Chapter 34 Nutrition and Disorders of Gastrointestinal and Hepatic Function
Serious gastrointestinal complications are rare following cardiac surgery; the overall incidence is about 1%.1–3 However, when they do occur, they result in prolonged stays in the intensive care unit (ICU) and hospital, increased treatment costs, and high mortality rates. Early diagnosis is essential to prevent adverse outcomes but may be difficult in critically ill patients in whom the signs of intraabdominal disease are masked by sedation, mechanical ventilation, and cardiac disease. In patients with unexpected multiorgan dysfunction, an intraabdominal cause should always be suspected. Aggressive abdominal investigation and early consultation with a gastrointestinal surgeon are recommended because early laparoscopy or laparotomy may be lifesaving.
NUTRITION
Critically ill patients have increased metabolic rates and undergo accelerated protein catabolism. Fever, sepsis, catecholamines, trauma, and major surgery all increase energy expenditure, whereas hypothermia, inactivity, sedation, neuromuscular paralysis, and β-adrenergic blocking drugs reduce it. Under the influence of high levels of cortisol and catecholamines and of reduced insulin effect (see Chapter 2), there is increased release of amino acids from skeletal muscle. These amino acids are used largely as substrate for hepatic gluconeogenesis, with the nitrogen being released as urea and excreted by the kidneys. In highly catabolic patients, urinary nitrogen losses can exceed 30 g/day, which corresponds to about 200 g/day of protein mass. Although nutritional support does not prevent enhanced protein catabolism, it does reduce net protein loss; that is, feeding increases protein synthesis but does not prevent catabolism. After routine cardiac surgery that includes hypothermic cardiopulmonary bypass, nitrogen losses are usually modest and similar to those observed in the nonstressed fasting state.4
Nutrition Requirements
In some critically ill patients, energy expenditure is greatly increased. For instance, values in excess of 40 kcal/kg/day have been reported in patients with trauma and sepsis.5 By contrast, energy requirements are not typically significantly elevated following routine cardiac surgery6 and in other forms of critical illness.7 Furthermore, high energy nutrition (>30 to 35 kcal/kg/day), particularly when administered parenterally, can lead to metabolic complications.8 Excess carbohydrate administration can cause hyperglycemia, hyperlipidemia, and the accumulation of fat in the liver. Hyperglycemia exacerbates hypercarbic respiratory failure and is associated with an increased risk of postoperative infection, particularly of sternal wounds.9,10 Excess protein administration exacerbates azotemia and may precipitate uremic encephalopathy and coagulopathy. It can also cause hypertonic dehydration and metabolic acidosis, particularly when urine-concentrating ability is impaired. Excess fat administration can cause hyperlipidemia and may predispose to infection.
In an individual patient, energy requirements may be estimated using weight-based empirical formulas, such as the Harris-Benedict11 or Schofield equations,12 or they may be measured using indirect calorimetry, in which energy expenditure is calculated on the basis of measured oxygen consumption and carbon dioxide production. However, these calculations are not usually necessary; for most patients, initial administration of 25 kcal/kg/day (about 100 kJ/kg/day) is adequate.13,14 Indirect calorimetry to rule out overfeeding is useful in patients who are difficult to wean from mechanical ventilation. It should also be performed in patients who require prolonged nutritional support because 25 kcal/kg/day may be inadequate late in the clinical course.14 Indirect calorimetry is easily performed at the bedside.
Energy is provided as a combination of nitrogen compounds (amino acids, proteins), carbohydrates (glucose, oligosaccharides, polysaccharides), and fat (fatty acids, triglycerides). Administration of 1.2 to 1.5 g/kg/day of protein is recommended13; this corresponds to 0.2 to 0.24 g/kg/day of nitrogen, or 15% to 20% of total calories. Lower levels of nitrogen (e.g., 0.15 g/kg/day) should be administered in patients with renal dysfunction (not requiring renal replacement therapy) or hepatic dysfunction, and higher levels (0.3 g/kg/day) may be given to severely catabolic patients. Fat should constitute about 20% to 30% of calories, with the remaining provided as carbohydrate.13 Fat should not exceed 2.5 g/kg/day and carbohydrate should not exceed 6 g/kg/day. Energy density is greatest with fat (~9 kcal/g) compared with carbohydrate and protein, which are both about 4 kcal/g. Nutritional support should also contain micronutrients: vitamins, minerals, and trace elements.15 Potassium, magnesium, and phosphate should be provided in sufficient quantities to maintain normal plasma levels.
Enteral Versus Parenteral Nutrition
Nutrition may be given enterally or parenterally. Enteral nutrition may be delivered into the stomach via a nasogastric tube or postpylorically into the small bowel via a nasojejunal tube. Enteral nutrition has the advantages of being simpler and cheaper than parenteral nutrition, and it is associated with fewer infectious complications in certain patient populations16–18 (although this has not been studied specifically in cardiac surgery patients). The reduction in infectious complications may be the result of the immunosuppressive effects of parenteral nutrition or because enteral nutrition may protect against the translocation of gastrointestinal microorganisms into the systemic circulation. Enteral nutrition also results in improved tolerance of carbohydrates compared with parenteral nutrition, with a reduced likelihood of developing hyperglycemia and its attendant complications.
Some clinicians consider enteral nutrition to be contraindicated in patients with severe circulatory compromise because of the potential for developing splanchnic oxygen supply-demand mismatch, in which the increased metabolic requirements of gut cannot be met by increased blood flow—a situation that could potentially cause mesenteric ischemia. However, in practice, this concern does not appear to be justified.19 Although gastric stasis is common, postpyloric delivery of enteral nutrition results in splanchnic vasodilation and nutrient absorption, even in postoperative cardiac patients with circulatory compromise.20–22 However, underfeeding is a possibility and caloric intake should be carefully monitored.22
Patients with persistent intolerance of enteral nutrition or gastrointestinal failure (e.g., due to small bowel ileus or mechanical obstruction) should receive parenteral nutrition. There may be a survival benefit in critically ill patients for early parenteral nutrition over delayed enteral nutrition.17 Combined support with enteral and parenteral nutrition may be considered in patients who are unable to achieve nutritional goals by means of enteral nutrition alone.
Enteral Nutrition
Composition
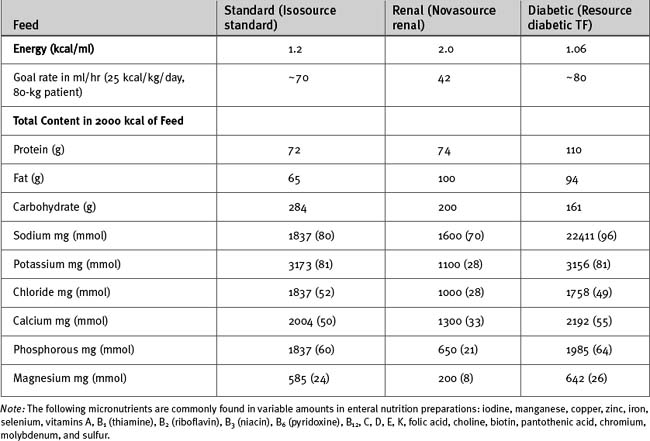
Most commercially available enteral feeds have an energy content of 1 to 2 kcal/ml; a value of 1.0 to 1.2 kcal/ml is typical (Table 34-1). Thus, a 70-kg patient receiving 25 kcal/kg/day has an obligatory fluid load of 1 to 2 l/day. Nitrogen is administered as protein (e.g., a soy isolate), carbohydrate as polysaccharides (e.g., corn syrup or hydrolyzed corn starch), and fat as triglycerides (e.g., canola oil). Micronutrients are added in appropriate quantities. Fiber and electrolytes are present in variable amounts.
A wide variety of different enteral feeds are available for use in specific circumstances; only some of them are clinically useful. Elemental feeds, in which the nitrogen content is provided as amino acids, are useful in patients with short bowel syndrome, pancreatic insufficiency, and radiation enteritis, but they offer no advantage in the critically ill. Renal formulations that are calorie dense (typically 2 kcal/ml) and low in volume, sodium, and potassium are available. They are useful in patients with oliguric renal failure but should not be used in lieu of renal replacement therapy when it is indicated (see Chapter 33). Formulations for patients with diabetes, in which the carbohydrate content is reduced and the protein and fat content is increased, are available. However, the high protein content may be inappropriate for patients with renal dysfunction, and hyperglycemia is best managed by intravenous insulin rather than by carbohydrate restriction. Thus, diabetic formulations are infrequently used in the ICU. The use of so-called immune-enhancing feeds is controversial. Guidelines do not support the use of arginine-enriched feeds, but they offer limited support for the use of feeds supplemented by glutamine (for patients with burns and trauma) and by fish oils, borage oils, and antioxidants (for patients with acute respiratory distress syndrome).16 Formulations that are high in fat (50% of calories) and low in carbohydrate and sodium are available for use in patients with acute respiratory distress syndrome; they may have a role in selected patients who have hypercarbic respiratory failure.
Route of Administration and Feeding Protocols
Nasogastric Feeding.
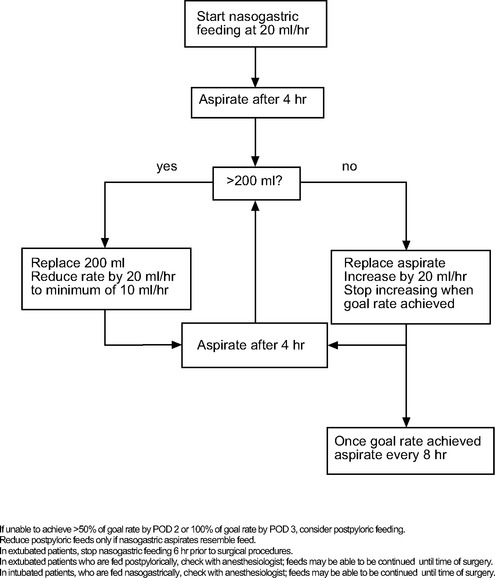
Consensus guidelines recommend starting enteral nutrition within 24 to 48 hours of ICU admission.16 Therefore, in all patients who are not expected to be extubated within 24 to 48 hours of surgery, a 12F or 14F nasogastric tube should be inserted on the first postoperative day. If the hemodynamic state permits, patients should be nursed at a 45-degree head-up position to help prevent aspiration of gastric contents and reduce the incidence of ventilator-associated pneumonia.23 Patients with intraaortic balloon pump in situ may be safely nursed at a 20-degree head-up position. Nasogastric feeding should be commenced at a low rate (e.g., 20 ml/hr). In critically ill patients who are receiving high doses of inotropic drugs, it may be prudent to maintain this low rate for the first 24 to 48 hours. Otherwise, the rate should be increased incrementally to the goal value according to protocol, an example of which is shown in Figure 34-1. The target rate should be determined on an individual basis. Aspirates in excess of 200 ml are indicative of gastric stasis. Metoclopramide (10 mg every 6 hours intravenously) may be used to promote gastric motility, but it is only weakly effective. Erythromycin is a more effective prokinetic than metoclopramide, but because it is potentially proarrhythmic and may promote antimicrobial resistance, its use for this purpose is not recommended.16
Nasojejunal Feeding.
If by postoperative day 3, gastric aspirates remain high and the target feeding rate has not been achieved, a fine-bore (10F to 12F), 135-cm nasojejunal feeding tube should be inserted, under endoscopic or fluoroscopic guidance, and postpyloric feeding commenced. Nutrition may be commenced at 20 ml/hr and gradually increased as for nasogastric feeding (see Figure 34-1). Regular flushing with water helps to prevent blockage of the tube, particularly when low rates of feed are utilized.
Adverse Effects
Apart from hyperglycemia—which should be treated with intravenous insulin—the metabolic consequences of overfeeding that were described earlier are uncommon with enteral nutrition. Diarrhea and constipation are common, each occurring in about 15% of enterally fed patients.24 Other commonly reported adverse effects include gastric stasis (39%), abdominal distention (13%), vomiting (12%), and regurgitation (5%).24 There is no difference in the incidence of aspiration between gastric and postpyloric feeding.25 Occasionally, a large volume (>1 to 2 l/day) of gastric fluid is aspirated from the stomach despite successful postpyloric feeding. If this fluid is discarded, hypovolemia and hypochloremic metabolic alkalosis can develop. The treatment is intravenous 0.9% saline. Intolerance of nasogastric nutrition is very common and is not necessarily a cause for concern; intolerance of postpyloric feeding is less common and may indicate intraabdominal pathology (see subsequent material).
Sinusitis can occur when nasogastric or nasojejunal feeding tubes are in situ for long periods. Polyurethane or silicone feeding tubes should be used when enteral feeding is anticipated to be used for longer than 2 weeks because polyvinylchloride tubes progressively degrade during contact with gastric contents.26
Parenteral Nutrition
Composition
Parenteral nutrition is provided in elemental form: nitrogen as L-amino acids, carbohydrate as glucose, and fat as fatty acids. The appropriate proportion of nitrogen, carbohydrate, and fat is the subject of debate, but a ratio similar to that used for enteral nutrition is common. The advantage of a relatively high proportion of fat is the avoidance of hyperglycemia secondary to high glucose administration; the disadvantage is a potentially increased incidence of nosocomial infection.27 If it is anticipated that parenteral nutrition will be required for fewer than 10 days in a patient who is not malnourished, withholding lipids should be considered.16 Vitamins and trace elements are added in appropriate amounts.
Variable amounts of sodium, potassium, calcium, magnesium, buffer, and water are added to suit patients’ needs. Many patients require water restriction, and a minimum volume of 1 liter per 24 hours is usually possible. Daily sodium and potassium requirements are discussed in Chapter 32. Many patients receiving parenteral nutrition have expanded extracellular spaces and require very little, if any, sodium. Potassium requirements may be high because of insulin treatment given for hyperglycemia or low due to renal failure. Anions are provided as a mixture of chloride and acetate, with a small amount of phosphate. Relatively more chloride may be used in patients with alkalosis, and relatively more acetate may be used in patients with acidosis.
Route of Administration
Parenteral nutrition is hypertonic and must be administered via a central venous catheter. To minimize the risk of infection, it should be a dedicated single-lumen catheter inserted either peripherally or into the subclavian vein (see Chapter 40). Three-way taps in the infusion set should be avoided, and the delivery tubing and any remaining nutrition should be changed each day using aseptic precautions.
Adverse Effects and Biochemical Testing
Hyperglycemia should be treated with intravenous insulin (see Chapter 36). Hypoglycemia can occur with abrupt cessation of treatment, so when it is no longer required, patients should be weaned from parenteral nutrition slowly over several hours. If for some reason the daily supply is interrupted, patients should be commenced on a dextrose infusion at a rate similar to the concentration in the parenteral nutrition.
Chronically malnourished patients who receive aggressive nutritional support, particularly via the intravenous route, are at risk of the refeeding syndrome.28,29 This condition is characterized by disturbances in the balance of body sodium and water (hypovolemia with predominantly protein feeding and hypervolemia with predominantly carbohydrate feeding), hypophosphatemia, hypokalemia, hypomagnesemia, and thiamine deficiency (manifested as Wernicke encephalopathy). Thiamine (100 mg intravenously daily for 3 days) should be given before commencing feeds if malnutrition is suspected. Profound hypophosphatemia can cause myopathy and rhabdomyolysis. Refeeding syndrome is very uncommon in postoperative cardiac surgery patients. Complications that involve vascular access devices are listed in Chapter 40.
INTRAABDOMINAL COMPLICATIONS
Diarrhea and Constipation
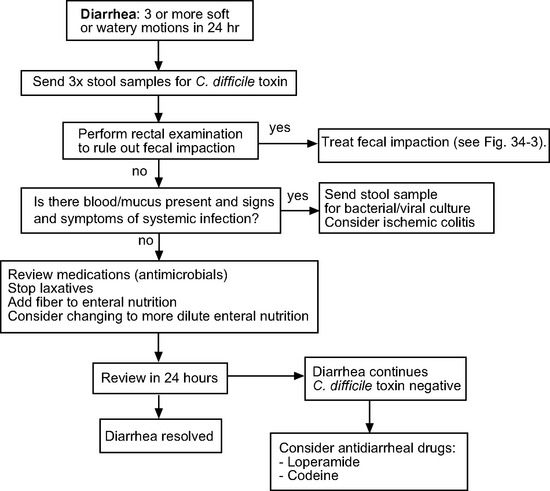
Diarrhea and constipation are common in hospitalized patients. Diarrhea is a common occurrence in patients receiving enteral nutrition,24 particularly when calorie-dense feeds (1.5 to 2.0 kcal/ml) are used. Adding nonabsorbable soluble fiber or changing to a more dilute feed may be beneficial. Once other causes of diarrhea have been excluded, antidiarrheal drugs such as loperamide or codeine may be used. An algorithm for treatment of diarrhea is provided in Figure 34-2.
Bacterial overgrowth by Clostridium difficile, a gram-positive, anaerobic bacillus, may require specific treatment. Clostridium colitis is diagnosed by identifying C. difficile toxin in the stool. Risk factors include prolonged ICU stay and treatment with antimicrobials (particularly clindamycin, cephalosporins, and fluoroquinolones) and proton pump inhibitors. Usually, the condition causes diarrhea only. Rarely, pseudomembranous colitis, an inflammatory condition of the large bowel that is associated with toxic megacolon and bowel perforation, can result. If diarrhea is mild, no treatment is required other than stopping the offending antimicrobial. However, if diarrhea is severe or there are other abdominal symptoms and signs, treatment with metronidazole or oral vancomycin is indicated.30
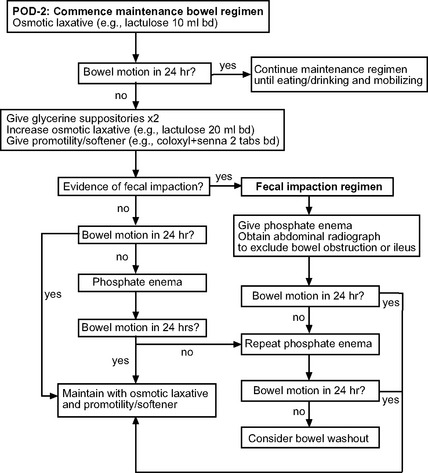
Reduced frequency of bowel motions is normal following cardiac surgery due to reduced oral intake and the effect of opioid analgesics. However, if it is persistent and untreated, severe constipation or fecal impaction can develop. If patients have not had a bowel motion by postoperative day 2, and in the absence of contraindications, laxatives should be commenced. A protocol for the prevention and treatment of constipation is provided in Figure 34-3. Signs of fecal impaction include abdominal distension and discomfort. Hard stools may be palpated on abdominal and rectal examination and may be visible on a plain abdominal radiograph. Patients may have overflow diarrhea. Rarely, a mechanical bowel obstruction develops (see subsequent material).
Bowel Obstruction and Ileus
Impaired gastrointestinal motility may arise from ileus, mechanical obstruction, or pseudoobstruction. These conditions can be difficult to distinguish: all are characterized by abdominal distension and intolerance of enteral nutrition. The presence or absence of and the character of abdominal pain may be unknowable in patients who are sedated and ventilated. The presence or absence of bowel sounds is nondiscriminatory. All three conditions may be mistaken for early mesenteric ischemia. Important diagnostic information may be obtained from supine and decubitus abdominal radiographs. Typical radiographic findings are listed in Table 34-2. Abdominal computed tomography (CT) with oral and intravenous contrast may provide additional diagnostic information.
Table 34-2 Characteristic Appearances of Ileus, Mechanical Obstruction, and Pseudoobstruction on Supine and Decubitus Abdominal Radiographs
| Ileus |
| Nonspecific gaseous distension of large and small bowel |
| Colonic bowel gas present through to rectosigmoid junction |
| Scattered air-fluid levels of variable sizes |
| Mechanical Obstruction |
| Gaseous distension localized to a specific region |
| Air-fluid levels of varying sizes; may be localized to a specific region |
| Colonic bowel gas typically not seen in rectosigmoid junction |
| Pseudoobstruction |
| Marked gaseous distension of large bowel, with gas extending through to rectosigmoid |
| Small bowel appearances relatively normal |
Ileus
Ileus is a reversible reduction in gastrointestinal motility resulting in delayed passage of enteric contents. Ileus may involve the stomach (gastric stasis), small bowel, or colon. Gastric stasis is common and was discussed earlier. The causes of small bowel ileus are listed in Table 34-3. Patients have abdominal distension and are intolerant of postpyloric feeding. Colicky abdominal pain is uncommon. Bowel sounds may be absent. The radiographic findings are listed in Table 34-2. Treatment is conservative, and the condition typically resolves within a few days.
| Hypokalemia | Pain |
|---|---|
| Hypocalcemia | Retroperitoneal hematoma |
| Hypomagnesemia | Opioid analgesics |
| Hypophosphatemia | Vasopressors |
| Acidosis | Anticholinergic drugs |
| Diabetes | Systemic inflammation |
| Hypothyroidism |
Mechanical Obstruction
Mechanical obstruction is uncommon following cardiac surgery but, because it may require surgical intervention, the diagnosis must always be considered. Causes of mechanical obstruction include volvulus (a twisting of the bowel upon itself, typically involving the ileum, cecum, or sigmoid colon), internal hernias, and fecal impaction. Patients may report colicky, cramping abdominal pain. Bowel sounds are usually present. A rectal examination should be performed to exclude fecal impaction. The diagnosis may be confirmed by abdominal radiography (see Fig. 34-2) or CT.
Pseudoobstruction
Pseudoobstruction is a syndrome of colonic atony, which results in severe dilation and functional obstruction. The condition typically develops in unwell, hospitalized patients. It is often self-limiting, but occasionally it results in progressive colonic dilation, with the attendant risk for ischemia and perforation.31 Pseudoobstruction is commonly confused with mechanical obstruction because both conditions result in colicky abdominal pain and tympanic abdominal distension. Abdominal radiographs may help to distinguish between the two conditions (see Table 34-2).
Treatment is initially conservative. If distension has not resolved after 24 to 48 hours of conservative management, treatment with intravenous neostigmine (2.5 mg)32 or colonoscopic decompression should be considered. Neostigmine is a cholinomimetic agent that increases colonic motility. A single dose may lead to copious diarrhea and sustained abdominal decompression. However, in the presence of mechanical obstruction, neostigmine can precipitate intestinal perforation. Thus, it is essential that mechanical obstruction be excluded before administering neostigmine. In general, neostigmine should not be given unless bowel gas can be seen in the rectum and sigmoid colon; the absence of gas in the distal colon may indicate a mechanical obstruction. Patients who fail to respond to these measures or who have cecal dilatation greater than 12 cm on an abdominal radiograph should be referred to a gastrointestinal surgeon.
Mesenteric Ischemia and Infarction
Mesenteric infarction is a rare but highly lethal complication of cardiac surgery. A wide variety of incidences and mortality rates have been reported, but figures of 0.5% and 75%, respectively, are typical.1,33,34
Pathogenesis
Mesenteric ischemia is not a single entity; it represents a continuum that ranges from self-limited, subclinical ischemia to fully developed infarction involving most of the gut. A number of mechanisms are recognized, including splanchnic hypoperfusion, arterial embolism, arterial or venous thrombosis, and gross bowel distension due to mechanical or pseudoobstruction. Many patients who develop mesenteric ischemia have experienced antecedent hemodynamic instability or shock. With shock, there is intense splanchnic arteriolar vasoconstriction, which can cause mesenteric hypoperfusion and ischemia. This may be exacerbated by exogenously administered vasopressors, particularly in the setting of hypovolemia. Mesenteric arterial atherosclerosis and high systemic (and thus portal) venous pressure, such as occurs with right ventricular failure and pericardial tamponade, also impairs splanchnic perfusion. Systemic arterial embolism may occur as the result of an intracardiac thrombus, a ruptured atherosclerotic plaque, or calcified debris from the aorta or aortic valve. Aortic dissection may involve the mesenteric arterial supply.
Ischemia begins within the mucosa and extends outward. Thus, early ischemia or infarction may be missed at laparotomy. The distribution of ischemia is varied but most commonly involves the territories of the superior (distal ileum to transverse colon) or inferior (transverse colon to sigmoid colon) mesenteric arteries. Infarction involving the distribution of the celiac axis (stomach and proximal small bowel) is uncommon. When ischemia involves the territory of the superior mesenteric artery (or celiac axis), full-thickness infarction commonly develops. Full-thickness infarction results in the release of multiple inflammatory mediators from the gut and the translocation of enteric bacteria into the circulation (see Chapter 2). This typically leads to vasodilatory shock and multiple organ dysfunction syndrome. Mortality rates are very high. By contrast, insufficiency involving the territory of the inferior mesenteric artery may result in ischemia or infarction that is limited to the mucosa or submucosa, in which case shock and multiple organ dysfunction syndrome are less likely to develop, and mortality rates are lower.
Risk Factors
The risk factors for mesenteric ischemia and infarction are outlined in Table 34-4. Mesenteric ischemia, particularly when it develops as a consequence of hypoperfusion, typically occurs in critically unwell patients. Such patients are usually receiving mechanical ventilation, high-dose inotropic support, renal replacement therapy and, occasionally, mechanical cardiac support.33 Rarely, mesenteric ischemia develops due to systemic arterial embolism in a patient who is otherwise making an uneventful recovery.
Table 34-4 Risk Factors for Mesenteric Infarction Following Cardiac Surgery
| Increased age |
| Prolonged mechanical ventilation |
| Prolonged cardiopulmonary bypass |
| Open-heart and complex procedures |
| Atrial fibrillation |
| Intraaortic balloon pump use |
| Renal replacement therapy |
| Emergency surgery |
| High requirements for inotropes or vasopressors |
From Venkateswaran RV, Charman SC, Goddard M, et al: Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication? Eur J Cardiothorac Surg 22:534-538, 2002; Garofalo M, Borioni R, Nardi P, et al: Early diagnosis of acute mesenteric ischemia after cardiopulmonary bypass. J Cardiovasc Surg 43:455-459, 2002; Gennaro M, James J, Sheikh A, et al: Acute mesenteric ischemia after cardiopulmonary bypass. Am J Surg 166:231-236, 1993; and Chaudhuri N, James J, Sheikh A, et al: Intestinal ischaemia following cardiac surgery: a multivariate risk model. Eur J Cardiothorac Surg 29:971-977, 2006.
Clinical Assessment
Often, the first sign of evolving mesenteric infarction in a sedated patient is hemodynamic deterioration in association with renal dysfunction and nonspecific abdominal signs. Presentation usually occurs between 3 days and 2 weeks after surgery, rarely earlier.33
Investigations
Abnormalities suggestive of mesenteric ischemia or infarction include leukocytosis, abnormal liver function tests, elevated amylase, and hyperphosphatemia. However, none of these findings are sensitive or specific for mesenteric ischemia, and all may be absent early in the clinical course. Lactic acidosis is a common finding with fully developed infarction, but it may be absent early in the clinical course. Other causes of lactic acidosis (see Chapter 31) and hyperlactatemia must be considered.
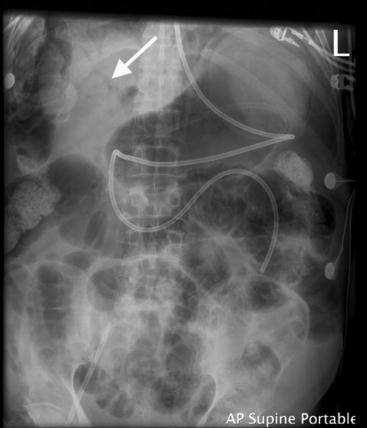
On the supine radiograph, the presence of gas within the bowel wall or the portal venous system is highly suggestive of mesenteric infarction (Fig. 34-4). However, these signs are easily missed or may be absent, particularly early in the clinical course. With ischemia, dilation of the bowel lumen and edema of the bowel wall are common findings but are nonspecific. Occasionally, free intraperitoneal air, suggestive of bowel perforation, is identified on a decubitus abdominal film or erect chest radiograph. (It is important to note that a small amount of subdiaphragmatic free air is a normal finding in postoperative cardiac surgery patients; it results from a breach in the peritoneum at the lower end of the sternal wound or from subxiphisternal chest drains.)
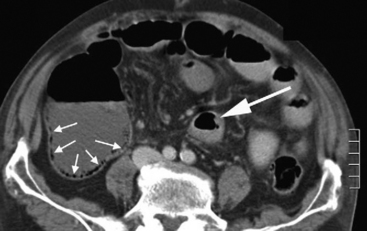
Confirmatory evidence of mesenteric ischemia or infarction may be obtained by means of an abdominal CT scan, ideally using oral and intravenous contrast and scanning in the arterial and portal venous phases (i.e., a mesenteric CT angiogram). The use of intravenous contrast must be carefully considered because concomitant renal failure is common. If CT scanning is performed without intravenous contrast, assessment of vascular patency is not possible. However, the identification of bowel wall edema or gas (Fig. 34-5), bowel dilatation, and free intraperitoneal air or fluid is still possible. CT scanning is useful in patients who are hemodynamically stable and in whom there is genuine diagnostic uncertainty. However, in patients with advanced abdominal signs and shock, it is more appropriate to proceed directly to laparotomy.
Treatment
Untreated, full-thickness mesenteric infarction is uniformly fatal. If there is a high index of suspicion, urgent referral to a gastrointestinal surgeon for laparoscopy or laparotomy is indicated. In stable patients, laparoscopy may be the intervention of choice; the bowel can be inspected and a decision to proceed to laparotomy made if appropriate. In patients with shock, insufflation of the peritoneal space to facilitate laparoscopy may cause hemodynamic collapse; such patients should undergo laparotomy. If infarcted bowel is identified, it should be resected. If segments of bowel appear hypoperfused but potentially viable, a second-look laparotomy 24 to 48 hours later may be appropriate. Massive infarction involving all or most of the intestines is uniformly fatal. In this situation, it is not appropriate to attempt resection, and the patient should be returned to the ICU and be provided with comfort cares (see Chapter 39). Patients with suspected ischemic colitis should undergo colonoscopy. If the diagnosis is confirmed, and assuming the patient remains stable, conservative management is appropriate. A repeat colonoscopy 24 to 72 hours later may be indicated to assess progress.
Fluid and inotropic therapy guided by invasive hemodynamic monitoring should be used to optimize global and splanchnic perfusion. In cases of vasodilatory shock, norepinephrine, either alone or in combination with dobutamine, is the inotrope of choice.35,36 Splanchnic perfusion appears to be worse with epinephrine than with norepinephrine.37 Vasopressin may worsen splanchnic perfusion38 and is best avoided. Low cardiac output should be treated by an inodilator such as dobutamine or milrinone. Severe lactic acidemia may be treated by hemofiltration using bicarbonate buffered substitution fluid (see Chapter 33).
Gastrointestinal Hemorrhage
Gastrointestinal hemorrhage occurs in about 0.3% to 3% of patients following cardiac surgery.1 The most common cause is gastritis or acute stress ulceration involving the stomach or duodenum. Occasionally, esophagitis or a lower gastrointestinal source (e.g., ischemic colitis or a bleeding diverticulum) is responsible.
Risk factors for acute stress ulceration are similar to those listed for mesenteric ischemia (see Table 34-4). Additional risk factors include peptic ulcer disease, anticoagulation, and coagulopathy.39
Diagnosis and Treatment
Patients with minor upper gastrointestinal bleeding should continue to be fed enterally and commenced on a proton pump inhibitor (e.g., omeprazole 40 mg daily). Bleeding that is associated with hemodynamic instability or the need for transfusion of four or more units of blood should be investigated by means of urgent upper gastrointestinal endoscopy. Not only is endoscopy diagnostic, it is also prognostic and may be therapeutic. The finding of an actively bleeding ulcer, a nonbleeding vessel in an ulcer, or clot in the base of an ulcer identifies patients at high risk for further bleeding. These patients should be given high-dose proton pump inhibitor therapy (e.g., 80 mg omeprazole followed by an infusion of 8 mg/hr for 24 hr).40 At the time of endoscopy, epinephrine may be injected into and around a bleeding ulcer, electrocoagulation can be used to cauterize bleeding sites, or hemoclips may be applied to a bleeding vessel. If upper gastrointestinal endoscopy is normal and there is persistent blood loss, colonoscopy or CT mesenteric angiography should be performed. Coagulopathy should be corrected as described in Chapter 30. In patients who have undergone anticoagulation by warfarin or heparin, the risks of thrombosis associated with the reversal of anticoagulation must be weighed against the risk of further bleeding.
Stress Ulcer Prophylaxis in Cardiac Surgery Patients
Acid prophylaxis in high-risk ICU patients reduces the risk for stress ulceration41 but leads to gastric colonization by enteric gram-negative microorganisms and increases the risk of developing ventilator-associated pneumonia.23 There are no data supporting routine acid prophylaxis in cardiac surgery patients, but therapy is probably justified in patients at high risk for stress ulceration, such as those who are or have been shocked, have been ventilated for longer than 48 hours, or have been treated with anticoagulants. If patients have been receiving ulcer prophylaxis preoperatively, it should be recommenced immediately after surgery. Sucralfate is associated with a lower incidence of ventilator-associated pneumonia than is ranitidine,23 but sucralfate is less effective than ranitidine in preventing stress ulceration.42 Proton pump inhibitors (e.g., omeprazole or pantoprazole) appear to be more effective than ranitidine for prophylaxis of stress ulcers,43,44 so they are the preferred agents.
Hepatic Dysfunction
Liver Function Tests
Between 15% and 20% of patients develop transient increases in plasma liver enzyme levels following cardiac surgery.1 Abnormal results of liver function tests may be seen because of preexisting liver dysfunction (e.g., viral hepatitis, cirrhosis, biliary tract disease) or may arise de novo during the postoperative period.
Two basic patterns of liver dysfunction are recognized: hepatocellular, characterized by elevated hepatic transaminases (aspartate aminotransferase and alanine aminotransferase); and cholestatic, characterized by elevated alkaline phosphatase and conjugated (direct) bilirubin. Jaundice is usually apparent when the bilirubin concentration exceeds 50 μmol/l (3.0 mg/dl).45 The common causes of abnormal results of liver function tests in ICU patients are listed in Table 34-5.
| Cause | Pattern of Abnormality |
|---|---|
| Intravascular hemolysis | Unconjugated hyperbiliru-binemia |
| Sepsis and multiple organ dysfunction syndrome | Predominantly cholestatic |
| Drugs | Cholestatic, hepatocellular, mixed |
| Intravenous nutrition | Cholestatic |
| Hepatic ischemia | Hepatocellular |
| Passive hepatic congestion | Mixed |
| Pancreatitis | Cholestatic |
| Cholecystitis (including acalculous) | Cholestatic |
Severe, chronic hepatic disease leads to reduced protein synthesis by the liver. This is manifested as hypoalbuminemia and an elevated prothrombin time. A low plasma albumin concentration also occurs with malnutrition, congestive heart failure, systemic inflammation, and gastrointestinal or renal protein loss. In cardiac surgery patients, preoperative hypoalbuminemia is a powerful predictor of postoperative adverse outcome,46 presumably because it represents poor nutritional state or severe chronic disease.
Drugs and the Liver
Various drugs are associated with the development of abnormal results of liver function tests (Table 34-6). Some drugs (e.g., flucloxacillin) typically produce a cholestatic pattern; others (e.g., fluconazole), a hepatocellular picture. With many drugs, both patterns can occur. Most drug-induced hepatic injury is idiosyncratic; that is, only some patients develop hepatotoxicity, and the effect is not directly related to the dose of the drug. Other drugs, notably acetaminophen, cause dose-dependent hepatotoxicity.
Table 34-6 Hepatotoxicity Associated With Drugs Commonly Used in the ICU
| Acetaminophen | Cyclosporine |
|---|---|
| Statins | Amoxicillin |
| Nonsteroidal antiinflammatory drugs | Flucloxacillin |
| Erythromycin | Tetracycline |
| Diltiazem | Nitrofurantoin |
| Phenytoin | Rifampicin |
| Chlorpromazine | Sulphamethoxazole |
| Amiodarone | Fluconazole |
Note: All agents except acetaminophen cause idiosyncratic hepatotoxicity. Acetaminophen hepatotoxicity results primarily from the cumulative effects of the drug. Idiosyncratic and cumulative hepatotoxicity occurs with amiodarone and cyclosporine.
Ideally, if a drug is implicated as a potential cause of liver dysfunction, it should be stopped. This is particularly the case for acetaminophen, which has the potential to cause fatal hepatotoxicity. For other drugs, such as antimicrobials, it may be reasonable to continue treatment and closely monitor liver function.
With liver dysfunction, dosage reductions must be made in a number of hepatically metabolized drugs (Table 34-7).47 Failure to do so may exacerbate hepatic dysfunction or result in an increased pharmacologic effect due to reduced hepatic clearance. Hepatically mediated drug interactions are listed in Table 4-3.
Table 34-7 Drugs That Should Be Given at Reduced Dosages or Avoided in Patients With Liver Dysfunction
| Acetaminophen | Lidocaine |
|---|---|
| Nonsteroidal antiinflammatory drugs | Verapamil |
| Morphine | Diltiazem |
| Meperidine (pethidine) | Propranolol |
| Pentazocine | Procainamide |
| Aztreonam | Diazepam |
| Clindamycin | Cimetidine |
| Erythromycin | Aminophylline |
| Metronidazole | Tacrolimus |
| Rifampacin | Sirolimus |
Modified from Chernow B: The Pharmacologic Approach to the Critically Ill Patient, ed 3. Baltimore, Williams and Wilkins, 1994, p. 98.
Hepatic Ischemia and Acute Liver Failure
Occasionally, profound hepatic ischemia results in acute liver failure, the features of which include reduced level of consciousness due to cerebral edema, vasodilatory shock, oliguric renal failure, coagulopathy, lactic acidosis, and hypoglycemia. The findings of markedly elevated transaminases in association with lactic acidosis and hypoglycemia are strongly suggestive of acute liver failure. Treatment of hypoglycemia, coagulopathy, acidemia, coma, and raised intracranial pressure may be required. Unremitting lactic acidosis may be treated with hemofiltration using bicarbonate-buffered replacement solution (see Chapter 33). Mortality rates are very high.
Cholecystitis
Acute cholecystitis is a rare but well-recognized occurrence following cardiac surgery.1,48 Patients typically present with right upper quadrant abdominal pain, fever, and cholestatic jaundice. The diagnosis is usually confirmed by ultrasonography of the liver and biliary tract. Gallstones are absent in about one third of cases.1 The pathologic mechanism of acalculous cholecystitis is edema and obstruction of the cystic duct. Treatment of cholecystitis includes nil by mouth and broad-spectrum antimicrobials. Perforation of the gallbladder may occur, so urgent referral to a hepatobiliary surgeon is indicated.
Hyperamylasemia and Pancreatitis
Hyperamylasemia is extremely common following cardiac surgery; it occurs in 30% to 60% of patients.49–51 Often, the amylase comes from the salivary gland50,51 and is of no clinical consequence, so it is essential that a pancreas-specific test, such as an examination of lipase or pancreatic isoamylase, be performed. In addition to pancreatitis, raised amylase levels also occur with peptic ulcer disease, mesenteric ischemia, cholecystitis, intestinal obstruction, renal failure, and diabetic ketoacidosis. A plasma amylase concentration greater than 1000 U/l most likely represents pancreatitis. If the amylase is between 300 and 1000 U/l, other abdominal pathologies should be considered.
In most circumstances, elevated pancreatic isoamylase levels are an incidental finding in postoperative cardiac surgery patients; presumably, they represent subclinical pancreatic cellular injury.49,51 In the absence of clinical signs of pancreatitis or other abdominal pathology, no specific treatment is required. However, clinically significant pancreatitis does occasionally occur following cardiac surgery.52 Symptoms and signs are nonspecific: abdominal distension with or without pain, intolerance of enteral nutrition, fever, and leukocytosis. Other biochemical features of pancreatitis include hyperglycemia, hypocalcemia, abnormal liver function tests, and elevated lactate dehydrogenase. Severe pancreatitis is associated with vasodilatory shock, marked third-space fluid losses, and acute lung injury—a clinical syndrome that is identical to that of septic shock. If clinically significant pancreatitis is suspected, referral to a gastrointestinal surgeon is indicated.
1 Sakorafas GH, Tsiotos GG. Intra-abdominal complications after cardiac surgery. Eur J Surg Acta Chirurg. 1999;165:820-827.
2 Zacharias A, Schwann TA, Parenteau GL, et al. Predictors of gastrointestinal complications in cardiac surgery. Tex Heart Inst J. 2000;27:93-99.
3 Tsiotos GG, Mullany CJ, Zietlow S, et al. Abdominal complications following cardiac surgery. Am J Surg. 1994;167:553-557.
4 Johnson DJ, Brooks DC, Pressler VM, et al. Hypothermic anesthesia attenuates postoperative proteolysis. Ann Surg. 1986;204:419-429.
5 Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27:1295-1302.
6 Taggart DP, McMillan DC, Preston T, et al. Effects of cardiac surgery and intraoperative hypothermia on energy expenditure as measured by doubly labelled water. Br J Surg. 1991;78:237-241.
7 Weissman C, Kemper M, Askanazi J, et al. Resting metabolic rate of the critically ill patient: measured versus predicted. Anesthesiology. 1986;64:673-679.
8 Klein CJ, Stanek GS, Wiles CE3rd. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc. 1998;98:795-806.
9 Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
10 Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352-360.
11 Frankenfield DC, Muth ER, Rowe WA. The Harris-Benedict studies of human basal metabolism: history and limitations. J Am Diet Assoc. 1998;98:439-445.
12 Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(suppl 1):5-41.
13 Cerra FB, Benitez MR, Blackburn GL, et al. Applied nutrition in ICU patients: a consensus statement of the American College of Chest Physicians. Chest. 1997;111:769-778.
14 Reid CL. Nutritional requirements of surgical and critically ill patients: do we really know what they need ? Proceed Nutr Soc. 2004;63:467-472.
15 Demling RH, DeBiasse MA. Micronutrients in critical illness. Crit Care Clin. 1995;11:651-673.
16 Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enter Nutr. 2003;27:355-373.
17 Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intens Care Med. 2005;31:12-23.
18 Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients ? A systematic review of the literature. Nutrition. 2004;20:843-848.
19 Rokyta RJr, Matejovic M, Krouzecky A, et al. Enteral nutrition and hepatosplanchnic region in critically ill patients—friends or foes ? Physiol Res. 2003;52:31-37.
20 Revelly JP, Tappy L, Berger MM, et al. Early metabolic and splanchnic responses to enteral nutrition in postoperative cardiac surgery patients with circulatory compromise. Intens Care Med. 2001;27:540-547.
21 Berger MM, Berger-Gryllaki M, Wiesel PH, et al. Intestinal absorption in patients after cardiac surgery. Crit Care Med. 2000;28:2217-2223.
22 Berger MM, Revelly JP, Cayeux MC, et al. Enteral nutrition in critically ill patients with severe hemodynamic failure after cardiopulmonary bypass. Clin Nutr. 2005;24:124-132.
23 American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
24 Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447-1453.
25 Esparza J, Boivin MA, Hartshorne MF, et al. Equal aspiration rates in gastrically and transpylorically fed critically ill patients. Intens Care Med. 2001;27:660-664.
26 Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(suppl 7):vii1-vii12.
27 Battistella FD, Widergren JT, Anderson JT, et al. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997;43:52-58. discussion 58-60
28 Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition. 2001;17:632-637.
29 Marinella MA. Refeeding syndrome and hypophosphatemia. J Intens Care Med. 2005;20:155-159.
30 Bricker E, Garg R, Nelson R, et al. Antibiotic treatment for Clostridium difficile -associated diarrhea in adults. Cochrane Database of Systematic Reviews. 2005:CD 004610.
31 Rex DK. Colonoscopy and acute colonic pseudo-obstruction. Gastrointest Endosc Clin North Am. 1997;7:499-508.
32 Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137-141.
33 Edwards M, Sidebotham D, Smith M, et al. Diagnosis and outcome from suspected mesenteric ischaemia following cardiac surgery. Anaesth Intens Care. 2005;33:210-217.
34 Ott MJ, Buchman TG, Baumgartner WA. Postoperative abdominal complications in cardiopulmonary bypass patients: a case-controlled study. Ann Thorac Surg. 1995;59:1210-1213.
35 Meier-Hellmann A, Specht M, Hannemann L, et al. Splanchnic blood flow is greater in septic shock treated with norepinephrine than in severe sepsis. Intens Care Med. 1996;22:1354-1359.
36 Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intens Care Med. 1997;23:282-287.
37 Martikainen TJ, Tenhunen JJ, Giovannini I, et al. Epinephrine induces tissue perfusion deficit in porcine endotoxin shock: evaluation by regional CO (2) content gradients and lactate- to-pyruvate ratios. Am J Physiol Gastrointest Liver Physiol. 2005;288:G586-G592.
38 van Haren FM, Rozendaal FW, van der Hoeven JG. The effect of vasopressin on gastric perfusion in catecholamine-dependent patients in septic shock. Chest. 2003;124:2256-2260.
39 van der Voort PH, Zandstra DF. Pathogenesis, risk factors, and incidence of upper gastrointestinal bleeding after cardiac surgery: is specific prophylaxis in routine bypass procedures needed ? J Cardiothorac Vasc Anesth. 2000;14:293-299.
40 Julapalli VR, Graham DY. Appropriate use of intravenous proton pump inhibitors in the management of bleeding peptic ulcer. Digest Dis Sci. 2005;50:1185-1193.
41 Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA. 1996;275:308-314.
42 Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
43 Levy MJ, Seelig CB, Robinson NJ, et al. Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Digest Dis Sci. 1997;42:1255-1259.
44 Stollman N, Metz DC. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care. 2005;20:35-45.
45 Kasper DL, Braunwald E, Fauci AS, et al. Harrison’s Principles of Internal Medicine, ed 16, New York: McGraw-Hill, 2005.
46 Rich MW, Keller AJ, Schechtman KB, et al. Increased complications and prolonged hospital stay in elderly cardiac surgical patients with low serum albumin. Am J Cardiol. 1989;63:714-718.
47 Chernow B. The Pharmacologic Approach to the Critically Ill Patient, ed 3, Baltimore: Williams and Wilkins, 1994.
48 Sessions SC, Scoma RS, Sheikh FA, et al. Acute acalculous cholecystitis following open heart surgery. Am Surg. 1993;59:74-77.
49 Fernandez-del Castillo C, Harringer W, Warshaw AL, et al. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991;325:382-387.
50 Ihaya A, Muraoka R, Chiba Y, et al. Hyperamylasemia and subclinical pancreatitis after cardiac surgery. World J Surg. 2001;25:862-864.
51 Rattner DW, Gu ZY, Vlahakes GJ, et al. Hyperamylasemia after cardiac surgery: incidence, significance, and management. Ann Surg. 1989;209:279-283.
52 Haas GS, Warshaw AL, Daggett WM, et al. Acute pancreatitis after cardiopulmonary bypass. Am J Surg. 1985;149:508-515.
53 Venkateswaran RV, Charman SC, Goddard M, et al. Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication ? Eur J Cardiothorac Surg. 2002;22:534-538.
54 Garofalo M, Borioni R, Nardi P, et al. Early diagnosis of acute mesenteric ischemia after cardiopulmonary bypass. J Cardiovasc Surg. 2002;43:455-459.
55 Gennaro M, Ascer E, Matano R, et al. Acute mesenteric ischemia after cardiopulmonary bypass. Am J Surg. 1993;166:231-236.
56 Chaudhuri N, James J, Sheikh A, et al. Intestinal ischaemia following cardiac surgery: a multivariate risk model. Eur J Cardiothorac Surg. 2006;29:971-977.