Chapter 163 Nuclear Replacement
Spinal surgeons contend with back pain on a daily basis. Back pain has acquired epidemic proportions in industrialized nations. It has a prevalence of 60% to 90% and is second only to the common cold as a reason for a physician visit.1,2 With the total costs associated with back pain ranging from 100 billion dollars to 200 billion dollars annually, back pain is a major health and socioeconomic burden.3 Most episodes of back pain are short in duration. However, many cases have a recurrent course, and further acute episodes affect 20% to 44% of patients in the working population within 1 year. The lifetime recurrence rate can be 85%.4
Lumbar disc degeneration is the most common cause of back pain. Disc degeneration generally results from reduced proteoglycan content in the nucleus and reduced nuclear hydration. Resulting biomechanical changes in the disc lead to loss of disc height and increasing biomechanical demand on the anulus with imbalance in the stress distribution across the disc space.5,6 When tension in the anulus is lost, ventral and dorsal instability of the motion segment can ensue. Increasing loads on the anulus can lead to anular tears with or without disc herniations. Continued loss of disc height can lead to osteophyte formation, facet arthrosis, and stiffness of the motion segment. Pain from disc degenerative disease (DDD) can occur at any stage of this degenerative cascade from early disc degeneration to instability and deformity.
Traditional treatment modalities for symptoms resulting from disc degeneration are focused on decompression with or without fusion. These treatment modalities do not attempt to halt the degenerative cascade and, in many instances, can lead to further progression of degeneration. Although short-term outcomes after lumbar discectomy have been shown to be superior compared with conservative care,7,8 long-term outcomes have been compromised by persistent back pain and a high risk of reoperations with a significant number of reherniations.9,10 Arthrodesis of the motion segment is still the “gold standard” for treatment of chronic disabling back pain of discogenic origin. However, it is difficult to predict the clinical response to arthrodesis. The outcome depends on multiple factors, such as the initial diagnosis, previous surgeries, prior fusion attempts, and the number of levels requiring fusion. Long-term studies have shown a fusion rate of 87% and clinical success rate of 76% for DDD.11,12 There are several disadvantages inherent to arthrodesis. Most importantly, arthrodesis can change the biomechanical loading of the adjacent segment, leading to accelerated degeneration.
Treatment of discogenic pain is fraught with difficulty and unpredictable outcomes. Additionally, in patients with early disc degeneration with minimal or no loss of disc height, there are no reliable methods for the diagnosis of the origin of discogenic back pain. Discography has been used to aid in the diagnosis; however, its reliability has been questioned.13–17
History of Nucleus Replacement
Early attempts at nuclear replacements consisted of injecting polymethylmethacrylate18,19 or self-curing silicone19 into the disc space after a nucleotomy. Clinical results were found to be similar to discectomy controls. Flow of the injected material and curing of the injected liquids were difficult to control. These variables led to abandonment of these procedures, but these initial attempts formed the theoretical basis of present-day preformed nuclear replacement systems.
Fernstrom20 was the first to use stainless steel balls as nuclear replacement by inserting them into the central cavity created after a discectomy. He implanted these balls in 125 patients at 191 lumbar levels. In 1966, Fernstrom20 reported that the outcome after this kind of nuclear replacement was better than discectomy alone and was similar to fusion. This procedure was largely abandoned owing to the subsidence of steel balls into the vertebral end plates. In 1995, McKenzie21 reported long-term outcomes of the Fernstrom ball. At 17 years of follow-up of 67 patients implanted with Fernstrom balls, he reported an 83% success rate in patients with one or more disc protrusions and a 75% success rate in patients with DDD. Although the Fernstrom ball never found mainstream acceptance as a nuclear replacement device, it did suggest that nuclear replacement can be a viable alternative to either a stand-alone discectomy or a fusion.
In 1981, Edeland22 suggested a design for an elastodynamic disc replacement device that would mimic the native disc biomechanically and biologically. Such a device would allow influx or efflux of water molecules in response to the load applied to it. Finding an elastodynamic material remained the most limiting factor. Attempts at finding such material in the 1980s and 1990s focused on either silicone polymers or a hydrogel nucleus composed of polyvinyl alcohol that was used to make soft contact lenses. Hou et al.23 designed a solid preformed silicone rubber horseshoe-shaped implant termed the lumbar intervertebral replacement prosthesis. Biomechanical cadaver studies and animal studies in monkeys showed restoration of the disc height and mechanical stability. Human studies with this implant have not been reported.
In 1991, Bao and Hingham patented a hydrogel nucleus made of polyvinyl alcohol that imbibed and extruded water and could replicate the biomechanical viscoelastic properties of the native nucleus. An animal study by Allen et al.24 showed no evidence of systemic or local toxicity but a high incidence of implant extrusion. Despite this setback, proven preclinical benefits with hydrogel acted as a springboard for other nucleus replacement technologies. With its first clinical use in 1996, the prosthetic disc nucleus (PDN) is the most well-known nucleus replacement device made of hydrogel.25 The device is made of hydrogel enclosed in a polyethylene terephthalate (PET) jacket to restrict expansion on hydration. Multiple design and material changes have followed after its first use (Fig. 163-1).
Current Nuclear Replacement Technology
HydraFlex (Raymedica, Bloomington, IN) is the latest version of the PDN; it is inserted in a dehydrated form that rehydrates on implantation (Fig. 163-2). The hydrogel component is a block copolymer of polyacrylamide-polyacrylonitrile (Hypan, Lipo Technologies, Vandalia, OH) formed by solution casting. The jacket is formed of a dense woven circular tube of PET, which is a thermoplastic polymer. The PET jacket is inelastic but flexible, which helps the hydrogel core to maintain its shape when subject to compressive forces. The hydrogel core absorbs water on implantation. Hypan80, which is currently being used, absorbs 80% of its weight by water, which makes it softer and flexible. HydraFlex takes approximately 32 hours to reach hydration equilibrium.

FIGURE 163-2 PDN replacement.
(From Berlemann U, Schwarzenbach O: An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. Eur Spine J 18:1706–1712, 2009.)
DASCOR (Disc Dynamics, Eden Prairie, MN) is a two-part in situ curable polyurethane. It consists of a polyether polyurethane core encapsulated and adhered to a polycarbonate polyurethane balloon, which has cavity expansion and conforming capabilities. The device is fabricated by mixing a two-part, liquid, pre–polyether polyurethane reactive system and injecting a liquid mixture in a polycarbonate polyurethane balloon delivery catheter placed within the nucleus cavity (Fig. 163-3). A custom electromechanical injection system is used to apply a computer-controlled pressure profile designed to deliver the liquid polymer.26
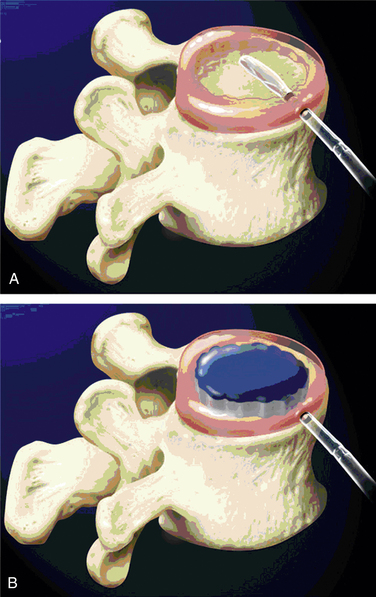
FIGURE 163-3 DASCOR nuclear replacement.
Implant placement (A) and deployment (B). (From Ahrens M, Tsantrizos A, Donkersloot P, et al: Nucleus replacement with the DASCOR disc arthroplasty device: interim two-year efficacy and safety results from two prospective, non-randomized multicenter European studies. Spine [Phila Pa 1976] 34:1376–1384, 2009.)
NuCore Injectable Disc Nucleus (Spine Wave, Shelton, CT) is an in situ curing protein polymer hydrogel that mimics the properties of the natural nucleus. The polymer chain is composed of silk and elastin components designed for elasticity and toughness. The polymer (P27K) is mixed with a cross-linking agent at the time of implantation and is injected as a fluid through the anular defect where it adheres to the surrounding intradiscal tissue as it cures. There is no measurable temperature increase produced during the cure process. NuCore has been studied as an adjunct to discectomy27 and is in the process of being studied for treatment of DDD (Fig. 163-4).
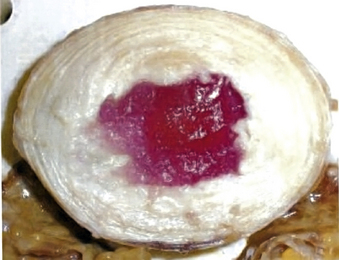
FIGURE 163-4 NuCore Injectable Disc Nucleus (red), shown interdigitating with normal disc after injection into nucleotomy defect.
(Used with permission of Spine Wave, Shelton, CT.)
The NeuDisc device (Replication Medical, Cranbury, NJ) is composed of a material that is a hydrolyzed polyacrylonitrile hydrogel (Aquacryl). In the absence of mechanical restrictions, the hydrogel imbibes 90% volume as a liquid. When implanted in a dehydrated state, this hydrogel implant is substantially smaller than the volume of resected nucleus and is easily placed though an incision in the anulus. After hydration, it expands anisotropically, in the axial direction principally, and becomes substantially larger than the incision. The axial expansion is not constrained by a jacket and results from its structure of polymer layers tied together with an internal substructure28 (Fig. 163-5; see also Fig. 163-1).
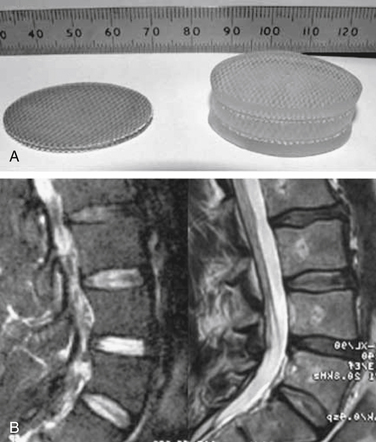
FIGURE 163-5 NeuDisc device shown clinically (preoperative and postoperative) (A) and on MRI (B).
(From Bertagnoli R, Karg A, Voigt S: Lumbar partial disc replacement. Orthop Clin North Am 36:341–347, 2005.)
Among nonelastomeric devices, Regain (Biomet, Warsaw, IN) is a modified form of the Fernstrom ball with a larger radius for larger end-plate contact surface that is manufactured from pyrolytic carbon. NUBAC (Pioneer Surgical Technology, Marquette, MI) is the only articulating nonelastomeric implant and is made of PEEK-OPTIMA (Invibio, Greenville, NC), which is a proven biostable and biocompatible polymer widely used for spinal fusion and shown to generate minimal wear. NUBAC comprises two plates with a large contact surface area in an anatomic shape similar to the native nucleus with an inner ball-and-socket articulation29 (Fig. 163-6).
Indications for Nucleus Replacement
The indications and contraindications for nucleus replacement are largely determined by the intended objectives and the unique design of the nucleus replacement devices. Although the indications and contraindications for the initial clinical studies were determined a priori, a better idea of these criteria cannot be established until at least limited clinical follow-ups are performed. The criteria will then be based on the outcomes of such studies and the risk-to-benefit and cost-to-benefit ratios.
Outcomes
To date, the most widely studied device with the longest history is the PDN. The PDN has shown favorable biologic compatibility and biomechanical stability. Fatigue testing performed with loads of 200 N to 600 N for 50 million cycles showed that the device continues to function with maintained disc height and structural integrity. It has been shown not to form polyethylene wear debris.30 Eysel et al.31 analyzed the effect of the PDN on restoration of segmental mobility after a nucleotomy in human cadaver spines. These investigators found that there was a statistically significant (P < .05) increase in segmental mobility in all directions after nucleotomy with an increased mobility of the segment of 38% to 100%. After the introduction of two PDN implants, there was a restoration of segmental mobility for all movement directions with no statistically significant difference compared with the intact segment before nucleotomy.
In clinical studies, the major concerns with the PDN device have been the risk of implant migration and the potential for end-plate changes and subsidence. Schonmayr et al.32 reported early clinical results with the PDN implant with a minimum 2-year follow-up in 10 patients with symptomatic lumbar DDD. They showed good functional outcomes with restoration of disc height and motion. However, three implants migrated, and one patient required a revision surgery for migration.
Multiple phases of implant trials following these early results involved changes made to the device shapes and to the surgical protocol to facilitate implantation and eliminate the high device migration rates. After these modifications, the success rate for the device improved significantly. Clinical data show excellent results with marked improvements in Oswestry Disability Index (ODI) and Prolo scores with disc height measurements being well maintained within normal physiologic ranges. Klara and Ray25 reported a cumulative 90% success rate with 10% of the patients requiring explantation. Following these early results, Bertagnoli and Vazquez33 described implanting the PDN device through an anterolateral transpsoatic approach to reduce the risk of implant migration. They reported the results in eight patients after using this technique. Four of the first five patients developed a transient psoas neurapraxia. Ventral migration of the PDN was detected in three patients and was thought to be due to preexisting defects in the anterior anulus unrelated to surgery. Because the patients were asymptomatic, no revision was attempted. One patient required a revision surgery with fusion for implant subsidence. Of the remaining seven patients, significant improvements were noted in the mean ODI and Prolo scores at 6 months and 12 months with maintained disc heights.
In earlier studies, two PDN implants were used per disc level. It was suggested that using two implants per level may lead to overstuffing of the disc space and increased risk of implant migration. Manufacturers have advocated the use of a single larger implant sufficient to occupy the cavity after a nucleotomy. In 2002, 45 patients were implanted with a single PDN implant for lumbar disc herniation. Follow-up was reported by Jin et al.34 for 33 patients at 6 months. Statistically significant improvements were noted in the ODI and Prolo scores with significant improvements in spinal mobility and disc heights. There were no cases of implant migration or failure in 6 months. Although Jin et al.34 did not report any end-plate changes and subsidence at 6 months, these have been reported on longer follow-up after PDN implantation.
Shim et al.35 followed 46 patients after PDN implantation for more than 6 months. Four patients (8.7%) showed device extrusion that needed revision surgery, and one patient had an infection. The investigators reported a 9.4% increase in the disc height at 1-year follow-up; 19.6% of patients showed some subsidence, 60.9% of patients showed end-plate sclerosis, and 82.8% of patients showed aggravation of Modic changes on MRI. Clinical results were excellent in 5 patients (10.9%), good in 31 (67.4%), fair in 3 (6.5%), and poor in 7 (15.1%), with an overall success rate of 78.3%.
Ma et al.36 reported on the midterm to long-term follow-up of PDN. From March 2002 to October 2003, 34 patients who underwent PDN replacement were followed for an average of 52.6 months (range 48 to 66 months). Although initial clinical results were encouraging, the patients did not do as well over the longer term. At 12 months after operation, a significant proportion of patients recovered from low back pain or leg pain, with an ODI score that decreased an average of 18.2% and pain on the visual analogue scale (VAS) that decreased to 1.8. The average increase of the postoperative disc height was 17.6%, with a range of motion of 9.2 degrees. At the final follow-up, the ODI increased from 18.2% 12 months after operation to 31.2%, VAS increased from 1.8 to 3.1, disc height improvement averaged to 13.5%, and range of motion decreased to 6.8 degrees. The rate of degeneration or breakages of the end plates was 64.7% (22 of 34), and implant device migrations were observed in 25 patients.
Published clinical data are limited for other devices. For the DASCOR device, 2-year follow-up data have been reported from European centers for 85 patients implanted with the device for single-level DDD between February 2003 and July 31, 2007. Mean VAS and ODI scores improved significantly after 6 weeks and throughout the 2-year follow-up. Radiographic results showed, at a minimum, maintenance of disc height with no device expulsion and, despite Modic type 1 changes, no subsidence. Analgesic medication use decreased 84.6% by 24 months from the preoperative average. Seven patients required explants for various reasons, but the device was in its intradiscal implant position in all patients, and no expulsions of the device had been observed.26
Berlemann and Schwarzenbach27 reported on the use of NuCore as an adjunct to discectomy to replace the loss of disc tissue after herniation and microdiscectomy. After a standard microdiscectomy procedure in 14 patients, the hydrogel material was injected into the nuclear void to replace what tissue had been lost to the herniation and surgery. Results showed significant improvement for leg and back pain and functional scores. No complications or device-related adverse events were observed. MRI controls confirmed stable position of the implants with no repeat herniations. Radiographic measurements indicated better maintenance of disc height compared with literature data on microdiscectomy alone.
A short follow-up has been reported after implantation of the NUBAC device. Ten patients in Mexico were implanted with NUBAC after discectomy and followed for 3 months. VAS score improved from 8.1 to 2.5 (P < .05), and the ODI improved from 58.2% to 24.2% (P < .05). Disc height before surgery was 9.4 mm and 3 months postoperatively was 12.5 mm with no complications, migration, or subsidence.29
Summary
Over the years, attempts at nuclear replacement were limited by the lack of material technology and a suitable design. With more recent advances, it has been possible to use materials that replicate the native nucleus properties and are able to imbibe and hold water. Multiple designs are in the process of development and in clinical studies. Current outcome data have been encouraging in terms of improved VAS and ODI scores. However, owing to lack of randomized controlled trials, it is unclear if these results are better and more durable than stand-alone discectomy or fusion procedures. Longer-term studies are required to assess the effects of nuclear replacement on the progress of degenerative cascade.
Ahrens M., Tsantrizos A., Donkersloot P., et al. Nucleus replacement with the DASCOR disc arthroplasty device: interim two-year efficacy and safety results from two prospective, non-randomized multicenter European studies. Spine (Phila Pa 1976). 2009;34:1376-1384.
Allen M.J., Schoonmaker J.E., Bauer T.W., et al. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine (Phila Pa 1976). 2004;29(5):515-523.
Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154-159.
Hamby W.B., Glaser H.T. Replacement of spinal intervertebral discs with locally polymerizing methyl methacrylate: experimental study of effects upon tissues and report of a small clinical series. J Neurosurg. 1959;16:311-313.
Ray C.D. The PDN prosthetic disc-nucleus device. Eur Spine J. 2002;11(Suppl 2):S137-S142.
Shim C.S., Lee S.H., Park C.W., et al. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech. 2003;16:324-330.
1. Brodke D.S., Ritter S.M. Nonsurgical management of low back pain and lumbar disk degeneration. Instr Course Lect. 2005;54:279-286.
2. National Center for Health Statistics. National Center for Health Statistics: National health interview survey. Hyattsville, MD: NCHS; 1988.
3. Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg [Am]. 2006;88(Suppl 2):21-24.
4. Bone and joint decade report. 2005. Available at www.boneandjointdecade.org
5. Nachemson A. The load on lumbar disks in different positions of the body. Clin Orthop Relat Res. 1966;45:107-122.
6. Edwards W.T., Ordway N.R., Zheng Y., et al. Peak stresses observed in the posterior lateral anulus. Spine (Phila Pa 1976). 2001;26:1753-1759.
7. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451-2459.
8. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008;33:2789-2800.
9. Osterman H., Sund R., Seitsalo S., et al. Risk of multiple reoperations after lumbar discectomy: a population-based study. Spine (Phila Pa 1976). 2003;28:621-627.
10. Loupasis G.A., Stamos K., Katonis P.G., et al. Seven- to 20-year outcome of lumbar discectomy. Spine (Phila Pa 1976). 1999;24:2313-2317.
11. Bono C.M., Lee C.K. The influence of subdiagnosis on radiographic and clinical outcomes after lumbar fusion for degenerative disc disorders: an analysis of the literature from two decades. Spine (Phila Pa 1976). 2005;30:227-234.
12. Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976). 2004;29:455-463.
13. Carragee E.J., Paragioudakis S.J., Khurana S. 2000 Volvo Award winner in clinical studies: lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976). 2000;25:2987-2992.
14. Carragee E.J., Tanner C.M., Khurana S., et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine (Phila Pa 1976). 2000;25:1373-1380.
15. Madan S., Gundanna M., Harley J.M., et al. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245-251.
16. Scuderi G.J., Brusovanik G.V., Golish S.R., et al. A critical evaluation of discography in patients with lumbar intervertebral disc disease. Spine J. 2008;8:624-629.
17. Willems P.C., Elmans L., Anderson P.G., et al. Provocative discography and lumbar fusion: is preoperative assessment of adjacent discs useful? Spine (Phila Pa 1976). 2007;32:1094-1099.
18. Hamby W.B., Glaser H.T. Replacement of spinal intervertebral discs with locally polymerizing methyl methacrylate: experimental study of effects upon tissues and report of a small clinical series. J Neurosurg. 1959;16:311-313.
19. Nachemson A. Some mechanical properties of the lumbar intervertebral discs. Bull Hosp Joint Dis. 1962;23:130-143.
20. Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154-159.
21. McKenzie A.H. Fernstrom intervertebral disc arthroplasty: a long term evaluation. Orthop Int. 1995;3:313-324.
22. Edeland H.G. Suggestions for a total elasto-dynamic intervertebral disc prosthesis. Biomater Med Devices Artif Organs. 1981;9:65-72.
23. Hou T.S., Tu K.Y., Xu Y.K., et al. Lumbar intervertebral disc prosthesis: an experimental study. Chin Med J. 1991;104:381-386.
24. Allen M.J., Schoonmaker J.E., Bauer T.W., et al. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine (Phila Pa 1976). 2004;29:515-523.
25. Klara P.M., Ray C.D. Artificial nucleus replacement: clinical experience. Spine (Phila Pa 1976). 2002;27:1374-1377.
26. Ahrens M., Tsantrizos A., Donkersloot P., et al. Nucleus replacement with the DASCOR disc arthroplasty device: interim two-year efficacy and safety results from two prospective, non-randomized multicenter European studies. Spine (Phila Pa 1976). 2009;34:1376-1384.
27. Berlemann U., Schwarzenbach O. An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. Eur Spine J. 2009;18:1706-1712.
28. Bertagnoli R., Sabatino C.T., Edwards J.T., et al. Mechanical testing of a novel hydrogel nucleus replacement implant. Spine J. 2005;5:672-681.
29. Alpizar-Aguirre A., Mireles-Cano J.N., Rosales-Olivares M., et al. [Clinical and radiological follow-up of Nubac disc prosthesis: preliminary report]. Cir Cir. 2008;76:317-321.
30. Ray C.D. The PDN prosthetic disc-nucleus device. Eur Spine J. 2002;11(Suppl 2):S137-S142.
31. Eysel P., Rompe J., Schoenmayr R., et al. Biomechanical behaviour of a prosthetic lumbar nucleus. Acta Neurochir (Wien). 1999;141:1083-1087.
32. Schonmayr R., Busch C., Lotz C., et al. Prosthetic disc nucleus implants: the Wiesbaden feasibility study: 2 years follow-up in ten patients. Riv Neuroradiol. 1999;12(Suppl 1):157-162.
33. Bertagnoli R., Vazquez R.J. The anterolateral transpsoatic approach (ALPA): a new technique for implanting prosthetic disc-nucleus devices. J Spinal Disord Tech. 2003;16:398-404.
34. Jin D., Qu D., Zhao L., Chen J., Jiang J. Prosthetic disc nucleus (PDN) replacement for lumbar disc herniation: preliminary report with six months’ follow-up. J Spinal Disord Tech. 2003;16:331-337.
35. Shim C.S., Lee S.H., Park C.W., et al. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech. 2003;16:324-330.
36. Ma Y.Z., Xue H.B., Chen X., et al. [The mid- or long-term clinical results of prosthetic disc nucleus replacement in the treatment of lumbar disc disease]. Chung Hua Wai Ko Tsa Chih. 2008;46:350-353.









