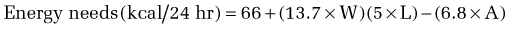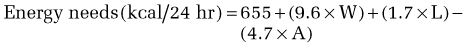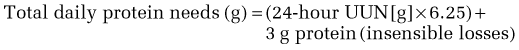CHAPTER 5 Nutrition in Gastrointestinal Diseases
NUTRITIONAL ASSESSMENT
Determining who is at risk for malnutrition is a complicated science. A proper nutritional assessment provides a mechanism whereby patients who may require nutritional support can be identified and also provides a gauge to monitor the effectiveness of such support (see Chapter 4).
MEDICAL HISTORY AND PHYSICAL EXAMINATION
Reading a patient’s chart, taking a history, and performing a physical examination allows a good understanding of a patient’s disease status and allows the diagnosis of some nutritional deficiencies. Inquiries regarding a patient’s usual body weight (UBW) versus ideal (IBW) or present body weight (PBW) should be noted at the initial patient encounter because these values have been shown in a number of studies to be predictors of morbidity and mortality.1,2 Although a simple tool, the most sensitive marker of nutritional risk is the percentage deviation from UBW over the past three to six months.
ANTHROPOMORPHIC MEASUREMENTS
Anthropomorphic measurements allow the estimation of body composition or body stores of energy using relatively simple and inexpensive equipment such as hand-held calipers (Fig. 5-1) and scales. Hand-held calipers allow measurement of a patient’s triceps skinfold (TSF), a marker of body fat stores, and midarm muscle circumference (MAMC), a marker of body protein stores. These measurements are compared with standardized tables to obtain percentages of normal values. Loss of body stores of protein (e.g., reduced MAMC) has been associated with poor patient outcomes.
Because of the obesity epidemic, emphasis has been placed on evaluating a patient’s weight and body habitus using the body mass index (BMI), which is defined as weight (in kilograms)/height (in meters squared). BMI values help categorize patients as underweight, normal weight, overweight, and obese (see Table 4-18).
BIOCHEMICAL MEASUREMENTS
The plasma proteins albumin, prealbumin, and transferrin are widely used to assess nutritional status. Albumin has been overrated as a predictor of protein malnutrition and, because it has a half-life of 21 days, it is a poor indicator of protein malnutrition. Infections, chronic medical conditions, liver disease, acute physiologic changes, and medications affect serum albumin levels through mechanisms not related to protein malnutrition.3 Low serum albumin levels (<3.5 g/dL) on hospital admission have been correlated with poor prognosis and poor surgical outcome.4
IMMUNOLOGIC TESTS
The serum total lymphocyte count has been correlated with changes in nutritional status, especially protein depletion.5 However, no prospective study has shown this to be a reliable marker of a patient’s nutritional status.
MUSCLE FUNCTION
An effective tool to evaluate a patient’s nutritional status over time is to measure his or her endurance or muscle strength. A practical method of assessing muscle strength involves hand grip strength,6 which is proportional to forearm lean muscle mass (Fig. 5-2). The reliability of this test is reduced for patients who are acutely ill or have hand or arm motor abnormalities.
GLOBAL ASSESSMENTS
To date, there is no single tool that is an accurate predictor of nutritional status. A variety of validated nutrition scoring systems, incorporating a number of features of the patient’s medical history and physical examination, has been developed to assess nutritional status. One of these, the subjective global assessment (SGA), is a comprehensive nutritional assessment tool that incorporates weight changes, dietary intake, functional capacity and preliminary medical diagnosis to categorize patients as well-nourished, moderately malnourished, or severely malnourished (see Table 4-24).7 The SGA has been validated in the oncology population.8 Other global nutritional assessment tools include the prognostic nutritional index (PNI), the instant nutritional assessment (INA), the Mini-Nutritional Assessment, the malnutrition universal screening tool, the nutritional risk score, and the nutritional risk index.9
CALORIC ASSESSMENT
Mathematical Equations
Calculation of energy requirements can be obtained through mathematical equations. Many different methods of estimating energy needs have been used over the years, including estimations based on body surface area, body weight, body height, and age of the patient. The most commonly used equation for calculating energy needs is the Harris-Benedict equation.10 The calculation is as follows:
where W = weight in kilograms, A = age, and L = height in centimeters.
This calculation of energy needs is multiplied by stress factors to arrive at a patient’s overall caloric needs (Table 5-1) Other caloric assessment formulas commonly used include the Ireton-Jones equation and the Mifflin-St. Jeor formula.11 Calculated energy needs, however, may over- or underestimate a patient’s true energy needs, especially in patients with complicated disease processes that can alter their metabolic rate.12 In these cases, direct measurements of overall energy needs may be more appropriate.
Table 5-1 Influence of Disease Severity (Physiologic Stress) on Resting Energy Expenditure (REE)
| DISEASE SEVERITY | REE (%) |
|---|---|
| Mild | 10 |
| Moderate | 25 |
| Severe | 50 |
Example: REE of 1500 kcal in a patient with moderate disease = (1500 kcal × 0.25) + 1500 kcal = 1875 kcal/day.
Indirect Calorimetry
Direct measurements of a patient’s energy needs can be performed using indirect calorimetry, which measures heat produced by oxidation. A ventilated hood is placed over the resting patient’s head and its oxygen and carbon dioxide content for two hours is analyzed. From this information, the resting energy expenditure (REE) is derived. True caloric needs are calculated by multiplying the REE by an activity or stress factor. In addition, a patient’s respiratory quotient (RQ) is derived. The RQ is equal to Vco2 (volume of CO2)/Vo2 (volume of O2). An RQ of approximately 0.7 or less is an indication of underfeeding whereas an RQ of approximately 1.0 or more is an indication of overfeeding.13 Indirect calorimetry plays a large role in obese or very malnourished patients, for whom determining caloric needs based on total body weight can be inaccurate.14 More recently, simple hand-held devices have been developed with an oxygen sensor for energy measurement. By breathing through the device for 10 minutes, the patient’s REE can be determined (Fig. 5-3).15 This tool has been shown to be as effective as the more expensive and laborious indirect calorimetry device in patients who can spontaneously breathe on their own volition, that is, those who are not on a ventilator.16
NUTRIENT SUBSTRATES
MACRONUTRIENTS
The macronutrients that fuel human metabolism include carbohydrates, fats, and proteins. The major source of energy in the human diet is carbohydrate, which constitutes almost half of the typical American diet. Carbohydrate is consumed as starch, sucrose, or lactose. Starch is made up of the polysaccharides amylopectin and amylose. Sucrose and lactose are disaccharides. Most digestion occurs in the duodenum and small intestine (see Chapter 100).17 Starch digestion begins in the mouth with the enzyme amylase and continues with the pancreatic enzyme alpha-amylase in the small intestine. Starch is broken down into oligosaccharides. The oligosaccharides and disaccharides are hydrolyzed by the small intestinal brush border to monosaccharides by glucoamylase, sucrase, and amylase. Glucose and galactose are absorbed across the small intestinal mucosa by active transport, whereas fructose moves across by facilitated diffusion.18
Dietary fat is primarily composed of triglycerides, which consist mainly of four long-chain fatty acids—palmitic, stearic, oleic, and linoleic—with smaller amounts of linolenic acid and medium-chain triglycerides (MCTs). Linoleic and linolenic acids are essential fatty acids; that is, they cannot be synthesized from nondietary sources. Essential fatty acid deficiency can result in a clinical syndrome consisting primarily of a scaly erythematous rash.19 Clinically, this may be seen in patients completely dependent on PN for their nutrient intake who receive no fat in their daily PN solution over a period of one to two weeks. Triglycerides are hydrolyzed to free fatty acids and beta-monoglycerides by pancreatic lipase and colipase.20 Because fats are insoluble in water, their digestion requires a unique environment involving an emulsification process whereby bile salts enhance their absorption (see Chapter 100). In water, bile salts form a micelle with a hydrophobic core and a hydrophilic periphery. Micellar contents diffuse across the water layer, intestinal mucosa, and cell membrane and enter the cell. Here they are reesterified to triglycerides and linked to an apoprotein to form a chylomicron.
Protein and amino acid metabolism are essential to provide the building blocks necessary to create a variety of body proteins and nitrogen-containing compounds. Proteins are composed of amino acids joined by peptide bonds. There are 22 amino acids, eight of which are essential: lysine, threonine, leucine, isoleucine, valine, methionine, phenylalanine, and tryptophan; two others, histidine and arginine, are essential for infants and growing children. Dietary proteins are partially digested by pepsin in the stomach to form polypeptides, but most protein digestion is performed in the duodenum and upper jejunum by pancreatic proteases, including trypsin, chymotrypsin, carboxypeptidase, and elastase (see Chapter 100).21 Peptides are further hydrolyzed in the small intestine by aminopeptidases, enzymes found in the brush border of the small intestine, to free amino acids and very small peptides. Large peptides must be hydrolyzed by brush border enzymes for absorption to occur, whereas dipeptides and tripeptides can move intact into mucosal cells.22
MACROMINERALS
Minerals account for only 4% of total body weight, yet they serve as essential cofactors, help maintain fluid osmotic pressures, and provide the proper environment for many chemical reactions (see Chapter 4).
Calcium
This is the most abundant cation in the human body. Bone and teeth contain about 99% of total body calcium. It is ingested in the form of an insoluble salt and must be released from its salt form to its ionized form for absorption (see Chapter 100).23 Calcium absorption occurs along the length of the small intestine and is vitamin D–dependent. In periods of restricted calcium intake, the colon may become more involved in calcium homeostasis by increasing its absorption. In periods of low serum calcium levels, renal excretion of calcium is reduced and bone calcium stores are released. Magnesium and calcium compete with one another for absorption. Unabsorbed dietary fat can interfere with calcium absorption by the formation of soaps in the intestine, which are excreted in the feces. The recommended daily intake of calcium is 800 mg/day for adults and 1200 mg/day for growing children. Calcium requirements are higher for pregnant women and older adults. In addition to bone mineralization, calcium also is important in blood clotting, muscle contraction, and the secretory activity of most endocrine and exocrine cells. Hypocalcemia may result in tetany, paresthesias, hyperreflexia, seizures, and mental status changes. Chronic calcium deficiency will result in rickets in children and osteomalacia in adults.
Magnesium
This is the second most abundant intracellular cation. Approximately 60% of magnesium is located in bone.24 Skeletal muscle also serves as another large source of magnesium storage. Magnesium absorption increases when magnesium intake is low. Vitamin D may affect absorption, although this relationship is not clear. Patients on a low-protein diet also can have difficulty with magnesium absorption. The recommended daily intake of magnesium is 300 to 500 mg/day. Magnesium is important in providing stability to the structure of ATP and is involved in numerous other enzyme systems. Magnesium deficiency can result in tetany, ataxia, myoclonus, coma, psychosis, cardiac dysrhythmias, and hypotension. Severe hypomagnesemia may be seen in patients with refeeding syndrome.
MICRONUTRIENTS
Essential micronutrients are present in minute or trace amounts in the body, sometimes in quantities less than 100 µg (see Chapter 4). Although trace elements are present in very small amounts, they often have dramatic effects; deficiencies are more common than toxicity. Deficiencies can result from reduced intake, decreased bioavailability, decreased transport proteins, excess excretion, or as the result of certain disease states. Many of these deficiencies develop in patients who are on long-term PN or who are severely malnourished. Assessment of trace element deficiency is extremely difficult (Table 5-2) because serum levels may not accurately reflect body stores. Therefore, clinicians may have to depend largely on physical signs and symptoms to detect micronutrient deficiency.
| TRACE ELEMENT | Requirement | |
|---|---|---|
| ENTERAL | PARENTERAL | |
| Chromium | 30 µg | 10-15 µg |
| Copper | 0.9 mg | 0.3-0.5 mg |
| Fluoride | 4 mg | Not well defined |
| Iodine | 150 µg | Not well defined |
| Iron | 18 mg | Not routinely added |
| Manganese | 2.3 mg | 60-100 µg |
| Molybdenum | 45 µg | Not routinely added |
| Selenium | 55 µg | 20-60 µg |
| Zinc | 11 mg | 2.5-5 mg |
From American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adults and pediatric patients. J Parenter Enteral Nutr 2002; 26:29SA-33SA.
Chromium
This is important in protein, carbohydrate, and lipid metabolism by serving as an important cofactor in enzymatic breakdown. It is crucial for the synthesis of glucose tolerance factor, a cofactor in insulin action.25 Chromium deficiency is manifested by glucose intolerance. The daily requirement is 50 to 100 µg/day.
Copper
This is important for normal body iron uptake. A microcytic hypochromic anemia can be seen with copper deficiency,26 a result of shortened red blood cell lifespan. Copper plays a major role in taste sensation and is important in reducing the potential injurious effects of free radicals. Copper is excreted in bile and should be replaced in those with external biliary drains or excessive diarrhea. The daily requirement is approximately 1.5 to 3 µg/day.
Zinc
This is an important component of many enzymes. It is involved in protein and lipid synthesis, and insulin activity. Approximately 25% of ingested zinc is absorbed daily in the duodenum and proximal jejunum. Excretion is through the biliary tract, skin, and feces. Zinc deficiency can result in a characteristic skin rash (acrodermatitis), poor wound healing, impaired taste, glucose intolerance, alopecia, depression, and diarrhea. Because body copper levels can be suppressed by zinc loading, zinc has been evaluated for the treatment of early Wilson disease.27 The daily requirement is approximately 10 to 15 mg/day.
VITAMINS
Vitamins (see Chapters 4, 100) are essential micronutrients involved in basic body functions such as growth, tissue maintenance, and metabolism. They are broadly classified as water-soluble or fat-soluble. Absorption of fat-soluble vitamins requires absorption and transport of lipids. Water-soluble vitamins, except vitamin C, are part of a B-complex group (Table 5-3).
Table 5-3 Daily Reference Intakes* and Sites of Absorption of Vitamins
| VITAMIN | RECOMMENDED DIETARY ALLOWANCE* | ABSORPTION SITES |
|---|---|---|
| C | 75-90 mg | Distal small intestine |
| B1 (thiamine) | 1.1-1.2 mg | Jejunum |
| Riboflavin | 1.1-1.3 mg | Proximal small intestine |
| Niacin | 14-16 mg | Stomach, small intestine |
| Pantothenic acid | 5 mg | Jejunum |
| Biotin | 30 µg | Unknown |
| Folic acid | 400 µg | Jejunum |
| B12 | 2-4 µg | Distal ileum |
| B6 | 1.3-1.7 µg | Jejunum |
| A† | 700-900 retinol equivalents | Proximal small intestine |
| D | 200-600 IU | Duodenum, terminal ileum |
| E | 15 mg/day | Mid–small intestine |
| K | 90-120 µg/day | Jejunum, ileum, colon |
* Daily Reference Intakes (DRI) were established by the Institute of Medicine between 1997-2001. They are quantitative estimates of nutrient intakes to be used for planning and assessing diets for healthy people. The DRIs include both recommended intakes and tolerable upper intake levels. The RDAs (Recommended Dietary Allowances) are a component of the DRIs and are defined as the daily intake of a nutrient considered sufficient to meet the requirements of 97% to 98% of adults.
† A retinol equivalent is 3.3 IU of vitamin A; 1 retinol equivalent = 6 µg β-carotene or 1 µg retinol.
Water-Soluble Vitamins
Niacin
This is found in animal foods such as beef, pork, and chicken and in cereal grains, especially wheat, rice, and bran. Niacin is hydrolyzed to niacinamide in the mucosal cells of the small intestine. It is important for the formation of the nucleotides nicotinamide adenine dinucleotide, reduced form (NADH) and NADPH, compounds that serve in a number of electron transport systems. Niacin is used in the treatment of hypercholesterolemia, although the mechanism whereby it reduces cholesterol levels is unclear. Side effects of therapeutic doses of niacin include flushing, liver injury, elevated uric acid levels, and dermatologic problems.28 Niacin deficiency may result in a constellation of symptoms referred to as pellagra, which is characterized by glossitis, coarse, scaly erythematous skin, diarrhea, and mental confusion.
Folic Acid
This is present in many foods including leafy vegetables, fruit, and liver. Polyglutamate forms of folate must be hydrolyzed by jejunal mucosal enzymes prior to absorption.29 Folate serves as a carbon donor in a number of synthetic reactions. The maturation of red blood cells and other short-lived cells is folate-dependent. Folate deficiency results in a macrocytic anemia because of decreased deoxyribonucleic acid (DNA) synthesis. Excessive folate ingestion may produce malaise, insomnia, and gastrointestinal distress.
Fat-Soluble Vitamins
Vitamin K
This is necessary for the synthesis of four of the 13 factors required for blood coagulation (II, VII, IX, and X). It also is important in the synthesis of four proteins involved in coagulation (C, Z, S, and M). It is found in plant and animal foods and is absorbed from the jejunum, ileum, and colon by an energy-dependent process or diffusion. Of the total ingested vitamin K, 8% is absorbed and its half-life is short, two to three hours. Bacteria in the digestive tract also can synthesize vitamin K. After liver conjugation, the excretion of vitamin K occurs in the bile, feces, and urine. The normal diet contains 300 to 500 mg of vitamin K/day; thus, deficiencies are rare, but can occur in patients with severe malnutrition, fat malabsorption, pancreatic insufficiency, cholestasis, or severe liver disease, or in patients receiving antibiotics. Toxicity is very rare and usually reported only in infants.30
NUTRITION IN SPECIFIC DISEASE STATES
INTESTINAL FAILURE (SHORT BOWEL SYNDROME)
Intestinal failure or short bowel syndrome results from loss or disease of the intestine, or both, to an extent that precludes adequate digestion and absorption (see Chapter 103); Crohn’s disease (see Chapter 111), intestinal trauma, and intestinal infarction (see Chapter 114) are the most common causes. The patient often presents with weight loss, diarrhea, dehydration, and weakness. Following extensive resection of the small intestine, intestinal rehabilitation (goal of resuming oral nutrition) of the remaining small intestine is more likely to be successful if the colon has been preserved and the ileocecal valve is maintained.31 The nutritional management of short bowel syndrome depends on the amount and location of small bowel removed. Initially, proton pump inhibitors are used to reduce gastric hypersecretion and anticholinergic agents are used to slow intestinal transit. Parenteral nutrition is prescribed to meet nutritional needs. Oral feedings are gradually started and the volume of PN is reduced as oral feedings are tolerated. If the patient has had a partial ileal resection and has an intact colon, cholestyramine can be used to reduce bile salt–induced diarrhea. In patients with a small amount of ileum remaining and an intact colon, however, the use of cholestyramine can increase diarrhea by creating a relative bile salt deficiency. Vitamin B12 should be given monthly. In patients with significant small bowel resections (80 to 100 cm remaining), a trial of a small-peptide, low-fat, enteral formula should be attempted to reduce the amount of PN the patient requires. Later, a polymeric enteral formula can be substituted. Patients with less than 80 cm of small intestine remaining and no colon often are PN-dependent. The use of somatostatin to reduce intestinal secretions and slow transit time remains controversial.32 Anticholinergic therapy should be initiated. Patients may require larger doses of anticholinergics than usually are recommended, because absorption of the oral medication is limited.
The use of growth hormone, glutamine, and a rice-based diet in an attempt to cause small bowel mucosal hypertrophy and better absorption is controversial. Early data have suggested very significant improvements in small intestinal absorptive function,33 not confirmed by subsequent studies.34 The use of a glycoprotein (GL-2) also has been postulated as a small intestine mucosal stimulator for improved absorption. A recent prospective evaluation of its efficacy noted a statistically significant reduction in PN use35; the effectiveness of this therapy is still being evaluated in additional studies.
PANCREATITIS
Nutritional support is imperative for patients with severe acute pancreatitis or relapsing chronic pancreatitis (see Chapters 58 and 59). Early PN appears to be associated with a reduction in the complications and mortality associated with acute pancreatitis compared with maintaining the patient on an NPO regimen.36 However, central line catheter sepsis rates are high and hyperglycemia is common. Enteral nutrition also has been used in patients with pancreatitis in contrast to previous beliefs that complete bowel rest was required. It appears that intrajejunal feedings are safe and well tolerated.37 A standard, fat-containing, polymeric enteral formula can be used.38 Randomized, prospective trials have shown a reduction in overall patient complications, hospital length of stay, and total hospital charges compared with the use of PN.39,40 The use of jejunal feeding in patients with chronic pancreatitis has been described to improve weight and reduce abdominal pain associated with eating.41 Gastric feedings also have been used successfully in patients with severe acute pancreatitis,42 but are still a topic of investigation.
CROHN’S DISEASE
Crohn’s disease (see Chapter 111) is sometimes associated with malnutrition.43 These patients often are hypermetabolic and may have anorexia because of nausea and abdominal pain. Deficiencies of magnesium, selenium, potassium, and zinc are common in inflammatory bowel disease (IBD) as a result of losses in diarrheal fluids and through fistula tracts.44 Dietary therapy in IBD always has been considered important, but no specific diet can be recommended. Fat restriction may be important in patients with ileal disease or those who have undergone an ileal resection. The use of EN can be an important component of IBD therapy for patients who cannot eat. Enteral nutrition has not proven superior to PN in inducing remissions in IBD,45 although it is less costly and associated with fewer complications. Enteral nutrition alone has not proven superior to drug therapy for the treatment of Crohn’s disease.46 The use of PN in IBD should be restricted to patients who have not responded to conservative medical therapy (EN and medications) or in whom EN cannot be delivered.
LIVER DISEASE
Nutritional deficiencies are common among patients with liver disease, mainly from decreased dietary intake, but also as a result of altered metabolism, decreased nutrient storage, and increased nutrient requirements (see Chapters 72, 92, and 93). Decreased nutrient intake is secondary to anorexia and nausea and is more common in patients with cirrhosis.47 Decreased bile salt production results in an intolerance to high-fat foods and the development of fat-soluble vitamin malabsorption. In addition, hypoalbuminemia results in edema of the mucosa of the small intestine, further compromising nutrient absorption. Depletion of muscle mass occurs secondary to a lack of adequate glucose stores and a dependency on protein stores for energy. Normal serum amino acid concentrations are altered, with a rise in aromatic amino acids (tyrosine, phenylalanine, and methionine) and a fall in branched-chain amino acids (valine, leucine, and isoleucine). The aromatic amino acids normally are removed by the liver; it is postulated that the rise in aromatic amino acids precipitates hepatic encephalopathy because they act as false neurotransmitters. Moreover, branched-chain amino acids are used preferentially as a protein source by patients in liver failure because they require minimal liver catabolism. Unfortunately, studies have failed to demonstrate improved outcomes in liver-failure patients fed a branched-chain amino acid–fortified diet or enteral solution.48 There is a general tendency to limit protein intake in patients with cirrhosis to prevent encephalopathy; however, these patients have an increased protein demand and further limiting their protein intake will only accelerate protein calorie malnutrition. It is preferable to feed patients according to their protein needs and treat encephalopathy with medications as it develops. Parenteral nutrition should be used with caution in patients with liver failure, because immune dysfunction places these patients at increased risk for catheter sepsis. In addition, the lack of liver glycogen stores can lead to episodes of hypoglycemia when patients are rapidly tapered off PN. Nutritional support prior to liver transplantation has been shown to improve patient outcome, especially in patients who are significantly malnourished prior to the transplantation.49
DIVERTICULAR DISEASE
Patients with diverticular disease (see Chapter 117) often are provided with incorrect nutritional information. They are told to avoid nuts or foods that contain seeds because of fear that the hard small particles may lodge in diverticula and precipitate diverticulitis. There are no clinical data to support this concept,50 and most data suggest that a high-fiber diet will reduce the occurrence of symptomatic diverticular disease.51 Patients hospitalized for complicated diverticular disease can remain symptom-free on a high-fiber diet.52 Fiber intake should be at least 25 g/day and should be provided as insoluble fiber, such as that contained in wheat bran, bran muffins, and fiber-based cereals. The use of probiotics has had some success in the treatment of and prevention of diverticulitis, although more work needs to be done in this area.53
DUMPING SYNDROME
Dumping syndrome is common after partial gastrectomy and vagotomy. Hypertonic gastric contents empty rapidly into the small intestine, and consequently up to 25% of plasma volume suddenly may be transferred to the small intestine.54 Nausea, cramping, diaphoresis, and palpitations develop. Nutritional therapy for dumping syndrome aims to deliver a lower osmolarity solution to the small intestine by the frequent ingestion of small meals containing fat, protein, and complex carbohydrates, but limited in simple sugars. Fluid intake should be restricted and separated from solid food intake to avoid rapid gastric transit. High pectin-containing foods (bananas, oranges) will slow gastric output.
CELIAC DISEASE
Small intestinal mucosal injury and consequent malabsorption in celiac disease (see Chapter 104) occur when a susceptible patient ingests gluten-containing foods such as wheat, barley, rye, or possibly oats. Patients, especially younger ones, present with classic signs of malabsorption, including diarrhea, cramping, and marked weight loss and often develop folate, iron, and fat-soluble vitamin deficiencies. The primary treatment is a gluten-free diet. Wheat starch free of gliadin forms the basis for most bread in a gluten-free diet. Corn, rice, and buckwheat are allowed. Most patients will improve with dietary management. The vast majority of commercially available EN products, if required, are gluten-free.
CANCER
Protein-calorie malnutrition is a common problem in cancer patients. Cancer cachexia is the consequence of multiple metabolic abnormalities induced by the tumor. Appetite stimulation has been used successfully in cancer patients with mild malnutrition.55 The routine use of aggressive nutritional support in all patients receiving chemotherapy and radiation is controversial. Prospective randomized studies have failed to show improved tolerance to chemotherapy with the use of nutritional support,56 and PN also has failed to show an improvement in morbidity from radiation therapy. Parenteral nutrition has been shown to be beneficial for patients with gastrointestinal obstruction from primary or metastatic tumors.57 Parenteral nutrition also has been found to be beneficial in patients following bone marrow transplantation who have developed severe gastrointestinal mucositis.58 Enteral nutrition has been used successfully in patients with head and neck cancer to prevent weight loss, reduce hospitalizations, and reduce interruptions in chemotherapy and radiotherapy. In summary, the use of nutritional support in the cancer patient should be restricted to those patients with a reasonable life expectancy who are likely to be unable to maintain their nutritional needs for a prolonged period. It is in these patients that an improved quality of life may occur.
OBESITY
Obesity is a disease that has had explosive growth, simultaneously affecting many individuals in a community without regard to age, gender, or ethnic origin (see Chapter 6).59 Traditionally, the gastroenterologist has been involved in the endoscopic management of postbariatric surgical complications, including stomal stenosis, gastrointestinal bleeding, and fistulization (see Chapter 7). Previous endoscopic technologies used to treat obesity endoscopically, such as the gastric balloon, had limited exposure in the United States and were removed from the market because of associated complications, such as balloon deflation with migration and resultant small intestinal obstruction. Internationally, however, balloon endoscopic therapy continues, with a number of favorable outcomes reported.60,61 Patients with obesity-related gastrointestinal disease, such as gastroesophageal reflux disease, always have been a staple of the gastroenterologist’s practice. Today, gastroenterologists find themselves on the front line with other physicians attempting to combat obesity and its associated complications, including assessment of patients for risk, identification of those who may benefit from weight loss therapy, and determination of the appropriate weight loss intervention, including management of therapy-associated complications.
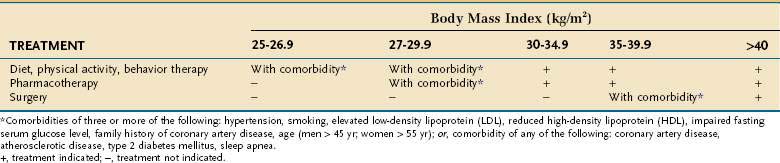
Assessing patients with obesity generally takes into consideration the BMI, waist circumference, and comorbid disease states. A person with a BMI more than 30 kg/m2 is considered obese. Patients who are overweight (BMI, 25 to 30 kg/m2) with comorbid diseases should be offered dietary management, exercise, and behavioral therapy. Patients with a BMI more than 30 kg/m2 also should be offered medical management (Table 5-4). If these patients have comorbid diseases, they also should be offered pharmacotherapy (weight loss medications). Patients with a BMI more than 35 kg/m2 should be offered medical management and pharmacotherapy. Surgical therapy is recommended for patients with a BMI more than 35 kg/m2 with significant comorbid diseases and who have not responded to medical management and pharmacotherapy. Patients with a BMI more than 40 kg/m2 should be offered medical management, pharmacotherapy, and surgery.
An enlarged waist circumference also places a patient at higher medical disease risk, separate from comorbid diseases, and correlates well with total body fat.62 Men with a waist circumference more than 40 inches or women with a waist circumference more than 35 inches have a higher risk, similar to the presence of significant comorbid diseases in a man with a BMI more than 30 kg/m2. These patients would be offered pharmacotherapy in addition to medical management, similar to the management of a patient with a BMI more than 30 kg/m2 with significant comorbid disease.
Surgical management of obesity in the United States typically involves a Roux-en-Y gastric bypass, vertical banded gastroplasty, or gastric banding (see Fig. 7-1). In general, most weight is lost in the first year.63 Gastric bypass surgery has a reasonable five-year outcome for maintenance of successful weight loss, but there is an associated mortality (0.5% to 2%) and complications associated with surgical therapy, some of them very significant.64,65
NUTRITIONAL THERAPY
PARENTERAL NUTRITION
PN delivers a solution consisting of water, electrolytes, amino acids, carbohydrates, fats, proteins, vitamins, and trace elements. These compounds are mixed and delivered over a period of time, usually 12 to 24 hours. Table 5-5 details a typical CPN formula. A CPN solution is six times more concentrated than blood (1800 to 2400 mOsm/L) and generally consists of approximately 30 to 50 g of protein and 1000 to 1200 cal/L. Determination of caloric and protein needs is based on a prior nutritional assessment.66 Table 5-6 provides an approximation of a patient’s daily protein and calorie needs based on the severity of the disease processes. These needs could be used instead of more complicated formulas presented earlier in this chapter for determining calorie and protein requirements. Overall, daily water requirements are estimated at 20 to 30 mL/kg.
| COMPONENT | AMOUNT |
|---|---|
| Amino acids | 220 kcal/L (55 g protein) |
| Dextrose | 555 kcal/L (163 g carbohydrates) |
| Lipids | 400 kcal/L (40 g of lipids), total: 1175 kcal/L |
| Sodium | 70 mEq/L |
| Potassium | 35 mEq/L |
| Calcium | 5 mEq/L |
| Magnesium | 5 mEq/L |
| Phosphorus | 15 mmol/L |
| Chloride, acetate | To balance |
| Multivitamins (MVI-13) | Industry standard |
| Trace elements (MTE-5) | Industry standard |
| Drug additives/L (heparin, insulin, H2 blockers) | Individualized |
* Volume: 2000 mL (83 mL/hr over 24-hr infusion).
Table 5-6 Calorie and Protein Needs Based on Degree of Physiologic Stress
| PHYSIOLOGIC STRESS | CALORIE NEEDS (KCAL/KG/DAY) | PROTEIN NEEDS (G/KG/DAY) |
|---|---|---|
| Mild | 25-28 | 0.8-1.0 |
| Moderate | 28-32 | 1.0-1.5 |
| Severe | 32-35 | 1.5-2.0 |
Central Parenteral Nutrition Formulation
To create a PN formula, one must first determine the protein, carbohydrate, and fat content required by the patient. A stepwise approach is effective (Table 5-7). Each component of PN has a defined caloric content, with protein = 4 kcal/g, carbohydrate = 3.4 kcal/g, and fat = 10 kcal/g. Once the macronutrient components of a PN formula have been defined, electrolytes, trace elements, multivitamins, and some medications such as insulin, heparin, and H2 blockers can be added. Any of the components can be increased or decreased based on a patient’s laboratory values and comorbid disease processes. In addition, serum glucose levels should be maintained below 120 mg/dL by the addition of insulin to the solution.67 Water is added to meet the daily volume needs of the patient.
Table 5-7 Stepwise Approach to Writing a Parenteral Nutrition Order*
| Caloric Contents of Nutrient Substances |
| Protein, 4 kcal/g |
| Fat, 10 kcal/g |
| Carbohydrates, 3.4 kcal/g |
| Estimated Daily Needs for This Patient |
| Calories: 30 kcal/kg = 2100 kcal |
| Protein: 1.2 g/kg = 84 g |
| Fluids: 30 mL/kg = 2100 mL |
| Steps |
| 1. Add protein (1.2 g/kg/day) to the PN mixture. |
| 84 g of protein needed |
| 1 g of protein = 4 cal (total, 326 kcal) |
| 2100 kcal − 326 kcal = 1774 kcal still required |
| 2: Add lipids (1.0-1.5 g/kg/day). |
| 70 g fat = 700 kcal |
| 1774 residual calories − 700 kcal = 1074 kcal still required |
| 3. Add carbohydrates (3-5 gm/kg/day). |
| 1074 kcal/3.4 cal/g carbohydrate = 315.8 g |
| 4. Make total volume. |
| 30 mL/kg = 2100 mL |
| Additional Additives |
| Electrolytes, minerals, vitamins added (see PN example formula for details) |
| Drug additives: Histamine 2 blockers, insulin, heparin |
PN, parenteral nutrition.
* Order is written for a 70-kg man with moderate physiologic stress (see Table 5-5).
Peripheral Parenteral Nutrition Formulation
PPN prescriptions account for an increasing percentage of all PN prescriptions written each day in this country, perhaps because hospital patients often are on short-term PN therapy. Fifty percent of patients in the hospital are on PN for less than 10 days and 75% for less than 14 days.68 Use of PPN would allow potential reduction of the risk and complications associated with central venous access because it is delivered via a peripheral vein.
Formulation of PPN requires more attention to the solution osmolarity than to its actual caloric or protein content. A hyperosmolar solution can cause a chemical thrombophlebitis, resulting in patient discomfort and loss of peripheral venous access. In general, PPN solutions should be maintained at 1000 mOsmol/L or lower.69 Of all of nutrient components, carbohydrates have the most significant impact on PPN osmolarity. Because of the osmolarity issues, the caloric content of PPN/mL is limited (Table 5-8). To approximate a patient’s daily calorie and protein needs, a larger volume of PPN than CPN must be delivered to maintain the solution’s lower osmolality. The addition of heparin, hydrocortisone, or lipids to the PPN solution may reduce the incidence of chemical thrombophlebitis.70
Table 5-8 Glucose Concentrations and Osmolarity of Intravenous Solutions
| GLUCOSE CONCENTRATION (%) | KCAL/L | OSMOLARITY (MOSM/L) |
|---|---|---|
| 5 | 170 | 252 |
| 10 | 340 | 505 |
| 20 | 680 | 1010 |
| 40 | 1360 | 2020 |
| 50 | 1700 | 2575 |
| 60 | 2040 | 3030 |
| 70 | 2380 | 3535 |
Administration
The average CPN solution is composed of approximately 25% to 30% solute. At initiation, the CPN formula should be infused over 24 hours. Patients with glucose intolerance or those at risk for refeeding syndrome (see complications below) should have their PN infused at half their daily caloric needs for the first 24 hours. This ratio may be increased to full caloric needs over the next 24 to 72 hours with monitoring of serum glucose, electrolyte, magnesium, and phosphate levels and fluid tolerance.71
Metabolic Complications
Metabolic complications may develop as a consequence of the glucose, amino acids, lipid, vitamin, electrolyte, or mineral content of the PN solution.72 Hyperglycemia is the most common complication and is directly related to the dextrose content of the PN, the patient’s glucose tolerance, and the rate of PN infusion. Critically ill patients and patients with preexisting glucose intolerance require the most aggressive monitoring of serum glucose. The serum glucose level should be maintained below 120 mg/dL. In the critical care setting, tight control of serum glucose levels has been shown to improve patient morbidity and mortality.73 Patients who develop hyperglycemia should first be maintained on a sliding scale of regular insulin. Of the total amount of sliding scale insulin required over 24 hours, two thirds should be added to the next day’s PN formula. Further adjustments in insulin dosing may be required on a daily basis and some patients in the hospital may require a separate insulin drip. Failure to control blood glucose levels results in an increase of infectious complications, such as catheter sepsis. In some hospitalized patients, blood glucose control may be difficult, even with an insulin drip. In such cases, the caloric load of the PN solution, specifically carbohydrate and fat calories, is reduced; this is known as permissive underfeeding.74 In these patients, the risk of hyperglycemia and its consequences has been determined to be more than the risk of temporarily underfeeding the patient. During permissive underfeeding, a patient’s full daily protein needs continue to be met.
Refeeding syndrome is a common metabolic consequence of PN resulting from sudden provision of a large amount of glucose calories to a patient who previously was malnourished. With PN infusion, the metabolism of these patients attempts to become rapidly anabolic. Insulin production is increased, pushing potassium, phosphorus, and magnesium into intracellular compartments, with resultant hypokalemia, hypophosphatemia, and hypomagnesemia.75 Sodium retention and large fluid shifts also can occur and may place the patient at risk of developing congestive heart failure.76
Abnormal liver biochemical test results are common after initiation of PN and typically feature elevations of serum aminotransferase levels up to twice normal. Greater elevations in aminotransferase levels and associated hyperbilirubinemia warrant investigation. Liver disease such as viral hepatitis, nonalcoholic steatohepatitis, sclerosing cholangitis, primary biliary cirrhosis, autoimmune hepatitis, and hemochromatosis need to be excluded. Right upper quadrant ultrasonography usually can eliminate the diagnosis of a liver mass, cholelithiasis, or biliary sludge. Acalculous cholecystitis needs to be excluded because the normal intestinal stimulation of gallbladder contraction is lost when a patient has no enteral intake. True PN-induced liver disease presents as a fatty infiltration of the liver that is especially prominent in the periportal areas, and may respond to a reduction in a patient’s total daily carbohydrate or total calorie infusion.77 It has been shown that repeated episodes of catheter sepsis will increase the probability of a deterioration in liver biochemical test results in patients on PN. Patients with short bowel syndrome are more likely to develop PN-associated liver failure, although the reason for this is unclear. Current research suggests that choline deficiency may play a role in the development of liver disease associated with PN use.79 Unfortunately, choline is not available for infusion in the United States. In addition, the lipid source of the PN solution is believed to contribute to this complication. In the United States, the lipid content of the PN solution is soy-based and composed of long-chain triglycerides (omega-6 fatty acids). In early reports, the use of an omega-3–containing lipid source (fish oil) had been shown to be effective in reversing PN-induced liver disease.80
Patients who develop significant complications with PN use may be candidates for small bowel transplantation. These complications include progressive liver failure, repeated catheter sepsis, or thrombosis of major venous systems that precludes obtaining central venous access. The arrival of tacrolimus as an immunosuppressive agent has improved small bowel transplant outcomes. Current five-year survival rates for patients receiving small bowel transplants are close to 50%.81 However, the five-year survival rate of patients on HPN are still better than the five-year survival rate for patients receiving small bowel transplants. Quality-of-life differences between HPN and small bowel transplantatation still are being explored.
Vascular Access Devices
Tunneled Silastic catheters (Hickman, Broviac; CR Bard, Murray Hill, NJ) commonly are used for longer term vascular access. They have a Dacron cuff to induce fibrotic tissue adherence, which is believed important in preventing bacterial migration up the catheter. The catheter tip should be positioned in the distal third of the subclavian vein, not in the right atrium. Implantable ports are placed subcutaneously, usually on the chest wall. They require a specialized access needle to allow blood drawing or fluid infusion. It is believed by some investigators that implantable ports are associated with a reduction in infectious and thrombotic complications compared with tunneled catheters.83 Because they are implanted in the chest wall, these ports require a more extensive procedure for bedside removal than tunneled catheters. Peripherally inserted central catheters (PICCs) have been used for PN infusion both in the hospital and at home. The PICC line is generally placed in an upper extremity. These catheters are long, allowing the tip of the catheter to be positioned into a central vein. PICC lines are associated with a reduction in major insertion complications, such as pneumothorax, compared with standard centrally inserted catheters; however, they have more complications, such as infection, thrombosis, and displacement.84 The use of PICCs for home PN infusion has not yet been evaluated in a prospective study.
Central Venous Catheter Complications
Central venous catheter complications occur, with incidence rates of 1% to 20%.85 Complications of subclavian vein catheterization include hemothorax, pneumothorax, brachial plexus injury, hematoma, and subcutaneous emphysema. Common long-term catheter complications include sepsis, thrombosis, and catheter occlusion.
Catheter infection generally occurs from touch contamination and often involves coagulase-negative staphylococcus. Currrent hospital environments also have led to a surge in vancomycin-resistant enterococcus. The major mechanism of catheter contamination is tracking of organisms from the skin to the subcutaneous tissues and catheter tip. Other organisms frequently causing catheter infection include gram-negative bacteria and fungi. In the home setting, the more time spent teaching the patient about the care and operation of the central venous access device, the less likely the patient is to develop complications.86
Diagnosing catheter infections can be difficult. The white blood cell count may not be elevated in a patient with a venous catheter infection. Peripheral blood cultures can be negative and catheter tip culture is a much more sensitive method of documenting catheter infections. Generally, bacterial infections of catheters can be treated with the catheter in place, whereas fungal catheter infections and tunnel infections of the catheter tract require catheter removal for effective treatment.87 Often, broad-spectrum antibiotic or antifungal treatment of catheter infections is initiated once the diagnosis is suspected and definitive therapy introduced when the organism is identified. The addition of heparin (1000 units/L) to each bag of PN solution can prevent subclinical thrombus formation to which bacteria or fungi can attach, thereby potentially reducing the risk of catheter sepsis. When treating central venous catheter infections, importance is given to the actual contact time between the bacteria or fungus in the catheter and the antibiotic or antifungal agent. Some believe in locking a small amount of antibiotic into an infected line to try and reduce central line decontamination.88 Others advocate flushing the catheter daily with absolute alcohol (100%) to sterilize the catheter and reduce infectious events.89
Catheter-induced thrombosis occurs secondary to irritation of the blood vessel wall. The thrombus usually is composed of fibrin. Precipitation of medication in the catheter occurs less commonly. Symptoms of central vein thrombus formation include neck pain, neck swelling, anterior chest wall venous distention, and reduced catheter function. Flushing of the central venous catheter with saline has proven as beneficial as flushing with heparin for the prevention of catheter occlusion with a thrombus.90 Treatment of fibrin thrombus formation requires a thrombolytic agent, such as streptokinase, given as a bolus or continuous infusion.91 Medication or precipitate occlusions can be treated by the instillation of small amounts of sodium hydroxide or hydrochloric acid, depending on whether the drug is acidic or basic, respectively.92
ENTERAL NUTRITION
Enteral Access
The radiologist, gastroenterologist, or surgeon usually places enteral access devices (Table 5-9). This can be done at the bedside, fluoroscopically, endoscopically, or in the operating room, depending on the specific device and the expertise available. Medicare trends from 1997 to 2000 have shown a significant increase in enteral access procedures in the United States. The greatest increase in enteral access procedures was among radiologists, closely followed by gastroenterologists. The percentage of surgeons performing enteral access has decreased over time.93
| TYPE OF ACCESS | PURPOSE | DURATION OF NEED (MO) |
|---|---|---|
| Surgical or Percutaneous | ||
| Gastrostomy | Gastric feeding | >1 |
| Gastric decompression | ||
| Gastrojejunostomy | Gastric decompression | >1 |
| Gastric feeding | ||
| Jejunal feeding | ||
| Jejunostomy | Jejunal feeding | >1 |
| Nasal or Oral | ||
| Nasal or oral gastric tube | Gastric feeding | <1 |
| Gastric decompression | ||
| Nasal or oral gastrojejunal tube | Gastric decompression | <1 |
| Gastric feeding | ||
| Jejunal feeding | ||
| Nasal or oral small bowel tube | Jejunal feeding | <1 |
Nasoenteric Tube Access
Nasoenteric tube placement techniques have been developed for placement at the bedside, with endoscopy, with fluoroscopy, or during surgery. These techniques all have their indications, benefits, and risks. The final position of an enteral access tube is the stomach for gastric feedings, or the jejunum for small bowel feedings. A patient who is intolerant of gastric feedings, such as a patient with gastroparesis, gastric outlet obstruction, or who has had the stomach surgically removed, will receive small bowel feedings. Nasogastric and nasojejunal tubes have similar complications (Table 5-10).
Table 5-10 Complications of Nasogastric or Nasojejunal Tubes
| Aspiration pneumonia |
| Nasal mucosal ulceration |
| Otitis media |
| Pharyngitis |
| Pneumothorax |
| Sinusitis |
| Tracheoesophageal fistula |
| Tube migration |
| Tube obstruction |
The use of small bowel feedings to prevent tube feeding aspiration events is a complicated and contentious issue. Some studies have shown fewer aspiration episodes in patients whose feedings were placed directly into the small bowel than when placed into the stomach.94,95 A prospective trial by Neumann and DeLegge96 directly compared the incidence of aspiration episodes with gastric feedings and small bowel feedings in the intensive care unit and found no difference. It took longer to initiate small bowel feedings, however, because of the difficulty in obtaining adequate tube position. A 2002 consensus conference determined that small bowel feeding is recommended for the prevention of aspiration pneumonia in critically ill patients,97 but this debate certainly will continue. Small bowel feedings should be initiated in patients known to have gastroparesis, patients who are intolerant of gastric feedings, or patients who have had a witnessed tube feeding aspiration event with gastric feedings.
Bedside nasoenteric tube placement is the most common enteral access technique used in the hospital and long-term care environments. A nasogastric (NG) or nasojejunal (NJ) tube may be placed, a decision based on concerns with tube feeding tolerance and aspiration risk. There are many techniques available for passing bedside NG tubes. Typically, an 8- to 12-Fr NG tube is lubricated and passed into the stomach with the patient’s head flexed; the patient ingests sips of water to assist in passing the tube into the stomach.98 Many centers advise bedside auscultation for confirmation of an adequate position of the NG tube before its use; however, this can be misleading because a tube in an inappropriate location (e.g., lung, pleural cavity, esophagus) may be misinterpreted as being in proper position by improper bedside auscultatory techniques. Roubenoff and Ravich99 have reported on a technique to avoid intrapulmonary placement of nasoenteric tubes in high-risk patients. They suggested measuring a length of the tube from the earlobe to the xiphoid process before insertion. Once the tube is passed to this length, an anterior-posterior plain film is obtained to determine that the tube is in the esophagus before passing it further into the stomach or small intestine. Every patient should have a plain film to confirm proper positioning of a NG or NJ tube before initiating feedings.100
The difficulty of passing a nasoenteric tube at the bedside blindly into the small intestine has prompted development of a variety of techniques. Thurlow has promoted the use of a stylet-filled NJ tube that is advanced with a corkscrew motion.101 Zaloga has confirmed the reliability of this technique, with a more than 90% success rate.102 Ugo and associates have reported on placing the patient in the right lateral decubitus position and tracking the position of the tube into the small bowel by using a stethoscope and auscultation, a technique that resulted with an 83% success rate.103 Lord and coworkers have promoted the use of unweighted feeding tubes for these bedside passages; their success rate for spontaneous small bowel placement was far more than that documented for weighted tubes (92% vs. 56%).104 The hypothesis here was that the weight at the tip of the feeding tube actually was an impediment to the tube’s spontaneous passage through the pylorus. In Lord’s study, metoclopramide was used to promote passage of the tube from the stomach into the small intestine. Others have reported on positioning a tube beyond the pylorus with the use of pharmacologic agents; results with metoclopramide ranged from no benefit105,106 to a 90% success rate.107,108 Silva and colleagues, in a comprehensive literature review, noted that metoclopramide given intravenously or intramuscularly was effective in promoting successful nasoenteric tube placement into the small intestine.109 Griffith and associates have confirmed the usefulness of another promotility agent, erythromycin, for promoting successful NJ tube placement at the bedside in critically ill patients.110
Newer technologies have been developed for NJ tube placement. Self-propelled NJ tubes have a spiral tip at the distal end. It is hypothesized that the stomach can propel this type of tip through the pylorus easier than with a standard straight tip. Berger and coworkers have reported a 50% success rate in passing the pylorus with the self-propelled feeding tube in 105 critically ill patients.111 Magnetically steered NJ tubes also have been reported to show reasonably successful rates of proper placement.112 Concurrent use of narcotics decreased the likelihood of successful tube placement.
If attempts fail to pass an NJ tube blindly at the bedside, fluoroscopic or endoscopic methods of passage are then required. The preference of technique is center-dependent. In centers with available C-arm fluoroscopy and modified fluoroscopy beds, fluoroscopic passage of NJ tubes can be done at the patient’s bedside. Success of fluoroscopic guidance of NJ tube passage can approach 100%.113 In institutions without bedside fluoroscopic capabilities, however, transport of patients to the radiology suite, especially critically ill patients, can be time-consuming, expensive, and hazardous.114 In these cases, bedside endoscopic passage of NJ tubes is preferable.
NJ feeding tubes can be placed endoscopically at the bedside with moderate sedation. Table 5-11 lists the techniques for endoscopic passage of nasoenteric tubes at the bedside. The “drag-and-pull” method has the longest history. In this technique, a suture or another grabbable item is attached to the end of a NJ tube and used to drag the NJ tube into position in the small intestine with a grasping forceps. Difficulty usually occurs in releasing the suture from the grasping forceps. A second common technique, the “over-the-guidewire” technique, requires the initial endoscopic placement of a guidewire into the small intestine. The endoscope is removed and the guidewire is left in place. A feeding tube subsequently is passed blindly or with fluoroscopic assistance over the guidewire and into position in the small intestine. Patrick and colleagues have reported a 94% success rate using this technique.115 Fang and associates have described the use of an ultrathin endoscope to perform nasal endoscopy and place a guidewire into the small intestine. After the endoscope is removed, a NJ tube is passed over the guidewire into position.116 With this technique, no sedation is required. Other methods of endoscopic NJ placement are used less frequently.
Table 5-11 Endoscopic Methods of Nasoenteric Tube Placement
| METHODS | TECHNIQUE |
|---|---|
| Drag-and-pull | Suture on end of a tube pulled with forceps into position |
| Over-the-guidewire | Tube pushed into position over a guidewire |
| Through-the-scope | Tube pushed through biopsy channel of endoscope into small bowel |
| Nasal endoscopy | Tube passed over guidewire placed through a nasal endoscope |
Nasoenteric tube placement is the most common method of enteral access. Unfortunately, nasoenteric tubes may fail early because of tube occlusion or dislodgment, with subsequent interruption of tube feeding and medication regimens. Therefore, nasoenteric tubes should be used in patients requiring NG or NJ access for less than one month. The tip of the NJ tube may be anchored onto the small bowel mucosa using an endoscopic clipping device, a practice that seems to add a few days to the projected longevity of NJ tubes, presumably by reducing the risk for NJ tube migration.117 Patients who have experienced repeated, early failure of nasoenteric tubes should receive more permanent enteral access, such as a percutaneous endoscopic gastrostomy, percutaneous endoscopic jejunostomy, surgical gastrostomy, or surgical jejunostomy.
Percutaneous Endoscopic Enteral Access
If a patient will require enteral access for longer than one month, percutaneous procedures are preferred. The endoscopic procedures include percutaneous endoscopic gastrostomy (PEG), percutaneous endoscopic gastrojejunostomy (PEG/J), and direct percutaneous endoscopic jejunostomy (DPEJ). All these procedures require the use of moderate or deep sedation and can be performed in the endoscopy suite, in the operating room, or at the bedside. In comparison to NG access, PEG has been shown to be a more reliable enteral access tube, allowing patients to receive more calories daily because of a reduction in tube dysfunction.118
Percutaneous Endoscopic Gastrostomy
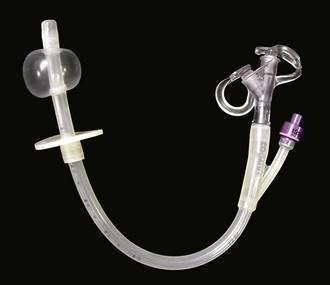
PEG was developed by Gauderer and coworkers in the early 1980s.119 The procedure involves the placement of a percutaneous gastrostomy tube after endoscopic transillumination of the abdominal wall for an appropriate gastrostomy site. The use of prophylactic antibiotics before the procedure is important to prevent postprocedure infections.120 Placement of a PEG tube can be accomplished by the Sachs-Vine (push) or Ponsky (pull) techniques, depending on physician preference. Prospective evaluations of PEG placement have found this procedure to be associated with few procedure-related complications.121 An older technique, the push-dilator technique, has started to make a resurgence. In this technique, the endoscope is used as a viewing port during PEG tube placement. T fasteners are placed percutaneously to attach the wall of the stomach to the abdominal wall; an incision is made in the abdominal wall and a fistula into the stomach is created and sequentially dilated. Ultimately, a PEG tube with a balloon internal bolster is passed through the newly created insertion site, as is done with balloon replacement tubes (Fig. 5-4).122
PEG tubes are indicated for patients who will be unable to consume sufficient nutrition for longer than one month, despite a functional gastrointestinal tract. Patients requiring PEG placement often are older and have numerous comorbid diseases. In one study of older patients referred for PEG placement, there was a 48% mortality at seven days if the patient had a prior aspiration episode or urinary tract infection, or was older than 75 years, compared with a 4% mortality if none of these risk factors was present.123 In addition to providing access for nutrition, PEG tubes are indicated for hydration and administration of medications as well as for gastric decompression. Some of the more common medical indications for PEG placement are described here.
Cancer
One area of oncology in which PEG tubes are beneficial is head and neck cancer. The benefit of PEG tubes in this setting was illustrated in a retrospective study that had 40% (32 of 88) of head and neck cancer patients receiving a PEG tube prior to chemotherapy and radiotherapy. Those who received a PEG lost an average of 3.1 kg compared with 7 kg of weight loss for those without a PEG. The same PEG group had significantly fewer hospitalizations for dehydration and malnutrition and had no interruption in treatment of their cancer compared with the group which did not receive PEG.124
Stroke
Data support the use of PEG tubes in patients with stroke-related dysphagia. In one study, the authors reported a 1-, 8-, and 48-month survival of 78%, 35%, and 27%, respectively, when the most common indication was a hemispheric stroke.125 Compared with NG feeding, early PEG placement was found to be associated with a lower incidence of ventilator-associated pneumonia.126 In contrast, Dennis and colleagues have reported an increase in the death rate and poor outcome in stroke patients randomized to PEG tube feedings as opposed to nasoenteric tube feedings.127 In this same study, patients receiving early tube feeding by any means had a reduction in overall mortality compared with patients who did not receive early enteral nutrition.
Dementia
Dementia is a frequent disorder of older adults and a common indication for referral for PEG. More than 36,000 older patients with dementia receive a PEG tube each year.128 The benefit of providing EN in these patients, however, is less clear.129 No large, prospective, randomized trials have been performed to evaluate the usefulness of PEG tube placement for this patient population. One retrospective analysis has suggested that mortality among dementia patients does not differ, regardless of whether a PEG tube is placed,130 although this study did not address whether the use of PEG tubes in this population for hydration and medication delivery would be appropriate. The use of PEG in the dementia population remains a subject of great debate131 and ultimately a prospective randomized trial will be required to answer the question definitively.
Disabling Neurologic Conditions
The use of PEG tubes for the patient with disabling motor neuron disease, such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS), has been examined. Both these diseases are associated with progressive dysphagia, leading to reduced oral intake, weight loss, and an increasing risk of oral aspiration. Langmore and coworkers did an extensive literature search of patients with ALS receiving PEG and only identified retrospective studies and prospective cohort studies.132 Using these data, they concluded that survival and nutritional status could be improved with PEG placement in ALS. There was a trend for improved quality of life although this required further study. Small case reports have demonstrated an improvement in comorbid disease states, such as pressure ulcer healing, in patients with MS and dysphagia who receive tube feedings.133
PEG Procedure
PEG kits are commercially available from a number of manufacturers. The most common sizes for adult patients range from 16 to 24 Fr. Most tubes are made of silicone, although some are constructed of polyurethane. In general, PEG tubes start to degrade one to two years after placement, usually from yeast implantation and degradation of the PEG tube wall.134 PEG tubes are less likely to clog than nasoenteric tubes because of their larger size. Obstructed PEG tubes may be cleared by flushing them with warm water. In some cases, pancreatic enzymes mixed in a bicarbonate solution also can be effective.135 There are no data to support the use of juices, soft drinks, or meat tenderizers to unclog a PEG tube. Commercially available PEG tube cleaning brushes also are available.
Relative contraindications for PEG placement include the presence of gastric varices, severe obesity, major gastric resection, significant disease of the gastric or abdominal wall, ascites, and coagulopathy. Absolute contraindications include the inability to transilluminate the anterior abdominal wall and an ineffective digital intrusion of the abdominal wall to locate a safe gastric access site.136
Once a PEG tube malfunctions, degrades, or is no longer needed, it can be removed at the bedside with a traction pull force of seven to 10 pounds; such removable PEG tubes are labeled as traction removal tubes.137 Some PEG tubes, labeled as endoscopic removal tubes, have a stiff internal bolster and can be removed only with an endoscope. Although there is an increase in cost with the use of endoscopic removal PEG tubes because of the need for a repeat upper endoscopy at removal, they may be safer to use in patients who are confused or combative and at risk for pulling their PEG tube out after initial placement. Some authors have suggested cutting the PEG tube at the abdominal wall level and allowing the remaining tube and internal bolster to pass through the gastrointestinal tract; this should not be done because there have been cases reported of these cut internal bolsters leading to a small bowel obstruction.138
Most post-PEG complications arise from a patient’s comorbidities, such as poor wound healing, aspiration, or coagulopathy.139–141 Caregivers must raise the head of the patient’s bed to 30 to 45 degrees during and for one hour after feeding to reduce the risk of aspiration.142 The most common complication is peristomal wound infection.143 Excessive tightening of the PEG tube external bolster against the abdominal wall can cause tissue ischemia, wound leakage, and necrotizing fasciitis.144 To minimize this complication, the external bolster of the PEG tube should be maintained 1 to 2 cm from the anterior abdominal wall to avoid tissue compression and wound breakdown.145 Peristomal wound infections often are treated for seven days with an oral antibiotic such as cephalexin to cover skin-related microorganisms. The infected area should also have daily topical cleansing with or without antibiotic ointment. The PEG tube should be removed in cases of worsening infection. Other common complications include pneumoperitoneum, fever, ileus, cutaneous or gastric ulceration, and tube extrusion or migration.146–148 Pneumoperitoneum is common after PEG placement and is not diagnostic of a perforation. Careful clinical evaluation of a patient after PEG placement with questionable peritonitis from perforation or leak should include a contrast study through the PEG tube to avoid unnecessary surgical exploration.149 Major complications are rare and include intra-abdominal bleeding or hematoma formation, peritonitis, necrotizing fasciitis, gastric or colonic perforation, and hepatogastric, gastrocolic, and colocutaneous fistula. A colocutaneous fistula results from the inadvertent placement of a percutaneous feeding tube through the colon before it enters the stomach. When a colocutaneous fistula is documented, the tube should be removed and the patient’s condition should be monitored for appropriate closure of the fistulous tract. If the tract does not heal, surgery may be warranted to repair the fistula.
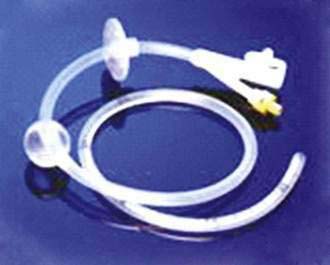
Replacement PEG tubes are divided broadly into two categories, replacement gastrostomy tubes and low-profile devices. Replacement gastrostomy tubes usually have a balloon-type internal bolster (Fig. 5-5). These balloon tubes can be inserted blindly through the gastrostomy site into the gastric lumen. The balloon is inflated to serve as the internal bolster and an external bolster is slid down the external tube against the abdominal wall to keep the PEG tube from migrating. There also are replacement PEG tubes with a distensible internal bolster (Fig. 5-6). The internal bolster is stretched with a stylet and pushed blindly through the gastrostomy site and the stylet is then removed, allowing the internal bolster to assume its previous shape. The direction of the gastrostomy tract should be known so that the stylet does not damage or rupture the tract.

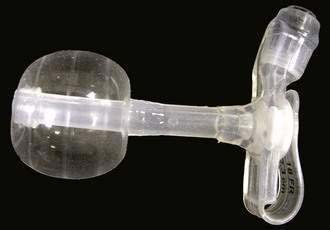
Figure 5-6. Replacement percutaneous endoscopic gastrostomy device with distensible internal bolster.
PEG tubes also may be replaced with low-profile gastrostomy devices (Fig. 5-7). These devices provide a skin level access to the gastric lumen, and may be particularly useful for disoriented patients who may habitually tug at their bedclothes and pull out their tube connections. The internal bolster of these low-profile devices may be an inflatable balloon or a distensible internal bolster that requires a stylet for placement. Low-profile PEG tubes come in predetermined lengths and the gastrostomy tract length must be measured in order to choose the correct length of the device. To access the low-profile device for feeding or gastric decompression, a separate access tube must be used to engage a valve in the top of the device. Although these tubes are cosmetically appealing, the small internal diameter of the access tubing and the valve make them more prone to valve and access tube occlusion.
Percutaneous Endoscopic Gastrojejunostomy
In patients in whom small bowel feedings are desired, endoscopic percutaneous small bowel access may be obtained by two methods. With the first method, percutaneous endoscopic gastrojejunostomy (PEG/J), a PEG is placed in the standard fashion, after which various techniques may be used to place a jejunal feeding tube through the PEG into the small bowel.150–152 Usually, a 9- or 12-Fr J tube is passed through the existing PEG and into position in the small bowel over a guidewire (Fig. 5-8). DeLegge and colleagues have reported a 100% success rate and no major complications using the over-the-guidewire technique for PEG/J placement, with a procedure time of 26 minutes.150 This PEG/J system allowed for concurrent gastric decompression and small bowel feeding. The average longevity of this tube system was approximately 120 days when patients who died from comorbid diseases were excluded from the analysis. A one-piece gastrojejunostomy (G/J) system does exist and is generally used as a replacement device that is passed through a preexisting PEG tube tract (Fig. 5-9). This tube can be dragged into position by a suture at the end of the G/J system during endoscopy or passed over a guidewire during endoscopy or fluoroscopy. The internal bolster on this system is a balloon.
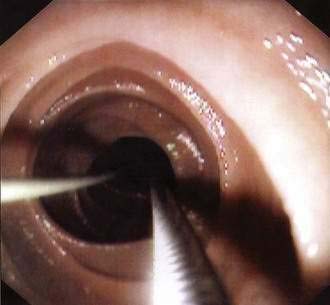
Figure 5-8. Percutaneous endoscopic gastrojejunostomy showing over-the-guidewire placement technique.
The management of PEG/J tubes is similar to that of PEG tubes. Jejunal tubes need to be flushed aggressively to avoid clogging. Reported clogging rates of J tubes have ranged from 3.5% to 35%.153,154 Administration of semidissolved medications and bulking medications such as fiber through the J tube, and checking J tube residuals, leads to an increased incidence of tube occlusion. In contrast, medications may be given through the gastrostomy tube because of its larger diameter; the gastrostomy tube also may be used for decompression of gastroparesis or gastric outlet obstruction.
Complications of PEG/J tubes include those already discussed for a PEG tube. In addition, the jejunal tube may migrate in a retrograde direction or become kinked so that it no longer functions. Tube migration occurs most commonly in patients who have persistent vomiting or in cases in which the J tube has been placed improperly.150,155
Direct Percutaneous Jejunostomy
The second method of jejunal access, direct percutaneous endoscopic jejunostomy (DPEJ), directly places a J tube into the small bowel using an endoscope. This procedure requires the use of an enteroscope or a pediatric colonoscope to reach a puncture position beyond the ligament of Treitz. Success with this procedure has been reported by Shike and associates;156,157 minor complications included local site infection, but no instance of peritonitis or bowel infarction. One of the difficulties with DPEJ placement is the frequent migration of the small bowel away from the introducer trocar needle once an adequate entry site on the abdominal wall has been located. Varadarajulu and DeLegge158 have resolved this problem with the use of a two-needle stick technique (Fig. 5-10). In this procedure, a smaller, sharper, 19-gauge needle (finder needle) first is passed through an appropriate abdominal site into the small intestine. This finder needle is grasped by a snare, thus anchoring the small bowel against the abdominal wall. The larger introducer catheter is passed alongside the 19-gauge needle into the small bowel without pushing the small bowel into the abdominal cavity. The snare is removed from the 19-gauge needle and placed around the introducer catheter. A guidewire is passed through the introducer catheter into the small bowel, where it is then grasped by the snare and pulled out via the oral cavity. A J tube is attached to the guidewire and pulled into place in the small bowel similar to how a PEG tube is placed. Adequate positioning of the internal bolster of the J tube is confirmed with endoscopic visualization.
Knowing what the patient’s needs are for jejunal access can help decide which enteral access is appropriate. Short-term access is probably best achieved by a nasojejunal (NJ) tube. Patients requiring jejunal access for less than six months or those requiring concomitant gastric decompression would do well with a PEG/J system. Long-term jejunal access (i.e., more than six months) is best achieved with a DPEJ tube. Comparisons of PEG/J and DPEJ for jejunal access have found fewer tube-related complications, such as jejunal tube migration and jejunal tube occlusion, with the DPEJ tube.159,160
Immediately after DPEJ placement, it is helpful to leave the J tube unclamped so that the substantial amount of air that was insufflated during the procedure may escape and thereby decompress the small bowel. Management of DPEJ tubes is otherwise similar to that of PEG tubes. Complications and technical failures have been presented in three retrospective series on DPEJ outcomes. Technical failure rates ranged from 12% to 28%. Complications included bleeding, abdominal wall abscess, colonic perforations, peristomal infections, enteric ulcers, small bowel torsion, and intraperitoneal leakage; tube-related malfunctions similar to those with PEG tubes also have occurred.157–159
Surgical Enteral Access
A number of studies have compared surgical gastrostomy with PEG and have shown cost savings, operative time savings, or a reduction in morbidity with PEG.160,161 In the standard surgical gastrostomy tube placement, a gastrotomy is formed and a gastric tube is placed into the gastric lumen. The gastric wall is then fixed to the abdominal wall. Surgical gastrostomy first was described by Stamm in 1894 and has not changed significantly since that description.162
Surgical jejunostomy was performed first by Bush in 1858 in a patient with nonoperable cancer163 and, in 1891, Witzel described the most well-known technique for jejunostomy, which subsequently has undergone a number of modifications.164 Laparoscopic placement of J tubes and G tubes began in the early 1990s because it was believed that these procedures would be associated with less morbidity and operative stress than the standard surgical jejunostomy and gastrostomy. It was soon learned that these laparoscopic techniques did not add any significant advantage over standard surgical gastrostomy or jejunostomy with regard to operative time or associated procedure morbidity. Needle catheter jejunostomy (NCJ) involves the placement of a 5- or 7-Fr catheter into the jejunum via a submucosal tunnel. It was hypothesized that this technique would have fewer procedure-related complications than standard jejunostomy because of the smaller entrance created to the jejunum. Multiple studies have reported reduced infectious complications of NCJ compared with standard surgical jejunostomy; however, there is a significant increase in tube occlusions and dislodgment with the smaller NCJ.
Fluoroscopic Percutaneous Enteral Access
Placement of percutaneous gastrostomy and gastrojejunostomy tubes with fluoroscopic guidance has continued to gain acceptance since the introduction of this technique in the early 1980s.165,166 These procedures usually are performed by radiologists in the fluoroscopy suite. After topical anesthesia to the abdominal wall, occasionally with additional moderate sedation, the inferior margin of the liver is identified by ultrasonography and marked on the patient’s abdominal skin surface. An NG tube is passed into the stomach, after which the stomach is insufflated and punctured with an introducer catheter; some but not all radiologists then attach the stomach to the anterior abdominal wall with T fasteners. A guidewire is placed into the stomach through the introducer and the puncture site is serially dilated over a guidewire to a size of 10 to 14 Fr. A gastrostomy tube is passed over the guidewire, through the dilated puncture site, and into the stomach or the small intestine if a gastrojejunostomy tube is desired.
This fluoroscopic approach to enteral access has a reported technical success rate of over 95%.167 Most of the complications involve inadvertent puncture of contiguous abdominal organs or separation of the abdominal and gastric wall during gastrostomy tract dilation; the latter may lead to peritonitis, intraperitoneal leakage, and even death. Frequent occlusion of these feeding tubes, because of their typically smaller internal lumen size, has been shown to be avoidable if larger gastrostomy tubes (18 to 22 Fr) are used.168
Enteral Feeding
For patients receiving gastric feedings, gastric residuals should be checked regularly and feeding intolerance monitored in all cases. Any gastric residual more than 400 mL should be followed closely.169 Repeated gastric residuals of more than 400 mL require the tube feedings to be stopped; in these cases, small bowel feedings may be necessary. Other indicators of tube feeding intolerance include persistent nausea and vomiting, abdominal distention, abdominal pain, and a dilated small intestine or colon seen on radiologic imaging.170 In general, it is a constellation of these signs, symptoms, and findings that should be present before intolerance to tube feeding is declared.
There is no good consensus on when to begin feeding a patient after PEG placement because it is thought that a patient develops transient gastroparesis or there is potential for intra-abdominal PEG tube leakage immediately after tube placement. Brown and coworkers randomized patients to begin feedings 3 or 24 hours after PEG placement and found no differences in tolerance or complications that required discontinuation of tube feedings. Wound infections were more common in the delayed feeding group, however.171
Enteral Formulations
Many formulations for enteral feeding are available including blenderized, polymeric, predigested, specialty, modular, and supplemental regimes (Table 5-12).
| TYPE | EXAMPLES |
|---|---|
| Fiber-containing (1.0 cal/mL) | Jevity |
| Free amino acids (elemental, low fat, 1.0 cal/mL) | Vivonex TEN |
| Polymeric (intact protein, 2.0 cal/mL) | Magnacal |
| TwoCal HN (high nitrogen) | |
| Polymeric (standard tube feedings, intact protein, 1 kcal/kg/day) | Nutren 1.0 |
| Isocal | |
| Osmolite HN (high nitrogen) | |
| Specialty Formulas | |
| Glucose intolerance (1.0 cal/mL, increased fructose component of carbohydrates) | Glucerna |
| Hepatic formulation (increased branched-chain amino acids) | Nutra-Hep |
| Immune-enhancing (1.0 cal/mL; arginine-, glutamine-, omega-3 fatty acid–fortified) | Impact |
| Crucial | |
| Oxepa | |
| Pulmonary (1.5 cal/mL, low carbohydrate) | Pulmocare |
| Renal (2.0 cal/mL, increased essential amino acids) | Amin-Aide |
Immune-Enhancing Formulas
If possible, administration of an immune-enhancing formula should be initiated for five to seven days prior to elective surgery. In such cases, feedings should be advanced as tolerated until 1500 mL are administered daily or more than 50% to 60% of calculated nutrient goals are met. Evidence suggests that, with their use, there is a reduction in subsequent infectious complications, antibiotic needs, ventilator days, episodes of multiple organ dysfunction, and decreased hospital stay.172 Some newer immune-enhancing formulas are showing promise in changing clinical outcomes for patients with acute respiratory distress syndrome.173
Enteral Feeding Complications
Gastrointestinal side effects of tube feedings are reported in 15% to 30% of patients and include nausea, vomiting, abdominal distention, abdominal cramping, and diarrhea. Nausea, vomiting, and abdominal distention often can be resolved by slowing the delivery rate of the feeding. Diarrhea is the most common complication.175 Its pathophysiology is complex. The most common cause of diarrhea in patients on enteral feeding is Clostridium difficile enterocolitis resulting from concurrent antibiotic use. The second most common cause of diarrhea in this group of patients is medications. Frequently, medications are changed from tablet to liquid form for easy instillation through the feeding tube. These liquid medications often have a sorbitol base, which is a known cathartic. Magnesium-containing medications, hypertonic medications, and promotility agents also may promote diarrhea. High-osmolarity tube feedings often are cited as a cause of diarrhea, although studies have demonstrated tolerance of tube feedings, the osmolality of which is as high as 600 to 700 mOsm/kg. There are no data to support the recommendation that commercial enteral formulations be diluted in an attempt to improve their gastrointestinal tolerance. For patients with small bowel malabsorption, especially fat malabsorption, predigested, lower fat formulas may improve absorption and reduce diarrhea. Hypoalbuminemia may lead to small bowel wall edema and resultant diarrhea. There are no data to support the use of intravenous albumin to improve diarrhea in these patients. In these circumstances, the use of anticholinergic agents to slow bowel motility may help improve absorption, thus decreasing diarrhea.
Fiber supplementation may improve diarrhea, although fiber’s effect on diarrhea remains controversial. Studies have provided evidence for and against fiber’s efficacy in treating diarrhea associated with tube feeding.176,177 There are a number of fiber-supplemented commercial enteral formulas available. Some fiber contained in enteral formulations may be termed a prebiotic.
Metabolic complications are less common with enteral than with parenteral feeding. Dehydration and fluid shifts may occur with formulas of high concentration, especially if insufficient water is supplied. Hyperglycemia may occur with high rates of carbohydrate delivery in patients with glucose intolerance. Medication delivery also may be affected by concurrent tube feeding. Phenytoin administration is affected because phenytoin binds to the enteral formula178 and forms a phenytoin–tube feeding complex that adheres to the wall of the feeding tube. Ciprofloxacin also has been shown to bind with tube feedings, reducing its absorption. Vitamin K, present in many enteral formulas, may make a patient more resistant to the effects of warfarin.
ORAL DIET THERAPY
A general diet is designed to provide optimal nutrition to patients who do not require a therapeutic diet. A healthful diet contains a variety of foods that are low in fat and cholesterol, contain moderate salt content, and has an abundance of fruits, grains, and vegetables. Low-carbohydrate diets have been promoted for weight loss,179 but the long-term outcomes of this dietary regimen for weight loss vary significantly among individuals (see Chapter 6).
Clear Liquid Diets
These supply fluid and energy in a form that creates a minimal amount of residue. They are meant to avoid a high osmolar delivery to the gastrointestinal tract, which would result in fluid shifts and associated nausea and diarrhea. Clear liquid diets generally contain an abundance of carbohydrates but little protein or fat. There is but sparse evidence to suggest that a clear liquid diet is better tolerated than any other diet following surgery. It is known that early feeding after abdominal or thoracic surgery reduces postoperative complications and hospital length of stay.180
Fiber- and Residue-Restricted Diets
These are used for patients with gastrointestinal strictures and are presumed to reduce the risk of obstruction while prolonging transit time.181 Carbohydrate intake is reduced and well-cooked vegetables, refined cereals, and breads are used. Although these diets are commonly prescribed, there are no data supporting their use in the gastrointestinal stricture patient population. High-fiber diets include soluble and insoluble fibers, which have a wide range of metabolic and physiologic effects. They are used to reduce intraluminal colon pressures in patients with diverticulosis, although no data support this concept. They also may be useful in diabetes by delaying glucose absorption, in cardiovascular disease by lowering serum cholesterol and serum triglyceride levels, and in the prevention of colon cancer. This diet emphasizes foods such as vegetables, fruits, legumes, and whole-grain breads and cereals.
Low-Fat Diets
These are used to minimize diarrhea and steatorrhea associated with fat malabsorption, especially in patients with pancreatic or biliary dysfunction. In patients who are to be on low-fat diets for prolonged periods, fat-soluble vitamins (A, D, E, K) need to be supplemented. MCTs may be used to substitute for some long-chain triglycerides. MCTs have 6- to 12-carbon fatty acid chains and high aqueous solubility, and do not require bile salts for absorption in the small intestine.182 Unfortunately, most MCT-based products are not very palatable.
Diabetic Diets
These are meant to maintain the blood glucose level as close to normal as possible and also to control serum lipid levels. Calories are provided to maintain a reasonable body weight. Carbohydrates represent 55% to 60% of total calories. Complex unrefined carbohydrates serve as the bulk of the fiber in the diet. Protein represents 12% to 20% of total calories. Fat is restricted because of related cardiovascular disease and should be less than 25% to 30% of total calories.183
PROBIOTICS
Probiotics adhere to the colon mucosa, thereby reducing sites at which pathologic bacteria can attach. Probiotics also may release bacteriocins that are detrimental to pathogenic bacteria, but not to the organisms of the ingested probiotic.184 Probiotics have been shown to decrease the production of inflammatory cytokines, such as tumor necrosis factor-α, interferon-γ, and interleukin-1, and to increase the production of the anti-inflammatory cytokines interferon-α and interleukin-10.185 Probiotics enhance the intestinal barrier function by binding with Toll-like receptors on the epithelial cells and activating protein kinase C, which results in tightening of the tight junctions of the epithelial cells.186
Probiotics mainly have been used as treatment in four areas of gastrointestinal disease: symptomatic diverticular disease and recurrent diverticulitis (see Chapter 117), irritable bowel syndrome (IBS; see Chapter 118), inflammatory bowel disease (see Chapters 111 and 112).
Diverticulitis
The theory behind the prophylactic or active treatment of diverticulitis with probiotics hinges on the concept that an altered microflora in an area of diverticulosis can set up an area of chronic inflammation and that probiotics can be used to alter this flora and reduce the recurrence rate of diverticulitis. One study using the Nissle 1917 strain of E. coli has demonstrated a reduction in diverticulitis episodes in patients with a history of relapsing diverticular disease.187 A study by Tursi and colleagues has compared the relapse rate of symptomatic diverticular disease in 90 patients randomized to mesalamine, Lactobacillus casei, or mesalamine and L. casei. The group receiving the combination of mesalamine and L. casei had significantly fewer recurrences of symptomatic diverticular disease than the group randomized to mesalamine or L. casei alone.188
Irritable Bowel Syndrome
The anaerobic breakdown of carbohydrates and protein by bacteria is known as fermentation. Fermentation is very active in the ascending colon and transverse colon and minimally active in the left colon.189 It is believed that fermentation substrates, especially hydrogen and carbon dioxide gas, are responsible for some of the cramping and pain associated with IBS. In an open label trial, Lactobacillus plantarum DSM9843 was administered to patients with IBS, with a reduction of flatulence and abdominal pain.190 Biopsies of the rectum were able to recover the probiotic in 34% of samples whereas culture of the feces recovered the probiotic in 83% of samples. In another open-label trial, VSL-3 (Lactobacillus, Bifidobacterium, and Streptococcus) was able to reduce pain in 81% of patients with IBS. In a prospective randomized trial, Saggioro evaluated 40 patients with IBS given probiotic A (Lactobacillus plantarum and Bifidobacterium breve), probiotic B (Lactobacillus plantarum and Lactobacillus acidophilus), or placebo.191 Both probiotic combinations A (49%) and B (45%) improved pain compared with placebo (29.5%) at four weeks. These findings were statistically significant.
Inflammatory Bowel Disease
In three controlled trials, capsules containing a nonpathogenic strain of E. coli (Nissle 1917) was shown to be as effective as mesalamine for maintenance of remission in patients with ulcerative colitis (UC).192,193 Total proctocolectomy with ileoanal anastomosis is a common surgical procedure for UC that may be complicated by pouchitis, an inflammatory process of the ileal reservoir. In one study, 40 consecutive patients were randomized to VSL-3 or a placebo immediately after this surgical procedure and treatment was continued for one year.194 Of patients on VSL-3, 19% had an occurrence of pouchitis in the first year following surgery compared with 40% treated with placebo (P < 0.05). More studies of this and other probiotics are indicated in IBD and for the postoperative management of these patients.
Infectious Diarrhea
The rationale for using probiotics in infectious diarrhea is that they act against enteric pathogens by competing for available nutrients and binding sites. They make the intestinal contents acidic, produce a number of chemicals that are bacteriostatic, and increase the specific and nonspecific immune systems. Allen and associates have reviewed the effect of probiotics in 23 studies of infectious diarrhea evaluating 1917 participants,195 and showed that probiotics reduce the risk of developing diarrhea after an initial exposure and reduce the duration of diarrhea in those with infectious diarrhea.
Abou-Abassi S, Craig K, O’Keefe SJD. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: Results of a randomized comparative study. Am J Gastroenterol. 2002;97:2256-62. (Ref 40.)
American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(Suppl):1SA-138SA. (Ref 71.)
Bistrian BR. Jonathan Rhoads lecture. Clinical aspects of essential fatty acid metabolism. JPEN J Parenter Enteral Nutr. 2003;27:168-75. (Ref 19.)
Buchman A. Total parenteral nutrition–associated liver failure. JPEN J Parenter Enteral Nutr. 2002;26(Suppl):S43-8. (Ref 79.)
Crook MA, Hally Panteli JV. The importance of refeeding syndrome. Nutrition. 2001;17:632-7. (Ref 75.)
DeLegge MH. Enteral access: The foundation of feeding. JPEN J Parenter Enteral Nutr. 2001;25(Suppl):S8-13. (Ref 116.)
DeLegge MH, Alsolaiman MM, Barbour E, et al. Short bowel syndrome: Parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007;52:876-92. (Ref 82.)
DeLegge MH, Drake LM. Nutritional assessment. Gastroenterol Clin North Am. 2007;36:1-22. (Ref 9.)
DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578-82. (Ref 64.)
Floch MH, White J. Diverticulitis: New concepts and new therapies. J Clin Gastroenterol. 2005;39:355-6. (Ref 53.)
Garrow D, Pride P, Moran W, et al. Feeding alternatives in patients with dementia: Examining the evidence. Clin Gastro Hep. 2007;5:1372-8. (Ref 131.)
Glynn CC, Greene GW, Winkler MF, Albina JE. Predictive versus measured energy expenditure using limits-of-agreement analysis in hospitalized, obese patients. JPEN J Parenter Enteral Nutr. 1999;23:147-54. (Ref 14.)
McClave SA, DeMeo MT, DeLegge MH, et al. North American summit on aspiration in the critically ill patient: Consensus statement. JPEN J Parenter Enteral Nutr. 2002;26:S80-5. (Ref 97.)
McClave SA, Lukan JK, Stefater JA. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33:324-30. (Ref 169.)
Morrill AC, Chinn CD. The obesity epidemic in the United States. J Public Health Policy. 2004;25:353-66. (Ref 59.)
Preiser JC, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:533-7. (Ref 67.)
Scolapio JS. Effect of growth hormone, glutamine and diet on body composition in short bowel syndrome. JPEN J Parenter Enteral Nutr. 1999;23:309-12. (Ref 34.)
1. Seltzer MH, Slocum BA, Cataldi-Betcher EL, et al. Instant nutritional assessment: Absolute weight loss and surgical mortality. JPEN J Parenter Enteral Nutr. 1982;6:218-21.
2. Roy LB, Edwards PA, Barr LH. The value of nutritional assessment in the surgical patient. JPEN J Parenter Enteral Nutr. 1985;9:170-2.
3. Hickman DM, Miller RA, Rombeau JL, et al. Relation of serum albumin and body weight as predictors of postoperative course in colorectal cancer. JPEN J Parenter Enteral Nutr. 1980;4:314-16.
4. Reinhardt GF, Myscofski JW, Wilkins DB, et al. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J Parenter Enteral Nutr. 1980;4:357-9.
5. Blackburn GL, Bistrian BR, Maini BS, et al. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr. 1977;1:11-22.
6. Pieterse S, Manandhar M, Ismail S. The association between nutritional status and handgrip strength in older Rwandan refugees. Eur J Clin Med. 2002;56:933-9.
7. Charney P. Nutrition assessment in the 1990s: Where are we now? Nutr Clin Pract. 1995;10:131-9.
8. Capra S, Ferguson M, Kristen R, et al. Cancer: Impact of nutrition intervention outcome—nutrition issues for patients. Nutr. 2001;17:769-72.
9. DeLegge MH, Drake LM. Nutritional assessment. Gastroenterol Clin North Am. 2007;36:1-22.
10. Harris JA, Benedict FG. A biometric study of basal metabolism in man. Publication No. 279. 1919, Carnegie Institute, Washington, DC
11. Mifflin MD, St. Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241-7.
12. Daily JM, Heymsfield SB, Head CA, et al. Human energy requirements: Overestimation by widely used prediction equation. Am J Clin Nutr. 1985;42:1170-4.
13. Matarese LE. Indirect calorimetry: Technical aspects. J Am Diet Assoc. 1997;97(Suppl 2):S154-60.
14. Glynn CC, Greene GW, Winkler MF, Albina JE. Predictive versus measured energy expenditure using limits-of-agreement analysis in hospitalized, obese patients. JPEN J Parenter Enteral Nutr. 1999;23:147-54.
15. Fields DA, Kearney JT, Copeland KC. MedGem hand-held indirect calorimetry is valid for resting energy expenditure measurement in healthy children. Obesity. 2006;14:1755-61.
16. Compher C, Hise M, Sternberg A, Kinosian BP. Comparison between MedGem and Deltratrac resting metabolic rate measurements. Eur J Clin Nutr. 2005;59:1136-41.
17. Gray GM. Carbohydrate digestion and absorption. Role of the small intestine. N Engl J Med. 1975;292:1225-30.
18. Crane RK. Hypothesis for the mechanism of intestinal active transport of sugars. Fed Proc. 1962;21:891-5.
19. Bistrian BR. Clinical aspects of essential fatty acid metabolism. Jonathan Rhoads lecture. JPEN J Parenter Enteral Nutr. 2003;27:168-75.
20. Mattson FH, Volpenhein RA. The digestion and absorption of triglycerides. J Biol Chem. 1964;239:2772-7.
21. Chung YC, Kim YS, Shadchehr A, et al. Protein digestion and absorption in human small intestine. Gastroenterology. 1979;76:1415-21.
22. Silk DB, Grimble GK, Rees RG. Protein digestion and amino acid and peptide absorption. Proc Nutr Soc. 1985;44:63-72.
23. Sheikh MS, Santa Ana CA, Nicar MJ, et al. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med. 1987;17:532-6.
24. Brady H, Ryan M, Horgan J. Magnesium: The forgotten cation. Ir Med J. 1987;80:250-3.
25. Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969;49:185-92.
26. Karpel JT, Peden VH. Copper deficiency in long-term parenteral nutrition. J Pediatr. 1972;80:32-40.
27. Brewer GJ, Johnson VD, Dick RD, et al. Treatment of Wilson’s disease with zinc. XVII: treatment during pregnancy. Hepatology. 2000;31:531-2.
28. Alhadeff LC, Gualtieri CT, Lipton M. Toxic effects of water-soluble vitamins. Nutr Rev. 1984;43:33-40.
29. Halstead CH. The intestinal absorption of folates. Am J Clin Nutr. 1979;32:846-55.
30. Olson JA. Recommended dietary intakes (RDI) of vitamin K in humans. Am J Clin Nutr. 1987;45:687-92.
31. Dudrick SJ, Latifi R, Fosnocht DE. Management of the short-bowel syndrome. Surg Clin North Am. 1991;71:625-43.
32. Harris AG. Future medical prospects for sandostatin. Metabolism. 1990;39(Suppl 2):180-5.
33. Byrne TA, Nompleggi DJ, Wilmore DW. Advances in the management of patients with intestinal failure. Trans Proc. 1996;28:2683-90.
34. Scolapio JS. Effect of growth hormone, glutamine and diet on body composition in short bowel syndrome. JPEN J Parenter Enteral Nutr. 1999;3:309-12.
35. Jeppesen PB, Sanguinetti EL, Howard L, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;4:1224-31.
36. Kalfarentzos FE, Karavias DD, Karatzas TM, et al. Total parenteral nutrition in severe pancreatitis. J Am Coll Nutr. 1991;10:156-62.
37. Kudsk KA, Campbell SM, O’Brien T, Fuller R. Postoperative jejunal feedings following complicated pancreatitis. Nutr Clin Pract. 1990;5:14-17.
38. Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe, acute, pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665-9.
39. McClave S, Greene L, Snider H, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild, acute pancreatitis. JPEN J Parenter Enteral Nutr. 1997;24:14-20.
40. Abou-Abassi S, Craig K, O’Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: Results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255-62.
41. Giger U, Stanga Z, DeLegge MH. Management of chronic pancreatitis. Nutr Clin Pract. 2004;19:37-49.
42. Eatock FC, Brombacher GD, Steven A, et al. Nasogastric feedings in severe acute pancreatitis may be practical and safe. Int J Gastrointest Cancer. 2000;8:23-9.
43. Greenburg GR, Fleming CR, Jeejeebhoy KN, et al. Controlled trial of bowel rest and nutritional support in the management of Crohn’s disease. Gut. 1988;29:1309-15.
44. Goldschmid S, Graham M. Trace element deficiencies in inflammatory bowel disease. Gastroenterol Clin North Am. 1989;78:579-87.
45. Malchow H, Steinhardt HJ, Lorenz-Meyer H, et al. Feasibility and effectiveness of a defined-formula diet regimen in treating active Crohn’s disease: European Cooperative Crohn’s’ Disease Study III. Scand J Gastroenterol. 1990;25:235-44.
46. Muller JM, Keller HW, Erasmi H, et al. Total parenteral nutrition as the sole therapy in Crohn’s disease—a prospective study. Br J Surg. 1983;70:40-3.
47. Morgan AG, Kelleher J, Walker BE, et al. Nutrition in cryptogenic cirrhosis and chronic aggressive hepatitis. Gut. 1976;17:113-18.
48. Okuno M, Nagayama M, Takai T, et al. Postoperative total parenteral nutrition in patients with liver disorders. J Surg Res. 1985;39:93-102.
49. Riordan SM, Williams R. Nutrition and liver transplantation. J Hep. 1999;31:955-62.
50. Strate LL, Liu YL, Syngal S, et al. Nuts, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907-14.
51. Brodribb AJM. Treatment of symptomatic diverticular disease with a high-fibre diet. Lancet. 1977;1:664-6.
52. Gary RC. Diet and diverticulosis. BMJ. 1971;2:773-778.
53. Floch MH, White J. Diverticulitis: New concepts and new therapies. J Clin Gastroenterol. 2005;39:355-6.
54. French AB, Cook HB, Pollard HM. Nutritional problems after gastrointestinal surgery. Med Clin North Am. 1969;53:1389-402.
55. Desport JC, Gory-Delabaere G, Blanc-Vincent MP, et al. Standards, options and recommendations for the use of appetite stimulants in oncology. Br J Cancer. 2003;89((Suppl)1):S98-100.
56. Brennan MF. Total parenteral nutrition in cancer patient. N Engl J Med. 1981;305:375-82.
57. Saito H. Parenteral and enteral nutrition in non-operative patients with intestinal obstruction. Japan J Clin Med. 2001;59:S554-7.
58. Iestra JA, Fibbe WE, Zwinderman AH, et al. Parenteral nutrition following intensive cytotoxic therapy: An exploratory study on the need for parenteral after various treatment approaches for haematologic malignancies. Bone Marrow Transplant. 1999;23:933-9.
59. Morrill AC, Chinn CD. The obesity epidemic in the United States. J Pub Health Policy. 2004;25:353-66.
60. Genco A, Bruni T, Doldi SB, et al. BioEnterics intragastric balloon: The Italian experience with 2,515 patients. Obesity Surgery. 2005;15:1161-4.
61. Hampel H, Abraham NS, El Serag HB. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211.
62. Heymsfeld SB, Allison DB, Heska S, et al. Assessment of human body composition. In: Allison DB, editor. Handbook of assessment methods for eating behaviors and weight-related problems. Thousand Oaks, Calif: SAGE Publications; 1995:515.
63. Gould JDC, Beverstein G, Reinhardt S, Garren MJ. Impact of routine and long-term follow-up on weight loss after laparoscopic gastric bypass. Surg Obes Relat Dis. 2007;3:627-30.
64. DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578-82.
65. Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity. 2007;15:1827-40.
66. Baxter JP. Problems of nutritional assessment in the acute setting. Proc Nutr Soc. 1999;58:39-46.
67. Preiser JC, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:533-7.
68. Payne-James JJ, Degara CJ, Grimble GK, et al. Artificial nutrition support in the United Kingdom—1994: Third national survey. Clin Nutr. 1995;14:329-35.
69. Quercia RA, Keating KP. Peripheral parenteral nutrition; an underused nutritional modality. Conn Med. 1997;61:737-40.
70. American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adults and pediatric patients. JPEN J Parenter Enteral Nutr. 1993;17(Suppl):1SA-52SA.
71. American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(Suppl):1SA-138SA.
72. Kudsk K, Heyland DK. Parenteral nutrition in the critically-ill patient: More harm than good. Proc Nutr Soc. 2000;59:457-66.
73. Van den Berghe G. Insulin therapy in the intensive care unit should be targeted to maintain blood glucose between 4.4 mmol/l and 6.1 mmol. Diabetologia. 2005;51:911-55.
74. Malone AM. Permissive underfeeding: Its appropriateness in patients with obesity, patients on parenteral nutrition, and non-obese patients receiving enteral nutrition. Curr Gastroenterol Rep. 2007;9:317-22.
75. Crook MA, Hally Panteli JV. The importance of refeeding syndrome. Nutrition. 2001;17:632-7.
76. Starker PM, LaSala PA, Forse QA, et al. Response to total parenteral nutrition in the extremely malnourished patient. JPEN J Parenter Enteral Nutr. 1985;9:300-2.
77. Chung C, Buchman AL. Postoperative jaundice and total parenteral nutrition-associated hepatic dysfunction. Clin Liv Dis. 2002;6:1067-84.
78. Steiger E, Jonathan E. Rhoads lecture: Experiences and observations in management of patients with short bowel syndrome. JPEN J Parent Enteral Nutr. 2007;31:326-33.
79. Buchman A. Total parenteral nutrition-associated liver failure. JPEN J Parenter Enteral Nutr. 2002;26(Suppl):S43-8.
80. Lee S, Gura KM, Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45:841-5.
81. American Gastroenterological Association. American Gastroenterological Association medical position statement: Short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1105-10.
82. DeLegge MH, Alsolaiman MM, Barbour E, et al. Short bowel syndrome: Parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007;52:876-92.
83. Ingram J, Wetzman S, Greenberg ML, et al. Complications of indwelling venous access lines in the paediatric hematologic patient: A prospective comparison of external venous catheters and subcutaneous ports. Am J Pediatr Hematol Oncol. 1991;13:130-6.
84. DeLegge MH, Borak G, Moore N. Central venous access in the home parenteral nutrition population—you PICC. JPEN J Parenter Enteral Nutr. 2005;29:425-8.
85. Hardway LC. An overview of vascular access devices inserted via the anterolateral vein. J Intravenous Nurs. 1990;13:297-306.
86. Santapari L, Pananisi F, Alfonsi L, et al. Prevention and treatment of implanted central venous catheters (CVC)–related sepsis after six years of home parenteral nutrition (HPN). Clin Nutr. 2002;21:207-11.
87. Dudrick SJ, Latifi R. Total parenteral nutrition. Part II administration, monitoring and complications. Pract Gastroenterol. 1992;7:29-39.
88. Ryder M. Evidence-based practice in the management of vascular access devices for home parenteral nutrition therapy. JPEN J Parenter Enteral Nutr. 2006;30:582-93.
89. Opilla MT, Kirby DF, Edmond MB. Use of ethanol lock therapy to reduce the incidence of catheter-related bloodstream infections in home parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 2007;31:302-5.
90. Smith S, Dawson S, Hennessey R, Andrew M. Maintenance of the patency of indwelling central venous catheters: Is heparin necessary? Am J Pediatr Hematol Oncol. 1991;13:141-3.
91. Steiger E. Dysfunction and thrombotic complications of vascular access devices. JPEN J Parenter Enteral Nutr. 2006;30:565-9.
92. Bader SG, Balke P, Jonkers-Schuitema CF, et al. Evaluation of 6 years of sodium hydroxide to clear partially occluded central venous catheters. Clin Nutr. 2007;26:141-4.
93. Duszak R, Mabry MA. National trends in gastrointestinal access procedures: An analysis of Medicare services provided by radiologists and other specialists. J Vasc Interv Radiol. 2003;14:1031-6.
94. Burtch CD, Shatney CH. Feeding jejunostomy (versus gastrostomy) passes the test of time. Am Surg. 1987;53:54-7.
95. Baskin AIN, Johansen JF. An improved approach to the delivery of enteral nutrition in the intensive care unit. Gastrointest Endosc. 1995;42:161-5.
96. Neumann DA, DeLegge MH. Gastric versus small bowel tube feeding in the intensive care unit. Crit Care Med. 2002;7:1436-9.
97. McClave SA, DeMeo MT, DeLegge MH, et al. North American summit on aspiration in the critically ill patient: Consensus statement. JPEN J Parenter Enteral Nutr. 2002;26:S80-5.
98. Caulfield KA, Page CP, Pestana C. Technique for intraduodenal placement of transnasal enteral feeding catheters. Nutr Clin Pract. 1991;6:23-6.
99. Roubenoff R, Ravich WJ. The technique of avoiding feeding tube misplacement. J Crit Illness. 1989;4:75-9.
100. Cataldi-Belcher El, Selzer MH, Slocumb BA, et al. Complications during enteral nutrition therapy: A prospective study. JPEN J Parenter Enteral Nutr. 1983;7:546-52.
101. Thurlow PM. Bedside enteral feeding tube placement into duodenum and jejunum. JPEN J Parenter Enteral Nutr. 1986;10:104-5.
102. Zaloga GP. Bedside method for placing small bowel feeding tubes in critically ill patients. Chest. 1991;100:1643-6.
103. Ugo PJ, Mohler PA, Wilson GL. Bedside postpyloric placement of weighted feeding tubes. Nutr Clin Pract. 1992;7:284-7.
104. Lord LM, Weiser-Mamone A, Pulhamus M, et al. Comparison of weighted vs unweighted enteral feeding tubes for efficacy or transpyloric passage. JPEN J Parenter Enteral Nutr. 1993;17:271-3.
105. Kittinger JM, Sandler RS, Heizer WD. Efficacy of metoclopramide as an adjunct to duodenal placement of small-bore feeding tubes: A randomized, placebo controlled, double-blind study. JPEN J Parenter Enteral Nutr. 1987;11:33-7.
106. Selfert CS, Cuddy PG, Pemberton B, et al. A randomized trial of metoclopramide’s effects on the transpyloric intubation of weighted feeding tubes. Nutr Supp Serv. 1987;11:11-13.
107. Whatley K, Turner WWJr, Dey M, et al. When does metoclopramide facilitate transpyloric intubation? JPEN J Parenter Enteral Nutr. 1984;8:679-81.
108. Kalafarentzos F, Alivizatos V, Panagopoulos K, et al. Nasoduodenal intubation with the use of metoclopramide. Nutr Supp Sev. 1987;7:33-4.
109. Silva C, Saconato H, Attalah AN. Metoclopramide for migration of naso-enteral tube. Cochrane Database Syst Rev 2004; (4):CD003353.
110. Griffith DP, McNally AT, Battey CH, et al. Intravenous erythromycin facilitates bedside placement of postpyloric feeding tubes in critically ill adults: A double-blind, placebo-controlled study. Crit Care Med. 2003;31:39-44.
111. Berger MM, Bollmann MD, Revelly JP, et al. Progression rates of self-propelled feeding tubes. Int Care Med. 2002;28:1768-74.
112. Gabriel SA, McDaniel B, Ashley DW, et al. Magnetically guided nasoenteral feeding tubes. A new technique. Am Surg. 2001;67:544-9.
113. Baskin WN, Johansen JF. An improved approach to the delivery of enteral nutrition in the intensive care unit. Gastrointest Endosc. 1995;42:161-5.
114. Lovell MA, Mudaliar MY, Klineberg PL. Intrahospital transport of critically ill patients: Complications and difficulties. Anaesth Intens Care. 2001;29:400-5.
115. Patrick PG, Marulenmdra S, Kirby DF, Delegge MH. Endoscopic naso-gastric-jejunal feeding tube placement in critically ill patients. Gastrointest Endosc. 1997;45:72-6.
116. DeLegge MH. Enteral access: The foundation of feeding. JPEN J Parenter Enteral Nutr. 2001;25(Suppl):S8-13.
117. Chang B-S, Hsu P-I, Lo G-H, et al. Clip-assisted endoscopic method for placement of a nasoenteric feeding tube into the distal duodenum. J Formos Med Assoc. 2003;102:514-16.
118. Park RH, Allison MC, Lang J, et al. Randomized comparison of percutaneous endoscopic gastrostomy vs nasogastric feedings in patients with persistent neurological dysphagia. BMJ. 1992;304:1406-9.
119. Gauderer MWL, Ponsky J, Izant RJJr. Gastrostomy without laparotomy: A percutaneous endoscopic approach. J Pediatr Surg. 1980;15:872-5.
120. Jain NK, Larson DE, Schroeder KW, et al. Antibiotic prophylaxis for percutaneous, endoscopic gastrostomy: A prospective randomized, double blind clinical trial. Ann Intern Med. 1987;107:824-8.
121. Hogan RB, DeMarco DC, Hamilton JK, et al. Percutaneous endoscopic gastrostomy-to push or to pull: a prospective, randomized trial. Gastrointest Endosc. 1986;32:253-8.
122. Giordano-Nappi JH, Ishioka S, Maluf-Filho F, et al. A new device for the introducer technique for percutaneous endoscopic gastrostomy placement. Endoscopy. 2007;3:E274-5.
123. Light VL, Siezak FA, Porter JA. Predictive factors for early mortality after percutaneous endoscopic gastrostomy. Gastrointest Endosc. 1995;42:330-5.
124. Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:871-5.
125. DeLegge MH. Percutaneous endoscopic gastrostomy (PEG) placement: Justifying the intervention. UpToDate 2001. Available at http://www.uptodate.com/patients/content/topic.do?topicKey=~55BGAyvklRk6I
126. Kostadima E, Kaditis AG, Alexopoulos EL, et al. Early gastrostomy reduces the rate of ventilator-associated pneumonia in stroke or head injury patients. Eur Respir J. 2005;26:106-1.
127. Dennis MS, Lewis SC, Warlow C. FOOD trial collaboration. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): A multicenter randomized controlled trial. Lancet. 2005;365:764-72.
128. Gillick MR. Rethinking the role of tube feeding in patients with advanced dementia. N Engl J Med. 2000;342:206-10.
129. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: A worse outcome in patients with dementia. Am J Gastroenterol. 2000;95:1472-5.
130. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163:1351-3.
131. Garrow D, Pride P, Moran W, et al. Feeding alternatives in patients with dementia: Examining the evidence. Clin Gastro Hep. 2007;5:1372-8.
132. Langmore SE, Kasarskis EJ, Manca ML, Olney RK. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2006;(4):CD004030.
133. Annoni JM, Vuagnat H, Frischknecht R, Uebelhart D. Percutaneous endoscopic gastrostomy in neurological rehabilitation: A report of six cases. Disabil Rehabil. 1998;20:308-14.
134. Marcuard SP, Finley JL, MacDonald KG. Large-bore feeding tube occlusion by yeast colonies. JPEN J Parenter Enteral Nutr. 1993;17:187-90.
135. Marcuard SP, Stegall KS. Unclogging feeding tubes with pancreatic enzymes. JPEN J Parenter Enteral Nutr. 1990;14:198-200.
136. Safadi BY, Marks JM, Ponsky JL. Percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin North Am. 1998;8:551-568.
137. DeLegge MH, Kirby DF. Enteral nutrition overview. Part 1: Enteral access devices. Nutr Clin Pract. 1992;15:21-6.
138. Schrag SP, Sharma R, Nikhil P, et al. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointest Liv Dis. 2007;216:407-18.
139. Steiner M, Bourges HR, Freedman LS, Gray SJ. Effect of starvation on tissue composition of the small intestine in the rat. Am J Physiol. 1968;215:75-7.
140. Alverdy J, Chi HS, Sheldon GF. The effect of parenteral nutrition on gastrointestinal immunity: The importance of enteral stimulation. Ann Surg. 1985;202:681-4.
141. Adams S, Dellinger EP, Wertz MF, Oreskovich MR, et al. Enteral versus parenteral nutritional support following laparotomy for trauma: A randomized prospective trial. J Trauma. 1986;26:882-91.
142. McClave SA, DeMeo MT, DeLegge MH, et al. North American summit on aspiration in the critically ill patient: Consensus statement. JPEN J Parenter Enteral Nutr. 2002;26(Suppl):S80-5.
143. Jain NK, Larson DE, Schroeder KW, et al. Antibiotic prophylaxis for percutaneous, endoscopic gastrostomy. A prospective, randomized double blind clinical trial. Ann Intern Med. 1987;107:824-8.
144. DeLegge MH, Lantz G, Kazacos E, et al. Effect of external bolster tension on PEG tube tract formation. Gastrointest Endosc. 1996;43:A349.
145. DeLegge MH, DeLegge R, Brady C. External bolster placement after percutaneous endoscopic gastrostomy tube insertion: Is looser better? J Parent Ent Nutr. 2006;20:16-20.
146. Foutch PG, Talbert GA, Waring JP, et al. Percutaneous endoscopic gastrostomy in patients with prior abdominal surgery: Virtues of the safe tract. Am J Gastroenterol. 1988;83:147-50.
147. Wolfsen HC, Kozarek RA, Ball TJ, et al. Tube dysfunction following percutaneous endoscopic gastrostomy and jejunostomy. Gastrointest Endosc. 1990;36:261-3.
148. Kirby DF, DeLegge MH, Fleming RC. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282-301.
149. Faias S, Buck G, DeLegge M. Peritonitis after percutaneous endoscopic gastrostomy and jejunostomy: Where there is smoke, there may not be fire. Endoscopy. 2006;38:745-8.
150. DeLegge MH, Patrick P, Gibbs R. Percutaneous endoscopic gastrojejunostomy with a tapered tip, nonweighted jejunal feeding tube: Improved placement success. Am J Gastroenterol. 1996;91:1130-4.
151. Baskin WN, Johansen JF. An improved approach to the delivery of enteral nutrition in the intensive care unit. Gastrointest Endosc. 1995;42:161-5.
152. Adler DG, Gostout CJ, Baron TH. Percutaneous transgastric placement of jejunal feeding tubes with an ultrathin endoscope. Gastrointest Endosc. 2002;55:106-10.
153. Meyers JG, Page CP, Stewart RM, et al. Complications of needle catheter jejunostomy in 2022 consecutive applications. Am J Surg. 1995;170:547-50.
154. Holmes JH, Brundage SI, Yeun PC, et al. Complications of surgical feeding jejunostomy in trauma patients. J Trauma Infect Crit Care. 1997;47:1009-12.
155. Gore DC, DeLegge MH, Gervin A, et al. Surgically placed gastro-jejunostomy tubes have fewer complications as compared to feeding jejunostomy tubes. J Am Coll Nutr. 1996;15:144-6.
156. Shike M, Berner YN, Gerdes H, et al. Percutaneous endoscopic gastrostomy and jejunostomy for long-term feeding in patients with cancer of the head and neck. Otolaryngol Head Neck Surg. 1989;101:549-54.
157. Shike M, Latkany L, Gerdes H, et al. Direct percutaneous endoscopic jejunostomies for enteral feeding. Gastrointest Endosc. 1996;44:536-40.
158. Varadarajulu S, DeLegge M. Use of a 19-gauge needle as a direct guide for percutaneous endoscopic jejunostomy (DPEJ) tube placement. Gastrointest Endosc. 2003;57:942-5.
159. Fan AC, Baron TH, Rumalla A, et al. Comparison of direct percutaneous endoscopic jejunostomy and PEG with jejunal extension. Gastrointest Endosc. 2002;56:890-4.
160. Steigman GV, Goff JS, Silas D, et al. Endoscopic versus operative gastrostomy: Final results of a prospective, randomized trial. Gastrointest Endosc. 1990;36:1-5.
161. Scott JS, De La Torre RA, Unger SW. Comparison of operative versus percutaneous endoscopic gastrostomy tube placement in the elderly. Am Surg. 1991;57:338-40.
162. Munro JC. Abdominal surgery. In: Keen WW, editor. Keen’s surgery. London: WB Saunders; 1908:937-8.
163. Gerndt SJ, Orringer MB. Tube jejunostomy as an adjuvant to esophagectomy. Surgery. 1994;115:164-9.
164. Meyers JG, Page CP, Stewart RM. Atlas of nutrition support techniques. Boston: Little Brown; 1989. p 167
165. Preshaw RM. A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet. 1981;152:659-60.
166. Ho CS. Percutaneous gastrostomy for jejunal feeding. Radiology. 1983;149:595-6.
167. Ho CS, Young EY. Percutaneous gastrostomy and transgastric jejunostomy. AJR Am J Roentgenol. 1992;158:251-7.
168. Laasch H-U, Wilbraham L, Bullen K, et al. Gastrostomy insertion: Comparing the options—PEG, RIG or PIG? Clin Radiol. 2003;58:398-405.
169. McClave SA, Lukan JK, Stefater JA. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33:324-30.
170. DeLegge MH. Enteral nutrition and gastrointestinal intolerance. When should we be concerned? Clin Nutr High. 2006;2:2-8.
171. Brown DN, Miedema BW, King PD, et al. Safety of early feeding after percutaneous endoscopic gastrostomy. J Clin Gastroenterol. 1995;21:330-1.
172. Kudsk KA, Moore FA, DeLegge MH, et al. Consensus recommendations from the U.S. summit on immune-enhancing enteral therapy. JPEN J Parenter Enteral Nutr. 2001;25:S61-3.
173. Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325-33.
174. DeLegge MH, Kirby DF. Enteral nutrition overview. Part 2: Enteral feedings. Formula, delivery and complications. Pract Gastroenterol. 1992;16:32-44.
175. Edes TE, Walk BE, Austin JL. Diarrhea in tube-fed patients: Feeding formula not necessarily the cause. Am J Med. 1990;88:91-3.
176. Shimoni Z, Averbuch Y, Shir E, et al. The addition of fiber and the use of continuous infusion decrease the incidence of diarrhea in elderly tube-fed patients in medical wards of a general regional hospital: A controlled clinical trial. J Clin Gastroenterol. 2007;41:901-5.
177. Nakaji S, Umeda T, Kumae T, et al. A tube-fed liquid formula diet containing dietary fiber increased stool weight in bed-ridden elderly patients. Nutrition. 2004;20:955-60.
178. Fleisher D, Sheth N, Kou JH. Phenytoin interaction with enteral feedings administered through nasogastric tubes. JPEN J Parenter Enteral Nutr. 1990;14:513-16.
179. Bravata DM, Sanders L, Huang J, et al. Efficacy and safety of low-carbohydrate diets: A systemic review. JAMA. 2003;289:1837-50.
180. Lewis SJ, Egger M, Sylvester A, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: Systemic review and meta-analysis of controlled trials. BMJ. 2001;323:773-6.
181. Kramer P. The prognosis of high- and low-residue diets. Gastroenterology. 1964;47:648-54.
182. Hubbard US, McKenna MC. Absorption of safflower oil and structured lipid preparations in patients with cystic fibrosis. Lipid. 1987;22:424-8.
183. Rosenstrock J, Strowig S, Cercone S, et al. Restriction in cardiovascular risk factors with intensive diabetes treatment in insulin-dependent diabetes mellitus. Diabetes Care. 1987;10:729-34.
184. Pothoulakis C, Kelly CP, Joshi MA, et al. Saccaromyces boulardii inhibit clostridium difficle toxin A binding and enterotoxicity in the rat ileum. Gastroenterology. 1993;104:1108-15.
185. Gill HS, Rutherford KJ, Prasad J, et al. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN01), LactobacIllus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000;83:167-76.
186. Madsen K, Cornish A, Soper P, et al. Probiotic bacterian enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-91.
187. Frick P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol. 2003;15:313-15.
188. Tursi A, Brandimarte G, Elisei W, et al. Mesalamine and/or Lactobacillus: Case for maintaining remission of symptomatic diverticular disease of the colon; a prospective, randomized study. Dig Liver Dis. 2005;37:520-2.
189. Cummings JH, Englyst HN. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45:1243-55.
190. Nobaek S, Johanssen ML, Molin G, et al. Alteration of intestinal microflora is associated with a reduction of abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-8.
191. Sagioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004;38:S104-6.
192. Kruis W, Fric P, Stolte M. The Multiflor Study Group. Maintenance of remission in ulcerative colitis is equally effective with Escherichia coli Nissle 1917 and with standard mesalamine. Gastroenterology. 2001;120:680A.
193. Renbacken BL, Scnelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalamine in the treatment of ulcerative colitis: a randomized trial. Lancet. 1999;354:635-9.
194. Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-9.
195. Allen SJ, Okoko B, Martinez E, et al. Probiotics for the treatment of infectious diarrhea. Cochrane Database Sys Rev 2004; (4):CD003048.