Nosocomial Infection in the Intensive Care Unit
GENERAL INFECTION CONTROL MEASURES
Hospital Infection Control Programs
Role of the Microbiology Laboratory
Architectural and Environmental Issues
Reliable Sterilization Procedures, Chemical Disinfectants, and Antiseptics
NOSOCOMIAL INFECTIONS AND SPECIFIC INFECTION CONTROL MEASURES
Intravascular Device–Related Bloodstream Infection
Ventilator-Associated Pneumonia
AVANT GARDE INFECTION CONTROL MEASURES
![]() APPROACH TO A NOSOCOMIAL EPIDEMIC
APPROACH TO A NOSOCOMIAL EPIDEMIC
PROTECTION OF HEALTH CARE WORKERS IN THE INTENSIVE CARE UNIT
![]() Additional online-only material indicated by icon.
Additional online-only material indicated by icon.
Intensive care units (ICUs) have contributed greatly to the survival of patients with trauma, shock states, and other life-threatening conditions1–3 but are associated with a greatly increased risk of nosocomial (hospital-acquired) infection. Rates of nosocomial infection in patients requiring more than 1 week of advanced life support within an ICU are three to five times higher than in hospitalized patients who do not require ICU care.4–8 Infection, usually nosocomial, is the most common cause of death, directly or indirectly, of patients who survive the early period after major trauma or full-thickness burns and is the most commonly identified cause of multiple-organ dysfunction syndrome.9–11
Published guidelines for prevention are now available, based increasingly on randomized trials that have established the efficacy of specific control measures. Knowledge and technology of asepsis with regard to surgery and high-risk medical devices are now sufficiently advanced that, if applied consistently, the risk of nosocomial infection can be greatly reduced.12–15
Incidence and Profile
Definitions
Obtaining meaningful data on rates of nosocomial infection that can form the basis for comparisons within a hospital and, especially, among hospitals and that can also be used to monitor secular trends and document the efficacy or lack of efficacy of control measures must begin with clear, unambiguous definitions. Although there are no standardized definitions for infection at specific sites that are universally accepted by clinicians or investigators, the Centers for Disease Control and Prevention (CDC) has published definitions for the purpose of surveillance of nosocomial infection within hospitals, which most U.S. centers and an increasing number of hospitals around the world have adopted (Box 50.1).16,17 For research purposes, more stringent definitions for specific infections will usually be necessary,18 especially for pneumonia.19
Incidence
The incidence of hospital-acquired infection is most commonly expressed as the number of infections per 100 patients hospitalized and is highest in burn units7,20 and surgical ICUs,5–7,20–23 with intermediate risk in medical ICUs,* and lowest risk in coronary care units.4,7,8,20
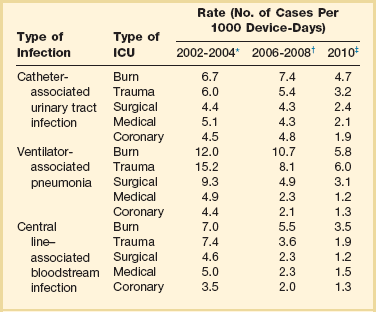
Recognizing that the risk of nosocomial infection within ICUs is heavily influenced by the length of stay and that the length of stay ranges widely among ICUs in the same hospital and among different hospitals, the CDC has advocated the use of rates expressed per 1000 patient-days to permit more meaningful intrainstitutional and, especially, interhospital comparisons.25–27 Furthermore, recognizing the powerful influence of exposure to invasive devices on susceptibility to infection28,29 and the great variation in use of devices among different ICUs in the same hospital and among different hospitals, the CDC has further recommended surveillance of device-associated nosocomial infections expressed as infections per 1000 device-days.25 Representative rates of device-associated nosocomial infection in U.S. hospitals, which can be used for intrahospital and interhospital comparisons, are shown in Table 50.1.25–27 In the future, device-associated infection rates will be sought in accreditation reviews by the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO)30 as this influential organization continues to move toward measurement of patient outcomes as the most effective way to improve patient care in the United States.
Table 50.1
*Data from National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85.
†Data from Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009;37:783-805.
‡Data from Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control 2011;39:798-816.
Profile and Secular Trends
Approximately 40% of endemic nosocomial infections within ICUs are catheter-related urinary tract infections, and 25% are pneumonias—most associated with endotracheal intubation and mechanical ventilatory support. Up to 10% of patients hospitalized in a medical-surgical ICU for more than 72 hours acquire a nosocomial bloodstream infection, most commonly from an intravascular device.25,31,32 Postoperative surgical site infections, Clostridium difficile infection, nosocomial sinusitis, and nosocomial meninigitis account for the remainder.4–8,25,33–37
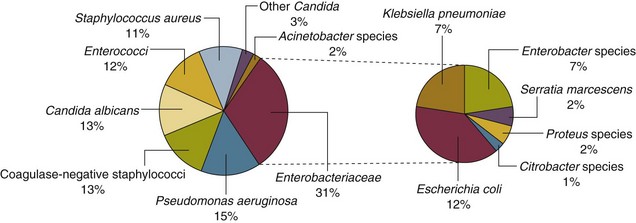
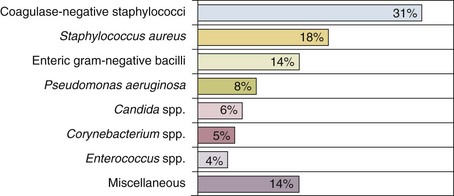
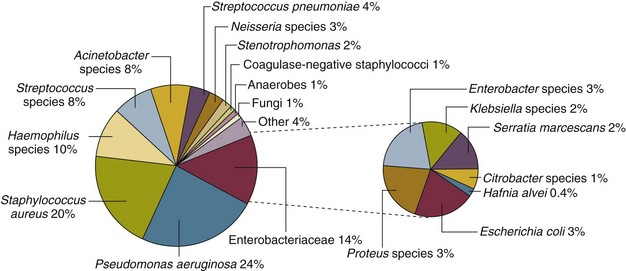
Nearly 50% of nosocomial infections in the ICU are caused by aerobic gram-negative bacilli, especially Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae, and 35% are caused by gram-positive cocci, most commonly coagulase-negative staphylococci, Staphylococcus aureus, and enterococci (Fig. 50.1).38,39 Almost 15% are caused by Candida species.38,39 Filamentous fungi such as Aspergillus and Zygomycetes are being increasingly encountered in patients with hematologic malignancy or those who received solid organ transplants.40–42 Legionella species account for up to 10% of nosocomial pneumonias in centers that make efforts to diagnose Legionella infections.43
The microbial profile of infections at individual sites in ICU patients is shown in Table 50.2.39 There has been an unrelenting increase in nosocomial infections caused by intrinsically resistant organisms, especially P. aeruginosa, Acinetobacter species, and other resistant gram-negative bacilli; coagulase-negative staphylococci, S. aureus, enterococci; and Candida.31,38,39,44,45 Moreover, the incidence of infection caused by organisms with acquired resistance, especially methicillin-resistant S. aureus (MRSA); enterococci resistant to vancomycin (VRE), ampicillin, or both drugs; and gram-negative bacilli resistant to extended-spectrum β-lactams and fluoroquinolones, has increased even more sharply over the past several decades (Fig. 50.2).39,46 The recent emergence of carbapenem-resistant K. pneumoniae has become a significant problem as there are limited therapeutic options to treat this pathogen.39,47
Table 50.2
Profile of Nosocomial Infections in the Intensive Care Unit (ICU)
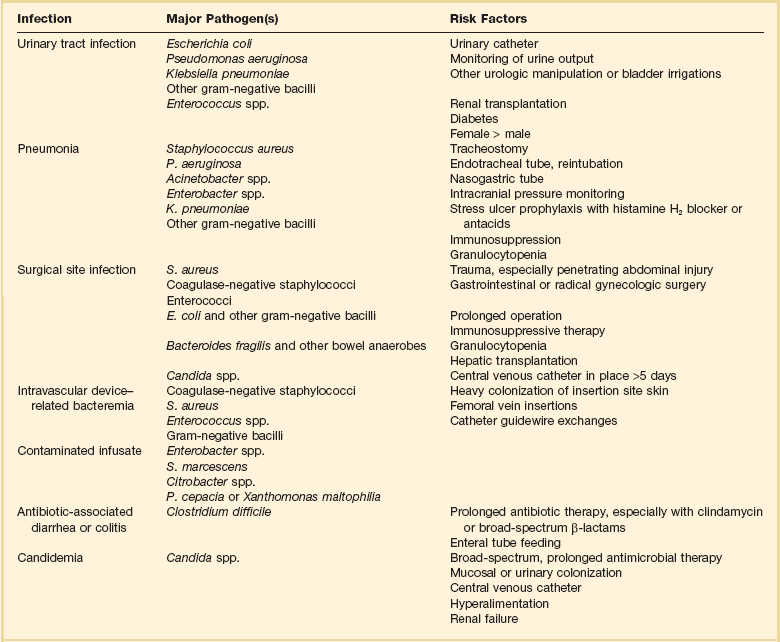
Modified from Maki DG: Nosocomial infection. In Parrillo JE (ed): Current Therapy in Critical Care Medicine, 2nd ed. Philadelphia, BC Decker, 1991.
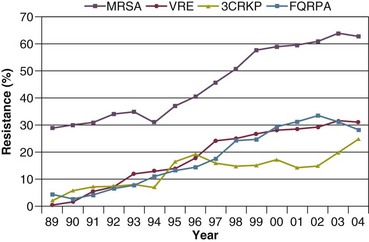
Figure 50.2 Temporal trends in the proportion of isolates resistant to antibiotics among pathogenically important bacteria in U.S. intensive care units (ICUs), National Nosocomial Infections Surveillance System (NNIS) 1989-2004. FQRPA, Pseudomonas aeruginosa resistant to fluoroquinolones; MRSA, methicillin-resistant Staphylococcus aureus; 3CRKP, Klebsiella pneumoniae resistant to third-generation cephalosporins; VRE, vancomycin-resistant enterococcus. (From Centers for Disease Control and Prevention: Trends in antibiotic resistance in National Nosocomial Infections Surveillance (NNIS) system hospitals, 1989-2004. Available at http://www.cdc.gov/ncidod/dhqp/pdf/ar/ICU_RESTrend1995-2004.pdf. Accessed January 15, 2007.)
Nosocomial infections acquired in the ICU clearly differ from infections acquired in non-ICU patient care units within the same institutions. Overall rates are two to three times higher, and rates of ventilator-associated pneumonia (VAP) and primary bacteremia—most of which originate from intravascular devices—are 10 times higher. A far greater proportion of ICU-acquired infections are caused by antibiotic-resistant bacteria because the intensive antimicrobial therapy characteristic of modern-day ICUs grossly distorts patients’ microflora. Moreover, more than half of all nosocomial epidemics now occur among the 10% of hospitalized patients confined to an ICU.20,31 Finally, the risk of occupationally acquired infection among health care workers (HCWs), particularly by bloodborne viruses and herpes simplex virus (HSV), is highest among ICU personnel, as contrasted with those who work in non-ICU patient care units (see Protection of Health Care Workers in the Intensive Care Unit later).
Morbidity and Economic IMPACT
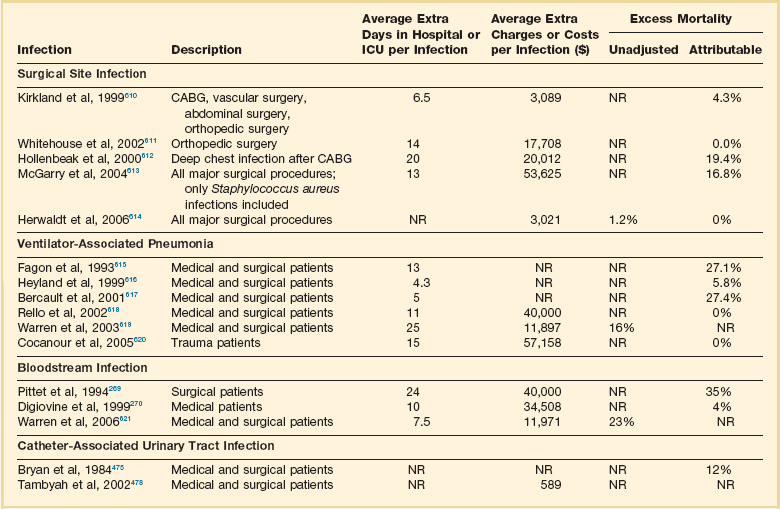
Nosocomial infections have a considerable impact on morbidity and mortality rates and are estimated to affect more than 2 million patients in U.S. hospitals annually.48 Table 50.3 summarizes major studies that have examined mortality, length of stay, and costs associated with the major nosocomial infections in U.S. hospitals.2–19 Nosocomial infections have been ascribed by the National Institute of Medicine to be responsible for more than 80,000 hospital deaths each year and in 1995 resulted in more than $5 billion in excess health care costs.48 Considering that nosocomial infections acquired by ICU patients account for nearly half of all infections in most hospitals, progress in reducing the incidence of infection acquired within ICUs could produce substantial economic benefits.
General Infection Control Measures
Hospital Infection Control Programs
Beginning in the late 1960s, scattered U.S. hospitals began to establish infection control programs to conduct surveillance, to develop infection control policies, and especially to try to implement control measures more consistently.116 In 1976 JCAHO added to its requirements for hospital accreditation the establishment of a formal infection control program.
In the early 1970s the CDC undertook determining the effectiveness of nosocomial infection surveillance and control programs in the United States through the auspices of the Study of the Efficacy of Nosocomial Infection Control (SENIC). The goals of SENIC were to determine the extent to which infection control programs had been adopted by U.S. hospitals and to ascertain how much these programs had reduced rates of nosocomial infection. SENIC was launched by a survey of all U.S. hospitals to determine the characteristics of infection control programs and was completed in 1975-1976 by a review of more than 339,000 patient medical records in 338 randomly selected hospitals.117
The SENIC found that hospitals reduced their nosocomial infection rates by approximately 32% if their surveillance and infection control program included four components: (1) emphasis on both surveillance and an infection control program, (2) at least one full-time infection control practitioner for every 250 beds, (3) a trained hospital epidemiologist, and (4) surveillance of surgical wound infections with feedback of wound infection rates to practicing surgeons.118 However, the relative importance of each component varied for the four major types of nosocomial infections (surgical wound infections, urinary tract infections, bloodstream infections, and pneumonia).118,119 SENIC suggests that nearly one third of all nosocomial infections are in theory preventable, whereas a 1983 survey of surveillance and control programs in a random sample of U.S. hospitals found that failure to implement all essentials of the program, particularly to have an adequate number of infection control practitioners or a trained hospital epidemiologist or to disseminate wound infection rates to surgeons, was greatly limiting the potential for prevention: U.S. hospitals were estimated to be preventing only 9% of all infections.120
It is hoped that surveillance and control programs will continue to evolve. Prevention of nosocomial infections is a major priority of the U.S. Public Health Service,121 JCAHO,30 and the Institute of Medicine.122 With the shift to prospective-payment reimbursement, hospitals now have a powerful financial incentive to reduce their rates of nosocomial infection,123 and it can be anticipated that efforts to prevent hospital-acquired infections will assume ever greater importance.
Pathogenesis and Epidemiology
Pathogenesis
The occurrence of nosocomial infection reflects the conjunction in space and time of a pathogenic microbe and a vulnerable patient, catalyzed by events associated with hospitalization and the patient’s care. Many patients admitted to an ICU are intrinsically more susceptible to infection because of underlying diseases or conditions associated with impaired immunity such as cancer, trauma,49 or advanced age50 or because of immunosuppression associated with malnutrition51 or therapy with corticosteroids,52 cancer chemotherapeutic agents,53 or other immunosuppressive drugs.54 Moreover, many drugs have indirect effects that increase susceptibility to infection, such as narcotics or sedatives that impair the capacity to protect the airway, or antacids or H2-histamine receptor antagonists that neutralize gastric acidity, producing gastric overgrowth by gram-negative bacilli,55 increasing the risk of nosocomial pneumonia.56 Even transfusion therapy produces immunosuppression and increases the risk of nosocomial infection.57
Moreover, most nosocomial pathogens exhibit resistance to antibiotics (see Figs. 50.1 and 50.2),44,45,47,58–61 and many are also more virulent because of (1) their capacity to subsist or even multiply in aqueous reservoirs for prolonged periods (e.g., pseudomonads62 or Legionella pneumophila63); (2) the elaboration of endotoxins (e.g., all of the gram-negative bacilli) or exotoxins (P. aeruginosa,64 C. difficile,65 or S. aureus66); or (3) the production of adhesions67 or exoglycocalyx68 (e.g., coagulase-negative staphylococci), conferring the capacity to adhere avidly and form biofilms on biologic and prosthetic surfaces resistant to host defenses69 and even antibiotics.70 Because most patients in ICUs receive broad-spectrum antibiotics, resistant nosocomial organisms have an enormous ecologic advantage and, in darwinian fashion, predictably supplant the normal cutaneous, respiratory, and gastrointestinal flora.
In most cases, colonization is the first step in the progression to nosocomial infection,71 especially if the patient is already vulnerable because of underlying disease, if the organism is more virulent or resistant to antibiotics, or if the patient has invasive medical devices that assist invasion by colonizing organisms, bypassing or further impairing host defenses.
Reservoirs and Transmission
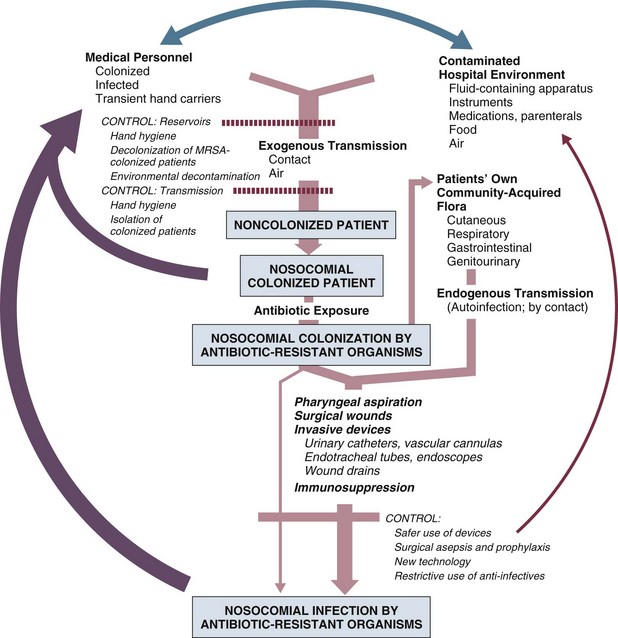
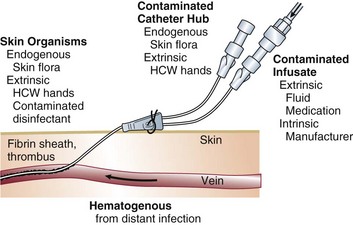
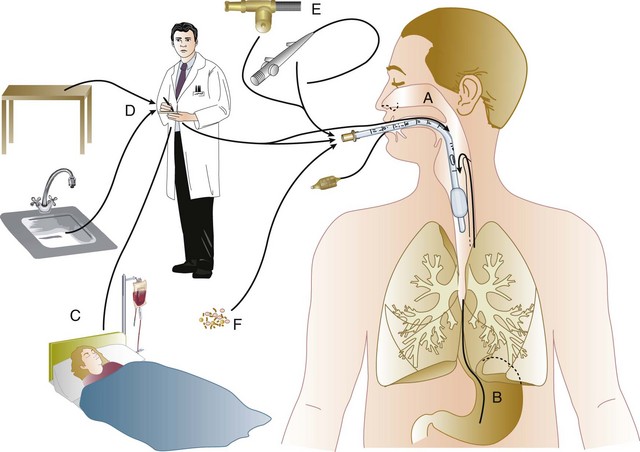
In the ICU the major reservoir of nosocomial organisms is the infected or colonized patient (Fig. 50.E1).28 Whereas Streptococcus pneumoniae,72 Mycobacterium tuberculosis,73–75 Legionella,43 Aspergillus and Zygomycetes,40–42 measles,76 rubella,77 and influenza A78 are transmitted by the airborne route, the best evidence suggests that most aerobic bacteria—particularly S. aureus,79 enterococci,29 and the enteric gram-negative bacilli80; many viruses such as hepatitis A, RSV,81 and rotaviruses82; C. difficile 83; and even Candida84—are spread in the ICU on the hands of medical personnel, who themselves are not infected or even permanently colonized. Surgery and exposure to invasive devices of all types greatly amplify transmission, colonization, and susceptibility to infection.28,85
Outbreaks of S. aureus86 or group A streptococcal infection87 usually indicate a health care provider who is a carrier of the epidemic strain. Airborne spread of gram-negative bacilli is probably rare unless unusual environmental circumstances generate massively contaminated aerosols.88
Increasing evidence suggests that many nosocomial infections acquired in the ICU derive from resistant organisms of enteric origin89–92 or that are present on skin89,90 or in the lower respiratory tract91 on admission to the ICU. This explains the failure of conventional infection control practices, based on the use of barriers, to prevent extrinsically acquired infection.93 Whereas food94 and even enteral feeding preparations95 are often heavily contaminated by microorganisms, studies have not conclusively linked such contamination to disease.
Nosocomial organisms originating from colonized or infected patients are readily perpetuated and spread in contaminated medical apparatus or devices28 such as urine-collection receptacles,96 respiratory therapy equipment,97,98 transducers used for hemodynamic monitoring,99 dialysis machines,100,101 and fiberoptic bronchoscopes and endoscopes.98,102–104 Given the implicit close proximity of vulnerable ICU patients and the HCWs who have repeated contact with them each day, it is almost predictable that the ICU is a milieu within the hospital uniquely conducive to the epidemic infection, especially infections caused by antibiotic-resistant pathogens.
Risk Factors
Risk factor analysis using statistical techniques such as multivariate analysis can identify variables that put a patient at increased risk for nosocomial infection and further guide the development of preventive strategies. Risk factors based on prospectively collected data and, in most cases, the use of multivariate analysis are listed in Table 50.2 for urinary tract infection,105,106 pneumonia,107,108 surgical site infection,109 intravascular device–related bloodstream infection,110 ventriculostomy-associated meningitis,111 C. difficile infection,33,112 and candidemia.113–115
Critical care medicine is synonymous with cutting-edge, high-tech medicine; mechanical ventilatory support; hemodynamic monitoring; total parenteral nutrition; hemodialysis; intracranial pressure monitoring; innovative forms of surgery; and a huge arsenal of drugs, especially anti-infectives of every genre. This technology, more than anything else, has forced critical care medicine to accept the necessity for nosocomial infection control. In general, invasive devices of all types are far more important in determining susceptibility to nosocomial infection than underlying diseases (see Tables 50.2 and 50.E1). However, this should be viewed as welcome news: There is far more hope for reducing nosocomial infections in the coming years by innovative improvements in aseptic technique and advances in the technology of invasive devices than by breakthroughs that will reverse the ravages of chronic organ failure or degenerative diseases such as type 1 diabetes mellitus.
Table 50.E1

*Relative risk or odds ratio: values >1 denote significantly increased risk of infection, and ratios <1, decreased risk, vis-à-vis a protective effect.
From Craven DE, Kunches LM, Lichtenberg DA, et al: Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med 1988;148:1161-1168.
JCAHO mandates that all hospitals have an active program for the surveillance, prevention, and control of hospital-acquired infections, which begins with an institutional infection control committee with representation from the major clinical services and hospital departments including the institution’s ICUs. The most essential members of the infection control program are the infection control practitioner(s), usually registered nurse(s), and the hospital epidemiologist, usually a physician with training in infectious diseases or microbiology, who implement the policies developed by the committee, educate hospital personnel about nosocomial infection control, and investigate suspected outbreaks (Box 50.2).
Surveillance of nosocomial infections is the cornerstone of an effective infection control program and offers numerous potential benefits119,124: (1) It permits determination of baseline (expected) infection rates, assisting recognition of outbreaks and evaluation of new policies and control measures; (2) it identifies institutional problems that require attention, permitting focused infection control efforts and education; (3) it provides reliable data that can be disseminated to individual departments, increasing awareness and involvement of individual staff members; (4) it increases the visibility of the infection control staff on patient care units, providing an opportunity for consultation and ad hoc education; and (5) it facilitates the earliest discovery of patients with communicable infections, permitting timely institution of isolation precautions to limit spread. Because total surveillance (of all infections) is labor intensive, most hospitals now focus their surveillance efforts on infections that are associated with high morbidity (e.g., nosocomial pneumonia), that greatly increase health care costs (e.g., post–cardiac surgery sternotomy infections), that are caused by antibiotic-resistant organisms with potential for spread (e.g., MRSA, C. difficile), or that are highly preventable (e.g., intravascular device-related bloodstream infections).119,125
The 1990s were characterized by major efforts by hospitals to apply the numerous facets of health care principles of quality improvement developed by industry. Hospital infection control programs had been working on quality improvement126 but, influenced by JCAHO, were probably too heavily focused on process, namely, policies and procedures, rather than documenting outcome vis-à-vis reduced infection rates. Infection control programs in most U.S. hospitals are now closely allied with their institutional quality improvement departments.126,127
Hospital infection control programs are also regulated by the Occupational Safety and Health Administration (OSHA) in terms of institutional standards and programs to protect HCWs from bloodborne pathogens128 and tuberculosis129; the Environmental Protection Agency130 has also published regulations in terms of disposal and tracking of medical waste—only a small fraction of which is truly biohazardous.131
Finally, it is essential that all health care personnel working in an ICU receive training in the epidemiology and control of nosocomial infections. This may be most important for house officers in teaching hospitals, who commonly enter the ICU with only the most rudimentary knowledge of asepsis but have hands-on contact with numerous patients each day. ICU physicians and nurses must be especially familiar with their hospitals’ guidelines for the management of invasive devices, particularly intravascular catheters of all types,132 urinary catheters,133,134 endotracheal tubes,135 and tracheostomies.135 Moreover, all physicians need to be made aware that broad-spectrum antimicrobial therapy greatly increases the risk of superinfection by antibiotic-resistant bacteria and Candida, as well as C. difficile.
Role of the Microbiology Laboratory
Accurate and timely diagnostic microbiology is as essential for nosocomial infection control as it is for the clinical management of patients’ infections. Although many infections can be diagnosed on the basis of clinical criteria alone, cultures and other laboratory tests allow infections to be diagnosed with much greater certainty, and certain infections such as bacteriuria, bacteremia, and fungal and viral infections cannot be diagnosed without cultures or other laboratory tests (see Box 50.1).16,17 Moreover, accurate antimicrobial susceptibility testing of clinical isolates is the only means of monitoring trends in antibiotic resistance of hospital organisms.136 Most importantly, identifying the microbial cause of nosocomial infections allows epidemiologic tracking of individual pathogens within the hospital, especially those that are commonly spread from patient to patient such as S. aureus, beta-hemolytic streptococci, enterococci, and the numerous gram-negative bacilli.
From an organizational standpoint, the institutional infection control program and clinical microbiology laboratory must have a close working relationship (see Box 50.2) to assist surveillance, which must be strongly laboratory based,119,137 and to permit the detection and resolution of potential problems. The laboratory director or a senior member of the laboratory staff should be a permanent member of the infection control committee.
The primary role of the clinical microbiology laboratory in any infection control program is to provide up-to-date clinical microbiologic data for use in the surveillance of nosocomial infections and identification of potential outbreaks.137 Protocols should be developed to ensure that laboratory staff immediately contact infection control personnel after the isolation of certain important pathogens such as MRSA or vancomycin-resistant enterococci (VRE) or the appearance of new resistance patterns in endemic organisms such as resistance of Klebsiella species to third-generation cephalosporins and carbapenems, or P. aeruginosa to aminoglycosides, fluoroquinolones, and carbapenems. Sifting through these data can be time consuming, and developing electronic information systems that streamline this process is essential to improving the efficiency of the infection control program. Commercial software programs that can automate this process are now available. Many of these programs automatically collate microbiologic data, provide rudimentary geographic information, and perform basic statistical analyses that can assist in the surveillance of nosocomial infections and identification of potential outbreaks.138,139
Reporting cumulative summaries of antimicrobial susceptibility data (antibiograms) is another essential responsibility of the clinical microbiology laboratory.140,141 When implemented appropriately, the timely dissemination of antibiograms helps guide the choice of empiric antimicrobials, pending the results of clinical cultures, and provides valuable data to help the infection control department monitor institutional antimicrobial resistance trends and identify potential outbreaks.142 The Clinical and Laboratory Standards Institute—formerly the National Committee for Clinical Laboratory Standards—recommends that institutional antibiograms be updated at least annually and has recently published standards for their content and format.143 Automated electronic systems for collating and disseminating nearly real-time antibiograms along with antibiotic-use decision support exist and, when implemented properly, have been effective in improving antimicrobial utilization within the hospital setting.144,145
Monitoring of sterilizers with spore tests, environmental sampling, and advanced microbiologic support for epidemiologic investigations are additional responsibilities expected of most clinical microbiology laboratories, although some university hospital programs have dedicated personnel within their infection control programs who perform these activities.137
The clinical microbiology laboratory is a key resource in the investigation of a suspected outbreak. One of the first and foremost actions when a nosocomial outbreak is suspected is to immediately retrieve all available isolates of the putative epidemic pathogen for possible subtyping.146 The need to move rapidly becomes apparent when it is realized that most hospital laboratories discard cultures as soon as the isolates have been fully characterized. All blood isolates should be routinely saved for at least 1 year.146 Laboratory personnel must be requested to save clinical isolates of any unusual organisms that are encountered for the first time or clusters of any organism and to inform infection control personnel of the findings and availability of the isolates.
The rapid evolution of molecular microbiology has revolutionized epidemiologic investigation of nosocomial outbreaks. Molecular-based tests for the rapid diagnosis of bacterial,147 viral,78,148 and fungal149 infections are now routinely available in most hospital-based and reference laboratories. Modern molecular tests can reliably detect minute numbers of organisms, allowing direct testing of clinical samples without the need for culture. In modern-day clinical virology, molecular tests based on polymerase chain reaction (PCR) for amplification of the pathogen’s DNA or RNA have supplanted tissue cultures and now allow rapid diagnosis of infections that would otherwise often not be identifiable by classic methods.
The availability of molecular subtyping systems has greatly strengthened investigations of outbreaks, as well as research on the epidemiology of nosocomial infections.150,151 The antimicrobial susceptibility pattern (antibiogram) or the detailed biochemical profile (biotype) is often useful for the initial epidemiologic subtyping of many bacteria and may be adequate for identifying an epidemic caused by an unusual pathogen. However, if an epidemic organism is a common species such as S. aureus, it can be difficult or even impossible to know with certainty that an outbreak derives from a common source using these techniques because they lack sufficient discriminatory power.150
The new molecular techniques of subtyping such as plasmid profile typing by agarose gel electrophoresis or the use of restriction endonuclease digests with pulsed-field electrophoresis (DNA fingerprinting)150 are now available in most infection control research laboratories but should be adaptable by many hospital laboratories. Genetic probes promise even more powerful tools for investigating outbreaks, particularly those caused by antibiotic-resistant organisms.147
Although molecular-based tests offer several advantages over traditional microbiologic techniques, they are not a panacea. A number of molecular diagnostic assays (e.g., analyte specific reagents [ASRs]) marketed for clinical practice do not require approval by the U.S. Food and Drug Administration.147 In the absence of published data on their accuracy and precision, the results of these tests must be interpreted with caution and should always undergo extensive inhouse validation before widespread adoption. Moreover, the exquisite sensitivity of many of these tests renders them more susceptible to false-positive results as a consequence of environmental contamination152,153 and mandates stringent quality control practices and procedures.
Architectural and Environmental Issues
The role of the inanimate environment on the transmission of nosocomial infections has been a subject of intense debate for decades. It has been shown that hospital surfaces are almost universally contaminated by potentially pathogenic bacteria such S. aureus,154 enterococcus,155 and gram-negative bacilli such as Acinetobacter baumanii.156 Prior to the 1970s, infection control personnel routinely sampled hospital surfaces. Despite this level of surface colonization, early studies found that the inanimate environment—surfaces, walls, and even air—does not contribute materially to the occurrence of most nosocomial infections,157 other than invasive infections caused by airborne Aspergillus and other filamentous fungi in seriously immunocompromised patients.40,41
Although inanimate surfaces may rarely be involved in the direct transmission of infection to patients, more recent evidence suggests that surfaces may well play an important role in the nosocomial acquisition of pathogenic bacteria, indirectly, through contact with HCWs’ hands and equipment (see Fig. 50.3). This indirect route of infection is of particular importance in the ICU, where all patients are heavily exposed to invasive devices and have a high risk of infection. In the ICU the inanimate environment may become a reservoir for the transmission of resistant nosocomial organisms such as MRSA,158,159 C. difficile,83,160 VRE,155,161 and gram-negative bacilli such as Klebsiella spp., Acinetobacter spp., and Enterobacter organisms.162,163 Studies have shown that enhanced surface decontamination with hypochlorite-containing cleaning solutions has been necessary to terminate outbreaks caused by C. difficile 164 and Acinetobacter baumanii.156
An ICU must be adequately staffed to allow the processes of care to be carried out but also assure a high level of compliance with essential infection control measures such as hand hygiene and barrier isolation. Adequate staffing cannot be overemphasized; numerous studies have found greatly increased rates of nosocomial infection when ICUs are staffed suboptimally or when staffing requirements are met with temporary personnel who are unfamiliar with ICU infection control policies and procedures.165,166 In a large nosocomial outbreak of Enterobacter cloacae infection in a neonatal ICU, Harbarth and colleagues167 found that infection rates during periods of understaffing were strikingly higher than during periods with adequate levels of staffing (relative risk [RR] = 6, 95% confidence interval [CI] = 2.2 to 16.4). The effects of understaffing are likely multiple; however, erosion of basic hygienic practices with excessive patient-to-staff ratios likely explains much of this phenomenon.168
Many of the published recommendations for ICU architectural design169 are empiric, and evidence that they reduce rates of nosocomial infection is, by and large, lacking. Although more research is necessary before specific features of ICU design achieve a level of evidence sufficient for an evidence-based guideline, certain facets of the ICU layout deserve attention:
• ICUs should be located in areas that limit traffic flow to essential ICU personnel.
• ICU facilities should be designed with ICU professionals in mind, ensuring appropriate space, resources, and environment for day-to-day operations.169 Recognizing the growing variety and complexity of life support equipment required for the care of many patients, each cubicle or room should provide a minimum of 11 m2 per bed.170 The area should be large enough to accommodate the bed and all equipment yet allow immediate access to the patient at all times from both sides of the bed. Adequate space must also be provided for storage of nursing supplies. Facilities for disposal of biohazardous waste (e.g., bedpan flushers); for cleaning, reprocessing, and storage of ICU equipment; and for storage of housekeeping supplies should be separate from patient care areas. Single-patient rooms may increase the likelihood of handwashing being done and improve compliance with isolation practices, reducing the risk of cross-infection. For example, Mulin and colleagues171 found that converting from an open unit to single rooms in their ICU greatly reduced rates of patient colonization with A. baumanii, and Shirani and colleagues172 found that renovation of their burn unit to include separate bed enclosures reduced rates of nosocomial infection by 48%.172
• Materials used for fixtures, furniture, and other surfaces should be smooth and easy to clean; surfaces made of porous materials foster bacterial colonization.173
• An adequate number of sinks must be available for convenient handwashing by ICU personnel. Ideally, a sink should be located at the entrance of each cubicle or patient room to encourage handwashing by all entering personnel who will have contact with the patient or the immediate environment.174,175 Separate sinks should be used for cleaning and reprocessing contaminated equipment. Sinks and sink drains are normally contaminated by pseudomonads,176 although their role in the epidemiology of nosocomial infection is as yet unclear. However, sinks should be designed to minimize aerosol formation and splashback.
• All ICUs should be equipped with one or more class A isolation rooms, which include an anteroom for gowning and handwashing and the necessary modifications (negative pressure, roofline exhaust) to permit it to be used for patients with tuberculosis or other airborne infections such as chickenpox, measles, disseminated HSV infection, or a highly contagious emerging pathogen such as the severe acute respiratory syndrome (SARS) human coronavirus. If an ICU treats bone marrow transplant patients or other patients with prolonged severe granulocytopenia, positive-pressure isolation rooms using high-efficiency particle-arrest (HEPA) filters should be available. Isolation rooms for patients with infections transmitted by the respiratory route or to protect profoundly granulocytopenic patients must be kept closed to maintain control over the direction of airflow.
• A centralized, filtered air-handling system that provides at least six room exchanges per hour is essential.170,177 Ideally, each patient’s room should have the capacity of being set at positive or negative pressure with respect to the rest of the unit; if it cannot be, the room should be maintained permanently at positive pressure.
A variety of microorganisms including bacteria, mycobacteria, fungi, and parasites can be isolated from hospital water and have been implicated in endemic and epidemic nosocomial infections.178 Many of these outbreaks were caused by bacteria typically thought of as “water” organisms such as P. aeruginosa,176 Stenotrophomonas maltophilia,179 and A. baumanii16,180,181; however, the most important and epidemiologically linked hospital water pathogen is the Legionella group.182
Nosocomial legionellosis was first described in 1979,183 and it is estimated that up to 50% of cases of legionellosis are acquired in the health care setting,184 with a mortality rate that approaches 30%.185 Contamination of hospital potable water remains underappreciated despite studies showing that Legionella species can be recovered from 12% to 70% of hospital water systems,186 and a number of studies in which nosocomial cases were identified only when specific diagnostic and surveillance methods were employed.187,188 Characteristics of hospital water systems that are associated with Legionella contamination include piping systems with dead-ends that facilitate stagnation, large-volume water heaters that result in inefficient heating of hospital water, sediment buildup, water heater temperatures less than 60° C and tap water temperatures less than 50° C, maintaining water pH greater than 8 and receiving municipal water untreated with monochloramine.189–191
Despite the ubiquity of water systems colonized with Legionella species and studies demonstrating a correlation between the level of colonization and risk of infection, the CDC does not recommend routine surveillance of hospital water systems,184 although this stance is controversial.186 Researchers from Pittsburgh, Pennsylvania, and the Allegheny County Health Department have recommended a more proactive stepwise approach that involves initial surveillance of hospital water for Legionella contamination, regardless of the presence or absence of institutional nosocomial legionellosis, followed by continued surveillance based on the level of water contamination found or the presence of institutional legionellosis.186
Legionella species are resistant to chlorine and heat, making it challenging to eradicate them from contaminated hospital water systems.191 Attempts to hyperchlorinate hospital water have been partially successful if chlorine levels are continuously maintained between 2 and 6 parts per million at all times but produce rapidly accelerated corrosion of water pipes and are expensive.192 Thermal eradication is feasible, using a “heat-and-flush” method to raise water tank temperatures to greater than 70° C and distal water sites to greater than 60° C for short periods of time.193 Although effective, superheating is labor intensive and there is the constant fear that patients or health care personnel may sustain scald injuries if they wash or shower with tap water during a flushing period. The use of technologies such as instantaneous steam heat for incoming water193 and ultraviolet light194 are technically feasible with newer hospital water systems but may be incompatible with older hospital water systems.
Perhaps the most attractive, effective, safe, and cost-efficient method for Legionella eradication may be the use of continuous copper-silver ionization systems to sterilize hospital water systems. These systems have been well studied over the past decade and have proved to be highly effective for reliably eradicating Legionella contamination of hospital water and, most importantly, for eliminating nosocomial legionellosis in institutions when other interventions have failed.195 In our own institution (Maki DG), two clusters of nosocomial legionellosis prompted a retrospective review that identified 10 cases over an 11-year period. Surveillance of the hospital water system found that 75% of all samples contained low levels of L. pneumophila, which were shown to be clonally related to the 10 cases of nosocomial legionellosis. Installation of a continuous copper-silver ionization system led to complete eradication of Legionella from water samples, and no further cases of nosocomial legionellosis have been identified at our institution since 1995, among 255,000 patients hospitalized.
Reliable Sterilization Procedures, Chemical Disinfectants, and Antiseptics
Reliable sterilization, disinfection, and antisepsis embrace virtually all measures aimed at prevention of nosocomial infection. Critical objects, which are introduced directly into the bloodstream or into other normally sterile areas of the body, such as surgical instruments, cardiac catheters, and implanted devices, must be reliably sterile and sterilized with steam, gas, hydrogen peroxide gas, or chemical sterilization. Semicritical items, which come into contact with intact mucous membranes, such as fiberoptic endoscopes, endotracheal tubes, or ventilator circuit tubing, can be decontaminated between patients by pasteurization or the use of high-level chemical disinfection with glutaraldehyde, peracetic acid, hydrogen peroxide, ethyl alcohol, or hypochlorite. Noncritical items, which normally come into contact only with intact skin, such as blood pressure cuffs or electrocardiograph electrodes, require hygienic cleansing or low-level disinfection with an iodophor, hypochlorite, quaternary ammonium or phenolic disinfectants, or alcohol.196,197 The lone exception to this classification scheme is devices that pose a risk of transmitting prion-related diseases, including Creutzfeldt-Jakob disease (CJD) and variant CJD (vCJD).198,199 Prions are not readily inactivated by conventional disinfection and sterilization procedures.196,199 As a result, devices that pose a risk for transmission of prion-related diseases should undergo special sterilization procedures after cleaning that involve sodium hydroxide followed by low-temperature autoclaving (121° C) or high-temperature autoclaving (132° C for 1 hour or 134° C for ≥18 minutes).197,199 Despite concerns that procedures involving semicritical items such as endoscopes and bronchoscopes may pose a risk for transmission of prion-related infections, there has not been a single report of CJD or vCJD associated with these devices. As a result, current guidelines recommend that only critical items and semicritical items that have come in contact with neurologic tissue (e.g., brain [including dura mater], spinal cord, eye, and pituitary tissue) should undergo special prion inactivation sterilization procedures.197,199,200
Numerous epidemics of gram-negative infection have been described in association with respiratory therapy equipment,97,98 diagnostic equipment such as bronchoscopes and endoscopes,98,102–104 and solutions used for cutaneous antisepsis.201,202 Most of these outbreaks were traced to improper procedures or malfunction of automated systems used for the disinfection and sterilization of medical devices, although a number of epidemics in years past arose as a result of extrinsic contamination of solutions used for cutaneous antisepsis.201,202 For these reasons, the importance of strict adherence to recommended policies and procedures for cleaning and reprocessing medical equipment used in the ICU cannot be overemphasized.
Endoscopes and bronchoscopes are essential diagnostic and therapeutic instruments in the ICU. Although most postendoscopy nosocomial infections are caused by inoculation of colonizing mucosal flora into normally sterile, vulnerable anatomic sites during the procedure, numerous epidemics have been traced to contaminated endoscopes.98,102–104 Following use for bronchoscopy, endoscopes are typically contaminated with 6×104 colony-forming units per milliliter (CFUs/mL).203 All endoscopes are considered semicritical medical devices by the Spaulding classification and therefore require high-level disinfection following use.200 In order to ensure their safe use, flexible endoscopes should be reprocessed with the following procedures: (1) physical cleaning to reduce microbial bioburden and remove organic debris; (2) high-level disinfection—glutaraldehyde and automated chemical sterilizing systems that use peracetic acid are most commonly used in the United States—with adequate contact time between the disinfectant and device surface; (3) following disinfection, rinsing with sterile or filtered tap water to remove disinfectant residue; (4) flushing of all channels with 70% to 90% ethyl or isopropyl alcohol; and (5) drying with forced air.200 Devices used with endoscopes that violate mucosal barriers, such as biopsy forceps, need to be reprocessed as critical medical items with full sterilization.200 Other devices used in the delivery of respiratory care are also considered semicritical under the Spaulding classification and therefore should be reprocessed in a manner similar to endoscopes prior to reuse.135
Iodophors (e.g., 10% povidone-iodine), until recently, have been the most common agents used for cutaneous disinfection in North America. However, a large, prospective, randomized trial of cutaneous antiseptics used for drawing blood cultures showed that chlorhexidine was superior to 10% povidone-iodine and was associated with a more than twofold reduced rate of contaminated blood cultures (odds ratio [OR] = 0.40, 95% CI 0.21 to 0.75, p = 0.004).204 Moreover, a meta-analysis examining the impact of different cutaneous antiseptic agents found that chlorhexidine was superior to povidone-iodine for both the prevention of intravascular catheter colonization and catheter-related bloodstream infection.205 On the basis of these and other recent studies,206,207 chlorhexidine-containing solutions are the preferred cutaneous antiseptics for insertion of intravascular devices in the ICU.132 Whatever agent is used, it is essential that it be applied with vigorous scrubbing for a minimum of 1 minute to allow adequate time for germicidal activity.
Hand Hygiene
The major reservoir of nosocomial infection in the ICU is infected or colonized patients, and the major mode of spread of most nosocomial bacterial pathogens, many viruses, and even Candida from patient to patient is by transient carriage on the hands of medical personnel (see Fig. 50.3). Studies of hand carriage of nosocomial pathogens by ICU personnel, using a simple rinse technique to quantify the transient flora,208 have shown that, on average, approximately 60,000 CFUs (or 4.6 logs) are recovered from the hands of ICU personnel randomly sampled (Table 50.4). Nearly half of persons cultured at any point in time will be found to be carrying gram-negative bacilli, and 10% will be carrying S. aureus.209 Serial culturing has shown that all ICU personnel, at various times, carry gram-negative bacilli and that nearly two thirds carry S. aureus. Carriage of both gram-negative bacilli and S. aureus is typically transient: sampling persons every other day over a prolonged period has shown S. aureus or the same gram-negative species in consecutive cultures only 16% of the time; prolonged carriage of a single gram-negative species seems to be rare—but has been reported.210
Table 50.4
Studies of Microorganisms Carried on the Hands of Hospital Personnel Working in a Neurosurgery Unit*

CFUs, colony-forming units; SD, standard deviation.
*At the University of Wisconsin Hospital.
†Based on 6 to 34 cultures obtained at random times from each of 25 employees working in the unit over a 4-month period.
From Maki DG: Control of colonization and transmission of pathogenic bacteria in the hospital. Ann Intern Med 1978;89:777-780.
Hygienic handwashing before undertaking invasive procedures, handling open wounds, or having manual contact with high-risk patients (e.g., newborns or patients in ICUs) or after touching a source or object likely to be contaminated has been recognized since the time of Semmelweis and Lister as one of the most basic and important infection control measures. Despite universal acknowledgment of handwashing as a cornerstone of nosocomial infection control programs, compliance rates much above 50% have been difficult to achieve, and handwashing rates among HCWs have ranged from 9% to 50% in numerous observational studies.168,211,212 Recent investigations have undertaken to better understand the reasons for poor compliance in the face of the compelling evidence that hand hygiene is essential for prevention of nosocomial infection,168 identifying cutaneous irritation, inconvenient sink location, time constraints, high workload, and understaffing. Of concern, risk factors for noncompliance with hand hygiene include being a physician (rather than a nurse); working in an ICU; and paradoxically, engaging in patient-care activities with a high risk of cross-transmission.168 Interventions to redress these deficiencies have included targeted education; feedback; convenient location of sinks and hand hygiene agents; use of alternative, less irritative hand hygiene agents; hand care lotions or creams213; and patient education.214
Studies done with working hospital staff have shown that hygienic handwashing with an antiseptic-containing agent reduces the count of microorganisms on the hands of the user far more effectively than handwashing with a nonmedicated soap.208 Repeated use of some antiseptics such as chlorhexidine has a cumulative suppressive effect on the transient hand flora. Routine use of an antiseptic-containing handwashing agent could, in theory, enhance the effectiveness of the handwashing that is done. Moreover, if an agent that exhibits prolonged antimicrobial activity, such as chlorhexidine, is used, it might also confer protection against contaminants acquired between handwashings.208 However, antiseptic-containing handwashing agents are more expensive and often more irritating to the skin. Irritation can result in dermatitis and, paradoxically, increased colonization by gram-negative bacilli.215
Clearly, antiseptic-containing soaps are more effective in removing microorganisms from the hands of users, but will routine use of these agents for hygienic handwashing reduce the incidence of nosocomial infection in patients? Discontinuation of hexachlorophene for handwashing by personnel and bathing of infants in the United States in 1973 was followed by a marked upsurge in S. aureus infections in nurseries,216 and use of chlorhexidine-containing handwashing agents was considered an essential measure for control of hospital outbreaks caused by multiply resistant Klebsiella217 and MRSA.218,219 However, since Semmelweis’s study, few studies have prospectively evaluated the efficacy of antiseptic-containing handwashing agents for reducing endemic nosocomial infections, particularly infections caused by gram-negative bacilli.215,220
In 1982 a comparative sequential trial of three handwashing agents—a nonmedicated tissue soap, 10% povidone-iodine (Betadine Scrub), and 4% chlorhexidine (Hibiclens)—was undertaken in the trauma-surgical ICU of the University of Wisconsin Hospital.215 Each agent was used exclusively for approximately 6 weeks, during which time hand cultures of ICU personnel were done at random and surveillance of infection in patients was carried out. Risk factors for infection in patients hospitalized during the use of each agent were comparable: Nearly two thirds of the patients in each period required ventilatory support and hemodynamic monitoring, and almost all had urinary catheters. The incidence of nosocomial infection in all groups was expectedly high, but it was 30% lower during the use of the two antiseptic-containing handwashing agents than during the use of the nonmedicated soap (p < 0.001). Povidone-iodine was irritating to the hands of most staff, and chlorhexidine had a slightly drying effect but was well tolerated, comparable with the nonmedicated soap.
In a similar study at the University of Iowa Hospital, Massanari220 did not find significant differences in the rates of nosocomial infection when nonmedicated soap was used exclusively as compared with alternating cycles during which 4% chlorhexidine (Hibiclens) was used in surgical ICUs; however, the incidence of infection in the medical ICU was 50% lower during use of chlorhexidine (p < 0.05).
In the largest multiple-crossover prospective study—1894 adult patients in three ICUs—of the relative efficacy of antiseptic-containing handwashing agents used by personnel in ICUs, Doebbeling and colleagues221 found that the use of 4% chlorhexidine (Hibiclens) was associated with a 30% reduction in nosocomial infections (OR = 0.73), as contrasted with rates when a 60% alcohol hand-rinsing agent (Cal-Stat) was used. Both regimens were well tolerated.
Recently, alcohol-based waterless hand rubs have become the agents of choice for hand hygiene and are now universally used in U.S. hospitals because of their convenience and broad-spectrum activity.214 Alcohols have the most rapid and pronounced bactericidal action and greatly reduce the time needed for hand disinfection. A vigorous 1-minute rubbing with a sufficient volume of alcohol to wet the hands completely has been shown to be highly effective at reducing the density of skin flora.222 Ethanol and iso- and n-propanol are the constituents of most commercially available alcohol-based hand rubs; at equal concentrations, n-propanol is most effective and ethanol the least. However, all have limited efficacy with gross soilage so that visibly soiled hands should always be washed with antiseptic soap and water.174 Moreover, at least 3 mL of an alcohol-based rub is necessary to completely coat the hands and achieve optimal degerming. The use of alcohol hand rubs or gels will be augmented by making conveniently located calibrated dispensers widely available. However, many HCWs prefer individual containers that can be carried in a pocket, which makes it difficult to ensure that an adequate volume is used with each application.
Few trials have been conducted to evaluate the efficacy of alcohol-containing hand rubs for reducing nosocomial infection. Most are quasi-experimental before-after studies, and most have shown a short-term reduction in nosocomial infection rates with use of alcohol-containing hand rubs.175,223,224
The major factor limiting acceptance of alcohol products for hand antisepsis in the past was desiccation and irritation of skin. This is now obviated by incorporating emollients into alcohol-based hand rubs, which has enhanced acceptance by HCWs and may augment antibacterial activity by slowing the evaporation of alcohol.225 A recent randomized clinical trial in 50 ICU HCWs compared a conventional 2% chlorhexidine gluconate wash with water to a waterless alcohol-based hand rub (61% ethanol with emollients) and showed that use of the waterless alcohol-based product produced significantly less skin scaling and irritation226; unfortunately, degerming was not assessed.
A recent review describes in detail the various hand hygiene agents available and their spectrum of activity.214 Recommendations for hand hygiene by the CDC have been published (Table 50.5),174 emphasizing hand antisepsis with an antiseptic-containing soap or detergent or an alcohol-based hand rub (1) before and after direct contact with patients or the environment and equipment in the immediate vicinity of the patient and (2) before performing invasive procedures such as insertion of an intravascular device or urinary catheter. Use of skin care products—lotions or creams—to minimize irritant contact dermatitis associated with frequent handwashing and improve compliance with hand hygiene practices is highly recommended.
Table 50.5

*Categorization of recommendations: IA: strongly supported for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies. IB: strongly recommended for implementation and supported by certain clinical or epidemiologic studies and by strong theoretical rationale. IC: required for implementation, as mandated by federal or state regulation or standard. II: suggested for implementation and supported by suggestive clinical or epidemiologic studies or by strong theoretical rationale. NR (no recommendation/unresolved issue): practices for which insufficient evidence or no consensus exists about efficacy.
Data from Boyce JM, Pittet D: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep 2002;16:1-45.
Institutional commitment is essential to improve compliance with recommended hand hygiene practices. The CDC guideline recommends that institutions (1) monitor and record adherence to hand hygiene by ward or service; (2) provide feedback to HCWs about their performance; and (3) monitor the volume of alcohol hand rubs used per 1000 patient-days.174
Isolation Precautions for Communicable Infections
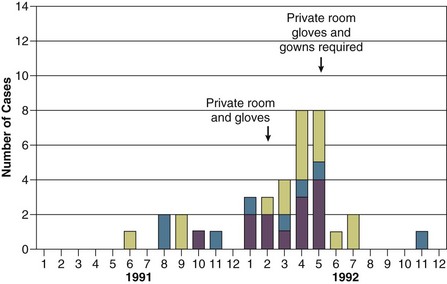
Isolation, the use of special precautions in the care of infected patients, is the only means of curtailing the spread of contagious microorganisms and preventing epidemics, especially in ICUs, where the risk of cross-infection is highest. Although requiring all persons entering an infected patient’s room to wear gloves and a gown, possibly even a mask, may seem ritualistic and almost archaic, each aspect of the isolation procedure is directed at interrupting a potential mode of spread and is based on the known epidemiology of the infecting organism.227 To be maximally effective, however, isolation procedures require compliance by each person coming into contact with the patient, including physicians. Isolation is also indicated, usually for the entirety of hospitalization, for all patients infected or known to be colonized by antibiotic-resistant nosocomial pathogens such as MRSA, multidrug-resistant gram-negative bacilli, or VRE; in such cases, isolation has been shown to be effective in reducing endemic infections228,229 (Figs. 50.3 and 50.4) and in controlling outbreaks.229
Isolation Systems
Most U.S. hospitals subscribe to one of two CDC isolation systems developed by panels of experts. The simplest system, category-specific isolation precautions, issued by the CDC in 1970,227 groups diseases in seven categories by infections for which similar precautions are indicated: wound and skin precautions, enteric precautions, discharge precautions, blood precautions, respiration isolation, strict isolation, and protective isolation. Guidelines for disease-specific isolation precautions, issued in 1983,230 consider each infectious disease individually, so only those precautions indicated to interrupt transmission of that specific disease are used. Disease-specific precautions minimize unnecessary isolation procedures; however, they are more complicated and may be implemented most effectively by a computerized system.
An alternative, simpler system, body substance isolation, has gained adherents and focuses on the isolation of potentially infectious body substances, such as blood, feces, urine, sputum, wound drainage, and other body fluids, of all patients through the use of simple barrier precautions—primarily gloves, gowns, plastic aprons, and masks or goggles. These barriers should be used when potentially infectious secretions are likely to soil or splash the clothing, skin, or face of the HCW.231 Body substance isolation provides sufficient flexibility to augment the basic precautions taken with each patient, as needed, and adds private rooms with masks for infections transmitted by the airborne route. A criticism of this simpler system has been the reduced emphasis on handwashing when gloves are removed.232
The most recent CDC guideline177 separates basic precautions into (1) standard precautions designed for the care of all patients in hospitals, regardless of their diagnosis or presumed infection status, and (2) additional transmission-based precautions designed for the care of specified patients who are known or suspected to be infected with highly transmissible or epidemiologically important pathogens. Standard precautions synthesize the major features of universal blood and body fluid precautions and are designed to reduce the risk of transmission of microorganisms from patient to patient and from patient to HCW, from both recognized and unrecognized sources of infection in the hospital. Transmission-based precautions are divided into three subgroups on the basis of the mode of transmission: contact precautions, droplet precautions, and airborne precautions. Contact precautions are recommended with multidrug-resistant bacteria that can be acquired by contact with the colonized patient or environmental surfaces or objects. Droplet precautions provide additional measures for transmission by large-particle droplets, such as during suctioning or bronchoscopy. Airborne precautions are added to standard precautions for the care of patients with tuberculosis and other microorganisms transmitted by the airborne route. In general, transmission-based precautions usually specify a private room—always for airborne precautions.
Special Issues in the ICU
An environmental issue pertaining to isolation may be most relevant in the ICU, namely, the greater potential for fomites or environmental surfaces to contribute to the spread of nosocomial infection, especially with antibiotic-resistant microorganisms. Although previous studies have not been able to demonstrate that the inanimate hospital environment, particularly surfaces, walls, or floors, contribute materially to the occurrence of nosocomial infection,157,158 accumulating evidence suggests that this may not necessarily be true for ICUs, where uniform exposure to invasive devices makes patients unduly susceptible. A number of careful studies of the epidemiology of ICU-acquired infection with resistant organisms such as MRSA,158,159 C. difficile,83,160 and VRE155,161,233 have shown heavy contamination of the inanimate environment immediately contiguous to the patient by strains implicated in nosocomial infections occurring in patients. Even if gloves are being worn as part of protective isolation or universal precautions, the possibility of transmission of microorganisms from the environment to patients on the gloved hands of HCWs is real. Prolonged wearing of gloves in the ICU, which is common, may increase the risk of nosocomial cross-infection, expanding the epidemiologic role of the inanimate environment with certain pathogens such as MRSA or VRE.233
Similarly, the use of common stethoscopes, sphygmomanometers, or electronic thermometers with multiple patients provides further opportunity for organisms to spread. Although stethoscopes are commonly contaminated by nosocomial organisms,234 their role in cross-infection is less clear.234 On the other hand, spread of VRE235 and C. difficile 236 has been traced to contamination of electronic thermometers. All surfaces contiguous to the ICU patient should be wiped down with the general hospital disinfectant at least daily, and each ICU patient should have a dedicated stethoscope and sphygmomanometer. The use of electronic temperature measuring devices on multiple patients within an ICU bears reevaluation, unless stringent efforts are made to assure reliable decontamination of the device after each use.
As discussed, many nosocomial infections appear to derive from organisms carried on the hands of ICU personnel, who during the working day have contact with multiple patients. To improve nursing care and reduce the risk of cross-infection, ICUs must have an adequate number of staff. Although the optimal nurse/patient ratio for patients in an ICU is not known, increased rates of infection and outbreaks have occurred when nurses have been assigned to multiple critically ill patients who require complicated nursing care.166 One-to-one nurse/patient ratios may significantly reduce the risk of cross-infection.
To contain the spread of certain resistant organisms in the ICU (e.g., MRSA, VRE), cohort nursing is strongly recommended. In cohort nursing, the care of patients known to be infected (or colonized) by the organism is provided by nurses (and respiratory therapists) who will not provide care during that shift for noninfected patients, and the nursing care of noninfected patients is restricted to personnel who will not have contact with infected patients, except in an emergency. Cohorting of patients known to be colonized or infected with MRSA is widely practiced but has not been adequately studied. In one recent prospective study, the authors found that there was no evidence of increased transmission of MRSA when patients were not cohorted.237
Tuberculosis
The upsurge in tuberculosis since 1985, particularly the numerous nosocomial outbreaks caused by multidrug-resistant strains,73–75,238,239 demonstrates the importance of isolation precautions to prevent the spread of tuberculosis within hospitals, especially within ICUs.129 Guidelines129 reemphasize the importance of air control by mandating the use of private negative-pressure rooms, combined with the use of ultraviolet lights or ventilatory modifications in which all air exiting the room is either filtered or exhausted directly to the roofline, away from hospital intake vents. Isolation room doors must be kept closed to maintain control over the direction of airflow, and all persons who enter a room in which tuberculosis isolation precautions are in effect must wear a disposable particulate respirator such as a dust-mist mask or a HEPA-filter mask. Gowns and gloves usually are not indicated. All ICUs should have one or more negative-pressure isolation rooms for the care of patients requiring respiratory isolation for tuberculosis and other airborne infections such as chickenpox or disseminated herpes zoster, disseminated HSV infection, or emerging, highly contagious airborne infections such as SARS. To reduce the risk of contaminating a ventilator or discharging M. tuberculosis into the environment, when mechanically ventilating a patient with suspected or confirmed pulmonary tuberculosis, a bacterial filter capable of filtering particles as small as 0.3 µm, with a filter efficacy of greater than 95%, should be placed on the patient’s endotracheal tube or at the expiratory side of the breathing circuit of a ventilator.129 ICU patients with tuberculosis not requiring mechanical ventilation should wear a surgical mask if leaving the negative-pressure isolation rooms for radiographic or other procedures.129
Standard Precautions
The world epidemic of AIDS and evidence that more than 1 million persons in the United States are silent carriers of the human immunodeficiency virus (HIV) have engendered great concern among HCWs regarding the risk of exposure to HIV in the workplace. In 1987 the CDC and the Department of Labor issued detailed guidelines for Universal Blood and Body Fluid Precautions240,241 to prevent exposure of HCW workers and patients to potentially hazardous blood or body fluids. Universal precautions were based on the concept that all blood and body fluids that might be contaminated with blood should be treated as infectious because patients with bloodborne infections can be asymptomatic or unaware they are infected. The relevance of universal precautions to other aspects of disease transmission was recognized, and in 1996 the CDC expanded the concept and changed the term to Standard Precautions.242 Standard precautions integrate and expand the elements of universal precautions into a standard of care designed to protect health care personnel and patients from pathogens that can be spread by blood or any other body fluid, excretion, or secretion. Standard precautions apply to contact with (1) blood; (2) all body fluids, secretions, and excretions (except sweat), regardless of whether they contain blood; (3) nonintact skin; and (4) mucous membranes.
Gloves are recommended for venipunctures, insertion of intravascular devices, and whenever it can be anticipated that the hands could become contaminated by blood or another high-risk body fluid. If there is potential for splatter or contamination of clothing, a gown is added. When there is potential for aerosolization of body fluids, such as during surgery, intubation, endoscopy, or insertion of an arterial catheter, a mask and eye shielding are included. Because the vast majority of occupationally related HIV infections have involved needle sticks or other sharps injuries, every effort must be made to avert such injuries that could result in percutaneous inoculation of HIV or other bloodborne viruses.240,243
Because prophylactic use of barrier precautions appears to be of some benefit for the prevention of nosocomial infection172,244,245 and all U.S. hospitals are currently mandated to follow standard precautions, it has been suggested that the use of gloves for all patient contacts, as is now common in many U.S. hospitals, should implicitly reduce the risk of nosocomial infection in general. However, this has not been demonstrated and there is concern that standard precautions might paradoxically increase the risk of nosocomial cross-infection.246 In most U.S. hospitals it is still common to observe ICU personnel, many of whom routinely wear gloves for all patient contacts to protect themselves, put on gloves, touch heavily contaminated areas (e.g., an open wound or tracheostomy), and then, without removing the gloves, proceed to write in the patient’s chart, answer the telephone, or care for another patient. This occurs because the health care providers have forgotten that although the gloves may protect themselves, the gloves must be immediately discarded after use to prevent cross-contamination of hazardous pathogens to other vulnerable sites on the same patient or transmission to other patients or the ICU environment. Before the era of AIDS and universal precautions, health care professionals were oriented toward protecting the patient and likely to wash their hands when exposed to potential contamination. Now the focus is centripetal, and many HCWs unfortunately view all precautions as measures to protect themselves. Thus prolonged wearing of gloves can result in heavy contamination of the gloves247 and increase the risk of nosocomial cross-infection among patients.233,248 It also puts the HCW at increased risk of dermatitis and allergic reactions to glove material.249 Standard precautions do not obviate the need for designated isolation precautions for patients with communicable infections. The greatly expanded use of gloves as part of standard precautions in hospitals must now be accompanied by educational programs on how to use gloves effectively and in a manner that will not jeopardize patients. Staff must be strongly encouraged to wash their hands after removing gloves, especially after performing a bloody procedure, because blood often penetrates defects in gloves and can be found on the hands of the wearer.250 Moreover, if the gloved HCW has had hands-on contact with a patient colonized by MRSA or VRE, the process of removing the gloves will result in contamination of the hands of the HCW by these organisms up to one third of the time.
Antibiotic Stewardship
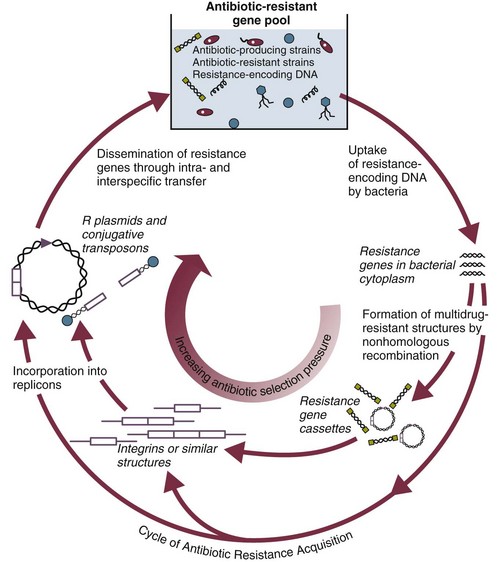
There is a world crisis in antibiotic resistance (see Fig. 50.2),251,252 which reflects in greatest measure the heavy use of systemic antibiotics worldwide over the past 30 years, especially in hospitals. Antimicrobial therapy has its greatest ecologic impact in the close confines of the ICU. Most nosocomial outbreaks caused by antibiotic-resistant microorganisms253,254 have occurred in patients hospitalized in an ICU. Antibiotic pressure, which promotes the exchange of genes encoding drug resistance by a variety of transfer mechanisms (Fig. 50.5),255 has been shown to be the single most important factor predisposing patients to nosocomial infection with resistant organisms. Modern-day ICUs are the breeding grounds for the multiply resistant bacteria that are now being encountered in hospitals throughout the world: MRSA; VRE; extended-spectrum β-lactamase-producing and carbapenemase-producing K. pneumoniae and E. coli, Enterobacter, Serratia, Citrobacter, and P. aeruginosa resistant to fluoroquinolones, aminoglycosides, or extended-spectrum β-lactams.47,58–61,251 Broad-spectrum antimicrobial therapy is the root cause of antibiotic-associated diarrhea and colitis caused by C. difficile.83
Clearly, antimicrobials are widely overused and misused; more than 75% of patients in U.S. ICUs, other than coronary care units, receive antimicrobial agents, whereas studies indicate that more than half of hospitalized patients receiving antimicrobial therapy have no evidence of infection or clear justification to be receiving antibiotics.256 Moreover, within ICUs, a high proportion of the antibiotics used are broad-spectrum/extended-spectrum penicillins, third- and fourth-generation cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, or vancomycin. Greater efforts must be directed to improving the use of systemic antibiotics, especially within ICUs.
JCAHO now mandates that hospitals periodically review their use of antimicrobial agents through the use of antimicrobial audits.30 Such audits should scrutinize the need for antimicrobial therapy—clear evidence of infection or clear justification for prophylactic use, the appropriateness of the regimen selected, and monitoring for therapeutic efficacy and side effects during therapy.257 Educational programs and institutional guidelines for antimicrobial use that permit the hospital staff to construct guidelines and policies based on local needs and judgments, aided by published criteria, have been shown to materially improve antimicrobial use within the hospital.256,258 Other important methods for controlling antimicrobial use include a restricted formulary, the policies of the clinical microbiology laboratory on reporting of susceptibility testing, and automatic stop orders for surgical prophylaxis.256 Many institutions also place expensive or the most broad-spectrum drugs (e.g., third- and fourth-generation cephalosporins, extended-spectrum penicillins [e.g., piperacillin-tazobactam], carbapenems, aminoglycosides, fluoroquinolones, vancomycin, linezolid, fluconazole, echinocandins, lipid formulations of amphotericin B, ganciclovir, etc.) on a restricted list, requiring physicians who wish to use the agents to justify their use to a representative of the institutional antibiotic review committee.259 Such programs greatly reduce use of restricted antibiotics and are gaining ever-wider acceptance.
Excellent resources,260–262 including other chapters in this book (Chapters 20, 51, 52), are available to guide the selection and use of anti-infective drugs in critically ill patients. However, several principles can reduce unnecessary antimicrobial therapy and improve the use of the drugs that are given:
1. Fever without other indications of infection should not mandate automatically beginning antimicrobial therapy in an ICU patient.
2. Unless antimicrobial therapy is being given for surgical prophylaxis, it is most likely being given for the treatment of suspected or proved infection. Gram-stained smears, cultures, and other appropriate diagnostic tests, as indicated, should be done without fail before beginning antimicrobial therapy for treatment of presumed infection in an ICU patient.
3. Whenever antimicrobial therapy is begun, the reason should be documented in the patient’s record (e.g., “for the treatment of pneumonia,” “for surgical prophylaxis”).
4. When possible, a single drug and the most narrow-spectrum drug or drugs should be used, especially if the infecting organism or organisms are known at the outset.
5. The need for continued antimicrobial therapy should be reassessed daily. If cultures identify the infecting microorganism or microorganisms, therapy should be modified, aiming for the most narrow-spectrum drug or drugs likely to be effective. If diagnostic studies are negative after 48 to 72 hours and the patient is not exhibiting signs of sepsis, antibiotic therapy should be discontinued, unless the patient is profoundly granulocytopenic.
6. Beyond monitoring for efficacy and adverse drug effects such as hypersensitivity or organ toxicity, it is essential that monitoring include surveillance for superinfection by resistant bacteria or Candida and for C. difficile infection.
7. Surgical antimicrobial prophylaxis should not extend beyond 24 hours postoperatively263,264 and in most operations can be limited to a single dose.263
Nosocomial Infections and Specific Infection Control Measures
As noted earlier, most nosocomial infections, especially in immunologically competent patients and in ICUs, are causally related to surgical operations or exposure to invasive devices of various types (see Tables 50.1 and 50.2). Comprehensive guidelines for the prevention of infection with procedures or devices that pose the greatest risk (urinary catheters,133,134,265 endotracheal intubation and mechanical ventilatory support,135 intravascular catheters and infusion therapy,132 hemodialysis,266 and surgery263) have been published and can form the basis for institutional policies and procedures. Health care professionals working in ICUs are obligated to be informed about prevention of infection associated with the procedures they perform and the devices with which they work daily.
Intravascular Device–Related Bloodstream Infection
Impact
Obtaining and maintaining reliable vascular access has become one of the most essential features of modern-day intensive care. Unfortunately, vascular access is associated with substantial and generally underappreciated potential for producing iatrogenic disease, particularly bloodstream infection (BSI) originating from infection of the percutaneous intravascular device (IVD) used for vascular access (i.e., IVD-related BSI), often referred to as “line sepsis.” Nearly 60% of all nosocomial bacteremias derive from vascular access in some form,18 and it is estimated that more than 500,000 IVD-related bloodstream infections occur in the United States each year.267,268 Early studies found that IVD-related BSIs are associated with excess attributable mortality rate ranging up to 35%269; however, subsequent case-control studies have not consistently found excess mortality rates, especially of this magnitude.270–272 This controversy aside, all studies examining the impact of IVD-related BSI on patient outcomes have found that IVD-related BSIs are associated with increased length of hospitalization and excess health care costs, averaging $30,000 per case.269–272
Definitions
IVDs are associated with both local and systemic infection. The CDC has published definitions for IVD-related infection (see Box 50.1).16,17 These definitions are useful for the purposes of surveillance but rely heavily on the construct, central venous catheter–associated BSI, which implicitly assumes that each primary BSI (i.e., a BSI without an identifiable local infection) originates from a central venous catheter (CVC). This practice results in an overestimation of the true risk of CVC-related infection because not all primary BSIs originate from a central venous device; some are secondary BSIs deriving from unrecognized postoperative surgical site or intra-abdominal infections or nosocomial pneumonias or originate from other vascular devices such as peripheral venous catheters or arterial catheters used for hemodynamic monitoring.
By applying molecular subtyping techniques110,273,274 to the results of semiquantitative or quantitative cultures of the removed IVD and blood cultures or the results of cultures of blood drawn through the IVD and a separate concomitant percutaneous peripheral blood culture, it is now possible to reliably determine whether an IVD was the source of a nosocomial BSI. Using these new diagnostic techniques allows the formulation of simple but more rigorous definitions for IVD-related infection (Table 50.6), which we believe bear consideration as the standard for randomized trials and epidemiologic studies of IVD-related infection.18 Recently published guidelines have outlined clinical definitions of IVD-related BSI similar to those listed in Table 50.7, although molecular subtyping methods are not recommended for routine clinical diagnosis of IVD-related BSI.275
Table 50.6
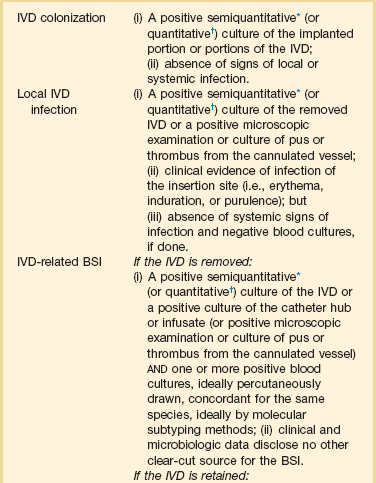
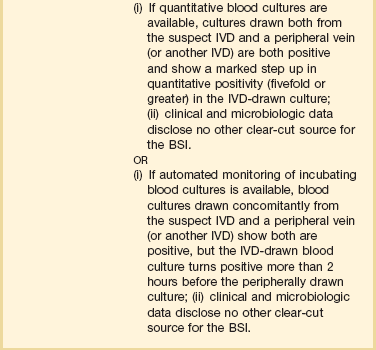
*Roll plate of cannula segment(s) >15 colony-forming units (CFUs).
†Sonication culture of cannula segment(s) ≥103 CFUs/mL.
Modified from Crnich CJ, Maki DG: The role of intravascular devices in sepsis. Curr Infect Dis Rep 2001;3:497-506.
Table 50.7
| Nonspecific | Suggestive of Device-Related Etiology |
| Fever | Patient unlikely candidate for sepsis (e.g., young, no underlying diseases) |
| Chills, shaking rigors* | Source of sepsis inapparent, no identifiable local infection |
| Hypotension, shock* | Intravascular device in place, especially central venous catheter |
| Hyperventilation, respiratory failure | Inflammation or purulence at insertion site |
| Gastrointestinal* | Abrupt onset, associated with shock |
| Abdominal pain Vomiting Diarrhea |
Bloodstream infection caused by staphylococci (especially coagulase-negative staphylococci), Corynebacterium, Candida, Trichophyton, Fusarium, or Malassezia species† |
| Neurologic* | Very high-grade (>25 CFUs/mL) candidemia |
| Confusion Seizures |
Cluster of cryptogenic infusion-associated bloodstream infections caused by Enterobacter cloacae, Pantoea agglomerans, or Serratia marcescens*† Sepsis refractory to antimicrobial therapy or dramatic improvement with removal of cannula and infusion* |
*Commonly seen in overwhelming gram-negative sepsis originating from contaminated infusate, peripheral suppurative phlebitis, or septic thrombosis of a central vein.
†Conversely, bacteremia caused by streptococci, aerobic gram-negative bacilli, or anaerobes is unlikely to derive from an intravascular device.
Modified from Maki DG, Mermel LA: Infections due to infusion therapy. In Bennett JV, Brachman PS (eds): Hospital Infections, 4th ed. Boston, Lippincott-Raven, 1998.
Recognition and Diagnosis
Clinical Features
Recent evidence-based guidelines provide the best current information on the evaluation of the ICU patient with fever or other signs of sepsis.34 Before any decision regarding initiation of antimicrobial therapy or removal of an IVD, the patient must be thoroughly examined to identify all plausible sites of infection including VAP, catheter-associated urinary tract infection, surgical site infection, IVD-related BSI, C. difficile infection, and other infections.34
Despite the challenge of identifying the source of a patient’s signs of sepsis,34 several clinical, epidemiologic, and microbiologic findings point strongly toward an IVD as the source of a septic episode (Table 50.7).267 Patients with an abrupt onset of signs and symptoms of sepsis without any identifiable local infection such as pneumonia or surgical site infection should prompt suspicion of infection of an IVD. The presence of inflammation or purulence at the catheter insertion site is now uncommon in patients with IVD-related BSI.276 However, if inflammation, especially any purulence, is seen in combination with signs and symptoms of sepsis, it is highly likely the patient has IVD-related BSI and should prompt removal of the device. Finally, recovery of certain microorganisms in multiple blood cultures, such as staphylococci, Corynebacterium or Bacillus species, or Candida or Malassezia, strongly suggests infection of an IVD.
Blood Cultures
Starting anti-infective drugs for suspected or presumed infection in the critically ill patient without first obtaining blood cultures from two separate sites, at least one of which is drawn from a peripheral vein by percutaneous venipuncture, is indefensible. The volume of blood cultured is essential to maximize the sensitivity of blood cultures for diagnosis of bacteremia or candidemia: in adults, obtaining at least 20 mL, ideally 30 mL, per drawing (each specimen, containing 10 mL or 15 mL, inoculated into aerobic and anaerobic media) significantly improves the yield as compared with obtaining only 5 mL at each drawing and culturing a smaller total volume.277,278 In adults, if at least 30 mL of blood is cultured, 99% of detectable bacteremias should be identified.277,279 Similar operating characteristics are achieved in the pediatric population using a weight-based graduated volume approach to blood cultures.280 Standard blood cultures drawn through CVCs provide excellent sensitivity for diagnosis of BSI but are less specific than cultures obtained from a peripheral vein.281,282 If the patient has a long-term multilumen catheter, it may be reasonable to obtain a specimen from each lumen of the catheter because studies have found discordance (≈30%) among cultures obtained from different lumens of the same catheter.283
Every effort must be made to prevent introduced contamination when drawing blood cultures because a single contaminated blood culture has been shown to prolong hospitalization by 4 days and increase the costs of hospitalization by $4100 to $4400.284,285 Tincture of iodine, isopropyl alcohol, chlorhexidine, or povidone-iodine combined with alcohol rather than povidone-iodine alone should be used for skin antisepsis prior to venipuncture for blood cultures, recognizing that studies have shown significantly reduced rates of contamination with use of these agents.204,285,286 Up to 30% of blood cultures positive for coagulase-negative staphylococcus represent true infection287,288; however, the majority of single positive cultures represent contamination,288 a finding that should reemphasize the need to obtain cultures from two separate sites whenever BSI is suspected.
Cultures of Removed Intravascular Devices
Removal and direct culture of the IVD has historically been the gold standard for confirming the presence of IVD-related BSI, particularly with short-term IVDs. Studies have shown that culturing catheter segments semiquantitatively on solid media289 or quantitatively in liquid media (e.g., removing the adherent organisms by sonication290) provides superior sensitivity and specificity for diagnosis of IVD-related BSI, with a strong correlation between high colony counts and line sepsis. Growth of greater than or equal to 15 CFUs from a catheter segment by semiquantitative culture or growth of greater than or equal to 103 CFUs from a catheter cultured after sonication with accompanying local inflammation or signs of sepsis indicates local catheter infection. Significant growth in the absence of local or systemic inflammation suggests colonization of the device; if continued vascular access is necessary, a new device should be placed in a new location rather than replacing it with a new one in the same location by guidewire exchange.
Although recent studies291 have suggested that quantitative methods (e.g., sonication) are superior to the semiquantitative methods (e.g., roll plate), other studies have shown them to be equivalent.292,293 Because hub contamination progressing to intraluminal colonization is the primary route of infection for long-term devices (e.g., devices in place >10 days), quantitative techniques may be superior to semiquantitative techniques in detecting infections from these types of devices because they remove organisms from both the internal and external surface of catheters.293 In contrast, semiquantitative methods may be preferred over quantitative methods in cases of suspected infection related to a short-term device (e.g., devices in place <10 days) because the primary route of infection in this setting is caused by extraluminal ingress of skin organisms at the catheter insertion site and the semiquantitative method is simple, less expensive, and allows identification of the infecting organisms a day earlier.
Direct and impression Gram stains294 or acridine orange stains293 of intravascular segments of removed catheters have shown excellent correlation with quantitative techniques for culturing catheters and can permit rapid diagnosis of catheter-related infection.
To rigorously identify the mechanism of IVD-related BSI in prospective studies, it is necessary to culture all potential sources of microorganisms at the time of catheter removal (Fig. 50.6): skin of the insertion site, each catheter hub, infusate from each lumen, as well as implanted catheter segments. If the results of these cultures appear to link a BSI with microorganisms isolated from one or more portions of the device by phenotypic criteria, efforts then need to be made to conclusively establish concordance, beyond speciation and antimicrobial susceptibility pattern, using one or more molecular subtyping systems such as multilocus enzyme electrophoresis, plasmid profile, or restriction-enzyme digestion of genomic DNA analyzed by pulsed-field electrophoresis.273,274,278,295
Diagnosis of Infection with Implanted Long-Term Intravascular Devices
The methods described earlier require removal of the device for confirmation of IVD-related BSI. This can pose formidable challenges to management with long-term, surgically implanted IVDs such as Hickman and Broviac catheters, cuffed and tunneled hemodialysis catheters, and subcutaneous central venous ports. Only 15% to 45% of long-term IVDs that are removed for suspected infection are truly colonized or infected at the time of removal.296–299 To avoid unnecessary removal of IVDs, methods have been developed to diagnose IVD-related BSI while allowing the device to remain in place: (1) paired quantitative blood cultures drawn from the IVD and percutaneously from a peripheral vein293 and (2) differential time to positivity (DTP) of paired standard blood cultures, one drawn from the IVD and the other from a peripheral vein.300
If a laboratory has available an automated quantitative system for culturing blood (e.g., Isolator lysis centrifugation system, Wampole Laboratories, Cranbury, NJ), quantitative blood cultures drawn through the IVD and concomitantly by venipuncture from a peripheral vein (or another IVD) can permit the diagnosis of IVD-related bacteremia or fungemia to be made with sensitivity and specificity in the range of 80% to 95%,293 without removal of the catheter, if empiric antimicrobial therapy has not yet been initiated. IVD-drawn cultures demonstrating 5- to 10-fold higher concentrations of microorganisms per milliliter, as compared with counts of the same microorganism obtained in a culture drawn from a peripheral vein, confirm the presence of IVD-related BSI.
The differential-time-to-positivity (DTP) of paired blood cultures, one drawn through the IVD and the second, concomitantly from a peripheral vein, has also been shown to reliably identify IVD-related BSI of long-term IVDs if the blood culture drawn from the IVD turns positive 2 or more hours before the culture drawn peripherally. In studies of patients with long-term IVDs, the sensitivity and specificity of DTP ranged from 82% to 94% and 88% to 91%, respectively.293,300 The performance of DTP in short-term IVDs has recently been examined, with disappointing results,301 a finding that is not entirely unexpected given the predominant extraluminal route of infection with these devices.
Detection of Contaminated Infusate
To diagnose infection caused by contaminated infusate, a sample of IV fluid, aspirated from the line, should be cultured quantitatively and qualitatively289; concordance with positive peripheral blood cultures, without another identifiable source for the patient’s BSI, definitively implicates infected infusate as the cause of the BSI. Anaerobic culture techniques are not necessary unless blood or another biologic product is involved.
Incidence
Prospective studies, in which every attempt was made to conclusively identify the presence of an IVD-related BSI, show that every type of IVD carries some risk of causing BSI; however, the magnitude of risk varies greatly, depending on the type of device (Table 50.8).302 The device that poses the greatest risk of IVD-related BSI today is the CVC in its many forms (see Table 50.8): short-term, noncuffed, single-lumen or multilumen catheters inserted percutaneously into the subclavian or internal jugular vein have shown rates of catheter-related BSI in the range of 3% to 5% (2 to 3 per 1000 IVD-days).302 Far lower rates of infection have been encountered with surgically implanted cuffed Hickman or Broviac catheters and subcutaneous central venous ports (1 and 0.2 per 1000 IVD-days, respectively).302 Contrary to popular belief, peripherally inserted central catheters (PICCs) used in inpatients and arterial catheters are associated with rates of catheter-related BSI approaching those seen with short-term, noncuffed, and nontunneled, multilumen CVCs—up to 2.1303 and 3.4304 BSIs per 1000 IVD-days, respectively.
Table 50.8
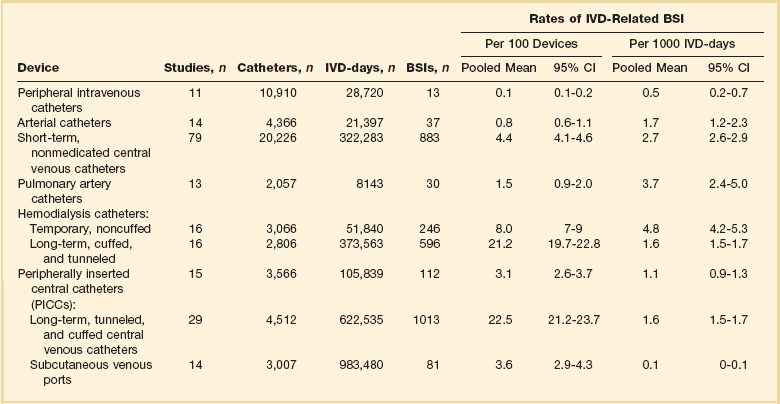
Modified from Maki DG, Kluger DM, Crnich CJ: The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159-1171.
Pathogenesis and Risk Factors
Two major sources of IVD-related BSI exist: (1) colonization of the IVD, catheter-related infection, and (2) contamination of the fluid administered through the device, infusate-related infection.267 Contaminated infusate is the cause of most epidemic IVD-related BSIs; in contrast, catheter-related infections are responsible for most endemic IVD-related BSIs.18
In order for microorganisms to cause catheter-related infection, they must first gain access to the extraluminal or intraluminal surface of the device, where they can adhere and become incorporated into a biofilm that allows sustained infection and hematogenous dissemination.305 Microorganisms gain access to the bloodstream by one of three mechanisms (see Fig. 50.7): (1) skin organisms invade the percutaneous tract, probably assisted by capillary action, at the time of insertion or in the days following; (2) microorganisms contaminate the catheter hub (and lumen) when the catheter is inserted over a percutaneous guidewire or later manipulated; or (3) organisms are carried hematogenously to the implanted IVD from remote sources of local infection such as pneumonia.
With short-term IVDs (e.g., in place <10 days) such as peripheral IV catheters; arterial catheters; and noncuffed, nontunneled CVCs, most device-related BSIs are of cutaneous origin, from the insertion site, and gain access extraluminally, occasionally intraluminally at insertion with the guidewire.306,307 In contrast, contamination of the catheter hub and luminal fluid is the predominant mode of invasive infection with long-term IVDs (e.g., in place >10 days) such as cuffed Hickman- and Broviac-type catheters, subcutaneous central ports, and PICCs.308,309
Also important is recognizing that infusate (parenteral fluid, blood products, or IV medications) administered through an IVD can also occasionally become contaminated and produce device-related BSI. Contaminated fluid is fortunately an infrequent cause of endemic infusion-related infection with most short-term IVDs; it is, however, an important cause of BSIs with arterial catheters used for hemodynamic monitoring and long-term IVDs such as Hickman or Broviac catheters, cuffed hemodialysis CVCs, and subcutaneous central venous ports.307,310,311
Most nosocomial epidemics of infusion-related BSI have been traced to contamination of infusate by gram-negative bacilli, introduced during its manufacture (intrinsic contamination) or during its preparation and administration in the hospital (extrinsic contamination).146,312 If an epidemic is suspected, the epidemiologic approach must be methodical and thorough yet expeditious, directed toward establishing the bona fide nature of the putative epidemic infections (i.e., ruling out “pseudoinfections”)246 and confirming the existence of an epidemic; defining the reservoirs and modes of transmission of the epidemic pathogens; and most importantly, controlling the epidemic quickly and completely. Control measures are predicated on accurate delineation of the epidemiology of the epidemic pathogen. The essential steps in dealing with a suspected nosocomial outbreak have recently been reviewed (and are discussed later).267
In recent years the factors associated with an increased risk of IVD-related BSI have become better delineated (Table 50.9). Prolonged hospitalization and severity of illness clearly influence the risk, and clinical states such as granulocytopenia, AIDS, and bone marrow transplantation have been associated with fourfold to sixfold increased rates of IVD-related BSI.313,314 However, the features of the IVD, its insertion, and its maintenance appear to have far greater impact on the overall risk of infection. In 289 patients, Merrer and colleagues315 found that insertion of an IVD in the femoral versus the subclavian vein was associated with a greatly increased risk of infection (20 versus 3.7 BSIs per 1000 IVD-days, p < 0.001) and thrombotic complications (21.5% versus 1.9%, p < 0.001).315 Moreover, Robert and colleagues316 found that patients with primary BSI were more likely to have received care during times when there was a lower nursing-to-patient ratio and a higher proportion of temporary (“float”) nurses rather than the full-time nursing staff.316
Table 50.9
Risk Factors for Intravascular Device–Related Bloodstream Infection with Short-Term Use
| Risk Factor (with No. of Studies) | Relative Risk or Odds Ratio |
| Underlying Disease | |
| AIDS (2) | 4.8 |
| Neutropenia (2) | 1-15.1 |
| Gastrointestinal disease (1) | 2.4 |
| Surgical service (1) | 4.4 |
| ICU/CCU placement (3) | 0.4-6.7 |
| Extended hospitalization (3) | 1-6.7 |
| Other intravascular devices (2) | 1-3.8 |
| Systemic antibiotics (3) | 0.1-0.5 |
| Active infection at another site (2) | 8.7-9.2 |
| High APACHE III score (1) | 4.2 |
| Mechanical ventilation (1) | 2-2.5 |
| Transplant recipient (1) | 2.6 |
| Features of Insertion | |
| Difficult insertion (1) | 5.4 |
| Maximal sterile barriers (1) | 0.2 |
| Tunneling (2) | 0.3-1 |
| Insertion over a guidewire (8) | 1-3.3 |
| Insertion Site | |
| Internal jugular vein (6) | 1-3.3 |
| Subclavian vein (5) | 0.4-1 |
| Femoral vein (2) | 3.3-4.8 |
| Defatting insertion site (1) | 1.0 |
| Use of a multilumen catheter (8) | −6.5 |
| Catheter Management | |
| Routine change of IV set (2) | 1.0 |
| Staffing in SICU (nurse-to-patient ratio) (1) | |
| 1 : 2 | 61.5 |
| 1 : 1.5 | 15.6 |
| 1 : 1.28 | 4.0 |
| 1 : 1 | 1.0 |
| Inappropriate catheter usage (1) | 5.3 |
| Duration of catheterization >7 days (5) | 1-8.7 |
| Colonization of catheter hub (3) | 17.9-44.1 |
| Parenteral nutrition (2) | −4.8 |
Modified from Safdar NS, Kluger DM, Maki DG: A review of risk factors for catheter-related infection caused by percutaneously inserted, noncuffed central venous catheters: Implications for preventive strategies. Medicine 2002;81:466-479.
Microbiology
Figure 50.7 summarizes the microbial profile of IVD-related BSI from 159 published prospective studies.317 As might be expected from knowledge of the pathogenesis of these infections, skin microorganisms account for the largest proportion of these infections.
Treatment
Treatment of IVD-related BSI is discussed in Chapter 54 (Specific Infections with Critical Care Implications), under “Device-Related Endovascular Infections.”
Strategies for Prevention
Recommendations for the prevention of IVD-related BSIs were recently published by the Hospital Infection Control Practices Advisory Committee (HICPAC).132 Table 50.10 summarizes the recommendations of the 2011 HICPAC guideline for the prevention of IVD-related BSI and scores each recommendation on the basis of the quality of the available scientific evidence. It must be reaffirmed that measures for prevention of any nosocomial infection must, wherever possible, be based on the best understanding of pathophysiology and epidemiology and, whenever possible, controlled clinical trials.
Table 50.10
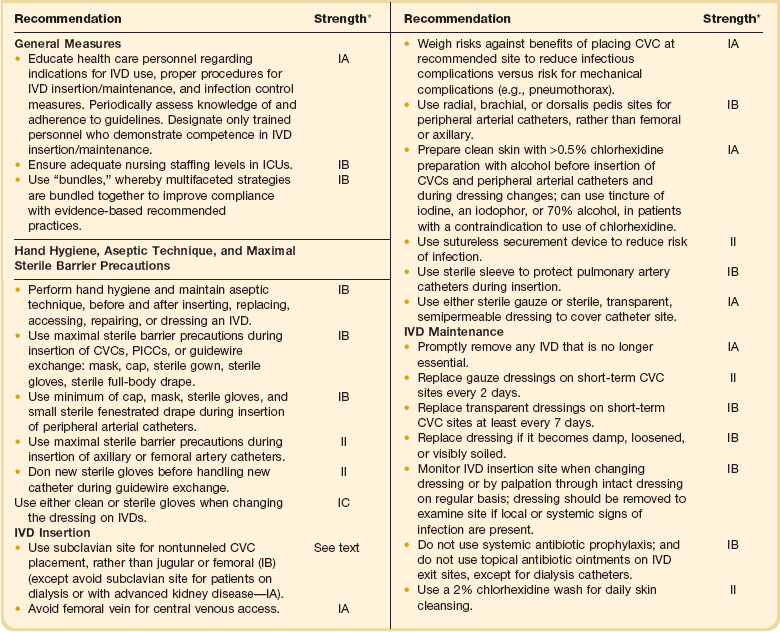
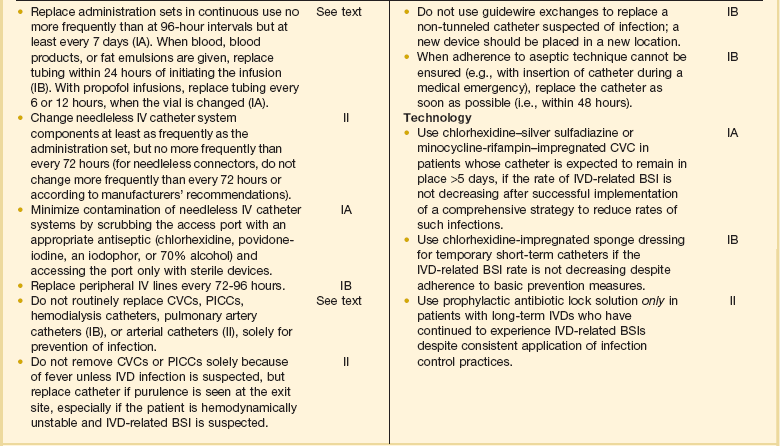
*Taken from CDC/HICPAC system of weighting recommendations based on scientific evidence: IA, strongly recommended for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies. IB, strongly recommended for implementation and supported by some experimental, clinical, or epidemiologic studies and a strong theoretical rationale; or an accepted practice (e.g., aseptic technique) supported by limited evidence. IC, required by state or federal regulations, rules, or standards. II, suggested for implementation and supported by suggestive clinical or epidemiologic studies or a theoretical rationale.
Data from O’Grady NP, Alexander M, Burns LA, et al: Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52(9):e162-93.
At-Device Insertion
1. Choice of catheter and site of device insertion: Obviously, the choice of IVD inserted into a patient will be guided primarily by that patient’s particular needs (e.g., hemodialysis versus fluid administration). However, the astute clinician can mitigate much of the risk associated with vascular access by choosing the best device for the task at hand and inserting the IVD in a location associated with the least risk of infection. Studies suggest that multilumen IVDs are associated with a higher risk of infection than single-lumen catheters.318 That said, if a patient has need for multiple infusions, inserting several single-lumen catheters will pose greater risks than a single multilumen catheter.
To date, there have been no randomized studies designed to evaluate the optimal location for placement of short-term CVCs. However, the data accumulated from numerous observational studies suggest that the lowest risk of IVD-related BSI is seen with subclavian vein insertion and the highest risk with femoral vein insertion, with an intermediate level of risk associated with jugular vein insertions.307,315
The femoral vein is often used for central venous access, especially on nonsurgical services, because of the ease of cannulation and the lower risk of mechanical complications from insertion (i.e., bleeding or pneumothorax). Unfortunately, prospective studies evaluating the risk of femoral vein device placement have shown that CVCs placed in the femoral vein are more likely to be colonized at the time of removal than catheters placed in the internal jugular vein (RR = 4.7, CI = 2 to 8.8, p = 0.0001)319 and are associated with an increased risk of IVD-related BSI when compared with CVCs placed in the subclavian vein (4.4% versus 1.5%, p = 0.07).315 Furthermore, prospective studies have found higher rates of catheter-related deep vein thrombosis with femoral catheters, in the range of 7% to 25%.314,315 In general, we believe femoral access should be used only if emergent access is required, the inexperience of the operator limits placement in the upper body, or there is a contraindication to placement in the upper body (no available sites, an extensive burn, or severe coagulopathy). If a short-term CVC must be placed in the femoral vein or artery, we believe it is important that the catheter insertion site be located at least 2 inches (5 cm) below the inguinal crease or an intertriginous area, which is heavily colonized with bowel organisms and yeasts; this also allows a more secure protective dressing to be affixed.
In contrast to short-term CVCs, observational studies of hemodialysis catheters have not been able to confirm a lower rate of infection with catheters inserted in the subclavian vein as compared with those inserted in the internal jugular vein,320 although there is still excess risk associated with femoral vein placement.321 More importantly, prospective studies of catheters used for hemodialysis have demonstrated a significant risk of great vein thrombosis and stenosis in catheters inserted into the subclavian vein that approaches 40% to 50% as compared with rates of 0% to 10% with catheters inserted into the internal jugular vein.322,323 On the basis of these data, internal jugular vein insertion is preferable to subclavian vein insertion for central access for hemodialysis.
2. Barrier precautions: Hand hygiene with an antiseptic-containing preparation, either conventional handwashing with chlorhexidine (2% to 4%) or with a waterless alcohol rub or gel,174 must always precede the insertion of an IVD and should also precede subsequent handling of the device or its administration set.132 A new pair of disposable, nonsterile gloves, using a “no-touch” technique, is adequate for the placement of peripheral IV catheters in most patients; however, sterile gloves should be used during insertion in high-risk patients such as those with granulocytopenia. Sterile gloves are strongly recommended for placement of all other types of IVDs that are associated with a 1% or higher risk of associated bacteremia, specifically arterial catheters and all types of centrally placed devices including PICCs.132
Studies have shown that the use of maximal barriers including a long-sleeved, sterile surgical gown, mask, cap and large sterile drape, and sterile gloves significantly reduces the risk of CVC-related BSI (0.08 BSIs with maximal barriers versus 0.5 BSIs per 1000 IVD-days without maximal barriers, p = 0.02).324 The use of maximal barriers has further been shown to be highly cost effective.324 Considering that of all IVDs, CVCs are most likely to produce nosocomial BSI, a strong case can be made for mandating maximal barrier precautions during the insertion of all central IVDs.132 They are not necessary, however, for arterial catheters used for hemodynamic monitoring, during which sterile gloves and a sterile fenestrated drape will suffice, because using maximal sterile barrier precautions does not appear to reduce the risk of arterial catheter-related BSI.325 However, recently published guidelines for the prevention of IVD-related BSI132 recommend that in addition to a using a minimum of a cap, mask, sterile gloves, and a small sterile fenestrated drape during the insertion of all peripheral arterial catheters, maximal sterile barrier precautions be used for axillary and femoral artery catheter insertion (see Table 50.10), although the evidence to support this recommendation is not strong.326
3. IV teams: Good technique is also essential. Studies have shown that the use of special IV therapy teams, consisting of trained nurses or technicians who can assure a consistent and high level of aseptic technique during catheter insertion and in follow-up care of the catheter, have been associated with substantially lower rates of catheter-related BSI and are cost effective.327,328 But even if an institution does not have an IV team, it can greatly reduce its rate of IVD-related BSI by formal education of nurses and physicians and stringent adherence to IVD care protocols.329,330
4. Cutaneous antisepsis: Given the evidence for the importance of cutaneous microorganisms in the pathogenesis of short-term IVD-related infections, measures to reduce colonization of the insertion site would seem of the highest priority, particularly the choice of chemical antiseptics for disinfection of the site. Nine randomized, prospective trials comparing a chlorhexidine-containing antiseptic to either povidone-iodine or alcohol for preparation of the skin prior to insertion of a short-term IVD have been reported.205–207,331 In the largest study to date, a randomized trial in 1050 CVCs and arterial catheters placed in a university hospital ICU, cutaneous antisepsis with 1% tincture of chlorhexidine showed a highly significant reduction in IVD-related BSIs compared with an iodophor (RR = 0.35, p < 0.01).331 More recently, a meta-analysis that examined results from eight of the nine aforementioned studies found that use of chlorhexidine was associated with a nearly 50% reduction in the risk of IVD-related compared with povidone-iodine (RR = 0.49, 95% CI = 0.28 to 0.88).205
Insertion Site Care and IVD Maintenance
1. IVD dressings: IVDs can be dressed with sterile gauze and tape or with a sterile transparent, semipermeable, polyurethane film dressing. The available data suggest that the two types of dressings are equivalent in terms of their impact on IVD-related BSI with peripheral IVs and short-term CVCs.332–334 In contrast, results from studies of arterial catheters have found that polyurethane dressings greatly increase the risk of IVD-related BSI.332,335 As a result, polyurethane dressings should probably not be used on arterial catheters until future studies confirm their safety.
2. Topical antimicrobial ointments: In theory, application of a topical antimicrobial agent to the catheter insertion site should confer some protection against microbial invasion. Clinical trials of a topical combination antibacterial ointment containing polymyxin, neomycin, and bacitracin with peripheral IVs have shown marginal benefit,336 but the use of polyantibiotic ointments has been associated with a fivefold increased frequency of Candida infection, limiting their utility.336,337 One recent double-blind, placebo-controlled, randomized clinical trial in hemodialysis patients with permanent tunneled cuffed dialysis catheters showed that the use of topical bacitracin, gramicidin, and polymyxin B at the catheter exit site as compared with topical placebo was associated with a reduction in catheter-related bacteremia (0.63 versus 2.48 per 1000 IVD-days, p = 0.0004) and mortality rate (3 versus 13 deaths, p = 0.004), without an increase in Candida infection.338
The topical antibacterial mupirocin, which is active primarily against gram-positive organisms, was shown in one study to significantly reduce colonization of internal jugular catheters without increasing colonization by Candida spp.,339 and a study by Sesso and colleagues340 showed significant reductions in hemodialysis catheter colonization (3.17 versus 14.27 per 1000 IVD-days, p ≤ 0.001) and S. aureus IVD-related BSIs (0.71 versus 8.92 BSIs per 1000 IVD-days, p ≤ 0.001).340 Unfortunately, resistance of S. aureus341 and coagulase-negative staphylococci342 rapidly emerges during wide-scale mupirocin use,343 which contravenes its use as a topical agent for the prevention of IVD-related BSI at this time.132
Three prospective studies of topical povidone-iodine ointment applied to central venous catheter sites have failed to show a statistical benefit to its use,336,344,345 but a single comparative trial in subclavian hemodialysis catheters showed that the use of topical povidone-iodine ointment was associated with a fourfold reduction in the incidence of IVD-related S. aureus BSI.346
Based on these data, if a topical agent is to be used with hemodialysis catheters, either an iodophor or topical bacitracin, gramicidin, and polymyxin B (not currently available in the United States) may be most desirable.132
3. Replacement of the device: Studies have shown that peripheral IVs may be safely left in place for up to 96 hours if the patient and the insertion site are monitored closely.347 Studies have suggested that the duration of peripheral catheterization may be prolonged even further,348 but viewing reports of increasing nosocomial S. aureus bacteremias linked to prolonged peripheral venous catheterization,349 more studies are required before this can become considered acceptable routinely.
Scheduled replacement of short-term, noncuffed, nontunneled CVCs has long been practiced in many centers; however, some studies have called this practice into question.350 Moreover, a meta-analysis found no benefit to routine replacement of short-term CVCs.351 On the basis of these data, there appears to be no indication for scheduled replacement of short-term CVCs that are functioning well and show no clinical signs of infection.
4. Guidewire exchanges of CVCs: The management of CVCs that must be replaced, either because of mechanical malfunction or suspected infection, deserves special attention. Replacement of CVCs by guidewire exchange is associated with a reduced risk of mechanical complications350,351; however, it is also associated with an increased risk of the newly placed CVC becoming infected and causing CVC-related BSI.350 As a result, if circumstances necessitate guidewire exchange for placement of a new catheter (e.g., the patient has limited sites for access, is morbidly obese, or is at high risk of mechanical complications because of underlying coagulopathy), the same strict aseptic technique, which includes full barrier precautions, must be used. However, the tip or intracutaneous segment(s) of the removed CVC should routinely be sent for culture to determine whether the insertion tract is colonized. If it is, the newly inserted CVC should be promptly removed and a new CVC placed percutaneously in a new site. If the tract is not colonized, the newly exchanged CVC can remain in the old insertion site.
Although small studies have found some utility of guidewire exchange in the management of CVCs suspected of being infected,352,353 we believe that, in the absence of randomized studies demonstrating its safety, guidewire exchange generally should not be performed if there is suspicion of IVD-related BSI, especially if there are signs of local infection such as purulence or erythema at the insertion site or signs of systemic sepsis without a source. In these cases the old catheter should be removed and cultured, and a new catheter should be inserted in a new site.
5. Replacing the delivery system: Whereas most infusion-related BSIs are caused by infection of the device used for vascular access, infusate can occasionally become contaminated and cause endemic BSIs.307,354 If an infusion runs continuously for an extended period, the cumulative risk of contamination increases, and there is further risk that contaminants can grow to concentrations that could produce BSI in the recipient of the fluid. For more than 25 years, most U.S. hospitals have routinely replaced the entire delivery system of patients’ IV infusions at 24- or 48-hour intervals355 to reduce the risk of BSI from extrinsically contaminated fluid. Prospective studies indicate that IV delivery systems need not be replaced more frequently than every 72 to 96 hours, including infusions used for total parenteral nutrition or any infusions in ICU patients347,356; extending the duration of use can permit cost savings to hospitals.356
Four clinical settings might be regarded as exceptions to using 72 hours as an interval for routine set change356: (1) administration of blood products, (2) administration of lipid emulsion, (3) arterial pressure monitoring, and (4) suspicion of an epidemic of infusion-related BSI. In these circumstances, it may be most prudent for administration sets to be changed routinely at 24- or 48-hour intervals.
Arterial infusions used for hemodynamic monitoring appear to be more vulnerable to becoming contaminated during use and producing endemic354 or epidemic septicemia,99 caused by gram-negative bacilli. If the infusion for hemodynamic monitoring is set up so that the fluid flows continuously through the system, thus eliminating a blind stagnant column of fluid, extrinsic contamination appears to be greatly reduced and may even eliminate the need to replace the administration set, transducer assembly, and other components of the system at frequent intervals.357,358 If disposable transducers are used, there appears to be no need to replace the transducer assembly and other components of the delivery system more frequently than every 4 days,357 and it may be safe to replace them even less frequently.358
6. Anticoagulation: Thrombus formation on an intravascular device is associated with an increased risk of infection.359,360 Two prospective studies have been performed to examine the efficacy of warfarin anticoagulation for reducing rates of IVD-associated thrombosis with long-term IVDs.361,362 Both studies found that use of warfarin in a dose of 1 mg/day was associated with significantly reduced rates of thrombosis with long-term IVDs, although no data were provided on rates of IVD-related BSI.
The use of prophylactic heparin for reducing rates of IVD-related thrombosis and infection has been evaluated in a meta-analysis.363 Examining a variety of different administration techniques in 14 randomized controlled studies, Randolph and colleagues363 concluded that systemic heparinization significantly reduced the risk of IVD-associated thrombosis (RR = 0.43, CI = 0.23 to 0.78) and device colonization (RR = 0.18, CI = 0.06 to 0.6) but failed to show a reduction in IVD-related BSIs. Heparin-bonded pulmonary artery catheters may be less prone to IVD-related BSI than nonheparinized catheters.307,364,365
On the basis of these studies, low-level anticoagulation with warfarin is warranted for long-term IVDs as long as there is no contraindication (bleeding diathesis, brain tumor, or predilection to falls) and the INR (international normalized ratio) is maintained below 1.6.361 For short-term IVDs, the use of low-dose subcutaneous heparin is more appropriate; it is commonly given to patients with CVCs or arterial lines as part of ICU thromboembolism prophylaxis.
Novel Technology
Despite compliance with recommended guidelines, many centers continue to have high rates of IVD-related BSI. Novel technology holds much promise (Box 50.3). Innovative technologies designed to reduce the risk of IVD-related BSI have proved not only to be effective but also to reduce health care costs, both with short-term and long-term IVDs.305,366
1. Novel securement devices: In a randomized trial of a novel sutureless device for securing noncuffed vascular catheters (StatLock, Venetec International), premature loss of pediatric PICCs caused by accidental extrusion and PICC-associated thrombosis was significantly reduced,367 and in two additional trials the incidence of catheter-related BSI was significantly reduced with the use of the novel securement device, both in adults and children with PICCs.367,368
The promise of this device for reducing infection may derive from elimination of a festering skin suture wound contiguous to the newly inserted catheter and minimizing to-and-fro movement of the catheter, which may promote invasion of the tract by cutaneous microorganisms through capillary action.369
2. Novel dressings: Studies of polyurethane dressings, which contain antiseptics such as povidone-iodine or ionized silver, have been disappointing. However, on the basis of demonstrated superiority of chlorhexidine for cutaneous disinfection of access sites, a novel chlorhexidine-impregnated sponge dressing has been developed (Biopatch, Johnson and Johnson Medical, Inc.). It maintains a high concentration of the antiseptic on the insertion site under the dressing. The largest study to date found that use of the chlorhexidine-impregnated sponge dressing was associated with a 60% reduction in catheter-related BSI (RR = 0.37, p = 0.01).370 Although there were no adverse side effects associated with the use of this dressing in this trial in adults, a pediatric trial found that 15% of low-birth-weight neonates developed local dermatotoxicity.371
3. Anti-infective impregnated catheters: Intravascular devices directly coated or impregnated with antimicrobials or antiseptics have been intensively studied over the past several decades. Eighteen randomized trials evaluating the efficacy of chlorhexidine-silver-sulfadiazine–impregnated or minocycline-rifampin–impregnated CVCs have been published in full article or abstract form since 1994.273,274,295,372,373
Of the 16 published studies that examined the effect of antimicrobial-impregnated CVCs on rates of CVC-related BSI, 12 found either a statistically significant reduction or a strong trend toward a reduction in rates of CVC-related BSI.372,373 Aggregate analysis of the 15 studies that compared antimicrobial-impregnated CVCs with nonimpregnated CVCs,372,373 encompassing a total of 4250 CVCs, shows that antimicrobial-impregnated CVCs are associated with a 40% reduction in CVC-related BSI (61 BSIs/2129 devices vs. 101 BSIs/2118 devices, OR 0.60, 95% CI = 0.44 to 0.82, p = 0.001), a result remarkably similar to the findings of three published meta-analyses.305,374,375
Finally, two rigorous and sophisticated economic analyses have found that antimicrobial-impregnated CVCs are cost effective.376,377 Veenstra and colleagues showed that antimicrobial-impregnated CVCs remained cost effective even if the cost of a CVC-related BSI was as low as $687 per case; cost savings were $196 per antimicrobial-impregnated CVC when a more realistic cost of a CVC-related BSI of $9738 was used in the analysis.376 Shorr and colleagues377 showed that use of antimicrobial-impregnated CVCs was associated with a cost savings of $9600 per CVC-related BSI prevented and that $165 to $280 would be saved for every patient who received an antimicrobial-impregnated CVC.
On the basis of this large body of data, two national advisory panels have recommended the use of antimicrobial-impregnated CVCs in clinical settings where, despite rigorous application of other preventive interventions, rates of IVD-related BSI remain unacceptably high (i.e., ≥3.3 BSIs per 1000 IVD-days).132,378
4. Antimicrobial lock solutions: Given the importance of hub contamination and intraluminal colonization in the genesis of IVD-related BSI with long-term IVDs, intraluminal instillation of an antibiotic or antiseptic solution has the potential to reduce the risk of BSI associated with these devices. Six randomized, prospective trials have examined a vancomycin-containing antibiotic lock solution for the prevention of IVD-related BSI, the largest of which found that use of a vancomycin or vancomycin/ciprofloxacin lock solution reduced the risk of IVD-related BSI nearly 80% (p = 0.005), with no evidence that the use of the lock solution promoted colonization or infection by vancomycin-resistant bacteria or fungi.379,380 Yet concern about the emergence of resistance with prophylactic antibiotic-containing lock solutions has limited their wider acceptance to date. However, the use of prophylactic antibiotic lock solution is considered acceptable in the 2011 HICPAC Guideline if a patient with an essential long-term IVD has continued to experience recurrent IVD-related BSIs despite consistent application of infection control practices.132
Various other prophylactic lock solutions have been studied as a means of preventing IVD-related BSI including trisodium citrate/gentamicin,381 minocycline/ethylenediaminetetraacetic acid (EDTA),382 ethanol,383 and taurolidine-containing solutions.384 Concerns about increased IVD complication rates384 and drug-related toxicity381 associated with the use of certain types of lock solutions, combined with the limited number of patients who have been studied while receiving these agents, precludes their routine use at this time.
5. Catheter hubs: A novel catheter hub that contains a chamber filled with iodinated alcohol has been shown to be effective in preventing colonization of IVDs in an animal model.385 Use of this same hub model in some clinical studies has demonstrated significantly lower rates of IVD colonization compared with IVDs with control hubs.386,387 One clinical trial has also demonstrated reduced rates of IVD-related BSIs with use of this hub (4% versus 16%, p < 0.01). A subsequent study also showed a reduction in hub-related IVD-related BSIs (1.7% versus 7%, p < 0.049), but overall rates of IVD-related BSIs in both groups were similar.387 Another study was unable to find any benefit with regard to IVD colonization or IVD-related BSI with use of the novel hub.388 This device is not yet available in the United States and until further studies more conclusively demonstrate its benefit, its use cannot be recommended at this time.
Ventilator-Associated Pneumonia
Incidence and Impact
Hospital-acquired pneumonia (HAP) is defined as pneumonia that develops more than 48 hours after hospitalization.389 VAP is a subset of HAP and is defined as pneumonia that occurs more than 48 to 72 hours after initiating mechanical ventilation.389 Nearly 300,000 episodes of HAP occur in U.S. hospitals each year.390 More than 90% of HAPs occur in patients undergoing mechanical ventilation, and 10% to 20% of mechanically ventilated patients will develop VAP.391 Incidence rates of VAP are highest in trauma, burn, neurosurgical, neurologic, and surgical ICUs (see Table 50.1).25 VAP increases length of hospitalization by 6.1 days and health care costs by $10,019 when compared with matched control subjects who had not developed VAP.391 More importantly, VAP is associated with more nosocomial deaths than is infection at any other site392—at least 50,000 deaths in U.S. centers annually—and increases hospital mortality rate at least twofold in affected individuals.391
Pathogenesis
In the normal nonsmoking host, multiple host defense mechanisms contribute to protection against pneumonia.393 The respiratory tract above the vocal cords is normally heavily colonized by bacteria, but unless the person has chronic bronchitis or has had respiratory tract instrumentation, the lower respiratory tract is normally sterile; although healthy adults aspirate frequently during sleep, the lower airways and pulmonary parenchyma of healthy, nonsmoking persons without lung disease are remarkably free of microbial colonization.394 The major defense mechanisms include anatomic airway barriers, the cough reflex, mucus,395 and mucociliary clearance.396 Below the terminal bronchioles, the cellular and humoral immune systems are essential components of host defense.397 Alveolar macrophages and leukocytes remove particulate matter and potential pathogens, elaborate cytokines that activate the systemic cellular immune response, and act as antigen-presenting cells to the humoral arm of immunity.398 Immunoglobulins and complement opsonize bacteria and bacterial products within the respiratory tract, assisting phagocytosis.
In the mechanically ventilated patient, numerous factors conspire to compromise host defenses: Critical illness, comorbid conditions, and malnutrition impair the immune system.399,400 Endotracheal intubation thwarts the cough reflex; compromises mucociliary clearance; injures the tracheal epithelial surface; and provides a direct conduit for bacteria from the mouth, hypopharynx, and stomach to gain direct access to the lower respiratory tract.401 Moreover, the cuff of the endotracheal tube allows pooling of oropharyngeal secretions in the subglottic region, forming an ideal medium for microbial growth, which periodically leaks around the cuff into the trachea. It would probably be more accurate pathogenically to rename VAP as “endotracheal intubation–related pneumonia.” This combination of impaired host defenses and continuous exposure of the lower respiratory tract to large numbers of potential pathogens through the endotracheal tube puts the mechanically ventilated patient at great jeopardy of developing VAP.
In order for microorganisms to cause VAP, they must first gain access to the normally sterile lower respiratory tract, where they can adhere to the mucosa and produce sustained infection. Microorganisms gain access by one of four mechanisms (Fig. 50.8): (1) aspiration of microbe-laden secretions, either from the oropharynx directly or, secondarily, by reflux from the stomach into the oropharynx, then into the lower respiratory tract402–404; (2) inhalation of contaminated air or medical aerosols405; (3) direct extension of a contiguous infection such as a pleural space infection406; or (4) hematogenous carriage of microorganisms to the lung from remote sites of local infection such as an IVD-related BSI.407
Although numerous epidemics of VAP have been caused by contaminated aerosols or medical respiratory devices,97,98,103 the preponderance of evidence suggests that most endemic VAPs derive from aspiration of oropharyngeal organisms41,408:
• The oropharynx of critically ill patients is rapidly colonized with the pathogens that cause VAP, especially aerobic gram-negative organisms and S. aureus.399
• Studies in which multiple anatomic sites are cultured simultaneously over time have shown that the pathogenic microorganisms implicated in VAP are usually first recovered from the oropharynx and later from the tracheobronchial tree and stomach.402–404,409 Moreover, heavy oropharyngeal colonization is a powerful independent predictor of subsequent tracheobronchial colonization and VAP.404
• Reducing oropharyngeal colonization with topical antimicrobials and antiseptics has been shown to significantly reduce the risk of VAP.410–413
Microbiology
Pathogens causing VAP may be part of the host’s endogenous flora at the time of hospitalization or may be acquired exogenously after admission to the health care institution from the hands, apparel, or equipment of HCWs; hospital environment; and use of invasive devices (see Fig. 50.9). The normal flora of the oropharynx in the nonintubated patient without critical illness is composed predominantly of viridans streptococci, Haemophilus species, and anaerobes. Salivary flow and proteins (immunoglobulin, fibronectin) are the major host factors maintaining the normal flora of the mouth (and dental plaque). Aerobic gram-negative bacilli are rarely recovered from the oral secretions of healthy patients.414 During critical illness, especially in ICU patients, the oral flora shifts dramatically to a predominance of aerobic gram-negative bacilli and S. aureus.399 Bacterial adherence to the orotracheal mucosa of the mechanically ventilated patient is assisted by reduced mucosal IgA and increased protease production, exposed and denuded mucous membranes, elevated airway pH, increased numbers of airway receptors for bacteria because of acute illness, and antimicrobial use.
Early-onset VAP, which manifests within the first 4 days of hospitalization, is most often caused by community-acquired pathogens, such as S. pneumoniae and Haemophilus species (Fig. 50.9).415 However, the microbial spectrum of VAP shifts to typical nosocomial pathogens with increasing lengths of mechanical ventilation and exposure to broad-spectrum antimicrobials (see Fig. 50.10).415 That the preponderance of episodes of VAP have a late onset is supported by the fact that the most common pathogens recovered from mechanically ventilated patients with pneumonia are P. aeruginosa, S. aureus, and the Enterobacteriaceae (Fig. 50.10).415,416 VAP is polymicrobial in up to 20% to 40% of cases. The role of anaerobic bacteria in VAP is not well defined.
Diagnosis
Hospitals participating in the CDC’s National Healthcare Safety Network (NHSN) use a standardized definition for clinically defined HAP16,17 (see Box 50.1) on the basis of clinical criteria developed empirically more than 3 decades ago417: (1) systemic signs of infection—fever, tachycardia, and leukocytosis; and (2) a new or worsening infiltrate on chest radiograph. Positive qualitative cultures of endotracheal aspirates are used to support the clinical diagnosis of HAP. Unfortunately, even when used in combination, the specificity of clinical criteria is poor, with an overall diagnostic accuracy of approximately 60% in published studies.418,419
Laboratory-defined HAP (see Box 50.1) relies on more specific criteria. Most experts have advocated routine use of invasive procedures when VAP is suspected—bronchoalveolar lavage (BAL), cultures of protected specimen brush (PSB) samples obtained by bronchoscopy, or blind (mini)-BAL, on the grounds that these diagnostic techniques have comparable sensitivity, greater specificity, and superior accuracy than clinical criteria alone.416,420–423 Whether more rigorous clinical criteria such as the clinical pneumonia infection score (CPIS),424 for example, or the use of quantitative cultures of endotracheal aspirates improve diagnostic accuracy without the need for invasive procedures is an unsettled issue.425
Although invasive procedures—BAL, PSB, and mini-BAL—are clearly more specific than clinical criteria, their impact on patient outcomes is much less clear.426,427 Fagon and colleagues426 found that patients with suspected VAP who were managed using an invasive diagnostic approach—bronchoscopic-guided PSB or BAL—had a significantly reduced 14-day mortality rate, reduced antibiotic-days, and reduced 28-day mortality rate on multivariate analysis, compared with patients managed using a clinical diagnostic approach (hazard ratio [HR] = 0.65, 95% CI = 0.46 to 0.91, p = 0.01).426 However, Heyland and colleagues427 found in a large multicenter Canadian trial that 28-day mortality rate and targeted antimicrobial use were identical among patients randomized to an invasive versus a clinical diagnostic approach. This study has been criticized for its exclusion of subjects at high risk for infection with antimicrobial-resistant pathogens.428 In the absence of definitive data demonstrating the superiority of either approach, the American Thoracic Society–Society of Critical Care Medicine–Infectious Disease Society of America joint guideline acknowledges that both diagnostic approaches are useful and acceptable when evaluating patients with suspected VAP. This puts great weight on an initial Gram stain of a deep tracheal aspirate; however, if no microorganisms are seen, it can be concluded that it is unlikely the patient has bacterial VAP.389
Risk Factors
A number of independent risk factors have been shown to increase the likelihood of developing VAP (Table 50.11).135,416 In general, these risk factors can be categorized as (1) factors that increase the likelihood or duration of mechanical ventilation, (2) factors that increase colonization of the oropharynx and gastric mucosa, (3) factors that increase the likelihood of aspiration, and (4) host factors that increase susceptibility to infection.
Table 50.11
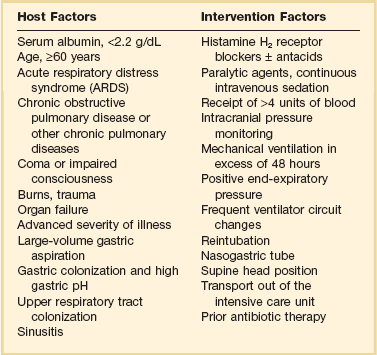
Modified from Chastre J, Fagon JY: Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903.
Prolonged mechanical ventilation, or reintubation, or both are the most powerful predictors of developing VAP. Cunnion and colleagues429 found that mechanical ventilation in excess of 24 hours was associated with a 12-fold increased risk of developing VAP, and Trouillet and associates found that ventilation longer than 7 days was associated with a sixfold increased risk.430 Emergent reintubation also carries a high risk of aspiration and was associated with a sixfold increased risk of VAP in a retrospective study.431
Poor dental hygiene increases the bacterial burden in the oropharynx and is an independent risk factor for nosocomial pneumonia.432 Likewise, a high gastric pH (>5) is associated with greatly increased bacterial colonization of the gastric contents,433 as well as an increased risk of VAP.434 A number of studies have found that exposure to antacids or H2-blockers is associated with an increased risk of VAP,56 although this has not been a universal finding.435
Depressed levels of consciousness, nasogastric tubes, and endotracheal tubes are ubiquitous in the ICU and all increase a patient’s risk of aspiration. That an altered level of cognition is associated with an increased risk of aspiration is supported by surveillance data showing increased rates of VAP in trauma and neurosurgical ICUs.25 Joshi and colleagues436 found that the use of a nasogastric tube was an independent predictor of VAP in a multivariate analysis (OR 6.5, 95%; CI 2.1 to 19.8). Finally, as noted, endotracheal tubes allow pooling of hypopharyngeal secretions that can leak around the cuff directly into the trachea, and a supine position appears to increase the risk of aspiration around the cuff.437
Host factors also contribute to an increased risk of developing VAP (see Table 50.11). Conditions such as advanced age, increased severity of illness, and the postsurgical state are rarely modifiable. However, poor nutritional status,403 oversedation,438 transfusion therapy,439 and exposure to broad-spectrum antimicrobials430 are associated with an increased risk of VAP and are under the control of the clinician.
Treatment
Treatment of VAP is discussed in Chapter 42 (Pneumonia: Considerations for the Critically Ill Patient), under “Therapy.”
Prevention
With an understanding of pathogenesis and epidemiology in hand, clinicians caring for mechanically ventilated patients can implement preventive strategies that can materially reduce the risk of VAP (Table 50.12). Both the CDC HICPAC and Canadian Critical Care Trials Group offer evidence-based guidelines for the prevention of VAP.135,440 Their recommendations are very similar, with minor differences. The Canadian guideline focuses exclusively on specific interventions for the prevention of VAP,440 whereas the HICPAC guideline incorporates additional guidance for the prevention of nosocomial influenza, legionellosis, and invasive filamentous fungal infections in the hospital.135 Recommendations from both guidelines can be divided into general, nonpharmacologic, and pharmacologic preventive measures (see Table 50.12).441 The general measures employed to reduce VAP including education, infection control, hand hygiene, and reliable disinfection and sterilization of respiratory care equipment are discussed elsewhere in this chapter.
Table 50.12
Recommendations for the Prevention of Ventilator-Associated Pneumonia
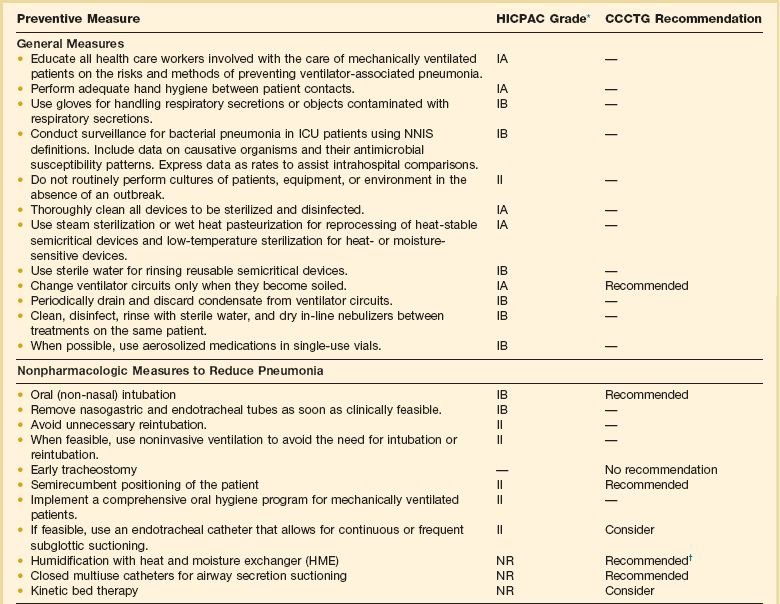
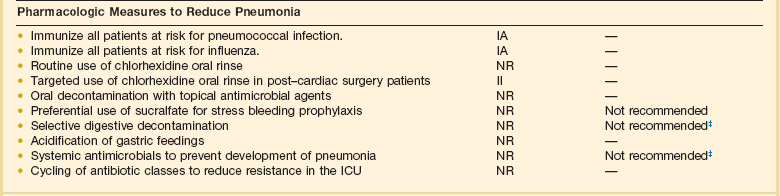
*Taken from CDC/HICPAC system of weighting recommendations based on scientific evidence. IA, strongly recommended for implementation and supported by well-designed experimental, clinical, or epidemiologic studies. IB, strongly recommended for implementation and supported by some experimental, clinical, or epidemiologic studies and a strong theoretical rationale. IC, required by state or federal regulations, rules, or standards. II, suggested for implementation and supported by suggestive clinical or epidemiologic trials or a theoretic rationale. Unresolved issue, an unresolved issue for which evidence is insufficient or no consensus regarding efficacy exists. NR, no recommendation for or against at this time.
†Recommended in patients without hemoptysis or high minute ventilation. Exchanger should be replaced weekly.
‡Topical or systemic antimicrobial agents alone are not recommended. Insufficient evidence on antibiotic resistance and cost-effectiveness exists to recommend combination topical and systemic therapy.
Modified from Tablan OC, Anderson LJ, Besser R, et al: Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53(RR-3):1-36; and Dodek P, Keenan S, Cook D, et al: Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med 2004;141:305-313.
Nonpharmacologic Preventive Measures
Avoiding prolonged intubation and reintubation—if avoiding intubation altogether is not feasible—offers the greatest promise for reducing an individual patient’s risk of developing VAP.431 The use of noninvasive ventilation in order to avoid endotracheal intubation has been shown to be successful in reducing rates of nosocomial pneumonia in a number of studies442,443 and may abrogate the need for reintubation in selected patients who prematurely extubate themselves.135 The implementation of weaning protocols has also been shown to significantly reduce the duration of mechanical ventilation,444,445 health care costs,444,445 and institutional rates of VAP.446,447 Early tracheostomy—within 1 week of intubation—has been advocated as a method for reducing the risk of VAP in patients likely to require prolonged mechanical ventilation. However, randomized trials, admittedly of limited power, have not found significant benefit with this approach448 and early tracheostomy is not currently recommended by most authorities.135,440
As noted earlier, supine positioning of the mechanically ventilated patient’s head has been shown to increase the risk of gastroesophageal-pharyngeal aspiration.437 A simple solution to this threat is to elevate the head of the patient’s bed 35 to 45 degrees. Drakulovic and colleagues449 found that patients whose torso and head were kept elevated at 45 degrees had much lower rates of microbiologically confirmed pneumonia compared with patients cared for in a 0-degree supine position (5% versus 23%, p = 0.018).449 In reality, maintaining elevation of the head in excess of 45 degrees on a consistent basis is actually quite difficult and uncommonly achieved in practice. A recent randomized study that sought to maintain head elevation above 45 degrees for 85% of the study period found that head elevation in the intervention arm only averaged 28.1 degrees.450 Perhaps as a result of failure to successfully achieve adequate elevation, no reductions in the rate of VAP were seen.
Although data on the effect that comprehensive oral care has on risk of infection are limited,451 maintaining adequate dental hygiene is considered an important component of VAP prevention.135 Binkley and colleagues452 found that although a majority of nurses caring for patients undergoing mechanical ventilation appreciated the importance of dental hygiene, the methods used to provide this varied considerably. Until more data are available on specific dental hygienic practices, it is recommended that mechanically ventilated patients have their teeth brushed daily, undergo oral cleansing every 2 to 4 hours, undergo routine suctioning to reduce accumulation of fluids in the oropharynx, and have a mouth moisturizer applied to their lips to prevent cracking.453 The periodic instillation of a topical oral antiseptic solution is an additional promising intervention453 and is discussed under pharmacologic preventive measures later.
The use of a modified endotracheal tube that has a separate ventral drainage tube for continuous or intermittent suctioning of subglottic secretions has been evaluated in a number of studies.454,455 Subglottic suctioning reduced the rate of VAP significantly in all but one of these studies.455 However, in this latter study, the time to onset of VAP was delayed significantly (5.9 days versus 2.9 days, p = 0.006),455 and recent evidence-based guidelines have recommended the use of endotracheal tubes that allow for suctioning of subglottic secretions.135,440 Nevertheless, the use of an endotracheal tube that allows for subglottic suctioning did not reduce the duration of mechanical ventilation or rate of ICU mortality in the studies done, which, coupled with the increased cost of the tube and propensity of the suction lumen to occlude, has limited wider adoption of this technology in practice.456
The evidence that heat and moisture exchangers (HMEs) are associated with a reduced risk of VAP is mixed. Only one of six published trials found a statistically significant reduction in VAP with use of HMEs (RR 0.41, 95% CI 0.20 to 0.86, p = 0.02).457 However, pooling data from a recent systematic review458 and a subsequently published randomized trial459 shows that HMEs reduce the risk of VAP by 38% (RR 0.62, 95% CI 0.43 to 0.89, p = 0.012). The use of HMEs has been recommended by authors of a systematic review460 and is currently recommended by the Canadian Critical Care Trials Group.440 However, HICPAC made no recommendation for the use of HMEs because five of six published trials failed to demonstrate a statistically significant reduction in the rate of VAP.135 Heat exchange moisturizers become readily occluded in patients with airway hemorrhage and can increase airway resistance. As a result, they should not be used in patients with hemoptysis or those requiring a high-minute ventilation.440 Finally, the membranes of HMEs can become colonized with bacteria and should be replaced weekly, according to current guidelines.440
The availability of in-line multiuse suction catheters abrogates the need to open and manipulate the endotracheal circuit, theoretically reducing the risk of exogenous contamination.461 Despite their theoretical benefit, prospective studies have not consistently showed that in-line suction catheters are associated with a reduced risk of VAP.462–464 Although in-line suction catheters do not appear to increase the risk of VAP, they are more time efficient for nursing personnel and respiratory therapists, and are more cost effective than open suction catheters.440 Kollef and colleagues465 found that rates of VAP were identical in patients randomized to as-needed changes of their inline suction catheter versus those who had their catheter changed every 24 hours (14.7% vs. 14.8%). As a result, there is no compelling evidence that in-line suction catheters should be periodically changed, unless clinically indicated.
Pharmacologic Preventive Measures
Antacids and H2-blockers have been used extensively in the ICU setting to prevent stress ulcer bleeding but have been associated with an increased risk of developing VAP because they lead to bacterial overgrowth of the gastric contents.56 Sucralfate prevents stress ulcer bleeding without reducing gastric pH but is more difficult to administer and is less effective than acid-reducing agents.435 The results of clinical trials examining these two competing strategies for preventing gastrointestinal hemorrhage in the ICU have been mixed, with earlier trials favoring the use of sucralfate.56 However, more recently published trials suggest only a small incremental increased risk of VAP with H2-blockers,435,466,467 and most experts feel that this risk is more than offset by their superior capacity to prevent stress ulcer bleeding.135,440
Selective digestive decontamination (SDD) is one of the most extensively studied preventive interventions in critical care medicine, yet the role for SDD continues to generate vigorous debate as to its overall benefit.468,469 A more detailed discussion on the risks and benefits of this intervention is provided later in this chapter. Most U.S. experts believe that SDD has the potential to increase infection caused by multiresistant bacteria, particularly in settings with high rates of endemic antimicrobial resistance.470,471 Until well-designed multicenter trials are done, proving that SDD does not adversely effect the ICU ecology, it is likely that North American guidelines will continue to discourage its use.135,440
The isolated use of parenteral antimicrobials for prevention of VAP has not met with much success,470 but selective antimicrobial decontamination of the oropharynx, without the use of enteral or systemic agents, reduced the risk of VAP nearly 70% (RR = 0.33, 95% CI 0.16 to 0.67, p = 0.001) in one trial.411 This study reemphasized the primary role of oropharyngeal colonization in the pathogenesis of VAP but engenders the same concerns as SDD over its potential for promoting antimicrobial resistance. However, it has facilitated the idea that topical decolonization of the oropharynx with nonantimicrobial agents might be able to materially reduce the risk of VAP without the potential for emergence of antimicrobial resistance. A meta-analysis of seven randomized trials that enrolled 914 mechanically ventilated patients found that topical chlorhexidine applied to the oropharynx reduced the risk of VAP by nearly 30% (RR = 0.74, 95% CI 0.56 to 0.96, p = 0.02), although there was no significant impact on mortality.413 The beneficial effects of chlorhexidine appear to be most pronounced in post–cardiac surgery patients,472,473 prompting HICPAC to recommend its use in this subpopulation.135
Catheter-Associated Urinary Tract Infection
Incidence and Impact
Each year, urinary catheters are inserted in more than 5 million patients in acute-care hospitals and extended-care facilities.474 Catheter-associated urinary tract infection (CAUTI) is one of the most common infections in ICUs, and incidence rates are highest in burn, neurologic, neurosurgical, and trauma ICUs, with intermediate risk in surgical and medical ICUs, and lowest risk in coronary care units (see Table 50.1).25
Nosocomial bacteriuria or candiduria develops in up to 25% of patients requiring a urinary catheter for more than 7 days, with a daily risk of 5%.474 CAUTI is the second most common cause of nosocomial bloodstream infection475; some studies have also found increased mortality rates associated with CAUTI.476 Although most CAUTIs are asymptomatic,477 rarely extend hospitalization, and add only $500 to $1000 to the direct costs of acute-care hospitalization,478 asymptomatic infections commonly precipitate unnecessary antimicrobial-drug therapy.479 CAUTIs comprise perhaps the largest institutional reservoir of nosocomial antibiotic-resistant pathogens, the most important of which are multidrug-resistant Enterobacteriaceae other than Escherichia coli such as Klebsiella, Enterobacter, Proteus, and Citrobacter; Pseudomonas aeruginosa; enterococci and staphylococci; and Candida spp.480
Pathogenesis
Excluding rare hematogenously derived pyelonephritis, caused almost exclusively by S. aureus, most microorganisms causing endemic CAUTI derive from the patient’s own colonic and perineal flora or from the hands of health care personnel and gain access to the patient’s urinary tract during catheter insertion or manipulation of the collection system.265 Organisms gain access in one of two ways. Extraluminal contamination may occur early, by direct inoculation when the catheter is inserted, or later, by organisms ascending from the perineum by capillary action in the thin mucous film between the external catheter surface and the urethral wall. Intraluminal contamination occurs by reflux of microorganisms gaining access to the catheter lumen from failure of closed drainage or contamination of urine in the collection bag. Recent studies suggest that CAUTIs most frequently stem from microorganisms gaining access to the bladder extraluminally,481 but both routes are important.
Most infected urinary catheters are covered by a thick biofilm containing the infecting microorganisms embedded in a matrix of host proteins and microbial exoglycocalyx.482 A biofilm forms on the intraluminal or extraluminal surface of the implanted catheter, or both, usually advancing in a retrograde fashion. The role of the biofilm in the pathogenesis of CAUTI has not been established. However, anti-infective–impregnated and silver-hydrogel catheters, which inhibit adherence of microorganisms to the catheter surface, significantly reduce the risk of CAUTI,483 particularly infections caused by gram-positive organisms or yeasts, which are most likely to be acquired extraluminally from the periurethral flora. These data suggest that microbial adherence to the catheter surface is important in the pathogenesis of many, but not all, CAUTIs. Infections in which the biofilm does not play a pathogenic role are probably caused by mass transport of intraluminal contaminants into the bladder by retrograde reflux of microbe-laden urine when a catheter or collection system is moved or manipulated.
Prevention
Several catheter-care practices are universally recommended to prevent or at least delay the onset of CAUTI265: most importantly, avoiding unnecessary catheterizations; considering using a condom catheter in a male or a suprapubic catheter; having trained professionals insert catheters aseptically; removing the catheter as soon as no longer needed; maintaining uncompromising closed drainage; ensuring dependent drainage as much as possible; minimizing manipulations of the system; and separating catheterized patients geographically on the patient care unit.
As noted earlier, technologic innovations to prevent nosocomial infection are most likely to be effective if they are based on a clear understanding of the pathogenesis and epidemiology of the infection. Novel technologies must be designed to block CAUTI by either the extraluminal or intraluminal routes, or both. Medicated catheters, which reduce adherence of microorganisms to the catheter surface, may confer the greatest benefit for preventing CAUTI. Two catheters impregnated with anti-infective solutions have been studied in randomized trials, one impregnated with the urinary antiseptic nitrofurazone484 and the other with a new broad-spectrum antimicrobial-drug combination, minocycline and rifampin.485 Both catheters showed a modest reduction in bacterial CAUTIs; however, the studies were small, and the risk of selection of antimicrobial drug–resistant uropathogens was not satisfactorily resolved. Silver compounds have also been studied for coating urinary catheters. A meta-analysis of eight randomized trials comparing silver oxide or silver alloy catheters with standard nonimpregnated catheters found that silver alloy, but not silver oxide, catheters were associated with a reduced risk of CAUTI.486 Recommendations for the prevention of CAUTI are summarized in Table 50.13.133
Table 50.13
CDC/HICPAC Guideline Recommendations for Prevention of Catheter-Associated Urinary Tract Infection
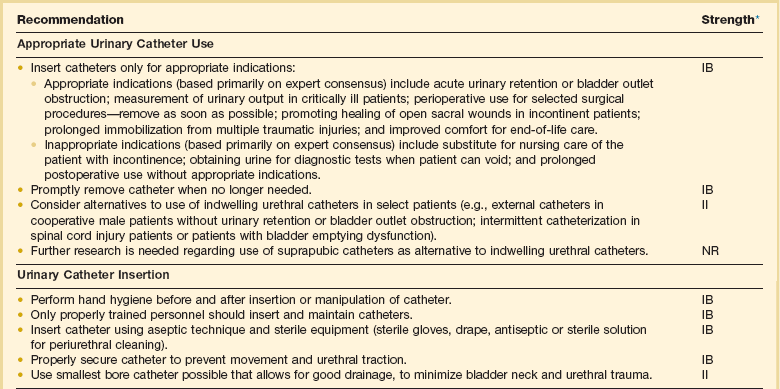

CAUTI, catheter-associated urinary tract infection.
*IA, strong recommendation supported by high- to moderate-quality evidence suggesting net clinical benefit or harm. IB, strong recommendation supported by low-quality evidence suggesting net clinical benefit or harm or an accepted practice (e.g., aseptic technique) supported by low- to very-low-quality evidence. IC, strong recommendation required by state or federal regulation. II, weak recommendation supported by any-quality evidence suggesting a trade-off between clinical benefit and harm. No recommendation/unresolved issue (NR), unresolved issue for which there is low- to very-low-quality evidence with uncertain tradeoffs between benefit and harm.
Data from Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010;31(4):319-26.
Control of Antibiotic Resistance
Evolution of Antibiotic Resistance in Intensive Care Units
In the early 1990s VRE burst onto the hospital and ICU scene in the United States and within a few years became entrenched in most tertiary medical centers (see Fig. 50.2). Heavy use of vancomycin, often as empiric treatment in response to concerns about MRSA, was probably the initial factor driving the emergence of VRE. In most settings, however, exposure to cephalosporins and antimicrobials with antianaerobic activity have emerged as the greatest risk factors for nosocomial colonization or infection by VRE. The mid-1990s witnessed growing problems with resistance in fungi and shifts to non–Candida albicans species, representing the effects of heavy empirical use of azoles such as fluconazole in hospitals during this period.
Forces Driving Resistance
To a large extent, the emergence of antimicrobial resistance reflects the combined effects of genetic selection, antibiotic pressures, and the frequency of cross-infection in ICUs.47 For some resistance mechanisms (e.g., extended-spectrum β-lactamases [ESBLs] that confer resistance to third-generation cephalosporins such as ceftazidime), a shift of a single amino acid in existing resistance genes can lead to new, inactivating enzymes. For other resistant bacteria, such as penicillin-resistant pneumococci, multiple resistance genes must be cobbled together in a specific, exacting sequence, which may take years to evolve, emerge, and spread.
Antibiotic pressures provide the necessary darwinian forces that amplify these genetic changes.487 Usually, resistance emerges to a specific agent that is used most heavily and, hence, provides the greatest pressure. In some instances, genetic linkage of resistance mechanisms to unrelated classes of antimicrobials results in the capacity of heavy use of one drug class to select for resistance to a different class. For example, use of trimethoprim-sulfamethoxazole has been associated statistically with the emergence of ceftazidime-resistant E. coli and K. pneumoniae as a result of linkage on a single plasmid of genes that encode production of ESBLs and trimethoprim-sulfamethoxazole resistance. A large proportion of extended-spectrum β-lactamase–producing gram-negative bacilli are also resistant to fluoroquinolones.488,489
In epidemiologic and clinical studies of antibiotic resistance, there is always a proportion of patients in whom resistance is found without exposure to the problem antibiotic. These patients usually have other important risk factors, such as increased severity of underlying disease, extremes of age, presence of invasive devices, recent surgery, or proximity to patients who are infected or colonized with antibiotic-resistant bacteria. In these cases the presence of antibiotic-resistant strains is most often the consequence of patient-to-patient spread, usually on the contaminated hands of HCWs; occasionally, spread results from a contaminated common source, such as an inadequately cleaned piece of equipment. Studies of HCW hand hygiene show that rates of handwashing between patient contacts range from 25% to 50%, at best, and are inadequate to control resistance, especially in ICUs, where the staff are extremely busy and less likely to be attentive to hand hygiene.168
Controlling Antimicrobial Resistance in the Intensive Care Unit
Third, the importance of hand hygiene must be stressed at all times. Aggressive hand hygiene campaigns, with adherence monitoring and feedback of ward and even individual results, may achieve compliance rates as high as 70%. For some situations (e.g., when there is extensive patient colonization by antibiotic-resistant bacteria), these levels of adherence may not be sufficient to control cross-infection. Response to this problem has been to encourage “universal gloving,” in addition to wider use of alcohol-based hand rubs (a “belt-and-suspender” approach) to bridge the gap left by incomplete attention to hand hygiene even in the best of circumstances. Use of universal gloving has been successful in controlling the spread of aminoglycoside-resistant gram-negative bacilli in ICUs and C. difficile–related diarrhea.228,245 Because patients’ intact skin and the environment in patient rooms may be a source of resistant bacteria, such as VRE, we recommend that disposable examination gloves be worn for all contact with ICU patients or their environment. Because gloves are not a total barrier, they must be removed and hands disinfected by an alcohol hand rub between patient contacts.
Fourth, antimicrobial stewardship is essential (Table 50.14).257 The primary goal of antimicrobial stewardship is to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use such as toxicity, emergence of resistance, and C. difficile–associated diarrhea. Because antimicrobial use drives antimicrobial resistance, the frequency of inappropriate antimicrobial use can be used as a surrogate marker for antimicrobial resistance. Both antimicrobial stewardship and a comprehensive infection control program are essential to limiting the emergence and transmission of antimicrobial-resistant pathogens. Most studies assessing the utility of antimicrobial stewardship have focused on adults in ICUs, where the burden of antimicrobial resistance is greatest.
Table 50.14
Recommendations for Developing an Institutional Program to Enhance Antimicrobial Stewardship

*Based on the Infectious Diseases Society of America grading system for ranking recommendations in clinical guidelines. A, good evidence to support a recommendation for use; B, moderate evidence to support a recommendation for use; C, poor evidence to support a recommendation for use; I, evidence from >1 properly randomized, controlled trial; II, evidence from >1 well-designed clinical trial, without randomization, from cohort or case-controlled analytic studies, or from multiple time-series; III, evidence from expert opinion.
Modified from Dellit TH, Owens RC, McGowan JE Jr, et al: Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159-177.
A comprehensive evidence-based stewardship program to combat antimicrobial resistance is typically a multifaceted, multidisciplinary program; the size and complexity of the management team and the specific measures applied to optimize prescribing vary on the basis of local antimicrobial use patterns, resistance trends, and available resources. The two core strategies that provide the foundation for a successful antimicrobial stewardship program are (1) prospective audits, with intervention and feedback, and (2) formulary restriction and preauthorization.257
Several studies have shown that prospective audits of antimicrobial use with intervention and feedback are an effective means of reducing inappropriate antimicrobial use.490,491 In a randomized trial conducted at a 600-bed tertiary teaching hospital, inpatients receiving parenteral antimicrobial therapy were randomized to an intervention group that received suggestions for optimal antimicrobial use from an infectious disease physician or to no interventions. Physicians in the intervention group implemented 85% of the suggestions they received, which resulted in 1.6 fewer days of parenteral therapy and $400 savings per patient. Similar results have been noted in trials undertaken in community hospitals.490 If daily review of antimicrobial use is not feasible, review of antimicrobial usage 3 days a week may still have a significant impact. Effective auditing with intervention and feedback can be undertaken most easily with automated computer surveillance of antimicrobial use, allowing the targeting of specific units where the problems are greatest.
Formulary restriction and preauthorization requirements for specific agents are now common in most hospitals. Antimicrobial restriction is unequivocally the most effective method of controlling antimicrobial use.492,493 However, it is unclear whether antimicrobial restriction achieves the more important outcome, reducing antimicrobial resistance. Several studies of outbreaks of C. difficile–associated diarrhea have shown abrupt cessation of the outbreak following restriction (and greatly reduced use) of one or more key antimicrobials such as clindamycin or third-generation cephalosporins.492 However, other studies have documented inexorably rising resistance rates in nosocomial pathogens despite a rigorous program of antimicrobial restriction.494 One explanation for this increase in resistance may be the compensatory increase in usage of broad-spectrum antimicrobials other than the restricted agent, thus counteracting any benefit of restriction. Furthermore, restricting the use of a single drug to reduce antimicrobial resistance may be ineffective because cross-resistance in bacterial species to more than one class of antimicrobials is the rule in nosocomial organisms.
Beyond the two major mechanisms of antimicrobial stewardship mentioned earlier, other elements that should be incorporated into an institutional antimicrobial stewardship program include education of health care providers; however, passive educational efforts such as conference presentations, teaching sessions, and provision of guidelines are only marginally effective in the absence of other active interventions.495 Clinical practice guidelines are being introduced with increasing frequency; however, the impact of these guidelines on provider behavior and clinical outcomes has been difficult to measure. Guidelines tailored to local antimicrobial resistance patterns and antimicrobial use trends may have more impact than a generic clinical pathway.
Interest has been sparked in ICUs by the reborn concept of antibiotic cycling.496,497 The most recent experiences have evaluated switch therapy498 for empiric antibiotic use, rather than actual cycling, and have shown beneficial reductions in resistance among gram-negative bacilli499 and in the prevalence of VRE. Such approaches, as well as true cycling through different antimicrobial classes, may be effective over limited periods in closed environments such as ICUs, by transiently reducing selection pressure and thus resistance to the restricted agent. Yet studies have thus far not shown a consistent long-term benefit with cycling, and mathematical models do not predict that cycling will be an effective measure to reduce antimicrobial resistance.500
Antimicrobial order forms reduce antimicrobial usage with automatic stop orders and the requirement for physician justification.501 Streamlining or de-escalation of therapy based on culture data is an essential component of appropriate antimicrobial use, with studies showing substantial reductions in days of antimicrobial use and cost savings.502,503
Computer order entry provides needed information at the moment in a neutral, nonjudgmental, fact-based format; this system is efficient, well accepted, and holds the promise to change prescribing behaviors materially.504,505
Effective antimicrobial stewardship programs can be financially self-supporting and improve patient care. Studies have shown reductions in antimicrobial usage from 22% to 36%, with annual savings of $200,000 to $900,000 in larger teaching hospitals and community hospitals. A recent guideline from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America provides detailed recommendations for developing institutional programs of antimicrobial stewardship, which are summarized in Table 50.14.257