retarding the skin’s natural aging through the use of cosmeceuticals. Especially during the past decade, research has demonstrated the efficacy of many topical nutrients, particularly antioxidants—some not synthesized by humans and therefore essential (vitamins C and E), some self-synthesized (α-lipoic acid, ubiquinone), and some exogenous (genistein). The challenge is to make topical formulations which attain percutaneous absorption of active forms and which maintain antioxidant activity. Such cosmeceuticals could protect as well as reduce and reverse manifestations of aging skin.
α-LIPOIC ACID
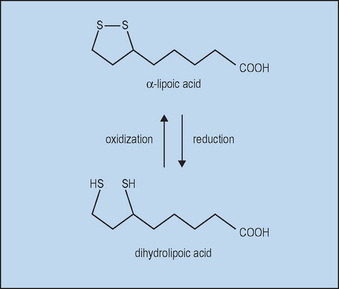
With oral supplements of free αLA, unbound αLA is transported to tissues. Free αLA is rapidly metabolized by the liver, so that the half-life in blood after absorption is only about 30 minutes, limiting the amount delivered. High tissue levels are short lived since most free αLA is rapidly reduced to dihydrolipoic acid (DHLA), as shown in Figure 18.1.
 As a small, stable molecule, it could successfully be percutaneously absorbed.
As a small, stable molecule, it could successfully be percutaneously absorbed.
 As a potent antioxidant it might protect from ultraviolet (UV) and other free radical environmental changes.
As a potent antioxidant it might protect from ultraviolet (UV) and other free radical environmental changes.
 Because it is soluble in both aqueous and lipid environments, it can interact with oxidants and antioxidants in many cellular compartments.
Because it is soluble in both aqueous and lipid environments, it can interact with oxidants and antioxidants in many cellular compartments.
Topical αLA with its metabolite DHLA could protect the skin from oxidative stress in several ways. Both αLA and DHLA are highly effective antioxidants as summarized in Table 18.1. DHLA is actually the more potent form. Both successfully scavenge reactive oxygen species (ROS) in vitro and in vivo. However, pro-oxidant activity has been observed. This occurs when an antioxidant reacts with a ROS scavenger, forming a product that is more harmful than the scavenged ROS. Fortunately, αLA can act as an antioxidant against the pro-oxidant activity of DHLA (Biewenga et al). Both αLA and DHLA further provide antioxidant activity by chelating Fe2+ and Cu2+ (αLA) and Cd2+ (DHLA).
| α-Lipoic acid | DHLA | |
| Antioxidant | + | ++ |
| Scavenges reactive oxygen species (ROS) | + | + |
| Chelates metals: | ||
| Fe2+, Cu2+ | + | − |
| Cd2+ | − | + |
| Regenerates endogenous antioxidants (vitamin E, vitamin C, glutathione, ubiquinol) | − | + |
| Repairs oxidatively damaged proteins | − | + |
| Pro-oxidant | + | + |
+ activity; ++ greater activity; − no activity. DHLA, dihydrolipoic acid.
Reproduced with permission from Biewenga GP, Haenen GRMM, Bast A 1997 The pharmacology of the antioxidant lipoic acid. General Pharmacology 29:315–331.
DHLA, unlike αLA, has the capacity to regenerate the endogenous antioxidants vitamin E, vitamin C, glutathione, and ubiquinol, as illustrated in Figure 18.2. This is clearly of great importance for skin, since UV exposure directly depletes ubiquinone and vitamin E in particular, as well as vitamin C, thereby stressing the other linked antioxidants. Regeneration of these major membrane and cytosol antioxidants gives cascading protection. Increases in the other important antioxidants (intracellular glutathione and extracellular cysteine) are noted when αLA is added to cell cultures. Vitamin E-deficient animals do not show symptoms (weight loss, neuromuscular abnormalities) when supplemented with αLA.
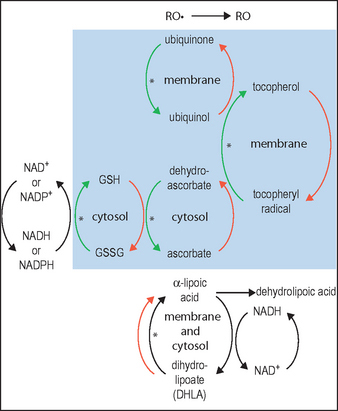
(Adapted from diagram in Podda & Grundmann-Kollmann 2001, incorporating information from Biewenga et al 1997)
Although αLA is a potent antioxidant, it provides no effective protection against UV-induced erythema or cell damage measured as sunburn cells. However, αLA (but not DHLA) acts as an anti-inflammatory agent by reducing the production and inhibiting the binding of transcription factors such as nuclear factor kappa B (NF-κB), thereby indirectly affecting the gene expression of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins. DHLA (but not αLA) can repair oxidatively damaged proteins, which in turn regulate the activity of proteinase inhibitors such as α1-AP, an inflammatory modulator. In vitro, both αLA and DHLA inhibit lipolysaccharide-induced nitric oxide (NO) and prostaglandin E2 (PGE2) formation and suppress inducible NO synthase (iNOS) but do not affect the expression of cyclooxygenase-2 (COX-2). In a mouse model, topical DHLA inhibits chemically induced activation of skin inflammation with a concomitant decrease in inflammatory modulators. Furthermore, topical DHLA (but not oral αLA) reduces chemically induced skin tumor incidence and multiplicity, and inhibits iNOS and COX-2 in a dose-dependent manner. As antioxidants, both αLA and DHLA are directly anti-inflammatory by virtue of their quenching oxidants secreted by leukocytes and macrophages at sites of inflammation.
αLA and DHLA have been shown to be effective depigmenting agents. Both depigment dark-skinned swine, inhibit tanning of light-skinned swine, and inhibit chemical and UVB-induced tyrosinase activity in melanocyte cultures. A recent new derivative of α-lipoic acid has been proven to be an effective depigmenting agent in melanoma cells in vitro. This depigmentation is achieved by formation of DAPA conjugate products.
UBIQUINONE (COENZYME Q10)
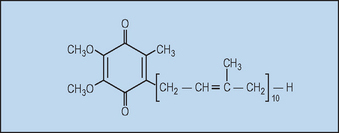
Ubiquinone (coenzyme Q10, Fig. 18.3) is so named because it is ubiquitous in virtually all living cells, excluding some bacteria and fungi, although the level is quite variable. Since most human tissues synthesize ubiquinone, it is not considered to be a vitamin.
Ubiquinone is primarily located in the inner mitochondrial membrane where it is essential for the production of the ATP required for all vital cellular functions. Until recently, ubiquinone was thought to function only in energy transduction; however, with the discovery that ubiquinone is also an antioxidant within subcellular membranes, new roles are now being recognized. Ubiquinone can regenerate reduced tocopherol, as depicted in Figure 18.2. In fact, within membranes the amount of ubiquinone is from three to thirty times that of tocopherol. Without ubiquinone, the regeneration of tocopherol would be very slow.
Ubiquinone’s antioxidant action in skin was confirmed in vitro by sophisticated ultra-weak photon emission (UPE). Increased antioxidants result in decreased UPE. Elderly volar skin demonstrated 33% reduction in antioxidant activity when compared with young skin. This was corrected after 1 week of twice daily topical application of 0.3% ubiquinone. After UVA irradiation, a decrease in antioxidant activity was noted; this loss was significantly corrected with topical 0.3% ubiquinone.
The efficacy of ubiquinol in reversing photoaging was further studied clinically. Ubiquinol cream (0.3%) was applied to one half of the face and placebo to the other once daily for 6 months. Casts were made of the periorbital rhytides. The improvement can be appreciated in the photographs shown in Figure 18.4. Quantitative microtopography demonstrated a 27% reduction in the mean wrinkle depth.
GENISTEIN
Topical genistein (10 μmol/cm2) protects against acute and chronic UV damage to the skin. After exposure of Skh:1 hairless mice to UVB, topical genistein blocked acute skin burns and inhibited UVB-induced cutaneous wrinkling, as demonstrated clinically in Figures 18.5 and 18.6. Histologic analysis confirmed that topical genistein blocks the signs of chronic photodamage—epidermal hyperplasia and reactive acanthosis with nuclear atypia (Fig. 18.7). At a molecular level, UV-induced damage to DNA (as measured by the biomarker 8-hydroxy-2′-deoxyguanosine) was reduced. Also, in Skh:1 mice, topical genistein inhibits UVB-induced pyrimidine dimer formation as well as UVB-generated suppression of repair of solar damage by proliferating cell nuclear antigen (PCNA). This photoprotection by genistein was further demonstrated in vitro with suppression of UVA-induced matrix metalloproteinases (MMPs) responsible for dermal destruction in photoaging. Inhibition of acute UV-induced erythema with topical genistein (5 μmol/cm2) was also demonstrated in humans: topical genistein (applied 30 minutes before UVB) inhibited by one minimal erythema dose (MED) the UVB-induced erythema as shown in Figure 18.8. Thus, topical genistein may protect human skin against photodamage.
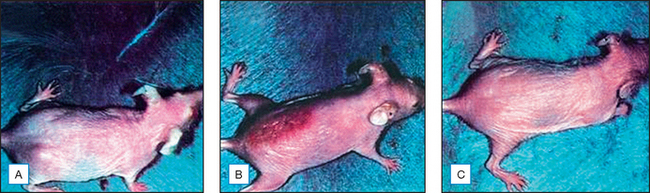
(Reproduced with permission from Wei H, Saladi R, Lu Y, et al 2003 Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition 133:3811S–3819S)
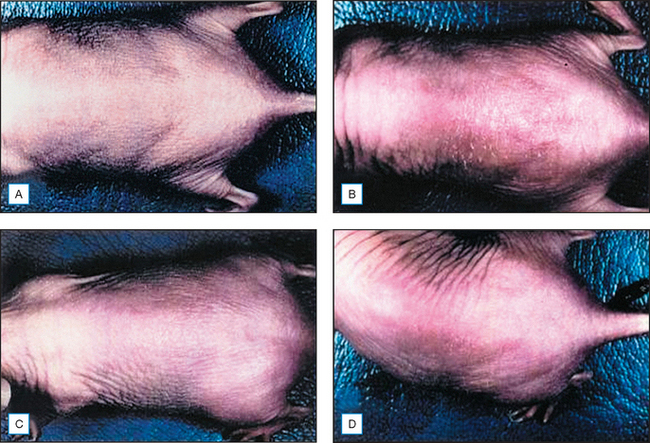
(Reproduced with permission from Wei H, Saladi R, Lu Y, et al 2003 Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition 133:3811S–3819S)
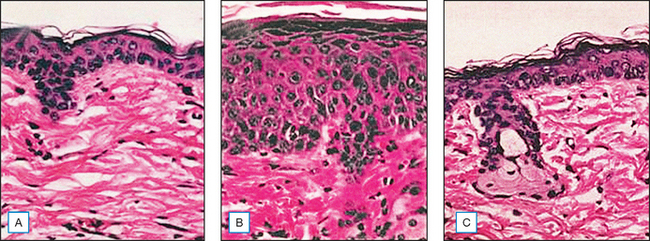
(Reproduced with permission from Wei H, Saladi R, Lu Y, et al 2003 Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition 133:3811S–3819S)
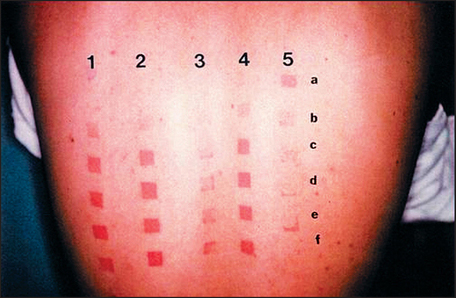
(Reproduced with permission from Wei H, Saladi R, Lu Y, et al 2003 Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition 133:3811S–3819S)
Equally impressive is the fact that topical genistein also inhibits skin cancer, a consequence of chronic UVB damage. Both the incidence and the multiplicity of UVB-induced skin tumors in Skh:2 hairless mice were reduced by about 90% after 25 weeks of UVB exposure. Figure 18.9 shows protection from carcinogenesis of representative mice treated with genistein before UVB exposure. Also, after chemical induction and promotion of skin tumors, topical genistein inhibited tumor cell number by 60–75%.
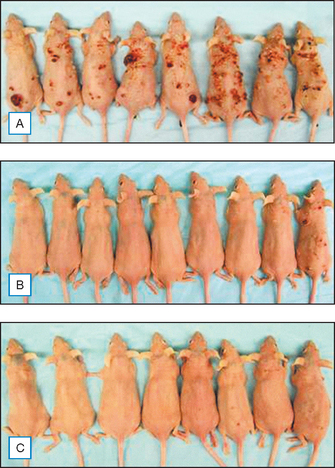
(Reproduced with permission from Wei H, Saladi R, Lu Y, et al 2003 Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition 133:3811S–3819S)
Another possible dermatologic benefit of genistein is as a phytoestrogen. The skin has both α- and β-nuclear estrogen receptors through which estrogen binding can regulate linked genes of proliferation and differentiation. Genistein has a 30-fold higher affinity for ERβ than ERα, but a greater ERα agonist activity than ERβ. Although estradiol has 700-fold more ERα and 45-fold more ERβ activity than genistein, the possible biologic effect of genistein through dietary soy isoflavones may be important. This estrogen activity of genistein has been further confirmed in vitro using B16F1 melanoma cells.
Aklyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. Journal of Biological Chemistry. 1987;262:5592–5595.
Beitner H. Randomized, placebo-controlled, double blind study on the clinical efficacy of a cream containing 5% alpha-lipoic acid related to photoaging of facial skin. British Journal of Dermatology. 2003;149:841–849.
Biewenga GP, Haenen GRMM, Bast A. The pharmacology of the antioxidant lipoic acid. General Pharmacology. 1997;29:315–331.
Crane FL. Biochemical functions of coenzyme Q10. Journal of the American College of Nutrition. 2001;20:591–598.
Han B, Nimni M. Transdermal delivery of amino acids and antioxidants enhance collagen synthesis: in vitro and in vivo studies. Connective Tissue Research. 2005;46:251–257.
Ho YS, Lai CS, Liu HI, et al. Dihydrolipoic acid inhibits skin tumor promotion through anti-inflammation and anti-oxidation. Biochemical Pharmacology. 2007;73:1786–1795.
Hoppe U, Bergemann J, Diembeck W, et al. Coenzyme Q10, a cutaneous antioxidant and energizer. BioFactors. 1999;9:371–378.
McDaniel D, Neudecker B, Dinardo J, et al. Clinical efficacy assessment in photodamaged skin of 0.5% and 1.0% idebenone. Journal of Cosmetic Dermatology. 2005;4:167–173.
Moore JO, Wang Y, Stebbins WG, et al. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis. 2006;27:1627–1635.
Pinnell SR, Lin J-Y, Lin F-H, et al. Alpha lipoic acid is ineffective as a topical photoprotectant of skin. Journal of Investigative Dermatology. 2004;123:996-998. (Poster presentation, 62nd Annual Meeting of the American Academy of Dermatology, Washington, DC)
Podda M, Grundmann-Kollmann M. Low molecular weight antioxidants and their role in skin ageing. Clinical and Experimental Dermatology. 2001;26:578–582.
Podda M, Traber MG, Packer L. Alpha-lipoate: antioxidant properties and effects on skin. In: Fuchs J, Packer L, Zimmer G, editors. Lipoic acid in health and disease. New York: Marcel Dekker; 1997:163–180.
Podda M, Zollner TM, Grundmann-Kollmann M, et al. Activity of alpha-lipoic acid in the protection against oxidative stress in skin. Current Problems in Dermatology. 2001;29:43–51.
Shyong EQ, Lu YH, Lazinsky A, et al. Effects of the isoflavone (genistein) on psoralen plus ultraviolet A radiation (PUVA)-induced photodamage. Carcinogenesis. 2002;23:317–321.
Stocker R. Coenzyme Q10. The Linus Pauling Institute Micronutrient Information Center Online. Available: http://lpi.oregonstate.edu/infocenter/othernuts/coq10/, 2003.
Thom E. A randomized, double-blind, placebo-controlled study on the clinical efficacy of oral treatment with DermaVite™ on aging symptoms of the skin. Journal of International Medical Research. 2005;33:267–272.
Tsuji-Naito K, Hatani T, Okada T, et al. Modulating effects of a novel skin-lightening agent, α-lipoic acid derivative, on melanin production by the formation of DOPA conjugate products. Bioorganic and Medicinal Chemistry. 2007;15:1967-1975.
Varila E, Rantalia I, Oikarinen A, et al. The effect of topical oestradiol on skin collagen of post-menopausal women. British Journal of Obstetrics and Gynaecology. 1995;102:985–989.
Wei H, Saladi R, Lu Y, et al. Isoflavone genistein: photoprotection and clinical implications in dermatology. Journal of Nutrition. 2003;133:3811S-3819S.