Nutrition
Notable features of nutrition in children are:
• The optimal nutrition for newborn infants is from breast-feeding.
• Inadequate nutrition in infants and young children rapidly leads to weight loss followed by growth failure, commonly called failure to thrive, which if severe and prolonged leads to malnutrition
• Whereas malnutrition is a major cause of morbidity and death in developing countries, obesity is the major nutritional problem in developed countries.
The nutritional vulnerability of infants and children
High nutritional demands for growth
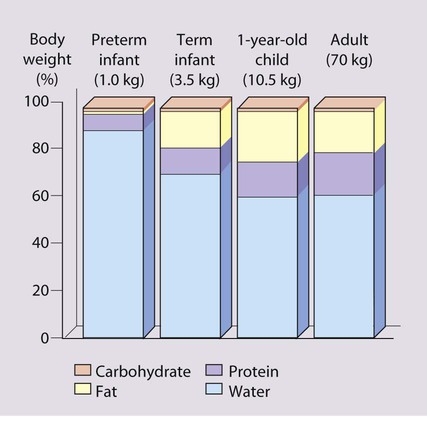
The nourishment children require, per unit body size, is greatest in infancy (Table 12.1), because of their rapid growth during this period. At 4 months of age, 30% of an infant’s energy intake is used for growth, but by 1 year of age, this falls to 5%, and by 3 years to 2%. The risk of growth failure from restricted energy intake is therefore greater in the first 6 months of life than in later childhood. Even small but recurrent deficits in early childhood will lead to a cumulative deficit in weight and height.
Table 12.1
Reference values for energy and protein requirements
| Age | Energy (kcal/kg per 24 h) | Protein (g/kg per 24 h) |
| 0–6 months | 115 | 2.2 |
| 6–12 months | 95 | 2.0 |
| 1–3 years | 95 | 1.8 |
| 4–6 years | 90 | 1.5 |
| 7–10 years | 75 | 1.2 |
| Adolescence | (male/female) | |
| 11–14 years | 65/55 | 1.0 |
| 15–18 years | 60/40 | 0.8 |
Rapid neuronal development
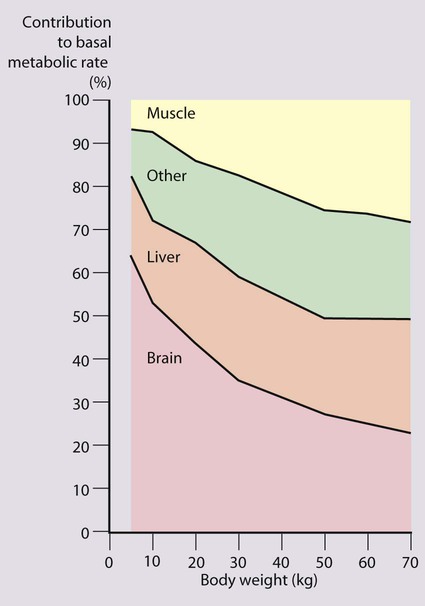
The brain grows rapidly during the last trimester of pregnancy and throughout the first 2 years of life. The complexity of interneuronal connections also increases substantially during this time. This process appears to be sensitive to undernutrition. Even modest energy deprivation during periods of rapid brain growth and differentiation is thought to lead to an increased risk of adverse neurodevelopmental outcome. This is not surprising when one considers that at birth the brain accounts for approximately two-thirds of basal metabolic rate, and at 1 year for about 50% (Fig. 12.2). Many studies have drawn attention to the delayed development seen in children suffering from protein-energy malnutrition due to inadequate food intake, although inadequate psychosocial stimulation may also contribute.
Long-term outcome of early nutritional deficiency
Linear growth of populations
Disease in adult life
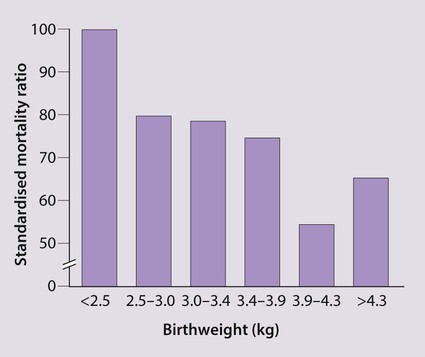
Evidence suggests that undernutrition in utero resulting in growth restriction is associated with an increased incidence of coronary heart disease, stroke, non-insulin-dependent diabetes and hypertension in later life (Fig. 12.3). There is also a similar but weaker association with low weight at 1 year of age. The mechanism is unclear, but it is recognised that fetal undernutrition leads to redistribution of blood flow and changes in fetal hormones, such as insulin-like growth factors and cortisol. Alternatively, it may be the rapid, postnatal growth (catch-up) seen in babies suffering from intrauterine growth restriction that is the causal factor.
Infant feeding
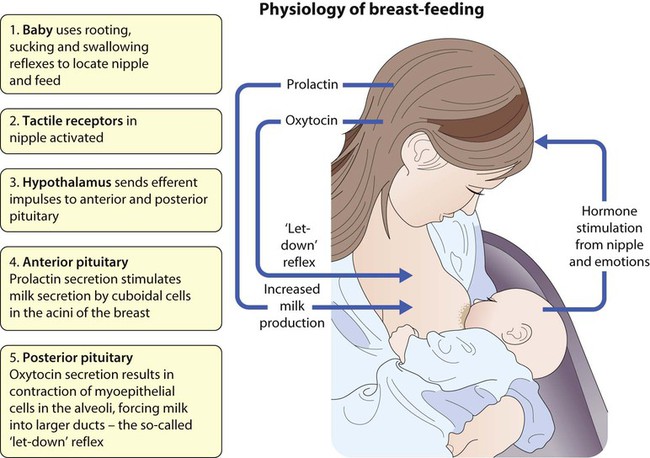
Breast-feeding
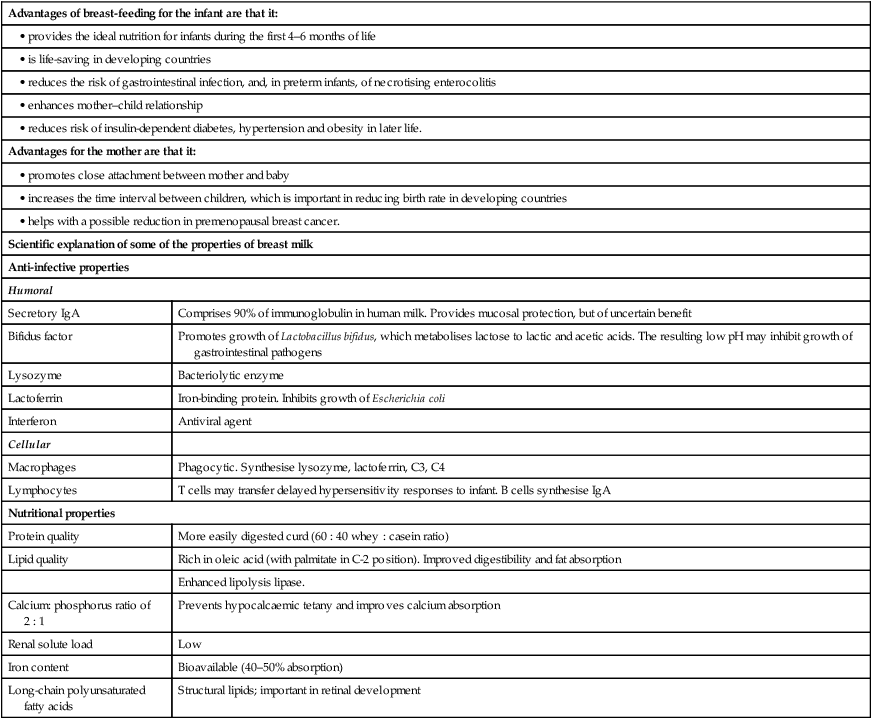
Potential complications (Box 12.2)
As one cannot readily tell how much milk a baby is taking from the breast, the baby’s weight should be checked regularly, every few days in the first couple of weeks, then weekly until feeding is well established. Successful breast-feeding of twins can be achieved, but is more difficult (Fig. 12.4). It is rarely possible to totally breast-feed triplets and higher-order births. Preterm infants can be breast-fed, but the milk will need to be expressed from the breast until the infant can suck. Maintaining the supply of milk can be a problem for mothers of preterm babies.

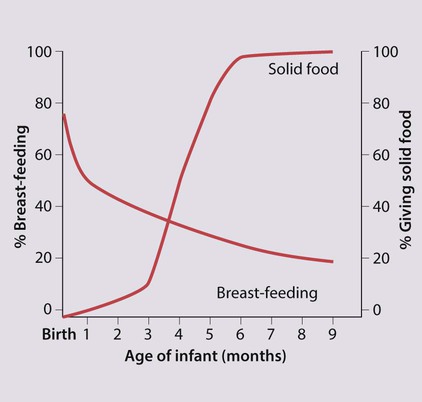
While two-thirds of mothers in the UK initially breast-feed, this proportion rapidly declines during the first few months (Fig. 12.5). Nearly 90% of social class I mothers start breast-feeding, but only 60% of mothers from social class V. Breast-feeding is restrictive for the mother, as others cannot take charge of her baby for any length of time. This is particularly important if she goes to work and may delay her return, which may cause financial hardship for the family. Facilities for breast-feeding in public places remain limited. Failure to establish breast-feeding will sometimes cause significant emotional upset in the mother.
Formula-feeding
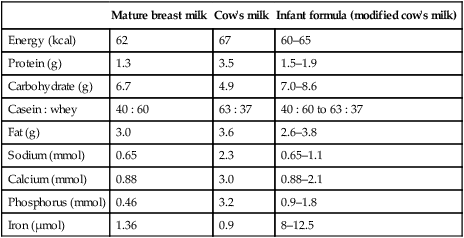
Infants who are not breast-fed require a formulafeed based on cow’s milk. Unmodified cow’s milk is unsuitable for feeding in infancy as it contains too much protein and electrolytes and inadequate iron and vitamins. Even after considerable modification, differences remain between formula feeds and breastmilk (Table 12.2)
Table 12.2
A comparison of human milk, cow’s milk and infant formula (per 100 ml)
| Mature breast milk | Cow’s milk | Infant formula (modified cow’s milk) | |
| Energy (kcal) | 62 | 67 | 60–65 |
| Protein (g) | 1.3 | 3.5 | 1.5–1.9 |
| Carbohydrate (g) | 6.7 | 4.9 | 7.0–8.6 |
| Casein : whey | 40 : 60 | 63 : 37 | 40 : 60 to 63 : 37 |
| Fat (g) | 3.0 | 3.6 | 2.6–3.8 |
| Sodium (mmol) | 0.65 | 2.3 | 0.65–1.1 |
| Calcium (mmol) | 0.88 | 3.0 | 0.88–2.1 |
| Phosphorus (mmol) | 0.46 | 3.2 | 0.9–1.8 |
| Iron (µmol) | 1.36 | 0.9 | 8–12.5 |
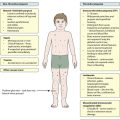
Failure to thrive
Differentiating the infant who is failing to thrive from a normal but small or thin baby is often a problem (Fig. 12.7). Normal but short infants have no symptoms, are alert, responsive and happy, and their development is satisfactory. The parents may be short (low mid-parental height) or the infant may have been extremely preterm or growth-restricted at birth. Any intercurrent illness may be accompanied by a temporary failure to gain weight.
Causes
Failure to thrive is usually classified as organic or non-organic (Fig. 12.8). Traditionally non-organic failure to thrive is believed to be associated with a broad spectrum of psychosocial and environmental deprivation. It is estimated that 5–10% of children with failure to thrive will be on a child protection register or be subjected to abuse or neglect, while in a larger proportion, socioeconomic deprivation is an important contributing factor.
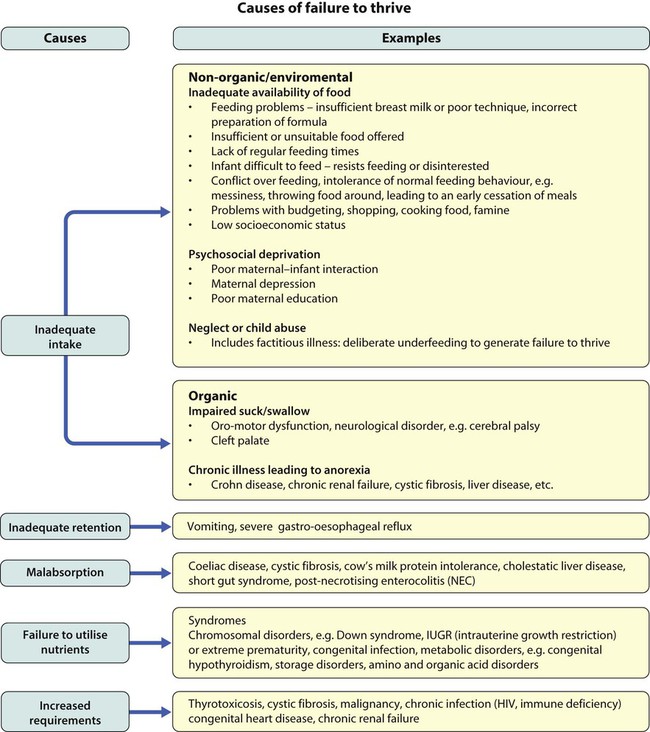
Organic causes are listed in Figure 12.8. Less than 5% of children with failure to thrive will be found to have an organic cause.
Clinical features and investigation
• A detailed dietary history, including a food diary over several days
• Feeding, including details of exactly what happens at mealtimes
• Is the child well with lots of energy or does the child have other symptoms such as diarrhoea, vomiting, cough, lethargy?
• Was the child premature or had intrauterine growth restriction at birth or any significant medical problems?
• The growth of other family members and any illnesses in the family
Further information about the child and family from the health visitor, general practitioner or other professionals involved with the family can be particularly helpful. Investigations to be considered are listed in Box 12.3.
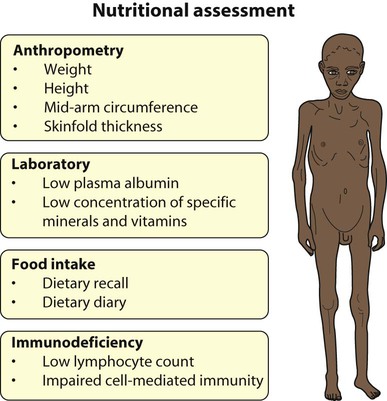
Malnutrition
Marasmus and kwashiorkor
The World Health Organization recommends that nutritional status is expressed as:
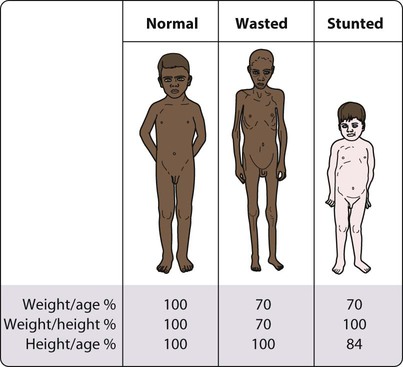
• Weight for height – a measure of wasting and an index of acute malnutrition (Fig. 12.11). Severe malnutrition is defined as a weight for height more than −3 standard deviations below the median plotted on the WHO standard growth chart. This corresponds to a weight for height <70% below the median.
• Mid upper-arm circumference (MUAC) – <115 mm is severe malnutrition
• Height for age – a measure of stunting and an index of chronic malnutrition
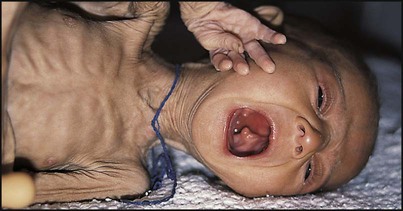
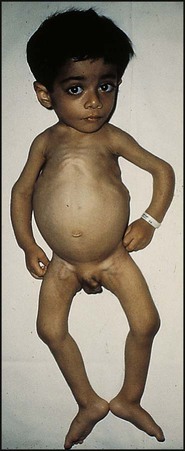
Severe protein-energy malnutrition in children usually leads to marasmus, with a weight for height more than −3 standard deviations below the median, corresponding to <70% weight for height, and a wasted, wizened appearance (Fig. 12.12). Oedema is not present. Skinfold thickness and mid-arm circumference are markedly reduced, and affected children are often withdrawn and apathetic.
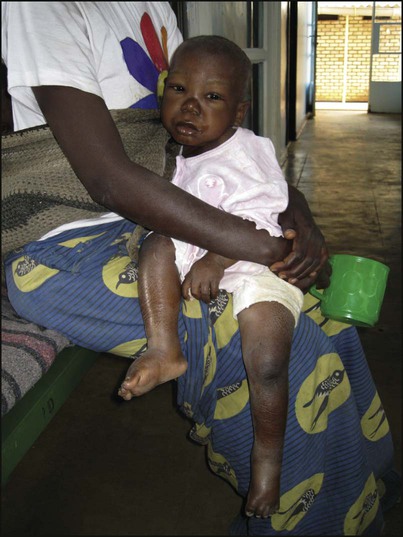
Kwashiorkor is another manifestation of severe protein malnutrition (Fig. 12.13), in which there is generalised oedema as well as severe wasting. Because of the oedema, the weight may not be as severely reduced. In addition, there may be:
• a ‘flaky-paint’ skin rash with hyperkeratosis (thickened skin) and desquamation
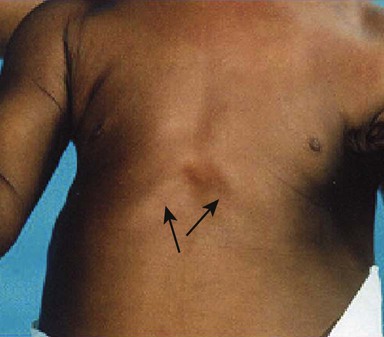
• a distended abdomen and enlarged liver (usually due to fatty infiltration)
• hair which is sparse and depigmented
Management
• Hypoglycaemia – common; correct urgently, particularly if coma or severely ill.
• Hypothermia – wrap, especially at night.
• Dehydration – correct, but avoid being overzealous with intravenous fluids, as may lead to heart failure.
• Electrolytes – correct deficiencies, especially potassium.
• Infection – give antibiotics; fever and other signs may be absent. Treat oral candida if present.
• Micronutrients – give vitamin A and other vitamins
• Initiate feeding – small volumes, frequently, including through the night.
Vitamin D deficiency
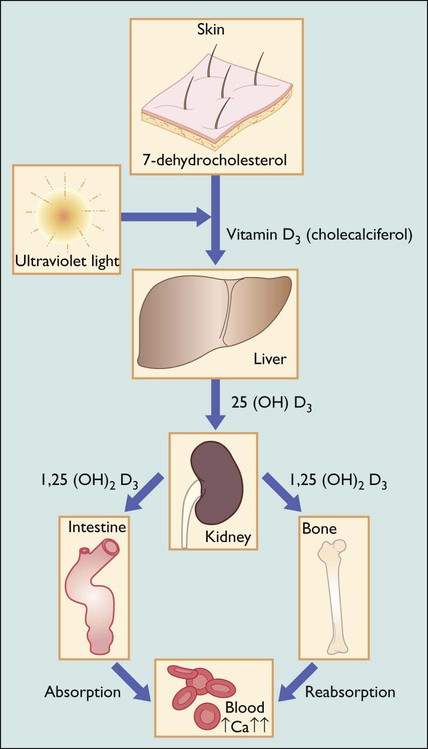
Vitamin D deficiency usually results from deficient intake or defective metabolism of vitamin D, causing a low serum calcium (Fig. 12.14). This triggers the secretion of parathyroid hormone and normalises the serum calcium but demineralises the bone. Parathyroid hormone causes renal losses of phosphate and consequently low serum phosphate levels, further reducing the potential for bone calcification.
Rickets
Aetiology
The causes of rickets are listed in Box 12.5 The predominant cause of rickets during the early twentieth century was nutritional vitamin D deficiency due to inadequate intake or insufficient exposure to direct sunlight. Nutritional rickets still remains the major cause in developing countries. In developed countries, nutritional rickets has become rare, as formula milk and many foods such as breakfast cereals are supplemented with vitamin D. However, nutritional rickets has re-emerged in developed countries in black or Asian infants totally breast-fed in late infancy. It is also seen in extremely preterm infants from dietary deficiency of phosphorus, together with low stores of calcium and phosphorus.
Clinical manifestations
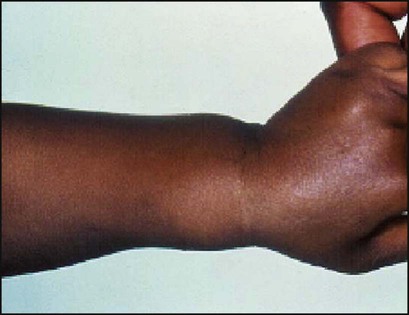
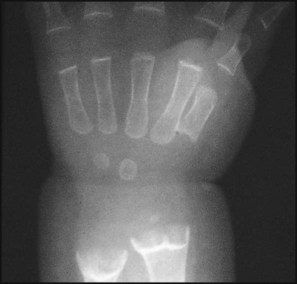
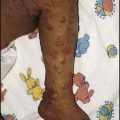
The earliest sign of rickets is a ping-pong ball sensation of the skull (craniotabes) elicited by pressing firmly over the occipital or posterior parietal bones. The costochondral junctions may be palpable (rachitic rosary), wrists (especially in crawling infants) and ankles (especially in walking infants) may be widened and there may be a horizontal depression on the lower chest corresponding to attachment of the softened ribs and with the diaphragm (Harrison sulcus) (Figs 12.15, 12.16). The legs may become bowed (see Fig. 12.15). The clinical features are listed in Box 12.6 (see also Case History 12.2).
Diagnosis
• Dietary history for vitamin and calcium intake
• Blood tests – serum calcium is low or normal, phosphorus low, plasma alkaline phosphatase activity greatly increased, 25-hydroxyvitamin D may be low and parathyroid hormone elevated.
• X-ray of the wrist joint – shows cupping and fraying of the metaphyses and a widened epiphyseal plate.
Obesity
Obesity is the most common nutritional disorder affecting children and adolescents in the developed world. Its importance is in its short- and long-term complications (Box 12.7) and that obese children tend to become obese adults.
Definitions
In children, the body mass index (BMI = weight in kg/height in metres2) is expressed as a BMI centile in relation to age and sex-matched population. By convention in the UK, the 1990 chart is used (Fig. 12.19). For clinical use, overweight is a BMI >91st centile, obese is a BMI >98th centile. Very severe obesity is >3.5 standard deviations above the mean; extreme obesity >4 standard deviations. For children over 12 years old, overweight is BMI ≥25, obese ≥30, very severe obesity BMI ≥35 and extreme obesity BMI ≥40 kg/m2.
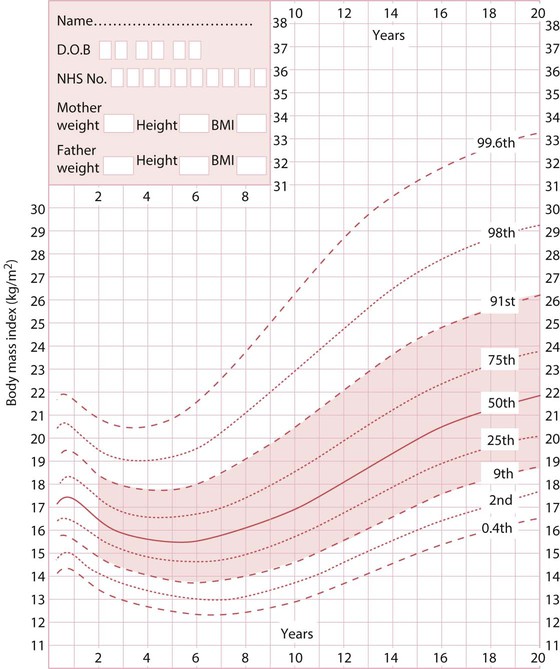
Endogenous causes
In children who are obese with learning disabilities, or who are dysmorphic, a syndrome should be considered. The commonest of these is Prader–Willi (obesity, hyperphagia, poor linear growth, dysmorphic facial features, hypotonia and undescended testes in males; see Chapter 8 and Figure 8.19. In severely obese children under the age of 3 years, gene defects, e.g. leptin deficiency, should be considered.
Management
Most obese children are managed in primary care. Specialist paediatric assessment is indicated in any child with complications (Box 12.7) or if an endogenous cause is suspected.
In the absence of evidence from randomised controlled trials, a pragmatic approach in any individual child based on consensus criteria has to be adopted (Box 12.8). Treatment should be considered where the child is above the 98th centile for BMI and the family are willing to make the necessary difficult lifestyle changes. Weight maintenance is a more realistic goal than weight reduction and will result in a demonstrable fall in BMI on centile chart as height increases. It can only be achieved by sustained changes in lifestyle:
• Healthier eating – no sugar-containing juices or fizzy drinks; decrease food portion size by 10–20%; increase protein- and non-carbohydrate-containing vegetables, discourage snacking and encourage family meals
• An increase in habitual physical activity to 60 min of moderate to vigorous daily physical activity
• Reduce physical inactivity (e.g. small screen time) during leisure time to less than an average of 2 h per day.
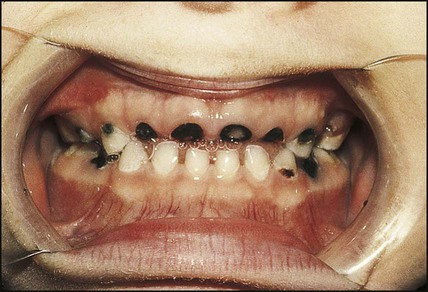
Dental caries
Dental caries occurs as a result of exposure to organic acids produced by bacterial fermentation of carbohydrate, particularly sucrose (Fig. 12.20). Prevalence is now rising in young children. It is strongly related to socioeconomic deprivation.
• A reduction in plaque bacteria (brushing and flossing)
• Less frequent ingestion of carbohydrates
• Regular inspection by a dentist
• An optimal intake of fluoride up to puberty to improve the resistance of the tooth to damage; water fluoridation is one of the most effective ways of achieving this.