CHAPTER 96 NOSOCOMIAL PNEUMONIA
The two broad classes of pneumonia are nosocomial and community-acquired pneumonia. Nosocomial pneumonia is often referred to as hospital-acquired pneumonia (HAP), defined as pneumonia occurring 48 hours or more after admission that was not incubating at the time of admission. Postoperative pneumonia is essentially HAP, except in a patient who has undergone a surgical procedure. Finally, ventilator-associated pneumonia (VAP) refers to pneumonia occurring 48 hours or more after initiating mechanical ventilation via endotracheal intubation or tracheostomy.
RISK FACTORS AND PREVENTIVE MEASURES
Nonmodifiable versus Modifiable Risk Factors
While the greatest risk factor for VAP is the duration of mechanical ventilation, there are many other independent predictors, including modifiable and nonmodifiable risk factors (Table 1).
| Nonmodifiable Factors | Modifiable Factors Affording Prevention Strategies |
|---|---|
ICU, Intensive care unit.
Putting All Risk Factors Together
Croce et al. recently reported a post-trauma VAP probability calculation formula incorporating many of these risk factors. The probability of VAP (PVAP) equals ef(x)/(1 + ef(x)), where f(x) = −3.08 – 1.56 ( mechanism of injury, penetrating = 1, blunt = 0) − 0.12 (Glasgow Coma Scale score) + 1.37 (spinal cord injury where yes = 1, no = 0) + 0.30 (chest abbreviated injury score) + 1.87 (emergency laparotomy where yes = 1, no = 0) + 0.67 (units of blood transfused in the resuscitation room) + 0.05 (Injury Severity Score) + 0.66 (intubation in either the field or the resuscitation room, where yes = 1, no = 0). Over 2 months, this formula was 95% accurate in predicting subsequent development of VAP.
DIAGNOSIS
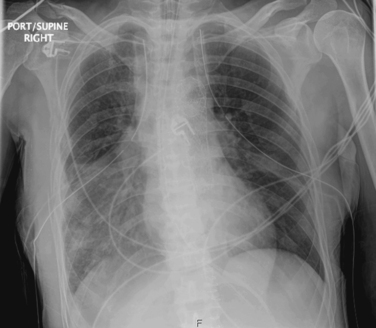
Pneumonia is suspected when a patient develops new or progressive radiographic lung infiltrates (Figure 1), along with a clinical scenario of pulmonary infection (i.e., fever, leukocytosis, purulent sputum, respiratory distress, and a worsening of oxygenation) (Table 2). Of note, in patients diagnosed with acute respiratory distress syndrome (ARDS), the suspicion of pneumonia should be especially high. Several studies have noted a higher incidence of pneumonia in patients with ARDS. For example, one study showed a pneumonia rate of 55% in patients with ARDS, versus 28% in those without. Another study noted a 60% incidence of pneumonia in patients with severe ARDS (PaO2/FIO2 ratio < 150 mm Hg).
Table 2 Centers for Disease Control and Prevention Criteria for Defining Hospital-Acquired Pneumonia
| Radiology | Signs/Symptoms | Laboratory |
|---|---|---|
| Two or more serial chest radiographs with at least one of the following: |
BAL, Bronchoalveolar lavage; PMN, polymorphonuclear leukocytes; PSB, protected specimen brush; WBC, white blood cells.
Diagnostic Strategies
However, ventilated ICU patients often have radiographic infiltrates, fever, and thick respiratory secretions in the absence of pneumonia. To assess the diagnostic efficacy of clinical criteria alone for VAP, one study reviewed 25 patients who died while on mechanical ventilation and used lung histology plus quantitative lung culture as the standard for pneumonia. The presence of radiographic chest infiltrates plus two of three clinical criteria (leukocytosis, purulent secretions, fever) had a sensitivity of 69% and a specificity of 75%. Thus, it is important to obtain sputum cultures to confirm the diagnosis of VAP; additionally, identifying the causative organism(s) aids in selecting appropriate antibiotics.
Methods of Obtaining Sputum Cultures
Samples for sputum culture may be obtained noninvasively, via tracheal aspiration, or invasively with bronchoscopy and either bronchoalveolar lavage (BAL) or a protected specimen brush (PSB). Positive tracheal cultures may reflect simple tracheal colonization, and overestimate the rate of pneumonia. Invasive cultures are more accurate in diagnosing pneumonia. In one multicenter, randomized trial of 413 patients, those receiving invasive, bronchoscopic management had a lower mortality at day 14, but not at 28, and lower mean sepsis-related organ failure assessment scores on days 3 and 7. At 28 days, the invasive management group had significantly more antibiotic-free days (11 ± 6 vs. 7 ± 7). A multivariate analysis showed a significant difference in mortality (hazard ratio 1.54, 95% confidence interval 1.10–2.16). Both BAL and PSB have sensitivities and specificities greater than 80%. Studies have shown these two techniques yield similar results (Table 3).
Table 3 Quantitative Cultures and Microscopic Examination of Lower Respiratory Tract Secretions in Diagnosis of Ventilator-Associated Pneumonia
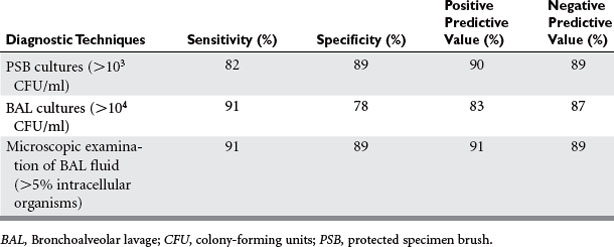
Most studies involving BAL have used 104 or 105 CFU/ml as the threshold for a positive culture. The presence of numerous squamous epithelial cells suggests upper pharyngeal contamination, and calls into question the utility of the specimen. The presence of intracellular organisms can be detected by Gram stain, and is particularly useful as it provides a rapid result with high predictive value (see Table 3).
Value of Clinical Pulmonary Infection Score in Trauma Patients
Lastly, the clinical pulmonary infection score (CPIS) is an attempt to optimize a noninvasive diagnostic approach by pooling several clinical indicators of pneumonia (Table 4). A CPIS greater than 6 has been shown to be highly suggestive of pneumonia and correlates with a high concentration of bacteria from invasive cultures. The main criticisms of the CPIS are that all elements are weighted equally even though some are stronger predictors of pneumonia, and that some elements are necessarily subjective, such as the interpretation of chest x-rays. Furthermore, most of the components of the CPIS may be altered by the systemic effects of trauma, and therefore simply reflect the systemic inflammatory response syndrome (SIRS). A recent study of 113 trauma patients with suspected pneumonia found the average CPIS score to be 7.0 in those with VAP confirmed by BAL versus 6.9 in those with a negative BAL. In this study the sensitivity and specificity of using a CPIS greater than 6 to diagnose VAP were only 65% and 41%, respectively.
| Variable | Finding | Points |
|---|---|---|
| Temperature (° C) | ≥365 and ≤384 | 0 |
| ≥385 and ≤389 | 1 | |
| ≥39 or ≤36 | 2 | |
| Blood leukocytes (n/mm3) | ≥4,000 and ≤11,000 | 0 |
| >4,000 or <11,000 | 1 | |
| Plus band forms ≥50% | Add 1 | |
| Tracheal secretions | Absent | 0 |
| Nonpurulent secretions present | 1 | |
| Purulent secretions present | 2 | |
| Oxygenation (PaO2/FIO2) | >240 or ARDSa | 0 |
| ≤240 and no ARDS | 2 | |
| Pulmonary radiography | No infiltrate | 0 |
| Diffuse (or patchy) infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Progression of pulmonary infiltrates | No radiographic progression | 0 |
| Radiographic progression (after CHF and ARDS excluded) | 2 | |
| Culture of tracheal aspirate | Pathogenic bacteria cultured in very low to low quantity or not at all | 0 |
| Pathogenic bacteria cultured in moderate or high quantity | 1 | |
| Same pathogenic bacteria seen on Gram stain | Add 1 |
ARDS, Acute respiratory distress syndrome; CHF, congestive heart failure; FIO2, fraction of inspired oxygen; PaO2, arterial oxygen tension; PAWP, pulmonary arterial wedge pressure.
a Defined as PaO2/FIO2 ≥200 and PAWP ≥18 mm Hg, with acute bilateral infiltrates.
MANAGEMENT
Adequate Initial Antibiotics
Effective treatment for HAP depends on rapid institution of an appropriate initial antibiotic. At least three separate studies have shown mortality to almost double when the initial choice of antibiotics was inadequate. Another study looked at patients receiving appropriate initial antibiotics, but with a delay of more than 24 hours from the time of meeting diagnostic criteria for VAP. In this group, VAPattributable mortality was 39.4%, compared to 10.8% in those receiving antibiotics in a timely manner.
The first step in treating pneumonia is to determine whether the responsible organism is likely to demonstrate antibiotic resistance. Hospitalization for 5 days or more and recent antibiotic or health care exposure are common risk factors for developing multidrug-resistant (MDR) pneumonia (Table 5).
Table 5 Risk Factors for Multidrug-Resistant Pathogens Causing Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia
After determining the likelihood of antibiotic resistance, an appropriate initial therapy is selected. If the likelihood of antibiotic resistance is low, suitable initial choices include a third- or fourth-generation cephalosporin, a fluoroquinolone, an antipseudomonal penicillin with a beta-lactamase inhibitor, or a carbapenem (Table 6). Also, knowing hospital-specific or even ICU-specific patterns of antibiotic resistance can be particularly useful in guiding antibiotic choices.
Table 6 Initial Empiric Antibiotic Therapy for Hospital-Acquired Pneumonia or Ventilator-Associated Pneumonia in Patients with No Known Risk Factors for Multidrug-Resistant Pathogens
| Potential Pathogens | Recommended Initial Antibiotics |
|---|---|
If the patient has risk factors for MDR pneumonia, initial antibiotics should include double coverage for Gram-negatives and an agent for MRSA. The necessity of double antipseudomonal coverage is controversial. Evidence of in vitro synergy with combination therapy has been inconsistently demonstrated, and proof of clinical relevance is lacking. Also, prevention of emergent drug resistance during therapy has not been well demonstrated. However, one good reason to initiate double coverage is simply to increase the odds that at least one of the drugs will have activity against the suspected MDR organism. For MRSA coverage, it is important to remember that vancomycin has relatively poor lung penetration, and serum drug levels should be measured to ensure adequate dosage. Linezolid is another option, and two retrospective analyses comparing vancomycin to linezolid in treating MRSA nosocomial pneumonia showed improved survival and clinical cure rates with linezolid therapy (Table 7).
Table 7 Initial Empiric Therapy for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia in Patients with Late-Onset Disease or Risk Factors for Multidrug-Resistant Pathogens
| Potential Pathogens | Combination Antibiotic Therapy |
|---|---|
| Acinetobacter speciesa | |
| Linezolid or vancomycin |
a If an ESBL+ strain, such as K. pneumoniae, or an Acinetobacter species is suspected, a carbepenem is a reliable choice.
b If L. pneumophila is suspected, the combination antibiotic regimen should include a macrolide (e.g., azithromycin) or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) should be used rather than an aminoglycoside.
ANTIBIOTIC PROPHYLAXIS AND TUBE THORACOSTOMY
Prophylactic Antibiotics for Chest Tube Placement
Multiple studies have investigated the efficacy of prophylactic antibiotics in reducing the incidence of pneumonia and empyema related to chest tube placement. An evidentiary review performed by the Eastern Association for the Surgery of Trauma (EAST) Practice Management group included nine prospective series and two meta-analyses. Their analysis showed that overall, the incidence of pneumonia was significantly reduced from 14% in the placebo group to 4.1% in the group receiving prophylactic antibiotic therapy, and the incidence of empyema was also significantly reduced from 8.7% in the placebo group to 0.6% in the antibiotic group.
American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588-2598.
Croce MA, Fabian TC, Schurr MJ, et al. Using bronchoalveolar lavage to distinguish nosocomial pneumonia from systemic infl ammatory response syndrome: a prospective analysis. J Trauma. 1995;39(6):1134-1139. discussion 1139–1140
Croce MA, Fabian TC, Waddle-Smith L, Maxwell RA. Identification of early predictors for post-traumatic pneumonia. Am Surg. 2001;67(2):105-110.
Croce MA, Tolley EA, Fabian TC. A formula for prediction of posttraumatic pneumonia based on early anatomic and physiologic parameters. J Trauma. 2003;54(4):724-729. discussion 729–730
Croce MA, Fabian TC, Mueller EW, et al. The appropriate diagnostic threshold for ventilator-associated pneumonia using quantitative cultures. J Trauma. 2004;56(5):931-934. discussion 934–936
Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118(1):11-18.
Etoch SW, Bar-Natan MF, Miller FB, Richardson JD. Tube thoracostomy. Factors related to complications. Arch Surg. 1995;130(5):521-525. discussion 525–526
Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275(11):866-869.
Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM. The incidence of ventilator-associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding: a randomized clinical trial. Crit Care Med. 2000;28(6):1742-1746.
Kollef MH, Von Harz B, Prentice D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest. 1997;112(3):765-773.
Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32(6):1396-1405.
Luchette FA, Barrie PS, Oswanski MF, et al. Practice management guidelines for prophylactic antibiotic use in tube thoracostomy for traumatic hemopneumothorax: the EAST Practice Management Guidelines Work Group. Eastern Association for Trauma. J Trauma. 2000;48(4):753-757.
Marik PE, Zaloga GP. Gastric versus post-pyloric feeding: a systematic review. Crit Care. 2003;7(3):R46-R51.
Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184-2193.
Shaw MJ. Ventilator-associated pneumonia. Curr Opin Pulm Med. 2005;11(3):236-241.
Spain DA. Pneumonia in the surgical patient: duration of therapy and does the organism matter? Am J Surg. 2000;179(Suppl 1):36-39.
Spain DA. Ventilator-associated pneumonia and surgical patients. Chest. 2002;121(5):1390-1391.
Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142(3):523-528.
Torres A, Gatell JM, Aznar E, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152(1):137-141.