Nuclear Cardiology
Myocardial Perfusion
Myocardial perfusion SPECT is useful for identification of fixed or stress-induced myocardial perfusion abnormalities in patients with a history of Kawasaki disease, transposition of the great arteries after an arterial switch operation, cardiac transplants, cardiomyopathy, chest pain, chest trauma, an anomalous left coronary artery from the right sinus of Valsalva, an anomalous right coronary artery from the left sinus of Valsalva, and a left coronary artery from the pulmonary artery. Other less frequent indications include hyperlipidemia, supravalvular aortic stenosis, syncope, coarctation of the aorta, and pulmonary atresia with an intact ventricular septum. In children with Kawasaki disease and coronary aneurysms (which has surpassed acute rheumatic fever as the leading cause of acquired heart disease in children in the United States), cardiac stress testing for reversible ischemia is indicated to assess the existence and functional consequences of coronary artery abnormalities (Figs. 68-1 and 68-2). It has been shown that myocardial perfusion SPECT is a safe and sensitive diagnostic method for identifying coronary stenosis in these children.
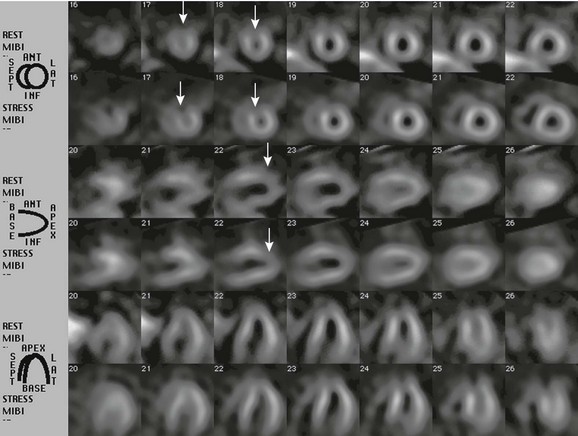
Figure 68-1 Single photon emission tomography images of a patient with Kawasaki disease.
An exercise stress test with technetium-99m MIBI was ordered for a 20-year-old man with a history of Kawasaki disease who had giant coronary artery aneurysms. Single photon emission tomography images were acquired after injection of the tracer material at rest, and the procedure was repeated during a period of stress on the same day. A small fixed perfusion defect on rest, along with stress images involving the distal anterior wall of the left ventricle and the apex (arrows), most likely represent an area of prior infarction. No evidence was found of additional stress-induced ischemia. Ant, Anterior; Inf, inferior; Lat, lateral; MIBI, hexakis-2-methoxyisobutylisonitrile; Sept, septal.
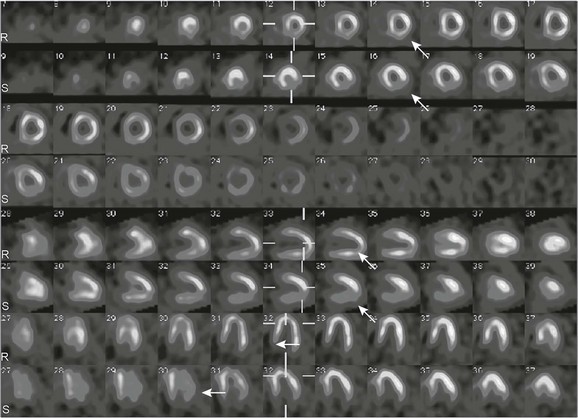
Figure 68-2 Single photon emission tomography images of a patient with Kawasaki disease.
A 4-year-old boy with a history of Kawasaki disease underwent exercise stress testing with technetium-99m MIBI. Short-axis images, as well as vertical and horizontal long-axis stress images (S rows of images), show a perfusion defect (arrows) in the inferolateral wall that is reversible on rest images (R rows of images), consistent with stress-induced ischemia. MIBI, hexakis-2-methoxyisobutylisonitrile.
Regional Pulmonary Blood Flow
A more frequent indication for pulmonary scintigraphy with 99mTc-MAA in pediatric nuclear cardiology is to assess regional pulmonary blood flow in children with congenital or acquired anomalies of the heart and great vessels. This rapid and safe technique often is used to quantify the percent distribution of total pulmonary blood flow in the left and right lungs before and after interventional procedures to relieve obstruction to pulmonary blood flow (Fig. 68-3). This technique is used, for example, before and after catheter or surgical arterioplasty in patients with tetralogy of Fallot and peripheral pulmonary artery stenosis, in patients with pulmonary vein stenosis, and to assess the effect of intravascular stent placement or coil occlusion of vascular communications.
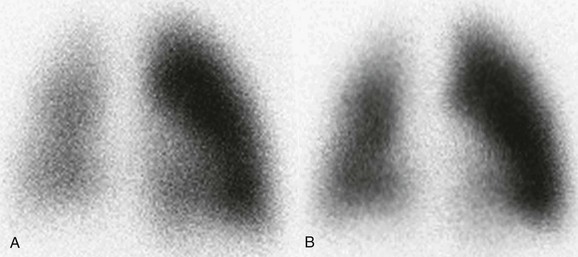
Figure 68-3 Lung perfusion scan images of a patient with tetralogy of Fallot.
After surgical repair, a 4-year-old girl with a history of tetralogy of Fallot underwent a lung perfusion scan to evaluate the distribution of regional pulmonary perfusion. A recent echocardiogram had revealed a slight size discrepancy between her branch pulmonary arteries. A, Her first lung scan showed an asymmetric perfusion of 80% to the left lung and 20% to the right lung. Because of this perfusion asymmetry, she was admitted for a hemodynamic cardiac catheterization study and balloon dilation of her right pulmonary artery branch. B, After intervention, the second lung scan demonstrates increased relative perfusion of the right lung, quantified as 34% to the right lung and 66% to the left lung.
Agarwala, S, Kumar, R, Bhatnagar, V, et al. High incidence of Adriamycin cardiotoxicity in children even at low cumulative doses: role of radionuclide cardiac angiography. J Pediatr Surg. 2000;35:1786–1789.
Askenazi, J, Ahnberg, DS, Korngold, E, et al. Quantitative radionuclide angiocardiography: detection and quantitation of left to right shunts. Am J Cardiol. 1976;37:382–387.
Hernandez-Pampaloni, M, Allada, V, Fishbein, MC, et al. Myocardial perfusion and viability by positron emission tomography in infants and children with coronary abnormalities: correlation with echocardiography, coronary angiography, and histopathology. J Am Coll Cardiol. 2003;41:618–626.
Hurwitz, RA, Treves, S, Kuruc, A. Right ventricular and left ventricular ejection fraction in pediatric patients with normal hearts: first-pass radionuclide angiocardiography. Am Heart J. 1984;107:726–773.
Jan, SL, Hwang, B, Fu, YC, et al. Usefulness of pharmacologic stress 201Tl myocardial tomography: comparison of 201Tl SPECT and treadmill exercise testing in patients with Kawasaki disease. Nucl Med Commun. 2000;21:431–435.
Kondo, C, Hiroe, M, Nakanishi, T, et al. Detection of coronary artery stenosis in children with Kawasaki disease. Circulation. 1989;80:615–624.
Maltz, DL, Treves, S. Quantitative radionuclide angiocardiography: determination of Qp:Qs in children. Circulation. 1973;47:1049–1056.
Miyagawa, M, Mochizuki, T, Murase, K, et al. Prognostic value of dipyridamole-thallium myocardial scintigraphy in patients with Kawasaki disease. Circulation. 1998;98:990–996.
Newburger, JW, Takahashi, M, Gerber, MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Circulation. 2004;110:2747–2771.
Quinlivan, RM, Robinson, RO, Maisey, MN. Positron emission tomography in paediatric cardiology. Arch Dis Child. 1998;79:520–522.
Rickers, C, Sasse, K, Buchert, R, et al. Myocardial viability assessed by positron emission tomography in infants and children after the arterial switch operation and suspected infarction. J Am Coll Cardiol. 2000;36:1676–1683.
Treves, ST, Blume, ED, Armsby, L, et al. Cardiovascular system. In Treves ST, ed.: Pediatric nuclear medicine, 3rd ed, New York: Springer-Verlag, 2007.






