CHAPTER 13 Nucleus disassembly
The evolution of phacoemulsification from the procedure described by Kelman1 in the late 1960s to the techniques that we currently practice represents one of the greatest success stories of modern medicine. Understanding this historical development engenders understanding of the fundamental forces at work during phaco; therefore, we begin this section with a historical review of phaco technique.
Divide and conquer technique
Divide and conquer phaco, described by Gimbel2, was the first nucleofractis (two-instrument) cracking technique developed. After adequate hydrodissection, a deep crater is sculpted into the center of the nucleus, leaving a dense peripheral rim that can later be fractured into multiple sections. A shaving action is used to sculpt away the central nuclear material, and when this material is no longer accessible to the phaco probe, the lens should be rotated and additional central phaco performed to enlarge and deepen the crater. The size of the central crater should be expanded for progressively denser nuclei. Enough of the dense material must be left in place, however, to allow the phaco probe and second instrument to engage the rim and fracture the lens into sections. Notably, the peripheral nuclear rim stretches the entire capsular bag and acts as a safety mechanism to prevent the posterior capsule from suddenly moving anteriorly and being cut by the phaco probe. Once fractured, the lens sections should be left in place within the rim both to maintain the circular rim and to hold tension on the capsule rather than emulsifying them as they are broken away. Leaving the sections in place also facilitates rotation and the progressive fracturing of the remaining rim. After the rim is fractured around the entirety of its circumference, each segment can then be brought to the center of the capsule for safe emulsification.
Phaco fracture technique
In phaco fracture, a technique described by Shepherd3, the surgeon sculpts a groove from the 12- to 6-o’clock position after performing hydrodissection. Using the phaco handpiece and a second instrument, he or she then rotates the nucleus 90° and sculpts a second groove perpendicular to the first, in the form of a cross. Sculpting continues until the red reflex is seen at the base of the grooves, often requiring additional rotations and removal of nuclear material. At this point, a bimanual cracking technique is used to create a fracture through the nuclear rim in the plane of one of the grooves. The nucleus is then rotated 90°, and additional fractures are made until four separate quadrants are isolated. These are then tumbled toward the center of the capsule. A short burst of phaco power is used to embed the phaco tip into the bulk of the isolated quadrant and then, with the use of aspiration, the quadrant is gently pulled into the center for safe emulsification.
Chip and flip technique
Introduced by Fine4, and useful for softer grades of nuclei, this procedure relies on a nucleus that rotates freely within the capsular bag. Initially, a central bowl is sculpted in the nucleus until a thin central plate remains (the chip). The second instrument introduced through the side port incision engages the subincisional nuclear rim to move the inferior nuclear rim toward the center of the capsule bag. Clock-hour pieces of the rim are then carefully emulsified as the nucleus is rotated. Once the entire rim is removed, the second instrument is used to elevate the chip, which is then emulsified. The epinucleus is engaged at the 6-o’clock position with aspiration alone. As the phaco tip is moved superiorly, the second instrument pushes the epinucleus toward the 6-o’clock position thereby tumbling the epinuclear bowl and permitting it to be aspirated (the flip).
Crack and flip technique
Fine and colleagues modified Shepherd’s phaco fracture technique by adding hydrodelineation, resulting in the crack and flip technique4. Sculpting with minimal vacuum, relatively low aspiration flow, and low phaco power can be used to create two deep grooves at right angles to each other that extend to the golden ring, or the perimeter of the endonucleus, and permits bimanual nucleus cracking. Due to hydrodelineation of the epinucleus, only the endonucleus cracks.
Phaco chop
Koch and Katzen5 modified this procedure because they encountered difficulty in mobilizing the nuclear fragments. They created a central groove or central crater, depending on the density of the nucleus, which permits ease of removing the nuclear fragments liberated by the phaco chop technique.
Choo choo chop and flip
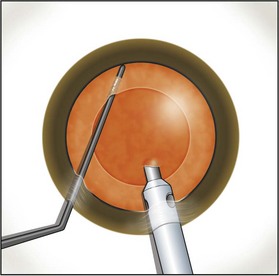
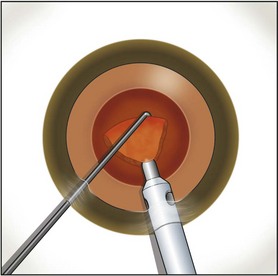
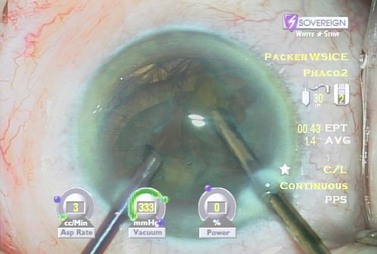
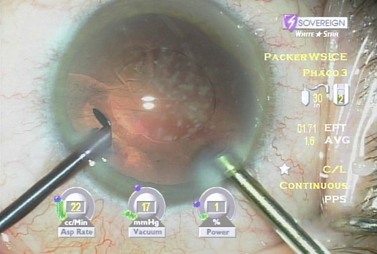
Fine described the ‘choo choo chop and flip’ technique in 19986. Subsequently, Fine, Packer, and Hoffman correlated the reduction of ultrasound energy with this technique to improvement in uncorrected postoperative day one visual acuity7. A 30° standard bevel down tip is used throughout endonuclear removal. After aspirating the epinucleus uncovered by the capsulorrhexis, a Fine/Nagahara chopper (Rhein Medical, Tampa, FL) is placed in the golden ring by touching the center of the nucleus with the tip and pushing it peripherally so that it reflects the capsulorrhexis. The chopper is used to stabilize the nucleus by lifting and pulling toward the incision slightly (Fig. 13.1), after which the phaco tip lollipops the nucleus. This can be done in either pulse mode at 2 pulses/second or 80 millisecond burst mode.
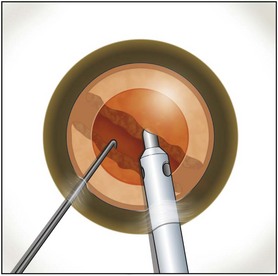
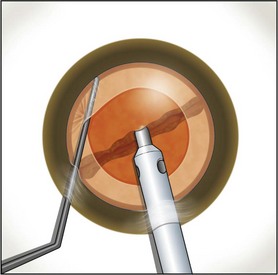
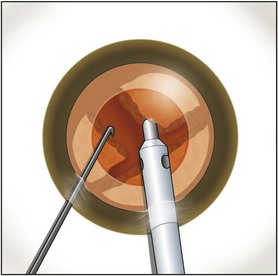
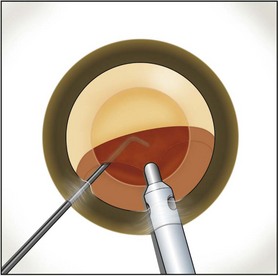
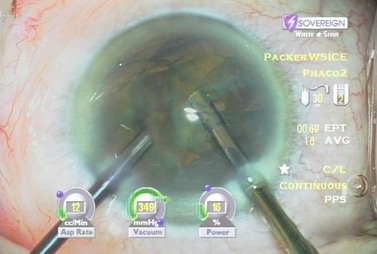
Burst mode is a power modulation that utilizes a fixed percentage power, a programmable burst width, and a linear interval between bursts. Phaco in burst mode or at this low pulse rate sounds like ‘choo-choo-choo-choo’; therefore, the name of this technique. With the energy delivered in this way, ultrasound energy into the eye is minimized and hold on the nucleus is maximized as vacuum builds between pulses or bursts. Because of the decrease in cavitational energy around the tip at this low pulse rate or in burst mode, the tunnel in the nucleus in which the tip is embedded fits the needle very tightly and allows excellent hold on the nucleus, thus maximizing control of the nucleus as it is scored and chopped (Fig. 13.2).The nucleus is scored by bringing the Fine/Nagahara chop instrument to the side of the phaco needle. It is chopped in half by pulling the chopper to the left and slightly down while moving the phaco needle to the right and slightly up. Then the nuclear complex is rotated. The chop instrument is again brought into the golden ring (Fig. 13.3), and the nucleus lollipopped, scored, and finally chopped with the resulting pie-shaped segment now lollipopped on the phaco tip (Fig. 13.4). The segment is then evacuated utilizing high vacuum and short bursts or pulse-mode phaco at 2 pulses/second. The nucleus is continually rotated so that pie-shaped segments, the size of which is driven by the density of the cataract, can be scored, chopped, and removed easily by the high vacuum and assisted by short bursts or pulses of phaco (Fig. 13.5).
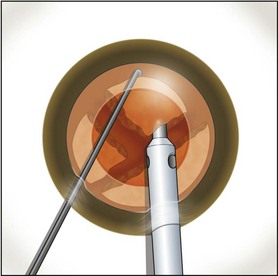
Fig. 13.5 An additional chop may be taken in the pie-shaped wedge if its density is relatively high.
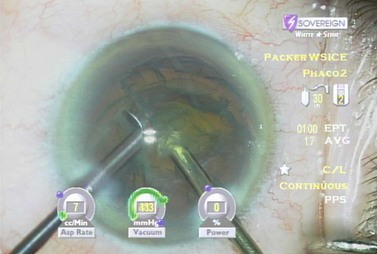
After complete evacuation of the first heminucleus, the second heminucleus is rotated to the distal portion of the bag and the chop instrument stabilizes it while it is lollipopped. It is then scored and chopped (Fig. 13.6). The pie-shaped segments can be chopped a second time to reduce their size (Fig. 13.7) if they appear too large to easily evacuate.
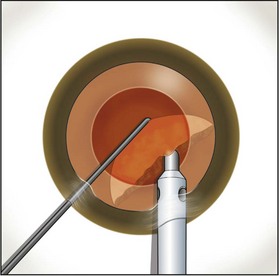
Fig. 13.6 After extraction of the first heminucleus, the remaining material is rotated distally and chopped.
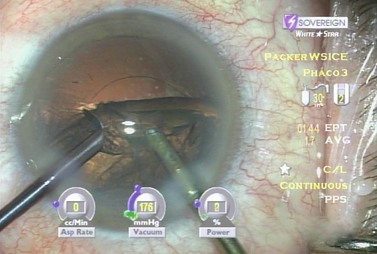
After evacuation of all endonuclear material, the epinuclear rim is trimmed in each of the three quadrants, mobilizing cortex as well. As each quadrant of the epinuclear rim is rotated to the distal position in the capsule and trimmed, the cortex in the adjacent capsular fornix flows over the floor of the epinucleus and into the phaco tip. The floor is then pushed back to keep the bag stretched until three of the four quadrants of the epinuclear rim and forniceal cortex have been evacuated while not permitting the epinucleus to flip too early. This prevents retaining a large amount of residual cortex after epinuclear evacuation. The epinuclear rim of the fourth quadrant is then used as a handle to flip the epinucleus (Fig. 13.8). As the remaining portion of the epinuclear floor and rim is evacuated from the eye, most of the time the entire cortex is evacuated with it.
Biaxial micro incision cataract surgery (B-MICS)
The historical development of biaxial micro incision cataract surgery began in the 1970s when Girard attempted to separate infusion from ultrasound and aspiration, but abandoned the procedure because of thermal injury to the tissue8,9. Shearing and colleagues successfully performed ultrasound phaco through two 1.0 mm incisions using a modified anterior chamber maintainer and a phaco tip without the irrigation sleeve10. They reported a series of 53 cases and found that phaco time, overall surgical time, total fluid use, and endothelial cell loss were comparable to those measured with their standard phaco techniques. Crozafon described the use of Teflon-coated phaco tips for bimanual high frequency pulsed phaco, and suggested that these tips would reduce friction and therefore allow surgery with a sleeveless needle11. Tsuneoka, Shiba, and Takahashi determined the feasibility of using a 1.4 mm (19-gauge) incision and a 20-gauge sleeveless ultrasound tip to perform phaco12. They found that outflow around the tip through the incision provided adequate cooling, and performed this procedure in 637 cases with no incidence of wound burn13. Additionally, less surgically induced astigmatism developed in the eyes operated with the bimanual technique. Agarwal and colleagues developed a bimanual technique, ‘Phakonit’, using an irrigating chopper and a bare phaco needle passed through a 0.9 clear corneal incision14. They achieved adequate temperature control through continuous infusion and use of ‘cooled balanced salt solution’ poured over the phaco needle.
Soscia, Howard, and Olson have shown in cadaver eye studies that phacoemulsification with the Sovereign WhiteStar system (AMO, Santa Ana, California), using a bare 19-gauge aspiration needle, does not produce a wound burn at the highest energy settings unless all infusion and aspiration are occluded due to the limited duration of energy pulses this technology delivers15,16.
Separation of irrigation from the aspirating phaco needle allows for improved followability by avoiding competing currents at the tip of the needle. In some instances, the irrigation flow from the second handpiece can be used as a gentle adjunctive surgical device – flushing nuclear pieces from the angle or loosening epinuclear or cortical material from the capsular bag while minimizing trauma to delicate intraocular structures. In refractive lens exchange the lens material may be washed completely out of the bag and extracted with aspiration and vacuum only, so that no ultrasound is used and no instrument enters the endocapsular space, increasing the safety profile of this demanding procedure17.
A separate infusion stream is also beneficial when scrubbing troublesome plaques from the posterior capsule (Fig. 13.9). Focusing the flow of fluid on the posterior capsule and putting the tissue on stretch facilitates capsule polishing with either a roughened or silicone-covered aspiration tip without entrapping the taut posterior capsule.
Perhaps the greatest advantage of the biaxial technique lies in its ability to remove subincisional cortex without difficulty. Brauweiler originally described switching infusion and aspiration hand pieces between two microincisions as a means of reaching 360° of the capsular fornices and making cortical clean-up quick and safe18. The ability to switch hands is also a significant advantage to instructors, who may find they must take over a case from a resident with opposite manual dominance.
While the advantages described above relate principally to separation of irrigation from aspiration, some of the other major advantages of B-MICS relate to incision size. For example, there has been an improvement in control of most of the steps involved in endocapsular surgery due to increase chamber stability. Since viscoelastics do not leave the eye easily through these small incisions, the anterior chamber is more stable during capsulorrhexis construction and there is much less likelihood for an errant rhexis to develop. This added margin of safety is particularly noticeable in cases of zonular compromise such as PXF, traumatic zonular dialysis and after glaucoma filtering surgery, as well as in cases of intumescent cataract and nanophthalmos with a very shallow anterior chamber (Figs 13.10 and 13.11). The added chamber stability can also make a difference in control of the capsulorrhexis in high myopia with an extremely deep anterior chamber and floppy capsule. Hydrodelineation and hydrodissection can also be performed more efficiently by virtue of a higher level of pressure building in the anterior chamber prior to eventual prolapse of viscoelastic through the microincisions.
As stated previously, the goal of cortical cleaving hydrodissection is to lyse the equatorial capsular-cortical connections, which will generally allow aspiration of the cortex, along with the mobilization of the epinucleus. Hydrodelineation is performed to allow free mobility of the endonucleus within the epinuclear shell, allowing endocapsular nuclear disassembly within the safety cushion of the epinucleus. Hydrodissection and hydrodelineation may be performed just as described above for standard small incision surgery; the microincisions do allow egress of viscoelastic during this step so that there is not an increased risk of blowing out the posterior capsule due to over-pressurization. Of note, we found that the intraocular pressure during hydrodissection, as measured in the vitreous cavity of a cadaver eye, varies around means of 78–223 mmHg regardless of whether a standard small incision or microincision is employed19. These were among the highest pressures we recorded during the entire phacoemulsification and IOL implantation procedure. Clearly, if viscoelastic is prevented from exiting the eye there is adequate pressure to rupture the capsule. This represents a special concern to users of high zero shear viscosity OVDs, who should ensure that a path for egress is prepared with a track of balanced salt solution from the cannula tip to the incision.
The surprising fact about horizontal and vertical chopping techniques with biaxial phaco is how little they differ in terms of hand movement from standard small incision coaxial phaco. Seeing the bulkier irrigating chopper in the eye and getting used to the heavier feel in one’s hand represent the major differences; the actual chopping techniques are the same. The stream of irrigation fluid from the chopper or manipulator can function as an efficient tool within the eye and is one of the most significant advantages of biaxial phaco, a key reason to abandon coaxial. In particular, washing subincisional endo- or epinuclear material into range of the phaco tip permits enhanced safety and control. An example is refractive lens exchange (RLE) with an accommodative IOL in high myopia, probably the situation in which we are most concerned about maintaining the integrity of the capsule. Not only would compromising the capsule increase the risk of posterior segment complications, but it may also result in an inability to implant the IOL of choice for the procedure. With biaxial RLE no instrument other than a cannula and a stream of fluid ever need enter the endolenticular, or endocapsular space; we can hydroexpress the soft lens material into the anterior chamber with the stream of irrigation fluid and carousel it safely from the eye with fluidics alone17. Cortical cleaving hydrodissection achieves a clean capsule without ever placing an aspiration tip below the level of the capsulorrhexis, and the absence of ultrasound energy allows for the safest, most minimally invasive procedure possible, both exemplifying the enhanced margin of safety with this approach.
If there is a breach of the posterior capsule, however, residual lens material can generally be removed while maintaining irrigation in the anterior chamber and preventing vitreous prolapse. With biaxial phaco it is possible to switch from a phaco tip to an aspirator to a vitrector if necessary without ever compromising chamber stability. The approach, once a tear is recognized, consists of continuous irrigation in the anterior chamber while lens material is removed from the bag. Once the bag is clean, a dispersive viscoelastic is injected at the level of the posterior capsule while irrigation is still maintained; only once the viscoelastic has fully tamponaded the break and filled the chamber is the irrigator removed. The IOL can then be inserted into the ciliary sulcus or the capsule through a standard temporal clear corneal incision. In the case of sulcus placement the optic is pushed posteriorly through the capsulorrhexis prior to final clean-up. Once viscoelastic is removed, the triamcinolone staining technique described by Burk and colleagues is utilized to ensure a completely vitreous-free environment in the anterior segment20.
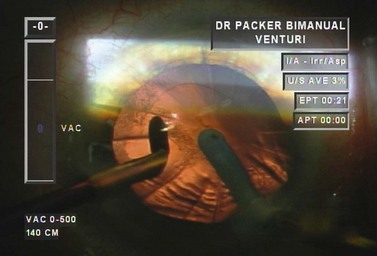
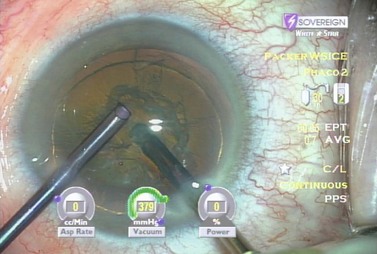
Examining each step in a model case enhances understanding. Following hydrodissection and hydrodelineation, the phaco needle is first embedded proximally with high vacuum and 40% power (Table 13.1). In the left hand is a vertical chopper which will be used to split the nucleus in two. As vacuum builds to occlusion a rapid rise time enables the phaco needle to quickly grasp the endonucleus. At the point occlusion is reached the aspiration flow rate drops to zero. We then move into foot position two so that high vacuum is maintained and the power goes to zero (Fig. 13.12). The blade of the irrigating vertical chopper is brought down just distal to the phaco tip as we lift up slightly with the phaco needle. As a full thickness cleavage plane develops, dividing the nucleus in two, we separate our hands to ensure a complete chop (Fig. 13.13).
Table 13.1 Dr Packer AMO Sovereign ‘PACKER BIMANUAL WS ICE*’ Whitestar v 6.1 Sov. Phacoemulsification parameters for biaxial microincision cataract surgery with the Sovereign WhiteStar (Abbott Medical Optics, Santa Ana, CA).
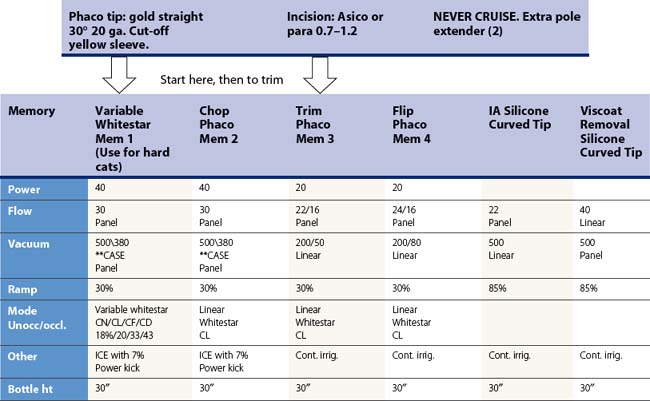
*ICE: Increased control efficiency.
**Case – [replaces occlusion mode when selected] – (Chamber stabilization environment).
Up threshold: 70%.
Down threshold: 50%.
Up time: 500 msec.
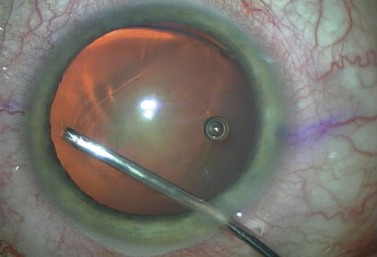
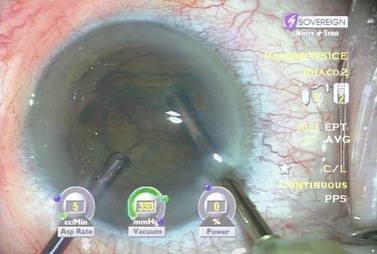
The lens can then be rotated with the irrigating chopper so that the first heminucleus can be chopped and consumed. If there is a disparity in size, the larger half is moved distally and a quadrant size piece is chopped off and consumed (Fig. 13.14). The remaining quadrant of the first heminucleus is then impaled with the phaco tip and aspirated (Fig. 13.15). Total effective phaco time (EPT), or the amount of time that ultrasound would have been turned on had it been running on 100% continuous power, is a useful parameter to follow and to this point was less than half a second. That means that we used about half a second of the maximum ultrasound power that the machine can produce to remove half of the nucleus thereby minimizing thermal injury and maximizing safety.
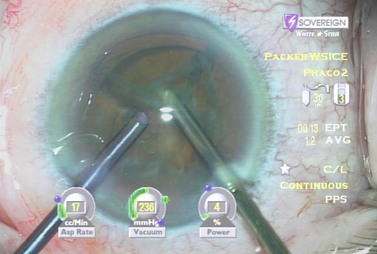
The second half of the nucleus is next addressed by rotating it with the irrigating chopper so that it is in the distal capsule. The phaco needle is embedded in the smaller heminucleus which is then subdivided with the irrigating chopper, again using high vacuum and low levels of power (Fig. 13.16). As the final quadrant is grasped and pulled centrally for aspiration, the sharp blade of the irrigating chopper is turned sideways as a safety precaution (Fig. 13.17).
When addressing the epinucleus, we reduce our settings, turn down the vacuum and flow rate and trim the rim of the epinucleus, preventing the epinucleus from flipping into the phaco needle with the stream of irrigation fluid or the irrigating chopper itself. The advantage of the trimming procedure lies in the aspiration of cortical material from behind the epinuclear shell. In most cases this step eliminates the need for I/A prior to IOL insertion. Once three quadrants of the epinuclear shell have been rotated and trimmed the final quadrant is used to flip the epinuclear bowl into the phaco needle (Fig. 13.18). Following aspiration of the epinucleus the capsule is entirely clean of cortex (Fig. 13.19). In conclusion, B-MICS with a vertical chop technique allows efficient lens extraction with rapid visual rehabilitation. This procedure demonstrates some of the tangible benefits of separating inflow from outflow, use of irrigation fluid as an instrument to mobilize material, and reduced Effective Phaco Time.
1 Kelman CD. Phacoemulsification and aspiration: a new technique of cataract removal: a preliminary report. Am J Ophthalmol. 1967;64:23.
2 Gimbel HV. Divide and conquer nucleofractis phacoemulsification: Development and variations. J Cataract Refract Surg. 1991;17:281.
3 Shepherd JR. In situ fracture. J Cataract Refract Surg. 1990;16:436.
4 Fine IH, Maloney WF, Dillman DM. Crack and flip phacoemulsification technique. J Cataract Refract Surg. 1993;19:797.
5 Koch PS, Katzen LE. Stop and chop phacoemulsification. J Cataract Refract Surg. 1994;20:566.
6 Fine IH. The choo-choo chop and flip phacoemulsification technique. Operative. Tech Cataract Refract Surg. 1998;1:61.
7 Fine IH, Packer M, Hoffman RS. Use of power modulations in phacoemulsification. J Cataract Refract Surg. 2001;27:188.
8 Girard LJ. Ultrasonic fragmentation for cataract extraction and cataract complications. Adv Ophthalmol. 1978;37:127.
9 Girard LJ. Pars plana lensectomy by ultrasonic fragmentation 1984, Part II: Operative and postoperative complications, avoidance or management. Ophthalmic Surg. 1984;15:217.
10 Shearing SP, Relyea RL, Loaiza A, et al. Routine phacoemulsification through a one-millimeter non-sutured incision. Cataract. 1985;2:6.
11 Crozafon P. The use of minimal stress and the Teflon-coated tip for bimanual high frequency pulsed phacoemulsification, presented at the 14th Meeting of the Japanese Society of Cataract and Refractive Surgery, Kyoto, Japan, July 1999.
12 Tsuneoka H, Shiba T, Takahashi Y. Feasibility of ultrasound cataract surgery with a 1.4 mm incision. J Cataract Refract Surg. 2001;27:934.
13 Tsuneoka H, Shiba T, Takahashi Y. Ultrasonic phacoemulsification using a 1.4 mm incision: Clinical results. J Cataract Refract Surg. 2002;28:81.
14 Agarwal A, Agarwal A, Agarwal S, et al. Phakonit: phacoemulsification through a 0.9 mm corneal incision. J Cataract Refract Surg. 2001;27:1548.
15 Soscia W, Howard JG, Olson RJ. Bimanual phacoemulsification through 2 stab incision. A wound-temperature study. J Cataract Refract Surg. 2002;28:1039.
16 Soscia W, Howard JG, Olson RJ. Microphacoemulsification with WhiteStar. A wound-temperature study. J Cataract Refract Surg. 2002;28:1044.
17 Fine IH, Hoffman RS, Packer M. Optimizing refractive lens exchange with bimanual microincision phacoemulsification. J Cataract Refract Surg. 2004;30:550-554.
18 Brauweiler P. Bimanual irrigation/aspiration. J Cataract Refract Surg. 1996;22(8):1013-1016.
19 Khng C, Packer M, Fine IH, et al. Intraocular pressure during phacoemulsification. J Cataract Refract Surg. 2006;32(2):301-308.
20 Burk SE, Da Mata AP, Snyder ME, et al. Visualizing vitreous using Kenalog suspension. J Cataract Refract Surg. 2003;29(4):645-651.