Chapter 27 Nosocomial Respiratory Infections
Pathogenesis
Prevention
Nosocomial respiratory infections are associated with high morbidity and mortality and constitute an important burden for the health care system; therefore, appropriate preventive strategies, summarized in Box 27-1, should be implemented to reduce overall incidence of those diseases. Approaches with proven efficacy in reduction of nosocomial respiratory infections should be grouped and implemented as a bundle, because together they are expected to result in a better outcome than when implemented individually.
Box 27-1
Preventive Strategies for Nosocomial Pneumonia
 Implementation, as a bundle, of nosocomial pneumonia–preventive strategies that have proven efficacy in reducing morbidity and mortality
Implementation, as a bundle, of nosocomial pneumonia–preventive strategies that have proven efficacy in reducing morbidity and mortality
 Implementation of educational programs for caregivers and frequent performance feedbacks and compliance assessment
Implementation of educational programs for caregivers and frequent performance feedbacks and compliance assessment
 Strict alcohol-based hand hygiene
Strict alcohol-based hand hygiene
 Avoidance of tracheal intubation and use of noninvasive ventilation when indicated
Avoidance of tracheal intubation and use of noninvasive ventilation when indicated
 Daily sedation vacation and implementation of weaning protocols
Daily sedation vacation and implementation of weaning protocols
 No ventilatory circuit tube changes unless the circuit is soiled or damaged
No ventilatory circuit tube changes unless the circuit is soiled or damaged
 Use of tracheal tube with cuff made of novel materials and shapes
Use of tracheal tube with cuff made of novel materials and shapes
 Use of silver-coated tracheal tube
Use of silver-coated tracheal tube
 Application of low level of PEEP during tracheal intubation
Application of low level of PEEP during tracheal intubation
 Aspiration of subglottic secretions
Aspiration of subglottic secretions
 Internal cuff pressure maintained within the recommended range and carefully controlled during transport of patients outside ICU setting
Internal cuff pressure maintained within the recommended range and carefully controlled during transport of patients outside ICU setting
 Avoidance of stress ulcer prophylaxis in patients at very low risk for gastrointestinal bleeding, with use of sucralfate considered when indicated
Avoidance of stress ulcer prophylaxis in patients at very low risk for gastrointestinal bleeding, with use of sucralfate considered when indicated
 Semirecumbent patient positioning
Semirecumbent patient positioning
 Continuous lateral rotation therapy
Continuous lateral rotation therapy
 Postpyloric feeding in patients who have impaired gastric emptying
Postpyloric feeding in patients who have impaired gastric emptying
 SDD for patients requiring mechanical ventilation for longer than 48 hours
SDD for patients requiring mechanical ventilation for longer than 48 hours
Diagnosis
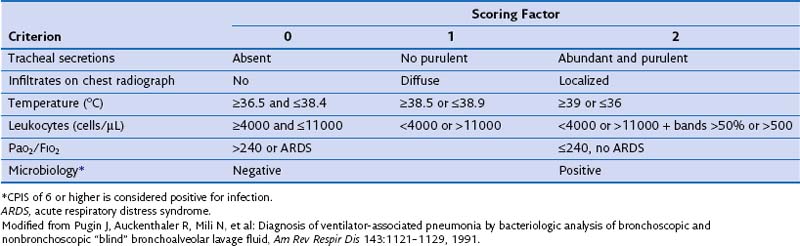
The presence of these clinical signs, without a new infiltrate on the chest film, suggests nosocomial tracheobronchitis. In patients managed in the ICU, clinical signs suggestive of pneumonia often are too nonspecific to be of diagnostic value. Moreover, the chest radiograph often is difficult to interpret in those patients; indeed, when infiltrates are evident, it is challenging to differentiate among cardiogenic and noncardiogenic pulmonary edema, pulmonary contusion, atelectasis, and pneumonia. Clinical variables often are evaluated as a group, to improve specificity of the clinical diagnosis. The Clinical Pulmonary Infection Score (CPIS) is based on clinical assessments, pulmonary radiographic findings, and semiquantitative culture of tracheal aspirate, each worth between 0 and 2 points (Table 27-1). A minimum value of 6 is the threshold to identify patients with pneumonia. Nevertheless, the value of CPIS remains to be validated in a large prospective study, especially in patients with bilateral pulmonary infiltrates.
Diagnostic Strategies for Nosocomial Respiratory Infection
• Accurately identify patients with true pulmonary infection and isolate the causative microorganisms, to allow prompt initiation of appropriate antimicrobial treatment and then optimization of therapy based on susceptibility studies of the pathogens
• Identify patients with extrapulmonary sites of infection
• Withhold and/or withdraw antibiotics in patients without infection
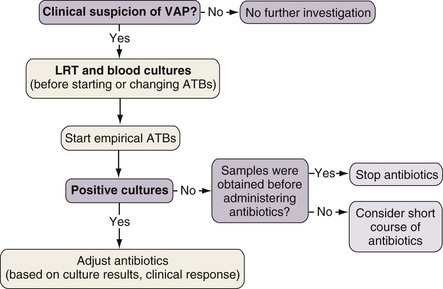
The diagnosis of nosocomial pneumonia begins with clinical suspicion triggered by suggestive findings. The presence of a new or progressively worsening radiographic infiltrate plus clinical criteria constitutes a firm basis for further investigation. Either of two diagnostic algorithms can be used: clinical or bacteriologic. The clinical approach recommends treating every patient suspected to have a pulmonary infection with new antibiotics even when the likelihood of infection is low (Figure 27-1). Nevertheless, samples of respiratory secretions such as endotracheal aspirate or sputum should be obtained before the initiation of antibiotic treatment. In this strategy, the selection of appropriate empirical therapy is based on risk factors and local resistance patterns. The etiology in each case of pneumonia is defined by semiquantitative cultures of endotracheal aspirates or sputum, often with additional microscopic examination of the Gram stain. Antimicrobial therapy is adjusted according to culture results or clinical response. Semiquantitative culture of tracheal aspirates has the advantage that no specialized microbiologic techniques are required, and the sensitivity is high. This clinical strategy provides antimicrobial treatment to a majority of the patients with suspected pneumonia and yields a low rate of false-negative results. Still, if the tracheal aspirate culture does not demonstrate pathogens and the patient has not received new antibiotics within the previous 72 hours, the diagnosis of pneumonia is unlikely. The main drawback of this strategy is that the high sensitivity of semiquantitative cultures of tracheal aspirates leads to overtreatment, with institution of unnecessary antibiotics in patients with false-positive results.
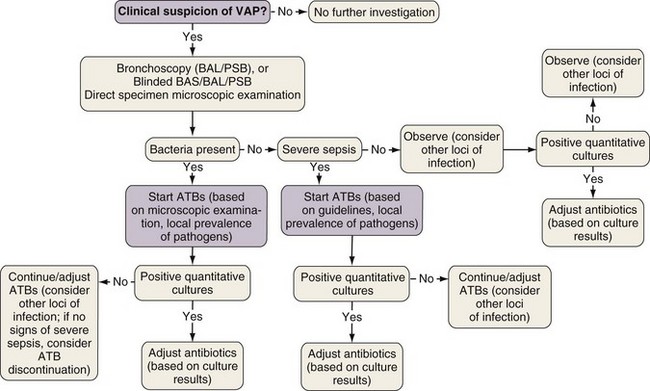
The bacteriologic strategy is based on the results of quantitative cultures of lower respiratory secretions (Figure 27-2). The procedure used to collect the samples (endotracheal aspirate, BAL fluid, or PSB) may be invasive (bronchoscopic) or noninvasive (blind procedures). The strategy reduces risks for overuse of antibiotics, because quantitative cultures yield fewer microorganisms above the threshold in comparison with semiquantitative cultures. Among the disadvantages of the bacteriologic strategy is the possibility of obtaining false-negative results, which leads to delayed antibiotic treatment in patients with pneumonia. Moreover, results obtained using the microbiology strategy may lack reproducibility, and often no microbiologic information is available at the time of initiation of empirical antibiotic therapy.
Practical Implementation of A Diagnostic Strategy in Suspected Nosocomial Respiratory Infection
In practice, the development of local clinical guidelines can combine both clinical and bacteriologic strategies. In mechanically ventilated patients, the presence of an infiltrate on the chest radiograph differentiates between the possible presence of pneumonia and tracheobronchitis. The next step is to obtain samples from the lower respiratory tract, before initiation of antibiotic treatment or change in regimen, even though it should not delay the administration of antibiotic therapy, particularly for septic patients. When indicated, additional samples should be collected for microbiologic analyses, such as blood, pleural fluid, and, in case L. pneumophila or S. pneumoniae infections are suspected, urine should be obtained. With clinical findings suggestive of pneumonia, CPIS should be calculated, to improve objective assessment of the clinical parameters (see Table 27-1).
Treatment
• The most likely etiologic microorganisms
• Choice of the empirical antimicrobials likely to be active against these microorganisms
• The adjustment of therapy after microbiologic results are in and with duration of treatment
The Likely Etiologic Microorganisms
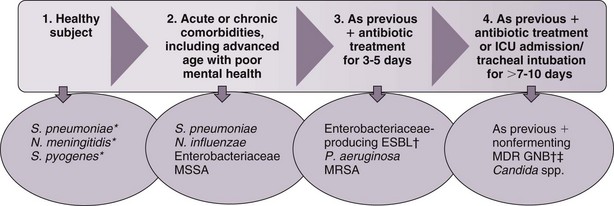
The selection of initial antimicrobial therapy needs to be tailored to the local prevalence of pathogens and antimicrobial patterns of resistance of the institution. The dynamics of change of oropharyngeal flora during hospital stay is depicted in Figure 27-3. Changes in oropharyngeal flora tend to occur progressively, so that the presence of microorganisms during one stage often overlaps with the next stage.
Choice of the Empirical Antimicrobials Likely to be Active Against Causative Microorganisms
The latest guidelines of the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) for the management of adult patients with nosocomial pneumonia recommend that the selection of empirical antibiotic therapy for each patient should be based on the timing of onset and presence of risk factors for MDR pathogens—antimicrobial therapy in preceding 90 days; current hospitalization of 5 days or more; high frequency of antibiotic resistance in the community or in the specific hospital unit; hospitalization for 2 days or longer within the preceding 90-day period; residence in a nursing home or extended care facility; home infusion therapy (including antibiotics); chronic dialysis within 30 days; home wound care; MDR pathogen carrier status or infection in a family member; and immunosuppressive disease and/or therapy. The antibiotics recommended in the current ATS/IDSA guidelines are shown in Tables 27-2 and 27-3. Broad-spectrum empirical antibiotic therapy should be rapidly deescalated as soon as microbiologic data become available, to limit the emergence of resistance in the hospital.
Table 27-2 Initial Empirical Antibiotic Treatment in Nosocomial and Ventilator-Associated Pneumonia of Early Onset in Patients Without Risk Factors for Infection by Multidrug-Resistant Pathogens
| Probable Pathogen | Recommended Antibiotic |
|---|---|
| Streptococcus pneumoniae | Ceftriaxone |
| Haemophilus influenzae | OR |
| Methicillin-sensitive Staphylococcus aureus | Levofloxacin, moxifloxacin OR Ampicillin/sulbactam OR Ertapenem |
| Enteric gram-negative bacilli | |
| Escherichia coli | |
| Klebsiella pneumoniae | |
| Enterobacter spp. | |
| Proteus spp. | |
| Serratia marcescens |
Modified from American Thoracic Society: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia, Am J Respir Crit Care Med 171:388–416, 2005.
Table 27-3 Initial Empirical Antibiotic Treatment for Nosocomial and Ventilator-Associated Pneumonia of Late Onset or in Patients with Risk Factors for Infection by Multidrug-Resistant Pathogens and Any Degree of Severity
| Probable Pathogen | Combined Antibiotic Treatment |
|---|---|
| Microorganisms from Table 27-2 PLUS: | Antipseudomonal cephalosporin (ceftazidime or cefepime)‡ OR Carbapenem (imipenem, meropenem)‡ OR Beta-lactam/beta-lactamase inhibitor (piperacillin-tazobactam)‡ + Antipseudomonal fluoroquinolone (ciprofloxacin, levofloxacin)§ OR Aminoglycoside§ (amikacin) ± |
| Pseudomonas aeruginosa | |
| Klebsiella pneumoniae (ESBL-positive)* | |
| Acinetobacter spp.* | |
| Other nonfermenting GNB | |
| Methicillin-resistant Staphylococcus aureus (MRSA) | |
| Legionella pneumophila† | |
| Linezolid or vancomycin¶ |
ESBL, extended-spectrum beta-lactamase; GNB, gram-negative bacilli; ICU, intensive care unit.
* If an ESBL-positive strain such as K. pneumoniae, or Acinetobacter, is suspected, a carbepenem is the agent of first choice.
† If L. pneumophila is the suspected pathogen, the combination antibiotic regimen should include a macrolide (e.g., azithromycin), or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) should be used rather than an aminoglycoside.
‡ The choice of beta-lactam is made as follows: Patients who have not received any antipseudomonal beta-lactam within the last 30 days should be administered piperacillin-tazobactam or an antipseudomonal cephalosporin. Patients who have received these drugs should be given empirical therapy with a carbapenem. Patients with infection by ESBL-producing microorganisms should be treated with carbapenem regardless of the results of the antibiogram.
§ For combined empirical therapy for multidrug-resistant GNB, an antipseudomonal fluoroquinolone should be given in cases of renal failure or with concomitant use of vancomycin. In other settings, combined empirical therapy with amikacin is initiated and maintained for a 5-day period.
¶ Empirical therapy aimed against MRSA is initiated in patients with proven colonization, previous infection by this microorganism, or implementation of mechanical ventilation for more than 6 days. The antibiotic of choice is either vancomycin (except in persons allergic to this medication, those with serum creatinine values of 1.6 mg/dL or greater, or patients presenting with signs of empirical treatment failure after 48 hours of antibiotic therapy) or linezolid. NOTE: For epidemiologic surveillance, nasal and perineal cultures should be performed on admission and at 1-week intervals thereafter during the ICU stay.
Patients with Late-Onset Pneumonia or Early-Onset Pneumonia with Risk Factors for Multidrug-Resistant Bacteria
Patients with late-onset pneumonia or early-onset pneumonia with risk factors for MDR bacteria may be infected with resistant gram-negative bacilli. Priority should be given to treatment with a beta-lactam. Choice of the beta-lactam agent should take into account the following factors: (1) in vitro susceptibility of P. aeruginosa in the ICU, (2) the prevalence of Enterobacteriaceae organisms producing ESBL, (3) the results of previous cultures, and (4) antibiotics already received by the patient. An antipseudomonal beta-lactam regimen would include a third-generation cephalosporin (ceftazidime or cefepime), piperacillin-tazobactam, or a carbapenem (imipenem or meropenem) (see Table 27-3).
Modifications of Therapy and Duration of Treatment
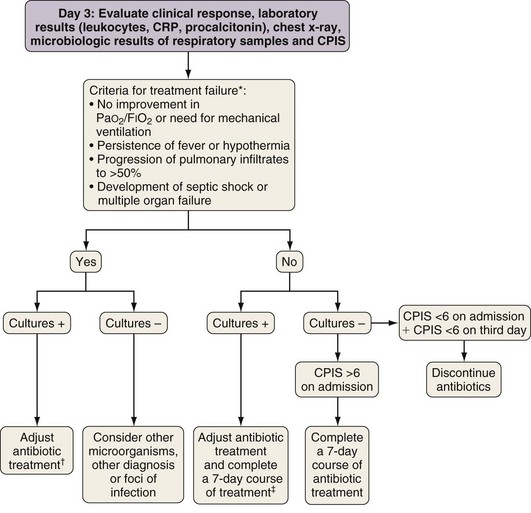
In patients originally suspected on clinical grounds to have ICU-acquired pneumonia but whose CPIS is lower than 6 on the third day of drug therapy, treatment may be withdrawn. In this setting, the patient probably did not have pneumonia, or the pneumonia was sufficiently mild that prolonged antibiotic treatment is not required (Figure 27-4).
American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
The Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630.
Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–S66.
Esperatti M, Ferrer M, Theessen A, et al. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010;182:1533–1539.
Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805–813.
Lacherade JC, De Jonghe B, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182:910–917.
Rello J, Sa-Borges M, Correa H, et al. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999;160:608–613.
Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–1276.
Torres A, Ewig S, Lode H, Carlet J, European HAP Working Group. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35:9–29.
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329.