Chapter 10 Nuclear Medicine
Positron Emission Tomography
Basic Physics of Positron Emission Tomography
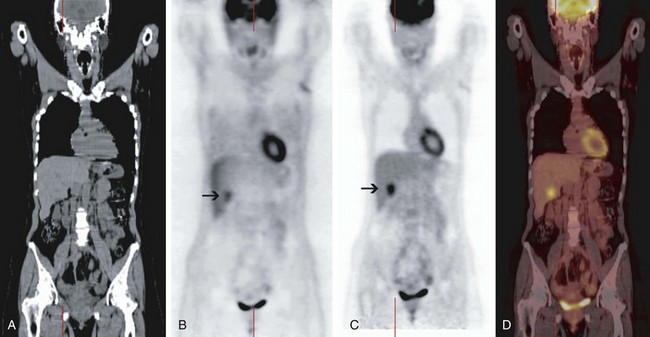
In addition to delivering anatomic information, the CT portion of PET/CT is used to measure the attenuation of the x-ray photons traveling through the patient to produce the so-called attenuation map and correct the PET data for tissue attenuation. During PET acquisition, photons from structures deep in the abdomen or pelvis are more strongly attenuated than those from superficial structures and the chest. The intensity of uptake in deeper structures is underestimated on non–attenuation-corrected PET images; the intensity of uptake in the deeper structures is normalized to the intensity of uptake in the superficial structures on the attenuation-corrected PET images (Fig. 10-1). Although correction of the PET data for tissue attenuation is indispensable, misalignment between PET and CT can cause mislocalization of lesions on the fused PET/CT images. This may be caused by the changed position of a body part (e.g., neck, legs) or physiologic changes in the position of an organ (e.g., respiratory movement) between the transmission scan (CT) and the emission scan (non–attenuation-corrected PET). Because the degree of misalignment and resulting mislocalization can be significant, the physician must be cautious when interpreting the attenuation-corrected PET/CT images or using PET/CT images for radiation therapy planning. The magnitude of this misalignment can be assessed by fusing the non–attenuation-corrected PET images with the CT images; this can be performed on any PET review station. In case of significant misalignment, the non–attenuation-corrected PET images should be reviewed without fusion with the CT images, and the metabolic findings of PET should be correlated side by side with the anatomic findings of CT (Fig. 10-2).
General Aspects of Tumor Visualization on 18F-Fluorodeoxyglucose Positron Emission Tomography
18F-fluorodeoxyglucose is also taken up in benign processes such as infection and inflammation because white blood cells and fibroblasts are highly avid for FDG. This is probably the most common reason for false-positive findings on PET scans obtained for oncologic indications. A false-positive reading on PET can partly be avoided by obtaining the patient’s medical history and determining the pretest likelihood of an infectious or inflammatory process. There are no published data on the time interval after an invasive procedure or radiation therapy in which a PET scan can be falsely positive, and the inflammatory cell reaction after such a procedure varies among organs. For example, there is evidence that only minimal reactive cell accumulation occurs in the liver within the first 3 days after radiofrequency ablation and that the reactive cells do not cause false-positive PET findings at least in the first 7 days after radiofrequency ablation.1
The standardized uptake value (SUV) is a semiquantitative measure of the tracer uptake in a region of interest that normalizes the lesion activity to the injected dose and body weight; SUV does not have a unit. Despite initial enthusiasm, it is generally accepted that SUV should not be used to differentiate malignant from benign processes, and that the visual interpretation of PET studies by an experienced reader provides the highest accuracy. There are many factors influencing the calculation of SUV, such as the body weight and composition, the time between tracer injection and image acquisition, the spatial resolution of the PET scanner, and the image reconstruction algorithm. Nonetheless, SUV may be useful as a measure to follow the metabolic activity of a tumor over time within the same patient and to compare different subjects within a research study under defined conditions. For example, the SUV of an individual tumor can be measured before and at different time points after therapy, and any change can be used as an index of therapeutic response. However, it is unclear whether this measure can be used for clinical decision making. Some indicate that the intensity of FDG uptake by itself can assess the aggressiveness of a tumor and therefore correlate with prognosis, regardless of the treatment modality. However, there is no firm evidence that patient management should be modified on the basis of intensity of uptake.2
Most Common Indications for 18F-Fluorodeoxyglucose Positron Emission Tomography in Oncology
Lung Cancer
Positron emission tomography has an overall sensitivity of more than 90% and a specificity of about 85% for diagnosing malignancy in primary and metastatic lung lesions; the sensitivity and specificity of PET for small cell lung cancer are similar. The sensitivity of PET for bronchoalveolar lung cancer and carcinoid of the lung is about 60%, and the specificity of PET for lung cancer is lower in areas with a high prevalence of granulomatous lung disease. PET is particularly useful in patients with a low (5% to 20%) or intermediate (20% to 70%) risk of lung cancer, as determined by an evaluation of symptoms, risk factors, and radiographic appearance. In these cases, PET is helpful in moving the patient to the very-low-risk (<5%) or high-risk (>70%) category.3 It is expected that the use of PET for diagnosing malignancy in indeterminate lung nodules will continue to grow as more patients are diagnosed with nodules on CT performed for other indications or as a screening test. Most current PET scanners are capable of detecting lung lesions as small as 6 mm, and the resolution of PET is likely to improve. Most of the literature in this regard is based on data from the 1990s, when the resolution of PET was above 1 cm; studies are being conducted to systematically assess the sensitivity and specificity of PET for detecting malignancy in subcentimeter lung lesions.
In mediastinal staging of non–small cell lung cancer (NSCLC), patients with clinical stage I and II disease have by definition a radiographically negative mediastinum. However, in patients with central tumors, adenocarcinoma, or N1 lymph node enlargement, the false-negative rate of CT for mediastinal involvement is 20% to 25%. It is unclear whether PET should be used instead of mediastinoscopy in staging the disease of these patients. In mediastinal staging of clinical stage III tumors, positive results of PET need to be confirmed by tissue diagnosis because of a relatively high false-positive rate (15% to 20%). The false-negative rate of PET and mediastinoscopy in assessing enlarged mediastinal lymph nodes is 5% to 10%, and some authorities therefore do not pursue biopsy in the case of a negative PET result for disease in the mediastinum, whereas others argue that mediastinoscopy can detect microscopic metastases, and they are not comfortable accepting a negative PET result.4 Practically, in larger centers, patients with stage III tumors undergo both PET to assess for distant metastases and mediastinoscopy, but a strong argument for staging of the mediastinum with PET can be made in communities without an experienced mediastinoscopy service.
For patients with clinical stage I peripheral tumors, most authorities do not request mediastinoscopy before surgery, because the rate of mediastinal or systemic involvement is very low (about 5%); however, this detection rate is based on investigations before implementation of PET, and PET may increase the detection rate. Among patients with clinical stage II tumors, the rate of metastatic disease is higher, and PET may be warranted in these patients to assess for systemic disease. For stage III tumors, the false-negative rate of clinical evaluation for systemic disease is 15% to 30%, and PET is justified instead of a battery of other tests (e.g., bone scan, CT, MRI) to assess for distant metastases.4 PET is more sensitive (90% vs. 80%) and more specific (90% vs. 70%) than bone scan in detecting bone metastases from NSCLC; PET has a sensitivity and specificity of greater than 90% in detecting adrenal metastases from NSCLC. Brain CT or MRI is still needed because PET cannot reliably detect brain metastases because of physiologically intense brain uptake of FDG. For patients with stage IV tumors, PET may be able to indicate the most accessible site for biopsy.
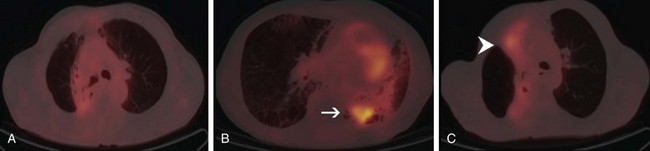
PET is also useful in restaging NSCLC. In particular, PET appears to be more sensitive than CT in differentiating postirradiation change from local recurrence, although differentiating these two entities remains a challenge. The postirradiation change in the chest can remain intense on PET for up to several years. In differentiating local recurrence from postirradiation change, the intensity of uptake and its shape should be taken into account (Fig. 10-3).
Head and Neck Cancer
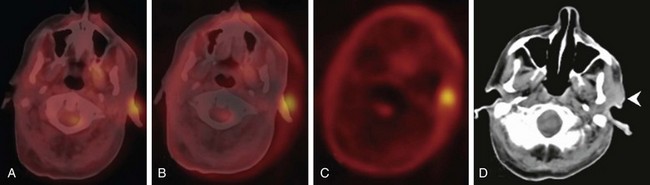
Most patients with head and neck cancer present for PET with a known diagnosis. However, cervical lymph node metastases from an unknown primary tumor constitute about 2% of newly diagnosed head and neck cancer cases; CT and MRI can identify up to 50% of the primary tumors in patients with no findings on physical examination. The overall PET detection rate in patients with negative results of physical examination, CT or MRI, and endoscopy is about 25%. This detection rate is based on studies in which stand-alone PET was used, and it is expected to be higher with fused PET/CT. PET/CT should probably be performed instead of CT or MRI before endoscopy.5 Knowledge of the variable physiologic uptake patterns in the head and neck region is essential to minimize false-positive interpretations.
In initial staging of head and neck tumors, PET has a sensitivity and specificity of about 90% for nodal staging, and PET therefore is more sensitive and specific than CT or MRI. A weakness of PET is its low sensitivity (30%) for nodal disease in patients with disease in the neck at clinical stage N0. Given the high specificity of PET in nodal staging, it appears reasonable to perform neck dissection in patients with a positive PET result, whereas those with a negative PET result may be able to undergo sentinel node localization and biopsy.5 In addition to local staging, PET can detect synchronous cancers and distant metastases. In initial staging of head and neck cancers, a PET scan is overall most helpful in patients with locally advanced disease because these patients have a risk of 10% or greater for distant disease.
For restaging of head and neck tumors after radiation therapy, PET is highly sensitive; however, the optimal time to perform PET is a matter of debate. There is a higher likelihood of false-positive findings when PET is performed earlier than 3 months after irradiation. Based on recommendations from large institutions in the United States, PET should be performed 3 months after radiation therapy; patients with a negative scan can be followed without intervention (i.e., high negative predictive value), but those with a positive scan need to undergo further evaluation.5
Colorectal Cancer
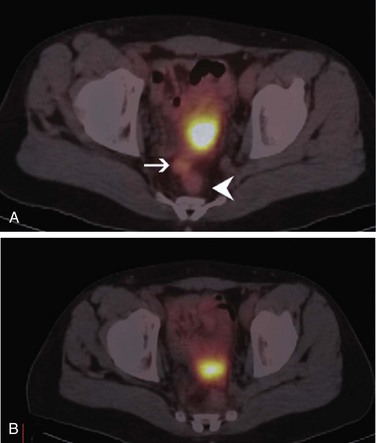
PET plays no role in the screening or diagnosing of colorectal cancer, and neither the depth of the tumor nor the status of local lymph nodes can be assessed by PET. However, PET is highly sensitive in detecting distant hepatic and extrahepatic metastases. A meta-analysis of the literature on detection of hepatic metastases from colorectal, gastric, and esophageal cancers by ultrasound, CT, MRI, and PET found that in studies with a specificity higher than 85%, the mean weighted sensitivity was 55% for ultrasound, 72% for CT, 76% for MRI, and 90% for PET. Results of pairwise comparison between imaging modalities demonstrated a greater sensitivity of PET than ultrasound (p = .001), CT (p = .017), and MRI (p = .055). The conclusion was that at equivalent specificity, PET is the most sensitive noninvasive imaging modality for the diagnosis of hepatic metastases from colorectal, gastric, and esophageal cancers.6 Considering the higher sensitivity of PET in detecting distant metastases and the introduction of intravenous contrast media to the CT portion of fused PET/CT, it is conceivable that PET/CT will be increasingly employed in preoperative staging of colorectal cancer; the contrast-enhanced CT portion of PET/CT can be used instead of conventional CT or MRI for evaluation of anatomic resectability of liver metastases. PET plays an important role in restaging of colorectal cancer and is even more important now that it is known that treatment of limited metastatic disease can be curative. PET can visualize the site of the local and distant disease when recurrence is suspected based on the clinical findings, findings on other imaging modalities, or an increasing carcinoembryonic antigen level with sensitivity and specificity higher than 90% (Fig. 10-4).
Breast Cancer
PET can increase the detectability of small primary breast cancers and may be especially useful in evaluating patients with dense breast tissue. Its role in routine patient care is under investigation. In evaluating the axillary lymph nodes, PET does not play any role because of its low sensitivity (60%) despite relatively high specificity (80%).7 In contrast, PET is relatively sensitive (85%) and specific (90%), and it is superior to CT (sensitivity of 54%, specificity of 85%) in evaluation of the internal mammary chain lymph node for metastases. The main role of PET in breast cancer lies in the investigation of distant metastases and response monitoring. Compared with CT, PET has a higher sensitivity (90% vs. 40%) but lower specificity (80% vs. 95%) in detecting metastatic disease. Overall, PET has the same sensitivity as bone scan in detecting bone metastases (both about 90%), but PET appears to be somewhat more sensitive than bone scan for osteolytic lesions and somewhat less sensitive than bone scan for osteoblastic lesions. PET has a higher specificity than bone scan in detecting bone metastases (95% vs. 80%). This may be explained by the fact that PET captures the metabolic activity of the tumor cells independently of changes in the bone, whereas bone remodeling seen on bone scan can result from metastatic disease and benign causes.
Targeted Imaging In Nuclear Medicine
Bone Scanning
An even older modality is now gaining a resurgence of interest. Several studies have shown that imaging with 18F-NaF using modern PET/CT systems is superior to 99mTc-labeled bisphosphonate imaging, including whole body SPECT.8 Furthermore, the imaging time involved in obtaining a tomographic whole body view with PET/CT is similar to the time it would take to obtain a whole-body planar scan with additional limited planar and/or SPECT views. Because 18F-NaF imaging can be started much earlier than traditional SPECT imaging, the procedure from injection time to the end of scan is a few hours shorter for the patient. Although 18F-NaF imaging has been approved by the U.S. Food and Drug Administration (FDA), it is currently being evaluated by the Centers for Medicare and Medicaid Services (CMS) for reimbursement as a routine clinical procedure.
Antibody-Based Imaging Agents
Carcinoembryonic antigen scans use a labeled murine antibody fragment (99mTc-arcitumomab) to detect recurrent colorectal carcinoma, and the modality can also be used for detecting breast cancer. OncoScint (111In-satumomab pendetide) is another antibody used for detection of recurrent colorectal and ovarian carcinomas. For colorectal malignancy, the sensitivity, specificity, and accuracy of extrahepatic disease recurrence detection for satumomab are 97%, 78%, and 92%, respectively, whereas those for CT scans are 72%, 89%, and 76%, respectively. For liver metastases, CT is better, with sensitivity, specificity, and accuracy values all about 92%; satumomab values are 85%, 92%, and 89%, respectively. When the two modalities are combined, the sensitivity is approximately 90%.9 Nevertheless, FDG-PET outperforms these modalities and is the scan of choice, although the antibodies have clinical value where FDG-PET is not available.
Neuroendocrine Tumor Imaging
Routine imaging of neuroblastomas and pheochromocytomas involves metaiodobenzylguanidine (MIBG), a norepinephrine analog taken up by the uptake 1 mechanism in receptors. Like octreotide, its uptake occurs in many similar cancers, although not with the same frequency. For imaging, MIBG may be labeled with 131I, 123I, or 124I, a positron emitter. The sensitivity for lesion detection can exceed 90%, and specificity approaches almost 100% for single-photon imaging.10 However, as tumors de-differentiate or metastasize to other sites, specifically the bones, MIBG becomes less sensitive, in which case a bone scan is useful. With the advent of 124I-MIBG used in PET/CT, it is possible that the increased resolution will allow better detection of disease, obviating the need for bone scans.
There is growing interest in being able to use newer tracers such as 3,4-dihydroxy-6-18F-fluoro-phenylalanine (18F-FDOPA) and PET in neuroendocrine imaging. These agents use neurohumoral pathways of uptake and trapping, similar in concept to that of FDG, to concentrate in relevant areas. Many studies show that this tracer has far greater accuracy for disease detection than either octreotide analogs or MIBG.11 Unlike the SPECT counterparts, the PET imaging can be done within an hour after injection; images are of the same quality as PET images. 18F-FDOPA is most useful in well-differentiated to moderately differentiated tumors, with FDG being superior for the more poorly differentiated ones. Other octreotide-based positron tracers labeled with 68Ga, such as [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-1-Nal3-octreotide (68Ga-DOTANOC), are showing promise as imaging agents.
Renal Cell Carcinoma
Clear cell varieties of renal cell carcinomas can constitute a substantial percentage of incidentally discovered renal masses, and methods to identify this aggressive cancer early and preoperatively could be useful. A recently developed agent, 124I-girentuximab, a radiolabeled antibody against carbonic anhydrase IX (CA IX), is reactive in more than 95% of immunohistochemical assays on fresh-frozen tissues. A phase I trial for this agent showed accuracy of more than 90% for the detection of primary clear cell renal carcinoma, although the accuracy for metastatic disease detection was not studied.12 A phase III trial of this agent has been concluded and is undergoing data analysis.
Multimodality Imaging
An intriguing concept is imaging of multiple tracers simultaneously, allowing a wealth of information to be obtained concurrently in a single imaging session. Whereas this can be done with different isotopes in the SPECT arena, it is much more limited with PET because all the positron isotopes emit the same energy photons (511 keV) and, therefore, different PET tracers cannot be distinguished. Whereas acquisition of separate images, such as FDOPA and FDG, may provide a wealth of molecular information about a disease, as well as diagnostic information, it would entail obtaining two separate scans, one for each tracer. Conversely, performing a scan with simultaneous administration of tracers would provide optimal diagnostic imaging and would require only one scanning session, although molecular information provided by the separate tracers would not be obtained. Similarly, other theoretical combinations with FDG/124I-cG250, FDG/F-choline, FDG/NaF, and others can be used in a variety of diseases to improve the overall performance of PET as a diagnostic tool, if that is all that is needed.13
Targeted Radionuclide Therapy
Thyroid Cancer Evaluation and Treatment
Thyroid cancer patients have traditionally undergone 4 to 6 weeks of LSM-thyroxine withdrawal prior to radioiodine scanning and treatment to stimulate tumors to take up iodine. The introduction of recombinant human thyroid-stimulating hormone (rhTSH) has revolutionized thyroid cancer evaluation. TSH is injected intramuscularly to stimulate residual thyroid cells to take up radioiodine. Results are available within a few days, which means that patients need not endure a prolonged hypothyroid state before diagnostic studies can be obtained, as they once did. Although approved for therapeutic administration of radioiodine for postsurgical remnant ablation in low-risk disease groups (based on data showing equivalent efficacy in outcomes),14 rhTSH is still not approved for patients who have moderate-risk or high-risk disease. However, the option to use it in patients who would otherwise be unable to tolerate hormone withdrawal, or would do so with difficulty, is a great step forward for many patients. Use with caution in the moderate-risk to high-risk groups because there are no long-term outcome studies showing the efficacy of rhTSH compared with the hormone withdrawal method.
Octreotide Derivatives in Neuroendocrine Cancers
A novel application is the use of octreotide in targeted radionuclide therapy. 111In-octreotide has been used in clinical trials, and initial results were encouraging, although objective response rates were a meager 0% to 8%, with 42% to 81% of patients having stable disease and 12% to 38% showing disease progression. The octreotide molecule has subsequently been modified to increase its specificity for tumor targeting, to decrease its affinity for nontargeting areas in other organs, and to try to improve its toxicity profile. 111In-octreotide has been altered to create [90Y-DOTA0]-Tyr3-octreotide (DOTATOC) and [177Lu-DOTA0]-Tyr3-otreotate (DOTATATE), with some improvement in their target-to-nontarget ratios. The DOTATOC trials showed improved objective responses ranging from 7% to 33%, with stable disease in 52% to 81% of patients and disease progression in 9% to 19%. Trials with DOTATATE showed similar objective response rates. Aside from marrow toxicity and myelodysplasia, nephrotoxicity was the other most significant long-term sequela; it could be decreased with infusion of amino acids before therapy. As in all radionuclide targeted therapies for solid tumors, hematotoxicity was seen.15
The success of DOTATOC and DOTATATE likely results from their better targeting of tumor compared with octreotide. The radiobiology of the beta emitters 90Y and 177Lu appears to be superior to that of the Auger emitter 111In. The newer trifunctional, somatostatin-based derivatives have prolonged cell retention, and they are internalized into the cell nucleus, making them optimal for Auger therapy, as 111In.16 Future trials with such compounds are of great interest in developing targeted radionuclide modalities.
Metaiodobenzylguanidine Therapy in Neuroendocrine Cancers
Metaiodobenzylguanidine (MIBG) has been used therapeutically as 131I-MIBG in neuroblastomas in children and in pheochromocytomas. Objective responses have ranged from 47% to 80% when MIBG is used as a single agent in neuroblastoma. The main toxicity is hematologic, and trials are being conducted with autologous stem cell reconstitution. Studies have also been conducted combining MIBG with chemoradiotherapy, with various responses.17
Other Uses of Targeted Radionuclide Therapies
Another therapy involves using microspheres embedded with 90Y for palliation in hepatocellular carcinoma, as well as other cancers such as colorectal carcinoma and neuroendocrine tumors that have metastasized to the liver. Microspheres are injected into the hepatic artery directly to the site of diseased liver, where they reside in the vascular space as the nuclides decay, delivering high local radiation doses. Because the product is not systemically delivered (although shunting can occur), side effects are well tolerated and may be less severe than with other therapeutic options. The data suggest palliative benefits, and initial evidence suggests promising survival times compared with systemic options.18 Similarly, 131I-lipiodol is emerging as a candidate for similar indications and has undergone trials with cisplatin showing a well-tolerated toxicity profile.19 On the horizon, newer therapies may use liposomes to deliver therapeutic radionuclides to micrometastases in various cancers to complement antibody-mediated therapies.20
Therapy-Enhancing Strategies
Therapy enhancement basically relies on trying to boost radionuclide uptake and prolong its cellular retention to improve tumor kill from the targeted radiation. In thyroid cancer, lithium has been shown to produce a modest increase in free iodine retention and to increase dose delivery to tissue.21 There is evidence to suggest that medications such as rosiglitazone, suberoylanilide hydroxamic acid (SAHA), and others may improve radioiodine uptake in selected tumors, but no clear clinical benefit has yet been demonstrated.22
The addition of gemcitabine to the 90Y-radiolabeled antibody 90YcPAM4 has shown some promise in pancreatic cancer in improving the efficacy of targeted therapy, presumably because of its action as a radiosensitizer to the effects of 90YcPAM4. Currently, phase I/II trials are under way for evaluation of this combination, but they use a humanized version of the antibody (90Y-hPAM4).23
1 Donckier V, Van Laethem JL, Goldman S, et al. [F-18] fluorodeoxyglucose positron emission tomography as a tool for early recognition of incomplete tumor destruction after radiofrequency ablation for liver metastases. J Surg Oncol. 2003;84:215-223.
2 Keyes JWJr. SUV. Standard uptake or silly useless value? J Nucl Med. 1995;36:1836-1839.
3 Detterbeck FC, Falen S, Rivera MP, et al. Seeking a home for a PET. Part 1. Defining the appropriate place for positron emission tomography imaging in the diagnosis of pulmonary nodules or masses. Chest. 2004;125:2294-2299.
4 Detterbeck FC, Falen S, Rivera MP, et al. Seeking a home for a PET. Part 2. Defining the appropriate place for positron emission tomography imaging in the staging of patients with suspected lung cancer. Chest. 2004;125:2300-2308.
5 Menda Y, Graham MM. Update on 18F-fluorodeoxyglucose/positron emission tomography and positron emission tomography/computed tomography imaging of squamous head and neck cancers. Semin Nucl Med. 2005;35:214-219.
6 Kinkel K, Lu Y, Both M, et al. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET). A meta-analysis. Radiology. 2002;224:748-756.
7 Wahl RL, Siegel BA, Coleman RE, et al. PET Study Group. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer. A report of the Staging Breast Cancer with PET Study Group. J Clin Oncol. 2004;22:277-285.
8 Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer. 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287-297.
9 Garcia-Fernandez R, Luna-Perez P, Hernandez-Hernandez DM, et al. Usefulness of scintigraphy images with 111In-CYT-103 in the diagnosis of colonic and rectal recurrence. Gac Med Mex. 2002;138(2):139-144.
10 Lumbroso JD, Guermazi F, Hartmann O, et al. Meta-iodobenzylguanidine (mIBG) scans in neuroblastoma. Sensitivity and specificity, a review of 115 scans. Prog Clin Biol Res. 1988;271:689-705.
11 Becherer A, Szabo M, Karanikas G, et al. Imaging of advanced neuroendocrine tumors with 18F-FDOPA PET. J Nucl Med. 2004;45:1161-1167.
12 Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses. A phase I trial. Lancet Oncol. 2007;8:304-310.
13 Iagaru A, Mittra E, Yaghoubi SS, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy. Results of the pilot-phase study. J Nucl Med. 2009;50:501-505.
14 Chianelli M, Todino V, Graziano F, et al. Low dose (2.0 GBq; 54 mCi) radioiodine postsurgical remnant ablation in thyroid cancer. Comparison between hormone withdrawal and use of rhTSH in low risk patients. Eur J Endocrinol. 2009;160:431-436.
15 Kwekkeboom DJ, Mueller-Brand J, Paganelli G, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):62S-66S.
16 Ginj M, Hinni K, Tschumi S, et al. Trifunctional somatostatin-based derivatives designed for targeted radiotherapy using Auger electron emitters. J Nucl Med. 2005;46(12):2097-2103.
17 Gaze MN, Wheldon TE, O’Donoghue JA, et al. Multi-modality megatherapy with 131I-metaiodobenzylguanidine, high-dose melphalan and total body irradiation with bone marrow rescue. Feasibility study of a new strategy for advanced neuroblastoma. Eur J Cancer. 1995;31A:252-256.
18 Stuart JE, Tan B, Myerson RJ, et al. Salvage radioembolization of liver-dominant metastases with a resin-based microsphere. Initial outcomes. J Vasc Interv Radial. 2008;19(10):1427-1433.
19 Raoul JL, Boucher E, Olivie D, et al. Association of cisplatin and intra-arterial injection of (131)I-lipiodol in treatment of hepatocellular carcinoma. Results of phase II trial. Int J Radiat Oncol Biol Phys. 2006;64(3):745-750.
20 Emfietzoglou D, Kostarelos K, Papakostas A, et al. Liposome-mediated radiotherapeutics within avascular tumor spheroids. Comparative dosimetry study for various radionuclides, liposome systems, and a targeting antibody. J Nucl Med. 2005;46(1):89-97.
21 Koong SS, Reynolds JC, Movius EG, et al. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999;84(3):912-916.
22 Kebebew E, Lindsay S, Clark OH, et al. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid. 2009;19(9):953-956.
23 Gold DV, Modrak DE, Schutsky K, et al. Combined 90Yttrium-DOTA-labeled PAM4 antibody radioimmunotherapy and gemcitabine radiosensitization for the treatment of a human pancreatic cancer xenograft. Int J Cancer. 2004;109:618-626.