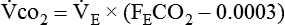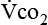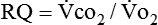Nutrition Assessment
After reading this chapter, you will be able to:
1. Recognize how nutrition and respiration and pulmonary status are interrelated.
2. Recognize the functional importance of oxygen in nutrition.
3. Identify the nutritional significance of measuring oxygen uptake.
4. Identify the value of determining the resting energy expenditure.
5. Recognize how starvation affects the following:
b. Muscle mass (diaphragm and other respiratory musculature)
c. Forced vital capacity, forced expiratory volume in 1 second, and diffusing capacity of the lung for carbon dioxide
6. Recognize how some respiratory treatment modalities may inhibit the nutritional status of patients.
7. Identify the by-products of anaerobic (without oxygen) metabolism.
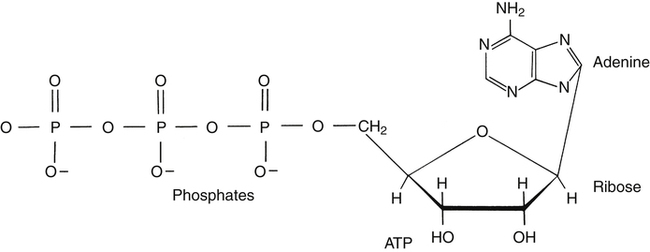
8. Identify oxygen’s importance in terms of adenosine triphosphate production.
9. Recognize how fat, carbohydrate, and protein metabolism affect the respiratory quotient.
10. Recognize the daily nutritional requirements for carbohydrate, protein, and fat.
11. Identify the protein requirements for normal and severely catabolic patients.
12. Recognize the significance of measuring nitrogen balance.
13. Recognize the problems associated with a low-protein diet.
14. Recognize the advantages and disadvantages of a high-carbohydrate diet in regard to the pulmonary system.
15. Identify the importance of vitamins and minerals in respiratory function.
16. Recognize the methods available for meeting nutritional requirements and their advantages and disadvantages.
17. Recognize the methods for assessing nutritional status.
18. Identify the role of the respiratory therapist in nutritional assessment in relation to inspection, auscultation, and laboratory and pulmonary function findings.
The pulmonary system has a synergistic relationship with nutrition throughout life, beginning with the fetus and extending through adulthood. Although nutritional status and pulmonary function are interdependent, a healthy pulmonary system supports the body through its ability to obtain oxygen needed for cellular demands of the metabolism of the three macronutrients: carbohydrates, proteins, and lipids. Provision of adequate nutrition to maintain optimal nutritional status assists in ensuring growth and development of the pulmonary system, including its supporting structures. The skeletal and respiratory muscles, as well as the nervous system and immune system, are supported and maintained through optimal nutrition. A person’s nutritional status and ability to metabolize carbohydrates, proteins, and fats is directly related to a healthy pulmonary system.1
Malnutrition and the Pulmonary System
Malnutrition adversely affects the structure, elasticity, and function of the lungs as well as the mass, strength, and endurance of muscles involved in the respiration process.2–4 Malnutrition-altered lung function includes decreased strength, power, and endurance of respiratory muscles and increased respiratory muscle fatigue. In addition, skeletal muscle relaxation slows, and muscle mass is diminished due to specific reduction in muscle fiber size and type. During starvation or malnutrition, respiratory muscles and skeletal muscles are subject to catabolism, providing energy to the body. The resultant reduction in the mass of the diaphragm, diminished inspiratory and expiratory muscle strength, and decreased vital capacity and endurance result in impaired pulmonary function.4–9 Inadequate nutrition or an increase in energy needs can result in malnutrition, leading to alterations in pulmonary muscle function.
Within days, protein deficits in the diet result in a decline in respiratory muscle function.7 Low levels of proteins in the blood (hypoalbuminemia) contribute to pulmonary edema as colloid osmotic pressure is decreased, allowing a fluid shift into the interstitial space. Extravascular lung water increases, resulting in a decrease in functional residual capacity and pulmonary reserve. Low serum albumin levels can result in an increase in extracellular fluid volume and a reduction in the intracellular space.7,9 Surfactant provides the low surface tension at the air-liquid interface, preventing the atelectasis, alveolar collapse, alveolar flooding, and severe hypoxia that result in respiratory distress. Even short periods of starvation result in a decreased synthesis and secretion of surfactant.10 Reduced surfactant, which is synthesized from proteins and phospholipids, contributes to the collapse of alveoli, resulting in an increased effort of breathing. Airway mucus is composed of glycoproteins, water, and electrolytes. Malnutrition results in depletion of liver and muscle glycogen and energy-rich compounds used to provide cellular energy, resulting in metabolic and muscular endurance dysfunction. This reduction in respiratory muscle function often coexists with increased energy requirements, resulting in a deterioration of gas exchange and an increased work of breathing, which can lead to pulmonary failure.7,9
Micronutrients consistent of vitamins and minerals. Micronutrient imbalances and deficiencies can affect pulmonary function in several ways. Iron deficiency can result in low hemoglobin levels, thus reducing the oxygen-carrying capacity of the blood. Low levels of other micronutrients, such as potassium, phosphorus, calcium, and magnesium, affect cellular processes. Collagen, composing the supporting connective tissue of the lungs, requires vitamin C for synthesis. Low levels of phosphorus result in neuromuscular dysfunction and can exacerbate pulmonary failure. A reduction in 2,3-diphosphoglycerate (2,3-DPG) in the red blood cells due to reduced phosphorus levels decreases oxygen delivery to tissues and decreases the contractibility of respiratory muscles. Muscle strength is reduced in the presence of magnesium deficiency.7 Deficiencies in vitamin A, pyridoxine, and zinc may impair immune status and increase risk for pulmonary infections.5,11 Antioxidant nutrients, including vitamins A, C, and E and flavonoids, have been reviewed for their relationship to the pathogenesis or exacerbations in patients with COPD. Reduced serum or tissue levels of antioxidant vitamins were found in people with COPD; however, other studies did not show significant effects.12 Malnutrition has been found to be an independent predictor of higher morbidity and mortality in respiratory disease.4
Interdependence of Respiration and Nutrition
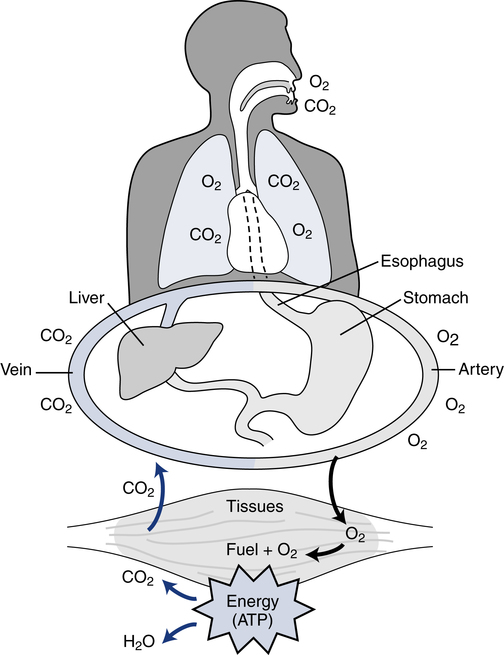
Respiration and nutrition are interdependent (Fig. 18-1). Air and food share common pathways during ingestion and then separate only briefly during “digestion,” with air going to the lungs for distribution and food to the stomach and intestinal tract for digestion and absorption. Oxygen and nutrients then combine in the blood and are distributed to the tissues of the body. The use of food for energy at the cellular level requires oxygen to support a controlled combustion process that produces energy molecules of adenosine triphosphate (ATP), which are used in all of the body processes for energy (Fig. 18-2).
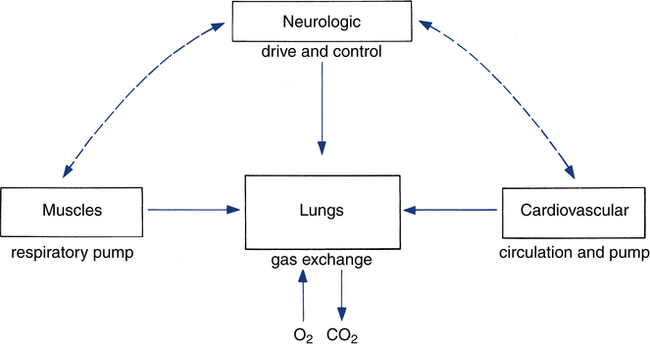
Titrating the proper amount of oxygen and eliminating carbon dioxide (the metabolic “smoke” of the combustion process) is the job of the respiratory system, coupled closely with the cardiovascular system. The respiratory system must be sensitive to the metabolic needs of the entire body. This process requires the integration of several organ systems. The respiratory system consists of neurologic components, cardiovascular components, respiratory muscles, and lungs (Fig. 18-3).
The metabolic rates of the tissues dictate the amount of oxygen needing to be picked up in the lungs. Oxygen uptake (< ?xml:namespace prefix = "mml" />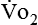 ) is a respiratory factor that can be measured in the laboratory or at the bedside using specialized equipment. Nutritionally speaking, it is this measure that indicates the patient’s energy requirement. If
) is a respiratory factor that can be measured in the laboratory or at the bedside using specialized equipment. Nutritionally speaking, it is this measure that indicates the patient’s energy requirement. If 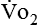 is measured while a person is in a resting, nonstressed state, the basal metabolic rate (BMR) or basal energy expenditure (BEE) can be calculated. The BEE is the measure obtained when a person is at absolute rest with no physical movement, which is not clinically possible in the hospitalized patient. The term resting energy expenditure (REE) is used when a person is at rest upon waking. REE is the measurement used in hospitalized patients and is about 10% higher than BEE13 because measurements are done when the patient is awake and at rest but not at basal conditions. A variety of predictive equations have been developed for use in estimating REE. For the purposes of predicting energy needs through predictive equations in nonobese critically ill patients when indirect calorimetry is not available, four equations were considered precise and unbiased in this population. In patients younger than 60 years and in those older than 60 years, respectively, the equations and their accuracy are as follows: Penn State Equation (69%, 77%), Brandi equation (61%, 61%), Mifflin-St. Jeor equation × 1.25 (54%, 54%), and Faisy equation (65%, 37%). The Penn State equation was validated in 2009 and is calculated as follows14:
is measured while a person is in a resting, nonstressed state, the basal metabolic rate (BMR) or basal energy expenditure (BEE) can be calculated. The BEE is the measure obtained when a person is at absolute rest with no physical movement, which is not clinically possible in the hospitalized patient. The term resting energy expenditure (REE) is used when a person is at rest upon waking. REE is the measurement used in hospitalized patients and is about 10% higher than BEE13 because measurements are done when the patient is awake and at rest but not at basal conditions. A variety of predictive equations have been developed for use in estimating REE. For the purposes of predicting energy needs through predictive equations in nonobese critically ill patients when indirect calorimetry is not available, four equations were considered precise and unbiased in this population. In patients younger than 60 years and in those older than 60 years, respectively, the equations and their accuracy are as follows: Penn State Equation (69%, 77%), Brandi equation (61%, 61%), Mifflin-St. Jeor equation × 1.25 (54%, 54%), and Faisy equation (65%, 37%). The Penn State equation was validated in 2009 and is calculated as follows14:
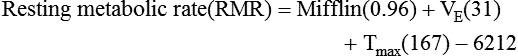
Energy production generates heat, and heat is measured in calories. Direct calorimetry directly measures the heat given off by the body in a carefully designed room. This measurement is not practical in clinical settings and cannot be used with compromised patient populations. Indirect calorimetry is the method most commonly used in clinical environments. Indirect calorimetry is the calculation of energy expenditure using measured respiratory parameters of oxygen consumption (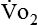 ) and carbon dioxide production (
) and carbon dioxide production (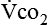 ).
).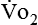 and
and 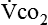 require precise measurements of inspired and expired gas concentrations and volume.
require precise measurements of inspired and expired gas concentrations and volume. 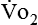 and
and 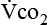 are converted to energy expenditure through the application of the abbreviated Weir equation13,16,17:
are converted to energy expenditure through the application of the abbreviated Weir equation13,16,17:
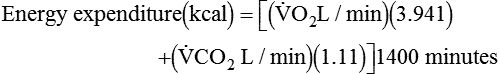
where 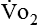 and
and 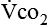 are expressed in liters per minute, and 1440 is the number of minutes in a day.
are expressed in liters per minute, and 1440 is the number of minutes in a day.
Because oxygen is not stored in the body, measuring oxygen uptake (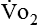 ) correlates directly with energy (ATP) creation and use. Metabolism (REE) then can be measured by oxygen consumption and is directly related to the energy (calories) used. Indirect calorimetry may be clinically beneficial in the identification of patients who will not be able to sustain spontaneous ventilation because of excessive pulmonary work.18 The work of breathing is attributable to about 2% to 3% of the REE in the normal adult; however, in the pulmonary-compromised patient, as much as 25% of the REE can be attributed to the work of breathing.19 The respiratory quotient (RQ) (
) correlates directly with energy (ATP) creation and use. Metabolism (REE) then can be measured by oxygen consumption and is directly related to the energy (calories) used. Indirect calorimetry may be clinically beneficial in the identification of patients who will not be able to sustain spontaneous ventilation because of excessive pulmonary work.18 The work of breathing is attributable to about 2% to 3% of the REE in the normal adult; however, in the pulmonary-compromised patient, as much as 25% of the REE can be attributed to the work of breathing.19 The respiratory quotient (RQ) (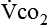 /
/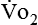 ) is also obtained from indirect calorimetry, which is used in the interpretation of net substrate use and as an indicator of test validity. The normal RQ range in humans is 0.67 to 1.2.17 Because energy measurements by indirect calorimetry are respiratory measurements, the RT is one of the members of the patient care team who commonly performs these measures in clinical settings. The RT who is trained to perform indirect calorimetry measurements is an important contributor to the assessment of nutritional needs of patients.
) is also obtained from indirect calorimetry, which is used in the interpretation of net substrate use and as an indicator of test validity. The normal RQ range in humans is 0.67 to 1.2.17 Because energy measurements by indirect calorimetry are respiratory measurements, the RT is one of the members of the patient care team who commonly performs these measures in clinical settings. The RT who is trained to perform indirect calorimetry measurements is an important contributor to the assessment of nutritional needs of patients.
Indirect calorimetry measurements are usually performed using a metabolic cart, although different types of calorimeters exist. A metabolic cart is a computer-controlled unit composed of oxygen and carbon dioxide gas analyzers and flow transducers. The cart automatically measures patients’ airflow and expiratory volumes, applies correction factors, and prints out and graphs the results. Different types of gas collection devices, such as a facemask, mouthpiece with nose clip, or canopy, can be used as long as rigorous control is used and no air leaks occur.14
1. Collect expired gas in a bag or Tissot spirometer for several minutes.
2. Analyze for carbon dioxide and oxygen (or oxygen and nitrogen).
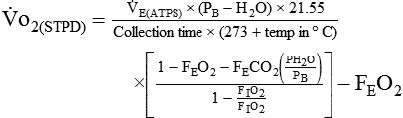
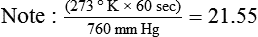
If gas is analyzed with desiccant (a drying agent) in line, then
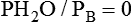
where 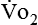 (STPD) = oxygen uptake, standard conditions
(STPD) = oxygen uptake, standard conditions
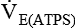 = minute volume; ambient temperature and pressure, saturated
= minute volume; ambient temperature and pressure, saturated
FEo2 = fraction of expired oxygen
In nutrition, energy is quantified in terms of kilocalories (kcal); 1 kcal is the amount of energy it takes to raise the temperature of 1 kg of water 1° C. (Although kilocalories have been used most frequently in clinical nutrition, the kilojoule [kJ] is often used in research because kJ is the international unit for energy. To convert kcal to kJ, multiply kcal by 4.184.) For approximately every 5 kcal burned, 1 L of oxygen is used by the tissues. Therefore, if a patient’s 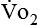 is measured as 300 mL oxygen/minute, then 300 mL oxygen × 60 minutes × 24 hours equals 432 L of oxygen required per day and 5 kcal × 432 L oxygen/day equals 2160 kcal/day that should be given to the patient. If less than this amount of energy is given, the patient must use body energy stores (glycogen, adipose tissue, and lean muscle mass), which are often already depleted in chronically ill patients. The abbreviated Weir equation is used for more precise conversion of
is measured as 300 mL oxygen/minute, then 300 mL oxygen × 60 minutes × 24 hours equals 432 L of oxygen required per day and 5 kcal × 432 L oxygen/day equals 2160 kcal/day that should be given to the patient. If less than this amount of energy is given, the patient must use body energy stores (glycogen, adipose tissue, and lean muscle mass), which are often already depleted in chronically ill patients. The abbreviated Weir equation is used for more precise conversion of 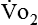 to kilocalories,16 as follows:
to kilocalories,16 as follows:
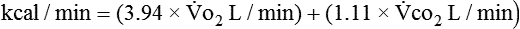
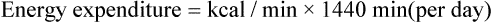
Nutritional Depletion and Respiration
Measures of REE are consistently higher in malnourished emphysematous patients.20 This increased REE leads to nutritional depletion and eventually malnutrition. Malnutrition can exacerbate symptoms of COPD by decreasing respiratory muscle strength and exercise tolerance and can compromise immune function, leading to increased respiratory infections. Energy expenditure is usually elevated related to pulmonary complications, including the degree of airway obstruction and resultant increased work of breathing.21 Respiratory inflammation, carbon dioxide retention, gas diffusing capacity, and other mediators, including hormones and cytokines, affect energy expenditure. In addition, insufficient absorption of some nutrients may lead to muscle wasting and malnutrition. Adequate protein intake is needed to maintain or restore lung and muscle strength in these patients.21 COPD presenting with chronic hypoxia and oxidative stress could be responsible for the catabolic state seen in these patients. Systemic inflammation, anorexia, and muscle dysfunction may all relate to the hypoxia. Correction of the hypoxia by oxygen supplementation seems to allow weight gain and in the short term improve exercise tolerance.22,23 This improvement is usually not sustained because the underlying metabolic increase is not reversed, and the patient’s appetite is not improved.
If an increased amount of food is consumed, weight can begin to normalize, but emphysematous patients are not comfortable eating larger quantities of foods. In one study, it was necessary to increase intake above 140% of the BMR before improvement of the nutritional status of COPD patients was achieved.24 If not continuously encouraged to do so, patients typically return to eating their normal amount, which is insufficient to maintain a normal weight. In addition, patients with chronic protein energy malnutrition (PEM), also known as protein-calorie malnutrition (PCM), experience higher morbidity and mortality rates. With loss of body protein, there is a subsequent loss not only of muscle and various enzyme systems but also of immunoglobulins (IgA, IgG, and IgM). Thus, susceptibility to respiratory infections is increased because of decreased immunocompetence.
Therapeutic Interactions of Respiration and Nutrition
The respiratory response to the body’s need for oxygen and carbon dioxide elimination is usually regulated by the carbon dioxide produced (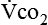 ). At the oxygen sensor level, increased hydrogen ion (H+) concentration in addition to carbon dioxide drives ventilation; this occurs when the amount of oxygen present is insufficient with respect to metabolic need, resulting in lactic acidosis. Oxygen levels, when low enough, become an important stimulus for breathing. A semistarved state can decrease hypoxic drive,25 compromising a patient even further.
). At the oxygen sensor level, increased hydrogen ion (H+) concentration in addition to carbon dioxide drives ventilation; this occurs when the amount of oxygen present is insufficient with respect to metabolic need, resulting in lactic acidosis. Oxygen levels, when low enough, become an important stimulus for breathing. A semistarved state can decrease hypoxic drive,25 compromising a patient even further.
Respiratory System and Nutritional Needs
For optimal ventilatory function, proper nutrition is needed for all components of the respiratory system (see Fig. 18-3).
Neurologic Component
The neurologic component drives and controls ventilation. The higher the 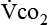 level, the greater the blood carbon dioxide concentration and therefore the greater the stimulus to the chemoreceptors.
level, the greater the blood carbon dioxide concentration and therefore the greater the stimulus to the chemoreceptors. 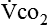 is increased in the body by metabolism, buffering of fixed acids, or both. This in turn increases the electrical activity in the respiratory centers of the central nervous system (CNS), resulting in increased minute ventilation. The nervous system’s fundamental requirement is for glucose. The energy derived from glucose is used to maintain an electrical charge across the nerve cell membrane, allowing for depolarization (action potential) and subsequent repolarization. The neurotransmitters at the synaptic ends are amino acids or derivatives of them, and their presence is necessary for the relay of information from one neuron to another and from nerve to muscle. Apparently, the sensitivity of the respiratory centers (either peripheral or central chemoreceptors) is affected by the amount and quality of protein ingested. The respiratory response to carbon dioxide or low levels of oxygen is increased with high protein intake.26 However, too much protein may make some patients too sensitive to gas partial pressure changes, thus increasing the work of breathing. Giving the optimal amount of protein is the task of the nutritional support team.
is increased in the body by metabolism, buffering of fixed acids, or both. This in turn increases the electrical activity in the respiratory centers of the central nervous system (CNS), resulting in increased minute ventilation. The nervous system’s fundamental requirement is for glucose. The energy derived from glucose is used to maintain an electrical charge across the nerve cell membrane, allowing for depolarization (action potential) and subsequent repolarization. The neurotransmitters at the synaptic ends are amino acids or derivatives of them, and their presence is necessary for the relay of information from one neuron to another and from nerve to muscle. Apparently, the sensitivity of the respiratory centers (either peripheral or central chemoreceptors) is affected by the amount and quality of protein ingested. The respiratory response to carbon dioxide or low levels of oxygen is increased with high protein intake.26 However, too much protein may make some patients too sensitive to gas partial pressure changes, thus increasing the work of breathing. Giving the optimal amount of protein is the task of the nutritional support team.