Chapter 62 Nosocomial infection
Nosocomial or hospital-acquired infections are a major problem in hospitals, affecting up to 9% of inpatients at any one time. Intensive care units (ICUs) represent 2–10% of hospital beds, but are responsible for 25% of all nosocomial blood stream and pulmonary infections. In the European Prevalence of Infection in Intensive Care (EPIC) snapshot of prevalence the infection rate in ICU was 20.62%.1 Nosocomial infection is, at least in theory, a preventable cause of morbidity and mortality (Table 62.1).
Table 62.1 Principles of diagnosis of nosocomial infections
EPIDEMIOLOGY
The prevalence of nosocomial infection is reported as being between 3 and 12% in most institutions but varies considerably between different sites within each institution.2 The vulnerability of the patient population, the nature of interventions and cross-infection are but three of many factors. This is seen clearly if one compares the range between ophthalmology and critical care – 0–23%.3
The impact of nosocomial infection is impressive. Ventilator-associated pneumonia (VAP) is common, has significant morbidity with increased length of stay, associated costs and a twofold increase in mortality.4 It has been suggested that blood stream infections, surgical wound infections and nosocomial pneumonia result in 14, 12 and 13 attributable extra hospital days respectively.5 Catheter-related blood stream infection (CR-BSI) was also associated with major morbidity although, curiously, not necessarily mortality.6 The mortality rates directly due to these infections are hard to separate from the mortality attributed to the presenting severity of illness, which in its own right may have predisposed to infection. What is clear is that nosocomial infection is associated with increased mortality, and huge financial and resource costs.2
THE MECHANISMS INVOLVED IN NOSOCOMIAL INFECTION
A range of factors come together to enable nosocomial infection to occur. Some may be risk factors in their own right whereas other may simply represent an identifier of a sicker and therefore more vulnerable population (Table 62.2).
| Patient |
| Severity of illness |
| Underlying diseases |
| Nutritional state |
| Immunosuppression |
| Open wounds |
| Invasive devices |
| Multiple procedures |
| Prolonged stay |
| Ventilation |
| Multiple or prolonged antibiotics |
| Blood transfusion |
| Environment |
| Changes in procedures or protocols |
| Multiple changes in staff; new staff |
| Poor aseptic practice – poor hand-washing |
| Patient-to-patient: busy, crowded unit, staff shortages |
| The organism |
| Resistance |
| Resilience in terms of survival |
| Formation of slime or ability to adhere |
| Pathogenicity |
| Prevalence |
HOST
The vulnerability of a potential host will be determined by several factors:
ENVIRONMENT
Local environmental pressures play their part. The combination of antibiotics, in particular multiple antibiotics, and cross-infection predisposes a vulnerable population to pseudomembranous colitis from Clostridium difficile toxin.8 Epidemiological patterns, such as the prevalence of Enterococcus faecalis as a common pathogen in the surgical population, may be linked to widespread cephalosporin usage. Much of the multiresistance problem probably originates from antibiotic pressures.9 Cross-infection is the biggest single problem in intensive care and transmission is by various means, but still the most common is by hands.10
ORGANISM
The host usually lives in synergistic or symbiotic tranquillity with a huge range of organisms (Table 62.3). Antibiotics suppress many normal organisms and allow the emergence and overgrowth of a usually insignificant organism or resistant organism of the same type. For example, an intrinsic organism such as Candida will flourish in the presence of broad-spectrum antibiotics and this overgrowth may result in symptomatic or even invasive candidiasis. Cephalosporin use may encourage the intrinsically resistant but quiescent enterococci to emerge as a dominant and problematic organism.
Table 62.3 Common commensals that may cause infection in a vulnerable host
| Site | Common commensal organisms |
|---|---|
| Skin | Staphylococcus epidermidis, streptococci, Corynebacterium (diphtheroids), Candida |
| Throat | Streptococcus viridans, diphtheroids |
| Mouth | Streptococcus viridans, Moraxella catarrhalis, Actinomyces, spirochaetes |
| Respiratory tract | Streptococcus viridans, Moraxella, diphtheroids, micrococci |
| Vagina | Lactobacilli, diphtheroids, streptococci, yeast |
| Intestines | Bacteroides, anaerobic streptococci, Clostridium perfringens, Escherichia coli, Klebsiella, Proteus, enterococci |
THE ORGANISMS
A vast range of organisms can cause nosocomial infection (Table 62.4). It must be emphasised that each hospital and each ICU will have its own local ecology and knowing this ecology is important. Regional, national and international surveys give indications of general trends but this does not supplant local knowledge.
Table 62.4 Organisms responsible for the majority of nosocomial infections
| Methicillin-resistant Staphylococcus aureus (MRSA) |
| Coagulase-negative Staphylococcus (CNS) |
| Enterococcus spp. (E. faecalis, E. faecium) |
| Pseudomonas aeruginosa |
| Acinetobacter baumanii |
| Stenotrophomonas maltophilia |
| Enterobacter spp. |
| Klebsiella spp. |
| Escherichia coli |
| Serratia marcescens |
| Proteus spp. |
| Candida spp. (C. albicans, C. glabrata, C. krusei) |
Other organisms may be a problem in the severely immunocompromised, such as those with acquired immunodeficiency syndrome (AIDS: see Chapter 60)
The combination of sick patients and widespread use of potent antibiotics selects out problematic organisms and, as this epitomises intensive care practice, it is in ICU where multiresistance is common.
MULTIRESISTANT ORGANISMS
Staphylococcal resistance to meticillin occurs due to an altered penicillin-binding protein, which has low affinity for all β-lactam agents. It is linked to a MecA gene. This gene does not develop readily and spread of meticillin resistance is by vector transmission, not de novo production of resistance7 (Table 62.5).
Table 62.5 The influence of extended-spectrum β-lactamases (ESBL) on resistance in Klebsiella
| Antibiotics | ESBL-negative (% resistant) | ESBL-positive (% resistant) |
|---|---|---|
| Gentamicin | 8 | 76 |
| Amikacin | 3 | 52 |
| Ciprofloxacin | 3 | 31 |
| All the above | 0 | 5 |
(Reproduced from Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother 1996; 38: 409–24.)
SOME COMMON ORGANISMS
See Table 62.4 for organisms responsible for the majority of nosocomial infections.
Escherichia Coli
E. coli was one of the first organisms to become multiresistant. The spread of its resistance is a model of how the problem develops in different environments. Currently in Europe, about 4% of E. coli is resistant to ceftazidime but in Turkey this figure is 26%. The trend towards an increase in resistance seems to correspond with the use of quinolones.11
Enterobacter species, cloacae and Aerogenes
Part of the normal intestinal flora, these species tend to develop ESBL relatively easily, making them multiresistant to a wide range of antibiotics, including ceftazidime. The prevalence of resistance in many western ICUs is running at about 35% and rising. This organism has been implicated in cross-colonisation in ICU and the relevant enzymes, including TEM-24, impart cross-resistance to multiple classes of antibiotics. Prompt recognition and treatment are usually effective.12
Pseudomonas
Pseudomonas are versatile opportunistic pathogens common in the critically ill; they may colonise patients with chronic lung disease. Resistance is able to develop in a range of ways and produces a very broad spectrum of resistance, making it potentially very difficult to treat and resulting in the increased popularity of combination therapy.13 It is associated with adverse outcome in the critically ill.14
Acinetobacter Baumanii
Acinetobacter baumanii is an increasing and major problem. It survives even in dry environments and, despite its name, spreads and cross-infects readily (a cineto: without movement). They are multiresistant and, although they were sensitive to carbapenems, they are increasingly resistant even to these agents, presumably through gene exchange. They become rapidly resistant and the profile of resistance is unpredictable but can be extremely broad, with some organisms recently only sensitive to colistin.15
Staphylococcus Aureus
This is a virulent pathogen causing a wide range of infections. Meticillin resistance started in the early 1960s, and although rates between countries and ICUs vary considerably, there has been an inexorable rise in its prevalence in most countries. It is easily identified. It both colonises and infects, but is relatively easily treated with glycopeptides. More recent reports of occasional glycopeptide-resistant organisms are therefore a major source of concern. It has been a model of the failure of some infection control methods. It is being increasingly assessed in terms of suppressibility with a selective decontamination of the digestive tract (SDD)-style approach to elimination.16
Enterococcus Faecalis and Faecium
These organisms emerged with increased third-generation cephalosporin use. E. faecalis is more commonly isolated and may still be sensitive to ampicillin but more than 30% are resistant to aminoglycosides. This contrasts with E. faecium, which has a resistance rate of 7% to vancomycin (and rising), 53% to ampicillin and 30% to aminoglycosides. Glycopeptide resistance is increasing and vancomycin-resistant enterococcus (VRE), unheard of 10 years ago, is now being regularly identified.17
Clostridium difficile
This originally emerged as an organism that became a problem after certain antibiotics, including clindamycin. It is now clear that in the critically ill it often emerges after almost any broad-spectrum antibiotics, although usually after several courses and some considerable time. Probably as important is the observation of case clusters, indicating that cross-infection is important. The organism produces a toxin that causes pseudomembranous colitis, but paradoxically, testing for the toxin provides a rapid diagnostic pathway that can facilitate early treatment. Aggressive pseudomembranous colitis is a potentially catastrophic disease and, although it usually settles on metronidazole or vancomycin orally, not only is recurrence common but it can also progress to severe colitis and necessitates radical surgery such as colectomy.18 Surgery carries a high mortality rate, quoted at 11% for total colectomy, and very high mortality for less aggressive surgery such as hemicolectomy in the presence of toxic megacolon.19
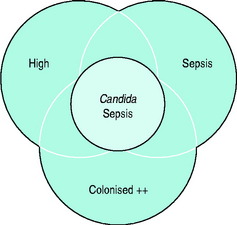
Candida Sepsis
In the next decade, three organisms will dominate nosocomial infection and one, MRSA, will provide a historical perspective. The organisms are Stenotrophomonas and Acinetobacter, which are rapidly becoming more common and less easy to treat. The third, although in relatively small numbers, relates to the rising incidence of multiresistant tuberculosis, which not only has potential but is already providing infection control challenges in the critically ill (Figure 62.1).
COLONISATION AND INFECTION
MRSA colonises very readily and causes infection in a significant proportion of cases but is often easily identified and treated. VRE is still relatively uncommon. It does colonise, although the incidence of overt infection appears low, at present, but is difficult to treat when it occurs. Acinetobacter colonises and infects very readily, may take days to identify and is difficult to treat.20
SITES OF INFECTION
NOSOCOMIAL PNEUMONIA
Nosocomial pneumonia is a common problem in the critically ill, particularly in ventilated patients, with an incidence of 15–30%.21–23
Aetiology
Patient factors predisposing to nosocomial pneumonia include acute severity of illness; chronic illness, especially chronic lung disease; diabetes; immunosuppression; advanced age; recent surgery to thorax or abdomen; intubation; and bronchoscopy. Environmental factors include broad-spectrum and prolonged use of antibiotics; potential pathogens in the vicinity; bacterial properties such as ability to adhere to surfaces; cross-infection; 24-hour ventilator tubing changes; and foreign bodies such as nasogastric tubes. Intubated and ventilated patients have a higher rate of nosocomial infection than patients receiving non-invasive ventilation. However, this may be due to different patient populations with different underlying problems and background morbidity.24 The role of neutralisation by H2 antagonists and proton pump inhibitors is still debated.25–27
Diagnosis
The general criteria for diagnosis are general signs of infection; clinical signs of a chest infection; purulent sputum; radiological evidence, such as new pulmonary infiltrates; and positive cultures, from sputum or blood.21,23,28,29 These produce a non-specific diagnosis. Expectorated sputum is difficult to assess, but should contain < 25 polymorphs and > 10 squamous epithelial cells per low-power field. In ICU it is more usual to provide samples from the endotracheal tube and then to use quantitative techniques. This can achieved with protected brush specimens (PBS) bronchoalveolar lavage (BAL) and protected BAL. These are difficult and time-consuming and it appears that non-bronchoscopic techniques such as blind tracheal aspirates through the endotracheal tube are both practical and reasonably effective from a clinical – if not a research – viewpoint23,30 (Table 62.6).
Table 62.6 Principles for the diagnosis of nosocomial pneumonia
| Crackles on auscultation or dullness to percussion on physical examinaion of the chest and any of the following:
• Isolation of pathogen from specimen obtained by transtracheal aspirate, bronchial brushing or biopsy
• Chest radiography examination shows no or progressive infiltration, consolidation, cavitation or pleural effusion
|
IgM, immunoglobulin M.
The Organisms
Aerobic Gram-negatives predominate, including Pseudomonas aeruginosa, Enterobacter spp., Klebsiella, Escherichia coli, Serratia marcescens and Proteus; between them, these account for about 70% of infections. Staphylococci, in particular MRSA, account for a small but significant number of nosocomial pneumonias. Early-onset VAP, between 48 hours and 5 days, may be due to community pathogens, such as methicillin-sensitive Staphylococcus aureus, Streptococcus pneumoniae and Haemophilus infiuenzae, as well as Gram-negative enteric bacilli (GNEB). This has been described as early endogenous infection. In contrast, later onset, after 5–7 days, involves more resistant species such as MRSA, Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia.31,32 This has been called secondary endogenous and exogenous infection.
Prevention
Prevention is by methods that reduce aspiration, cross-infection and contamination of respiratory devices.29 Several recommended methods are:
Treatment
The use of inappropriate or inadequate antibiotics leads to an increased mortality, so current trends are towards initial broad-spectrum antibiotics followed by reassessment and de-escalation.33,34 The principle is that an empirical broad-spectrum approach will cover the relevant organisms but once the specific organism is identified the antibiotics can be rationalised to focus on that organism; this is clearly harder to apply in practice than in theory. As the spectrum of resistance grows it is highly likely that it will be increasingly difficult to apply this broad-spectrum empiric treatment, especially with late-onset VAP. Surveillance of current colonisation, awareness of an individual ICU’s ecology and targeted treatment will replace empiric regimens. At present, combination therapy is recommended, but there is little evidence to support it over monotherapy.23,33,35–37
Another area of interest is duration of treatment with antibiotics. Many regimens are traditional rather then evidence-based and may result in unnecessarily prolonged treatment. There is a trend to reducing courses. For VAP an 8-day course may be adequate.30 There has not yet been a move towards using a clinical or bacterial response to treatment as an indicator for the duration of treatment, although it may be on the horizon.
The attributable mortality with nosocomial pneumonia is difficult to determine because patients requiring ventilation often have a high intrinsic mortality. Rates of 30% have been quoted: pneumonia accounts for 60% of deaths from nosocomial infections. In addition, nosocomial pneumonia increases length of stay by 12–23 days.4,38,39
WOUND INFECTION
RISK FACTORS
THE ORGANISMS
The organisms are frequently determined by the type of surgery and procedure. Skin commensals are common, such as staphylococci or streptococci, and are usually early infections. These are characterised by fever, pain and redness around the site. With streptococcal infection this may be quite confluent. Gram-negative organisms may also cause infection. Drainage is important.
ANTIBIOTIC PROPHYLAXIS
In many circumstances the evidence supporting prophylaxis is minimal, and frequently the antibiotics used are inappropriate to the perceived risk. Local factors, including the local ecology, will determine specific requirements. With grafts, mesh or prostheses the morbidity from infection is so great as to justify using prophylaxis even if the evidence is marginal (Table 62.7).
| Type of surgery | |
|---|---|
| Abdominal wall | Insertion of mesh. Staphylococcal or streptococcal cover. In the groin, Gram-negative cover may be needed |
| Cardiac | Protection of valves and grafts. Staphylococci may be resistant |
| Vascular | Protection of grafts. Staphylococci are a major consideration. If the groin is involved, it may require Gram-negative cover |
| Orthopaedic | Prostheses. Staphylococci are an increasing problem |
| Biliary upper GI | Usually Gram-negative and anaerobic cover, with an awareness of resistant enterococci |
| Colorectal | Protection against faecal flora. Cephalosporins and metronidazole have been popular but predispose to developing enterococci such as Enterococcus faecalis or E. faecium, which may be multiresistant |
| Gynaecological | Conventionally broad-spectrum involving cephalosporins or, currently, augmentin. Metronidazole is also favoured |
| Urological | Protection from instrumentation of the urinary tract, Gram-negative cover. Awareness of any existing infection |
GI, gastrointestinal.
LINE SEPSIS
The use of intravascular devices in hospital practice is ubiquitous. There is both a significant morbidity, resulting in prolongation of hospital stay, and an attributable mortality associated with their use due to infection.41–43
CENTRAL VENOUS CATHETERS
Colonisation of catheters is common and it is likely that this is a precursor of infection. Colonisation rates are in the order of 5–40%, determined in part by the risk factors involved (see below). Infection rates are approximately 10% of the colonised catheters.44
DEFINITIONS
The actual mechanics by which the organisms colonise the catheter is important. Some organisms, such as CNS, produce a polysaccharide film of ‘slime’ that develops on the catheter while the host’s proteins such as fibronectin may also provide a matrix in which the organism can adhere, and which may provide a protective barrier against both white cells and antibiotics. Polyvinyl chloride or polythene is more prone to this film developing than some other materials such as silicone (Table 62.8).
| Host risk factors |
| Site: subclavian is a lower risk than internal jugular and femoral |
| Catheter material: antibacterial catheters may reduce infection, antiseptic catheters reduce colonisation |
| Number of lumens: multilumen catheters increase the infection risk30 |
| Number of administrations through the lines |
| Dressing type: frequency of changes |
| Skin preparation |
| Experience of technique of personnel |
| Occurrence of bacteraemia |
| Tunnelling: often used for long-term access but the data are contentious31 |
TREATMENT OF CATHETER INFECTION
The most important aspect of treatment is a high index of suspicion that leads to removal of the device if infection is present either locally or systemically.42 Although fever and bacteraemia are likely to resolve rapidly after removal of the line, appropriate antibiotics are indicated. The recommended duration of treatment varies: 5–7 days for CNS, 10–14 days for S. aureus, Gram-negative organisms and fungi, and 4–6 weeks if there is evidence of endocarditis, infected thrombus or osteomyelitis, or clinical line sepsis is still present after 3 days.42 Lines removed should be cultured. If an infection is present it is best to avoid replacing the central venous catheter if possible for a few days. In situations where it is uncertain if the line is implicated in infection, some advocate replacing a new line over a wire. If the removed catheter is subsequently shown to be infected, then it must be removed.
PREVENTION
Important issues include adequate hand-washing; adequate skin disinfection; insertion under aseptic conditions; intravenous team to insert and manage lines; anchoring of lines to prevent excessive movement; closed systems with limited interruptions to the lines; application of sterile dressings to the insertion site; and daily inspection of catheter site. Replacement of intravenous administration sets at 72 hours is optimal. Stopcocks may be essential, but are portals of infection. Local antiseptics are advocated for site care, but there is little difference between gauze and transparent dressings, although efforts to reduce local humidity at the site may be important.45–47
METHODS OF INFECTION CONTROL
Each hospital has an infection control team that can employ techniques to reduce infection (Table 62.9).
| Surveillance and investigation of infection outbreaks |
| Education of staff |
| Review of antibiotic utilisation |
| Review of antibiotic resistance patterns |
| Review of infection control procedures and policies |
The most important aspects of preventing nosocomial infection and facilitating infection control are simple hygiene, such as hand-washing, and being aware that the problem exists. There are several ways in which the issue of nosocomial infection can be addressed. These include surveillance, screening, isolation, eradication and strategic planning.48
SELECTIVE DECONTAMINATION OF THE DIGESTIVE TRACT
SDD is based on the idea that overgrowth of colonising organisms in the gut predisposes to nosocomial infection so that reducing or eliminating the reservoir of organisms from the nasopharynx and from the gastrointestinal tract will prevent infection. To achieve this, oral non-absorbable antibiotics, such as polymyxin, tobramycin, gentamicin, neomycin and nystatin, are applied to the oropharynx and administered through nasogastric tubes. Parenteral antibiotics have been added to some regimens. Although the positive results outweigh the negative ones, the uptake of this technique has been limited.31,49,50 Concerns over generating resistance have not been substantiated nor completely refuted.51–53
A new controversial but interesting application has been in attempting to eliminate multiresistant organisms such as MRSA with an MRSA-targeted regimen, which is claimed to eradicate MRSA.16 It remains a controversial area.
1 Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639-644.
2 Rosenthal VD, Guzman S, Orellano PW. Nosocomial infections in medical–surgical intensive care units in Argentina: attributable mortality and length of stay. Am J Infect Control. 2003;31:291-295.
3 Sax H, Ghugonnet S, Harbarth P, et al. Variation in nosocomial infection prevalence according to patient setting: a hospital wide survey. J Hosp Infect. 2001;48:27-32.
4 Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184-2193.
5 Pittet D. Pneumonie nosocomiale: incidence, morbidité et mortalité chez le patient intube-ventilé. Schweiz Med Wochenschr. 1994;124:227-235.
6 Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591-1598.
7 Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302-2308. quiz 9
8 Ortiz R, Lee K. Nosocomial infections in neurocritical care. Curr Neurol Neurosci Rep. 2006;6:525-530.
9 Edgeworth JD, Treacher DF, Eykyn SJ. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit Care Med. 1999;27:1421-1428.
10 Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307-1312.
11 Hanberger H, Diekema D, Fluit A, et al. Surveillance of antibiotic resistance in European ICUs. J Hosp Infect. 2001;48:161-176.
12 Blot SI, Vandewoude KH, Colardyn FA. Evaluation of outcome in critically ill patients with nosocomial Enterobacter bacteremia: results of a matched cohort study. Chest. 2003;123:1208-1213.
13 Obritsch MD, Fish DN, MacLaren R, et al. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25:1353-1364.
14 Aloush V, Navon-Venezia S, Seigman-Igra Y, et al. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43-48.
15 Cisneros JM, Rodriguez-Bano J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect. 2002;8:687-693.
16 Wenisch C, Laferl H, Szell M, et al. A holistic approach to MRSA eradication in critically ill patients with MRSA pneumonia. Infection. 2006;34:148-154.
17 Weber SG, Gold HS. Enterococcus: an emerging pathogen in hospitals. Semin Respir Crit Care Med. 2003;24:49-60.
18 Gerding DN. Treatment of Clostridium difficile-associated diarrhea and colitis. Curr Top Microbiol Immunol. 2000;250:127-139.
19 Koss K, Clark MA, Sanders DS, et al. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006;8:149-154.
20 Theaker C, Azadian B, Soni N. The impact of Acinetobacter baumannii in the intensive care unit. Anaesthesia. 2003;58:271-274.
21 Guidelines for prevention of nosocomial pneumonia. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997;46:1-79.
22 Mehta RM, Niederman MS. Nosocomial pneumonia. Curr Opin Infect Dis. 2002;15:387-394.
23 Ostendorf U, Ewig S, Torres A. Nosocomial pneumonia. Curr Opin Infect Dis. 2006;19:327-338.
24 Girou E, Brun-Buisson C, Taille S, et al. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290:2985-2991.
25 Keenan SP, Heyland DK, Jacka MJ, et al. Ventilator-associated pneumonia. Prevention, diagnosis, and therapy. Crit Care Clin. 2002;18:107-125.
26 Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-313.
27 Cook D, Heyland D, Griffith L, et al. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med. 1999;27:2812-2817.
28 Depuydt P, Myny D, Blot S. Nosocomial pneumonia: aetiology, diagnosis and treatment. Curr Opin Pulm Med. 2006;12:192-197.
29 Flanders SA, Collard HR, Saint S. Nosocomial pneumonia: state of the science. Am J Infect Control. 2006;34:84-93.
30 Chastre J, Luyt CE, Combes A, et al. Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator-associated pneumonia to decrease resistance in the intensive care unit. Clin Infect Dis. 2006;43(Suppl. 2):S75-S81.
31 Silvestri L, Mannucci F, van Saene HK. Selective decontamination of the digestive tract: a life saver. J Hosp Infect. 2000;45:185-190.
32 Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996;153:1711-1725.
33 Bodmann KF. Current guidelines for the treatment of severe pneumonia and sepsis. Chemotherapy. 2005;51:227-233.
34 Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator-associated pneumonia. Chest. 2006;130:597-604.
35 Costa SF, Newbaer M, Santos CR, et al. Nosocomial pneumonia: importance of recognition of aetiological agents to define an appropriate initial empirical therapy. Int J Antimicrob Agents. 2001;17:147-150.
36 Rello J, Lorente C, Diaz E, et al. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest. 2003;124:2239-2243.
37 Alvarez-Lerma F, Alvarez B, Luque P, et al. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care. 2006;10:R78.
38 Beyersmann J, Gastmeier P, Grundmann H, et al. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol. 2006;27:493-499.
39 Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82:172-178.
40 Holzheimer RG, Haupt W, Thiede A, et al. The challenge of postoperative infections: does the surgeon make a difference? Infect Control Hosp Epidemiol. 1997;18:449-456.
41 O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23:759-769.
42 Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. J Intraven Nurs. 2001;24:180-205.
43 Mermel LA. New technologies to prevent intravascular catheter-related bloodstream infections. Emerg Infect Dis. 2001;7:197-199.
44 Hannan M, Juste RN, Umasanker S, et al. Antiseptic-bonded central venous catheters and bacterial colonisation. Anaesthesia. 1999;54:868-872.
45 Chatzinikolaou I, Raad II. Intravascular catheter-related infections: a preventable challenge in the critically ill. Semin Respir Infect. 2000;15:264-271.
46 Raad I, Hanna HA, Awad A, et al. Optimal frequency of changing intravenous administration sets: is it safe to prolong use beyond 72 hours? Infect Control Hosp Epidemiol. 2001;22:136-139.
47 Rizzo M. Striving to eliminate catheter-related blood stream infection; a literature review of evidence-based strategies. Semin Anesth Periop Med Pain. 2005;24:4.
48 Bearman GM, Munro C, Sessler CN, Wenzel RP. Infection control and the prevention of nosocomial infections in the intensive care unit. Semin Respir Crit Care Med. 2006;27:310-324.
49 Carlet JM. Controversies in the antibiotic management of critically ill patients. Semin Respir Crit Care Med. 2001;22:51-60.
50 Zwaveling JH, Maring JK, Klompmaker IJ, et al. Selective decontamination of the digestive tract to prevent postoperative infection: a randomized placebo-controlled trial in liver transplant patients. Crit Care Med. 2002;30:1204-1209.
51 Bonten MJ, Krueger WA. Selective decontamination of the digestive tract: cumulating evidence, at last? Semin Respir Crit Care Med. 2006;27:18-22.
52 Al Naiemi N, Heddema ER, Bart A, et al. Emergence of multidrug-resistant Gram-negative bacteria during selective decontamination of the digestive tract on an intensive care unit. J Antimicrob Chemother. 2006;58:853-856.
53 de Jonge E. Effects of selective decontamination of digestive tract on mortality and antibiotic resistance in the intensive-care unit. Curr Opin Crit Care. 2005;11:144-149.