Nose and Sinonasal Cavities
Development and Anatomy of the Sinonasal Cavities
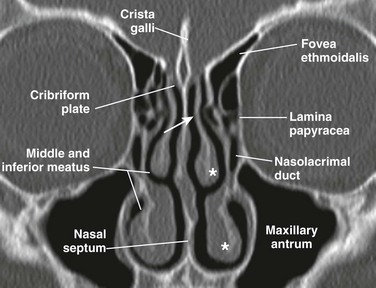
The nasal cavity is triangular and is separated in the midline by the nasal septum. The nasal cavity is composed of a cartilaginous portion anteriorly and an osseous portion posteriorly, which is formed by the perpendicular plate of the ethmoid posterosuperiorly and the vomer posteroinferiorly. The opening to each nasal cavity is known as the vestibule, which is bounded medially by the columella and nasal septum and laterally by the nasal alae. The cribriform plate is the roof of the nasal cavity, and the floor is the hard and soft palate. Posteriorly, the nasal cavity communicates with the nasopharynx via the choanae, after rupture of the oronasal membrane during the fetal period. From the lateral wall of the nasal cavity, three pairs of turbinates (superior, middle, and inferior) project into the nasal cavity, each with a corresponding meatus below them. The middle turbinate is attached to the cribriform plate via the vertical lamella and to the lamina papyracea via the basal lamella (Fig. 8-1).
Paranasal Sinuses
The paranasal sinuses form as diverticula from the walls of the nasal cavities and become air-filled extensions in the adjacent bones—maxilla, ethmoid, frontal, and sphenoid. The original openings of the diverticula persist as the ostia of the sinuses that communicate with the nasal cavity (Fig. 8-2, A and B).
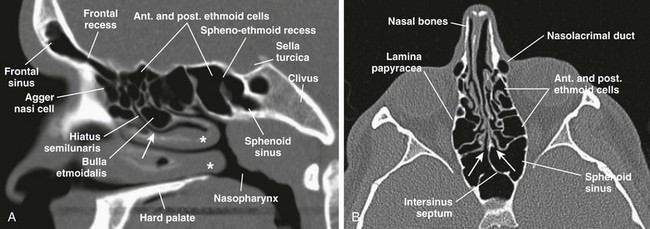
Figure 8-2 Normal sinus openings.
A, A sagittal computed tomography (CT) scan shows frontal recess of the frontal sinus, hiatus semilunaris, middle meatus (arrow), sphenoethmoid recess, and middle and inferior turbinates (asterisks). B, An axial CT view shows sphenoethmoid recesses (arrows).
The mucosal lining of the nasal cavities is contiguous with the paranasal sinuses and consists of pseudostratified, columnar, ciliated epithelium containing mucinous and serous glands,1 whereas the nasal septum is lined by squamous mucosa.2 In the human embryo, ethmoidal and maxillary sinus budding can be detected at 11 to 12 gestational weeks and at 14 to 15 gestational weeks, respectively.3 In general, only rudimentary maxillary and ethmoid sinuses, which continue to expand until puberty or early adulthood, are present at birth.4 The sinuses also are linked to facial growth and dentition and have been studied extensively by different anatomic and imaging methods (Fig. 8-3, A-C).4–11
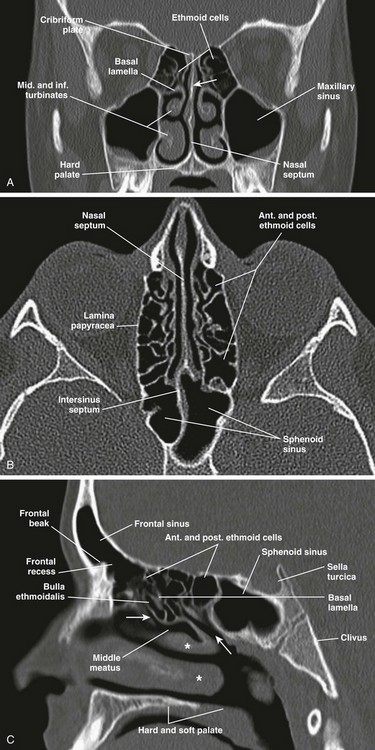
Figure 8-3 Normal anatomy of the sinuses demonstrated by fully developed sinuses in a 13-year-old boy.
A, A coronal computed tomography (CT) scan shows the perpendicular plate of the ethmoid bone (arrow). B, An axial CT image. C, A sagittal CT image shows hiatus semilunaris (arrow at left), drainage passage of posterior ethmoid cells (arrow at right), and middle and inferior turbinates (asterisks).
Ethmoid Sinuses
At birth the ethmoid sinuses are already developed in number and pneumatized, but they continue to expand, reaching adult proportions at about 12 years of age.12 The ethmoid sinuses consist of a paired group of a variable number of cells (3 to 18) within the lateral masses of the ethmoid bone, also known as labyrinths. In addition to the lateral masses, the ethmoid bone consists of the cribriform plate superiorly and the perpendicular plate, which is part of the nasal septum (see Fig. 8-3, A). The ethmoid sinus is bordered medially by the nasal cavity and laterally by the lamina papyracea; its roof is formed by the cribriform plate and fovea ethmoidalis (see Fig. 8-1). The anterior and posterior ethmoid air cells are separated by the basal lamella (see Fig. 8-3, C), which is the lateral attachment of the middle turbinate to the lamina papyracea. Drainage of the anterior ethmoid air cells occurs via the ethmoid bulla into the hiatus semilunaris and middle meatus. The posterior ethmoid air cells drain into the superior meatus and then into the sphenoethmoid recess (see Fig. 8-2, A).
The anterior ethmoidal artery arises from the ophthalmic artery in the orbit, pierces the lamina papyracea, and exits via its respective foramina by passing through the ethmoid roof in the superomedial wall of the orbit, 2 to 3 mm behind the anterior wall of the bulla ethmoidalis. Occasionally the anterior ethmoidal artery passes within bony septae of the ethmoid sinuses and is suspended in a mesentery without bony cover (e-Fig. 8-4).13
Anatomic Variants
The roof of the anterior ethmoid sinus is formed by the cribriform bone medially and the fovea ethmoidalis laterally (see Fig. 8-1). Any asymmetry in the height of the ethmoid roof should be documented by the radiologist because a higher incidence of surgical penetration occurs during endoscopic surgery on the side where the position of the roof is lower.14,15 An increased risk of inadvertent intracranial penetration may occur if the fovea ethmoidalis plane passes through the midorbital plane or below.
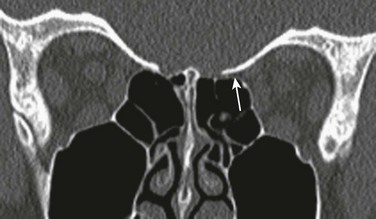
Concha bullosa refers to the pneumatization of the middle turbinate as a result of intramural extension of posterior ethmoid air cells (Fig. 8-5, B). A large concha bullosa eventually can cause obstruction of the airway.
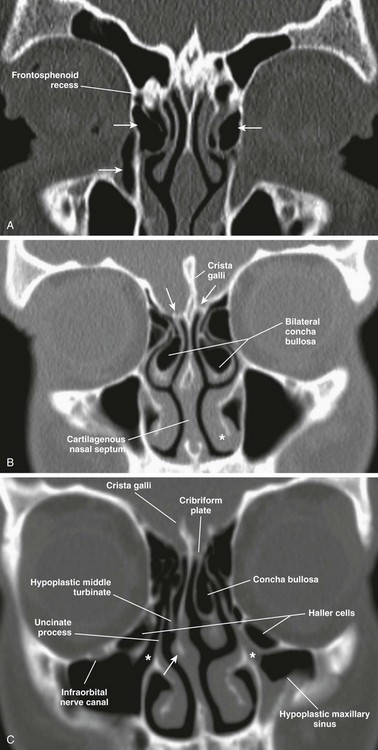
Figure 8-5 Anatomic variants.
A, A coronal computed tomography scan shows bilateral agger nasi cells (top arrows), the nasolacrimal canal (bottom arrow), and the right frontoethmoidal recess. B, Bilateral concha bullosa, asymmetric height of the ethmoid roofs (arrows), and the inferior turbinate (asterisk) are shown. C, A hypoplastic right middle turbinate, rightward nasal septal deviation, Haller cells, and infundibulum (asterisks) are shown.
Extramural expansion of ethmoid air cells can result in anatomic variants, including the following: Agger nasi cells (Fig. 8-5, A), which are the most anterior and inferior cells involving the lacrimal bone or maxilla; Haller cells, which are middle ethmoid air cells extending into the inferomedial floor of the orbit (Fig. 8-5, C); and Onodi cells, which are posterior sphenoethmoid air cells with prominent superolateral pneumatization in close relationship to the optic nerve canal.13
Maxillary Sinuses
The maxillary sinuses are very small and pneumatized at birth, measuring an ellipsoid volume (sinus volume index) of approximately 0.24 ± 0.36 cm3.4 During the first years of life, the maxillary sinuses undergo rapid inferolateral expansion, reaching full size between 15 to 18 years of age. The floor of the maxillary sinus usually is seen at the level of the middle meatus during infancy; it reaches the level of the nasal floor by 8 to 9 years of age, and by age 12 years, it is located at the level of the hard palate.6 However, variation exists in the final descent of the floor, which lies below the level of the nasal floor in 65% of adults (e-Fig. 8-6, A–D).16
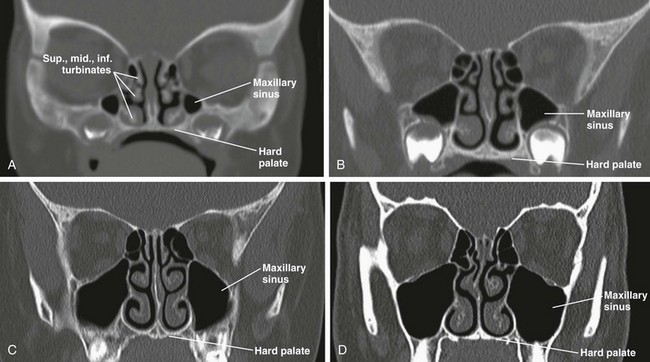
e-Figure 8-6 Maxillary sinus development.
Computed tomography images show maxillary sinuses at 1 month (A), 3 years (B), 7 years (C), and 15 years (D).
The maxillary sinus is the largest of the sinuses. Its roof is formed by the orbital floor, which carries the bony canal for the infraorbital nerve; the medial wall is formed by the lateral nasal wall. The posterior wall of the maxillary sinus forms the pterygopalatine fossa (Fig. 8-7, A). The maxillary sinus drains via the maxillary ostium located superomedially into the infundibulum, the hiatus semilunaris, and the middle meatus (Fig. 8-2, A).
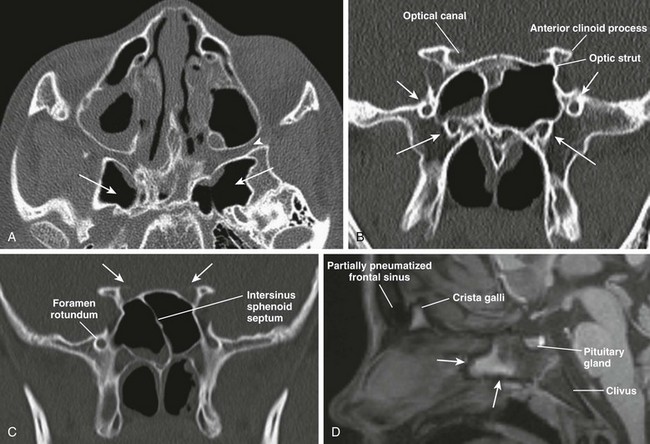
Figure 8-7 Sphenoid sinus anatomy and variants.
A, An axial computed tomography (CT) image shows hyperpneumatization of the sphenoid sinus involving the pterygoid plates bilaterally (arrows); the pterygopalatine fossa (arrowhead) also is shown. B, A CT image shows foramen rotundum (short arrows) and the vidian canal (long arrows) bilaterally. C, A coronal CT image shows insertion of the intersinus septum into the right optic canal (arrows). D, A sagittal T1-weighted magnetic resonance image demonstrates a nonpneumatized sphenoid sinus in an 11-year-old boy with fatty transformation of its anteroinferior portion (arrows).
Sphenoid Sinus
The sphenoid sinus begins to pneumatize in a ventrodorsal direction from 7 months to 2 years of age. A significant acceleration in sinus expansion occurs between 3 and 8 years of age, with complete pneumatization usually present by age 10 years (e-Fig. 8-8, A–E).9 Its border is formed superiorly by the sella turcica, posteriorly by the clivus, anteriorly by the ethmoid sinus, and inferiorly by the nasopharynx (see Fig. 8-2, A and B). This sinus drains anteriorly via the sphenoethmoid recess. Lateral recesses of the sphenoid sinus are formed from pneumatization of the pterygoid process in 44% of people (see Fig. 8-7, A). Benign sphenoid marrow variants, which sometimes are mistaken for lesions, can be seen adjacent to the pneumatized sphenoid sinus (e-Fig. 8-9, A-C).
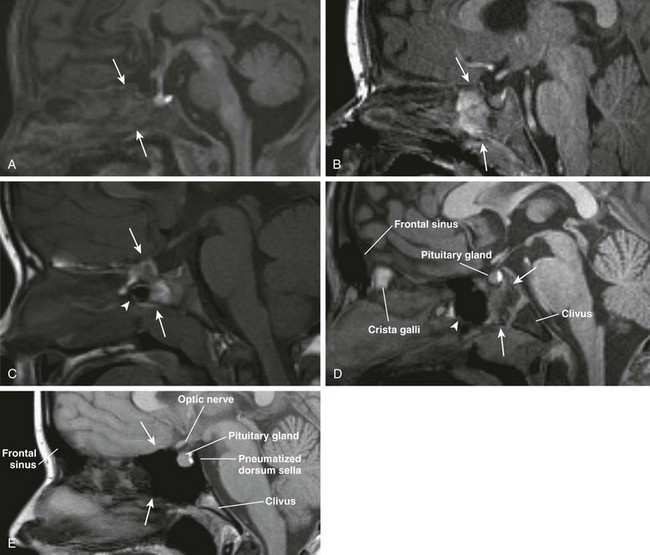
e-Figure 8-8 Sphenoid sinus development is shown in magnetic resonance sagittal T1-weighted images.
A, Hematopoietic marrow (T1 hypointense) of the sphenoid bone in a newborn (thin arrows). B, Fatty transformation of the anteroinferior aspect of the sphenoid bone in a 12-month-old (arrows). C, Pneumatization of the anteroinferior aspect of the sphenoid sinus in a 3-year-old (arrowhead) with fatty sphenoid marrow (arrows). D, Further pneumatization of the sphenoid sinus (arrowhead) and fatty transformation of the sphenoid bone (arrows). E, Fully pneumatized sphenoid sinus (arrows), including the dorsum sella in a 14-year-old boy.
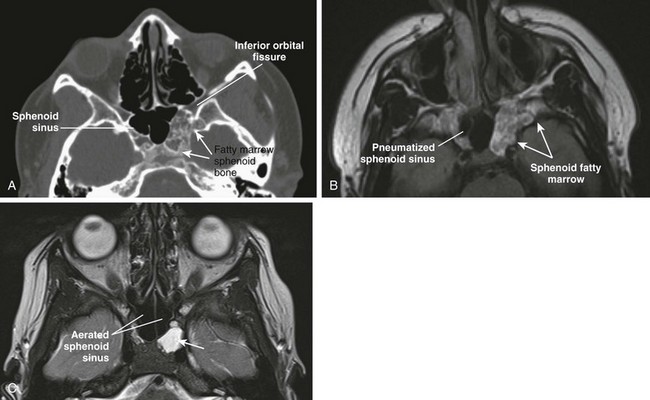
e-Figure 8-9 Benign sphenoid marrow variants.
A, An axial computed tomography (CT) image shows asymmetric fatty marrow of nonpneumatized sphenoid bone on the left side. B, An axial T1-weighted image shows correlation of T1-hyperintense normal marrow of the sphenoid bone, which can simulate mass (neoplasm/erosion). C, An axial CT image in another patient shows retained fluid in the left lateral recess of the sphenoid sinus.
Important anatomic relationships of the sphenoid sinus include the optic canal and nerve located superolaterally; the foramen rotundum with the maxillary nerve that courses along the inferolateral margin of the sphenoid sinus; the vidian canal, which usually runs along the floor of the sphenoid sinus; and the cavernous portion of the internal carotid artery, which protrudes laterally into the sinus (see Fig. 8-7, B). The sphenoid sinus septum usually is aligned anteriorly with the nasal septum, but the sinus septum can deviate posteriorly, forming unequal sphenoid cavities (see Fig. 8-7, C). Absent pneumatization in a child older than 9 years is usually abnormal and warrants clinical investigation (see Fig. 8-7, D).
Frontal Sinuses
The frontal sinuses are absent at birth and are the last to develop once bone marrow conversion has occurred. The frontal sinus is considered an extension of the anterior ethmoid air cells and pneumatizes between 2 to 8 years, with the most significant growth taking place between ages 1 to 5 years. The frontal sinuses can continue to expand up until the second decade of life.6,11 The frontal sinuses consist of paired, often asymmetric cells. The anterior wall corresponds to the outer cortical table of the frontal bone, and the posterior wall of the sinus separates it from the anterior cranial fossa. Drainage of this sinus occurs via the frontal recess, which is an hourglass-like narrowing between the frontal sinus and the anterior middle meatus (see Fig. 8-2, A). Hypoplasia and aplasia of the frontal sinus can be seen in 4% and 5% of the population, respectively.
Imaging of the Paranasal Sinuses
Although plain radiography is less costly and more widely available than computed tomography (CT), it has significant limitations in evaluating the paranasal sinuses. Specifically, plain radiography often overestimates or underestimates findings, it does not localize pathology well, and it does not provide important anatomic detail.17 Traditionally, the paranasal sinus series has consisted of four views (Caldwell, Waters, posteroanterior, and lateral) that are technically difficult to perform in children. The Waters view is obtained by angulating the beam in 5-degree increments per year (up to age 6 years) to compensate for the progressive enlargement of the maxillary antra throughout childhood (Fig. 8-10, A and B). Although this approach improves visualization of the maxillary sinuses by projecting them over the petrous pyramids, it also creates double contours that can simulate mucosal thickening. Radiographic density also is critical because overpenetration of the films can completely obscure density differences created by disease, whereas underpenetration can simulate disease. Air fluid levels also can be obscured as a result of beam angulation. Although some have suggested using only the Waters view radiograph, this modality has been shown to have 32% false-negative results and 49.2% false-positive results when compared with CT. Moreover, most of the abnormalities in the ethmoid and sphenoid sinus are not detected in the Waters radiograph.18 Ethmoid disease on the Caldwell projection can be overdiagnosed or underdiagnosed because of technical and anatomic factors. The lateral view for evaluating the sphenoid sinus is of very little value in children younger than 4 years.17,19
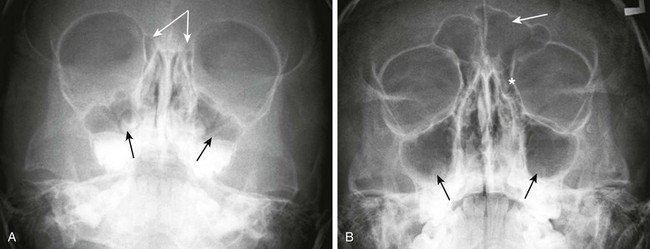
Figure 8-10 Plain radiograph—Waters view.
A, Maxillary sinuses (single arrows) and ethmoid air cells (attached arrows) in a 3-year-old child. B, Maxillary sinuses (bottom arrows), frontal sinus (top arrow), and ethmoid air cells (asterisk) in an 11-year-old child.
The American College of Radiology does not recommend the use of plain radiography in diagnosing sinusitis in children. Sinusitis is considered a clinical diagnosis that should not be made on the basis of imaging findings alone.20 Similarly, American Academy of Pediatrics (AAP) guidelines state that plain radiographs are unnecessary for diagnosing sinonasal disease in children younger than 6 years.21 Further, plain films do not play a role in evaluating sinonasal masses or complications of sinusitis.
Computed Tomography
In recent years, there has been heightened awareness of and concern about the potentially harmful side effects associated with radiation exposure, particularly in the pediatric population.22,23 For this reason, the practice of the “as low as is reasonably achievable” principle among the radiologic community is critical, with special attention given to CT protocols and parameters.22,24 In evaluating paranasal sinuses, it is possible to reduce radiation techniques for maxillofacial CT imaging, even to a level comparable to that used for standard radiographic images, without sacrificing diagnostic image quality.25
Magnetic Resonance Imaging
The normal nasal cycle of vasodilation and mucosal edema followed by vasoconstriction and mucosal shrinkage can produce signal changes that can create significant variability in findings and thus differing interpretations. This cycle varies from 50 minutes to 6 hours, and the signal intensity during the edematous phase is indistinguishable from that of inflammatory change. As with CT and plain radiographs, sinus MRI typically shows a high incidence of findings in asymptomatic persons (13% to 37%) and mucosal thickening of <3 mm that is likely insignificant.2 MRI can differentiate mucosal thickening from sinus secretions (Fig. 8-11, A–E).
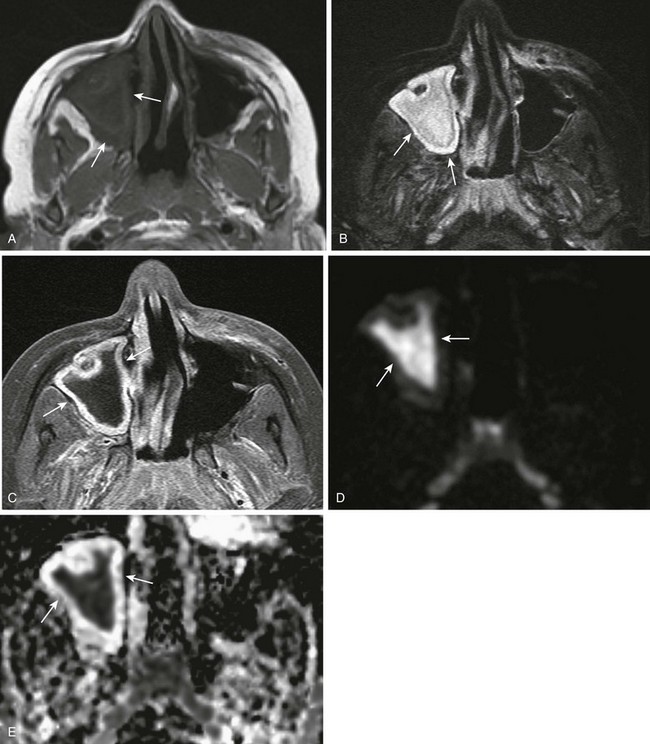
Figure 8-11 Acute bacterial sinusitis in a 10-year-old boy.
A, An axial T1-weighted magnetic resonance (MR) image shows hypointense material in the right maxillary sinus (arrows). B, An axial T2-weighted MR image shows corresponding T2-hyperintense material (arrows). C, An axial T1-weighted MR image with fat saturation and contrast shows circumferential mucosal enhancement. D and E, Corresponding decreased diffusivity in the right maxillary sinus is shown on diffusion-weighted imaging and apparent diffusion coefficient maps consistent with purulent material.
Imaging Anatomy of the Sinonasal Cavities
Regarded as an important anatomic region for potential surgical intervention, the ostiomeatal unit is a complex anatomic structure at the crossroads of mucociliary drainage from the frontal, anterior ethmoid, and maxillary sinuses.26 It includes the uncinate process, infundibulum, ethmoid bulla, hiatus semilunaris, and middle meatus (Fig. 8-12, A and B).13
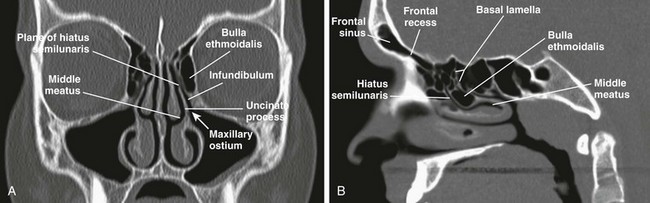
Figure 8-12 Ostiomeatal unit (OMU).
A, A coronal computed tomography (CT) image shows the OMU components: middle meatus, uncinate process, infundibulum, bulla ethmoidalis, maxillary ostium, and plane of the hiatus semilunaris. B, A sagittal CT image demonstrates the hiatus semilunaris.
The uncinate process arises from the upper medial maxillary wall and defines the wall of the infundibulum.27 The infundibulum is the channel defined laterally by orbit or Haller cells and medially by the uncinate process. The ethmoid bulla is a dominant middle ethmoid air cell that protrudes inferomedially into the infundibulum and hiatus semilunaris. The hiatus semilunaris is a semilunar region between the tip of the uncinate process and ethmoid bulla (see Fig. 8-12, A and B).
Congenital Lesions of the Nose
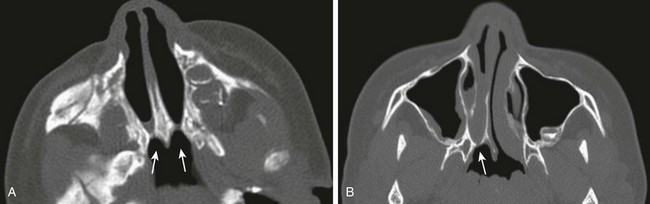
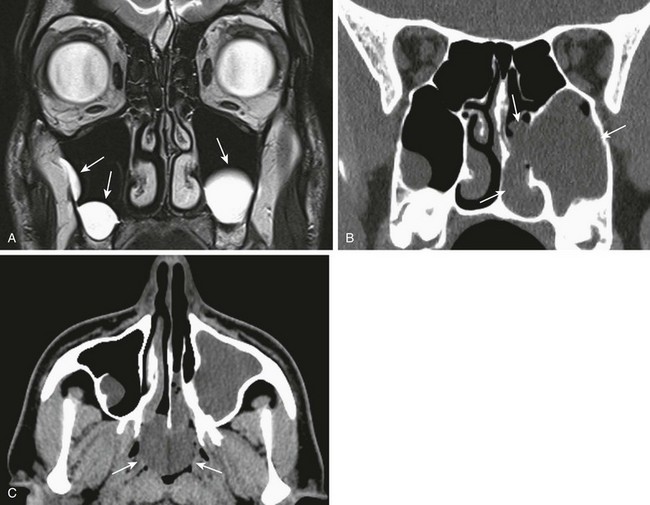
Choanal atresia, the most common congenital abnormality of the nasal cavity, is thought to result from failure of rupture of the oronasal membrane during the sixth week of fetal life. It consists of obstruction of the posterior opening of the nasal cavity, which is mixed bony-membranous in approximately 70% of cases and pure bony atresia in 30% of cases. The existence of a purely membranous atresia has been questioned, and evaluation with high-resolution CT often has failed to demonstrate a membranous atresia without a bony component.28 Choanal atresia can be unilateral (in 50% to 60% of cases) or bilateral, and it is more common in girls (the female : male ratio is 2 : 1).
Clinical Presentation: Bilateral choanal atresia presents with severe immediate onset of respiratory distress in the newborn, because infants are obligate nasal breathers. Symptoms are aggravated by feeding and relieved by crying. The inability to pass a nasogastric tube in a neonate with well-aerated lungs suggests the diagnosis. Unilateral choanal atresia usually is diagnosed later in childhood and presents with unilateral purulent rhinorrhea.
Imaging: Noncontrast CT is the imaging modality of choice. Nasal secretions should be suctioned before imaging is performed; administration of a nasal topical vasoconstrictor spray helps in reducing mucosal thickening. High-resolution imaging in bone algorithm will aid in delineating the bone margins. Findings consist of narrowing of the posterior choana (<0.34 cm in children <2 years of age) and enlargement and thickening of the vomer (>0.23 cm), which sometimes is fused to the maxilla.29 Medial bowing and thickening of the posterior aspect of the lateral wall of the nasal cavity also is seen. The nasal cavity often is filled with air, soft tissue, and fluid (Fig. 8-13, A and B). CT will identify other causes of bilateral nasal obstruction such as pyriform aperture stenosis and bilateral nasolacrimal duct cysts. Bilateral choanal atresia can be associated with other congenital abnormalities in 75% of cases, such as in persons with CHARGE syndrome.
Congenital Nasal Pyriform Aperture Stenosis
Congenital nasal pyriform aperture stenosis is a rare form of nasal obstruction that consists of narrowing of the nasal pyriform aperture as a result of bony overgrowth of the nasal process of the maxilla.30 It should be suspected in any neonate with upper respiratory obstruction and needs to be differentiated from choanal atresia and other congenital nasal masses.
Clinical Presentation: Symptoms depend on the severity of the stenosis and are similar to those in patients with choanal atresia, including cyanosis in the neonatal period that is exacerbated with feeding and relieved by crying. Congenital nasal pyriform aperture stenosis can present in association with other congenital anomalies such as holoprosencephaly, hypopituitarism, a single mega-incisor, hypotelorism, cleft palate, clinodactyly, or absent olfactory bulbs.31,32 A central mega-incisor often is associated with intracranial abnormalities, and thus further evaluation with MRI of the brain is indicated in these cases.
Imaging: Congenital nasal pyriform aperture stenosis is confirmed with CT and is characterized by overgrowth of the medial nasal process of the maxilla, resulting in narrowing of the anterior bony nasal cavity, and is usually bilateral. A pyriform aperture of less than 11 mm in a term infant is diagnostic.30 The choanae are usually normal in caliber, but choanal atresia may coexist.31 The hard palate is usually hypoplastic and in the shape of a triangle with the presence of a median maxillary single incisor tooth (mega-incisor) (Fig. 8-14, A–C).
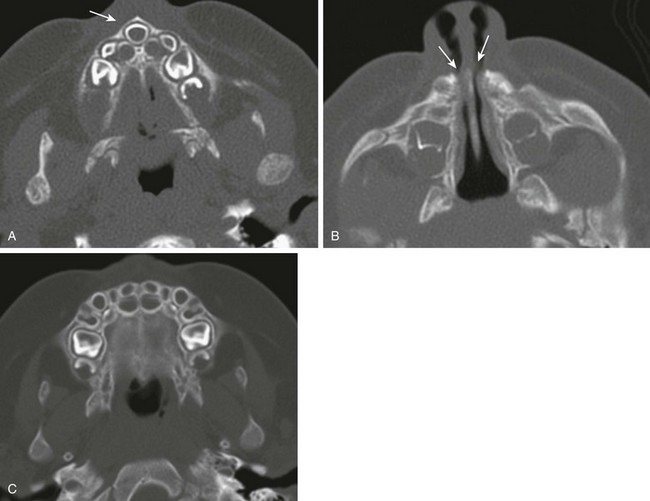
Figure 8-14 Congenital nasal pyriform aperture stenosis in a  -month-old infant with episodes of respiratory distress during breastfeeding.
-month-old infant with episodes of respiratory distress during breastfeeding.
A, An axial computed tomography (CT) image shows a triangular hard palate and solitary central maxillary mega-incisor (arrow). B, An axial CT image shows narrowing of the anterior and inferior nasal passages. C, A normal infant maxilla for comparison.
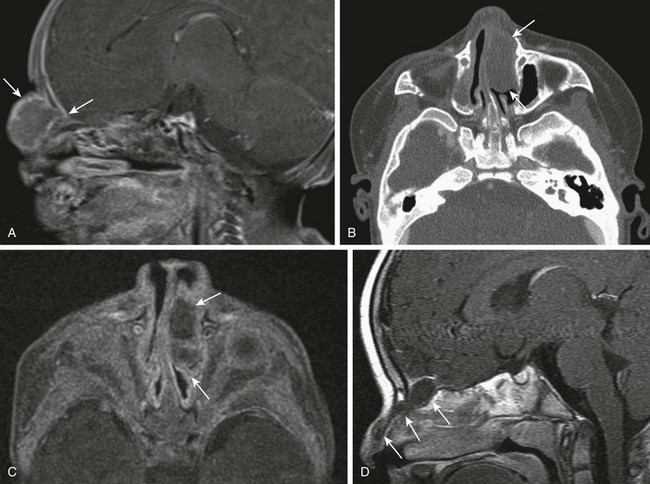
Congenital Nasolacrimal Duct Dacrocystoceles
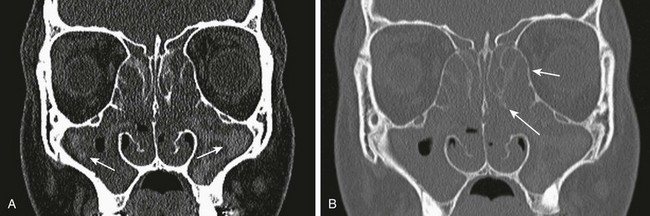
Clinical Presentation: A proximal lesion, a dacryocystocele, presents clinically as a round, bluish, medial, canthal mass usually identified at birth; it may vary in size from 5 to 10 mm. A distal lesion, a nasolacrimal duct cyst, usually presents in the neonatal period as an intranasal mass causing airway obstruction if it is bilateral. Neonates also can present with difficulty breathing while feeding.
Imaging: CT findings include a well-circumscribed, rounded, unilateral or bilateral hypodense “cystic” lesion in the medial canthus and/or anteroinferior nasal cavity. The cyst can communicate with an enlarged nasolacrimal duct (Fig. 8-15, A). Large cysts may cause nasal septal deviation. Association with choanal atresia has been described. Contrast-enhanced CT will show no enhancement or minimal wall enhancement. Infected dacryocystoceles present with thick rim enhancement.
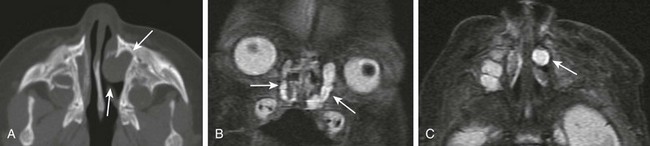
Figure 8-15 Congenital nasolacrimal duct dacryocystoceles in a 1-day-old neonate.
A, An axial computed tomography image shows a left nasal round soft tissue mass with enlargement of the ipsilateral nasolacrimal duct and canal (arrows). B and C, Coronal and axial magnetic resonance fast spin-echo inversion recovery images show bilateral cystic enlargement of the nasolacrimal sacs and ducts (arrows).
MR findings include a T1-weighted hypointense and T2-weighted hyperintense well-circumscribed mass at the medial canthus and/or nasal cavity with no enhancement or minimal wall enhancement (Fig. 8-15, B and C). Dacryocystoceles can be diagnosed prenatally with fetal ultrasound or MRI.33
Other Nasal Masses
Naso-orbital cephaloceles present as frontal, nasal, or medial orbital nonenhancing soft tissue masses contiguous with the intracranial brain, which extends through a bony defect. The adjacent meninges may enhance (e-Fig. 8-16, A).
A hemangioma of infancy is a well-defined, lobular mass that enhances diffusely and intensely.
A nasal glioma (nasal glial heterotopia) is a nonenhancing extranasal (along the nasal dorsum) or intranasal solid soft tissue mass without connection between the mass and intracranial contents (e-Fig. 8-16, B and C).
A nasal dermal sinus with or without a cyst presents as an enhancing tract from the tip of the nose to the foramen cecum (intracranially) just anterior to the crista galli with or without a dermoid/epidermoid cyst along its tract (e-Fig. 8-16, D). On diffusion-weighted imaging, the cyst will demonstrate high signal restricted diffusion.
Imaging of Specific Sinonasal Disease Entities
Rhinosinusitis is characterized by inflammation of the sinonasal mucosa, usually after an upper respiratory infection (URI). Children contract an average of three to eight viral URIs per year that are generally self-limited, with no antimicrobial therapy required.34 However, acute bacterial sinusitis (ABS) can complicate viral rhinosinusitis in up to 13% of affected children.21 Other predisposing conditions for sinusitis are allergic rhinitis, immunoglobulin deficiency, immotile cilia syndrome, cystic fibrosis (CF), and anatomic abnormalities causing obstruction of the drainage pathways. The AAP has defined five categories of bacterial rhinosinusitis: acute, subacute, recurrent acute, ABS superimposed on chronic disease, and chronic sinusitis.21
Bacterial sinusitis remains a challenging clinical diagnosis because the signs and symptoms of sinusitis typically seen in adults are noted less frequently in children. Because specific clinical signs in children are generally few, it often is difficult to differentiate ABS from viral rhinosinusitis. Signs and symptoms in children vary and may include cough, purulent nasal discharge, facial pain, fever, headache, and malodorous breath. A frequent cough and the urge to clear the throat usually is due to posterior nasopharyngeal drainage. Nasal congestion is frequent and leads particularly to nocturnal mouth breathing, which results in a sore throat upon awakening in the morning. Constitutional complaints are frequent, including malaise, poor appetite, easy fatigability, and irritability.34
The gold standard for diagnosis of acute or chronic sinusitis remains aspiration from the sinus cavities with the recovery of bacteria in a high density (>10 colony-forming units/mL).21 However, this method is impractical, invasive, and time-consuming and is rarely performed. For this reason, the diagnosis of sinusitis usually is based on clinical criteria; it is the duration rather than the severity of symptoms that raises suspicion for ABS. This diagnosis is considered when a URI with purulent discharge lasts for >10 days.
Acute Sinusitis
Imaging: Imaging of the paranasal sinuses in children with uncomplicated ABS is unnecessary and generally is not recommended because the results will not affect treatment decisions. Imaging cannot differentiate between viral and bacterial sinusitis. Mucosal abnormalities are reported routinely on radiographs, CT, and MRI in asymptomatic patients who undergo imaging for other indications. The incidence of abnormal findings is very high in infants and young children. For example, in a study of infants who had a cold in the 2 weeks preceding a head CT performed for reasons other than sinusitis, mucosal abnormalities were detected in the sinuses of 97% of the babies examined. Soft tissue changes can persist for months on MRI after an acute infection.20 For these reasons, clinical correlation is vital when evaluating imaging findings in children.
Chronic Sinusitis
The AAP defines chronic sinusitis as episodes of inflammation of the paranasal sinuses lasting >90 days. Patients have persistent residual respiratory symptoms such as cough, rhinorrhea, and/or nasal obstruction. Children who experience chronic sinusitis usually are older (at least 4 to 7 years of age) and often have a history of recurrent ABS. Other associated factors are recurrent viral URIs, allergic and nonallergic rhinitis, CF, immunodeficiency, ciliary dyskinesia, sinus and facial anatomic abnormalities, and gastroesophageal reflux.35
Clinical findings are similar to those of acute infection but are characterized mostly by a continuous nasal discharge and congestion with a persistent nighttime cough. Other symptoms include chronic headache, chronic halitosis, intermittent fever, sleep deprivation, and general malaise. In persons with chronic sinusitis, Staphylococcus aureus, coagulation negative Staphylococcus, anaerobic organisms, gram-negative bacteria, and fungi are the most commonly encountered colonizing organisms.35
Imaging: Radiography can show mucosal thickening or sinus opacification and, occasionally, sclerotic, thickened walls. CT findings include peripheral mucosal thickening in a hypodistended or contracted sinus with sclerotic thickened walls, although these findings are uncommon in children. Calcifications may be seen occasionally, and sinonasal polyposis also may be present. Secretions can be dense depending on their content. MR findings include mucosal thickening that enhances with administration of contrast material. Retained secretions will vary in signal intensity depending on content (see Fig. 8-11, A-E). Desiccated secretions are hypointense on T2-weighted imaging.
Treatment: Initial trial therapy includes environmental control, allergic therapy if indicated, antibiotics for 21 days directed to resistant organisms, saline solution irrigation, and topical nasal corticosteroids. Second-line therapy includes oral decongestants and oral corticosteroids. Endoscopic sinus surgery remains the most definitive therapy.35
Complications of Rhinosinusitis
Orbital and intracranial complications of rhinosinusitis can result from delayed diagnosis and treatment, antibiotic-resistant or aggressive organisms, or incomplete treatment. Signs and symptoms of orbital complications include periorbital edema, proptosis, chemosis, decreased visual acuity, and/or limited ocular motility. Potential intracranial complications should be suspected when a patient has a history of acute sinusitis combined with severe intractable headache, an altered level of consciousness, focal neurologic deficits, or signs of meningitis.36
Orbital complications usually result from the spread of ethmoid infection that occurs via valveless ethmoid veins that traverse the lamina papyracea. Extension of disease also can arise from the sphenoid, frontal, and maxillary sinuses in order of frequency. Complications include orbital cellulitis with inflammatory edema, preseptal cellulitis, postseptal cellulitis, subperiosteal abscess (Fig. 8-17) (see Chapter 6), orbital abscess (e-Fig. 8-18), optic neuritis, and cavernous sinus thrombosis.
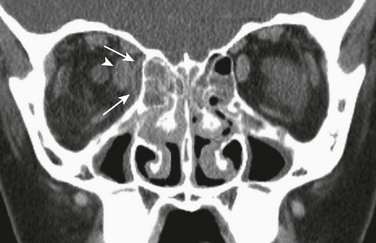
Figure 8-17 An orbital subperiosteal abscess in a 9-year-old girl.
A coronal contrast-enhanced computed tomography image shows a small, low-attenuation, rim-enhancing fluid collection consistent with an abscess (arrows) in the medial aspect of the orbit with mass effect on the medial rectus muscle (arrowhead), which is thickened. Bilateral ethmoid sinus disease with infiltration of the right retro-orbital fat is demonstrated.
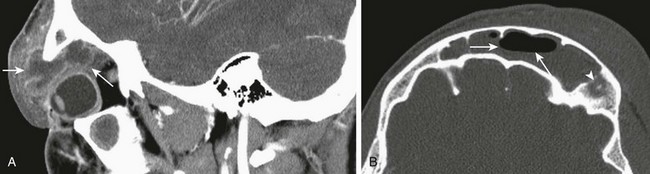
e-Figure 8-18 Intraorbital abscess and frontal bone osteomyelitis in a 14-year-old boy.
A, Contrast-enhanced sagittal computed tomography (CT) shows a large, multiloculated, intraorbital abscess with significant effect of the mass on the globe (arrows). B, An axial CT image shows an osteolytic lesion in the frontal bone consistent with osteomyelitis (arrowhead) and an air-fluid level in the frontal sinus (small arrows).
Intracranial complications include epidural empyema, subdural empyema, meningitis, cerebritis, and parenchymal abscess (e-Fig. 8-19, A-G). Subgaleal involvement such as with Pott puffy tumor and osteomyelitis also can occur (Fig. 8-20, A and B). Vascular complications are infrequent and include mycotic aneurysm, stenosis of the cavernous segment of the internal carotid artery (which, in very rare instances, can result in brain infarction37), and venous sinus thrombosis. Superior sagittal thrombosis usually results from frontal sinusitis, whereas sigmoid sinus thrombosis and cavernous sinus thrombosis are associated more often with sphenoid sinusitis (Fig. 8-21, C).
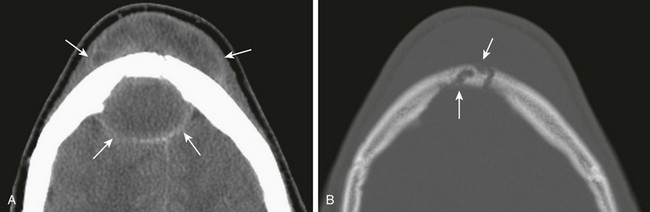
Figure 8-20 Pott puffy tumor and epidural abscess in a 16-year-old boy.
A, A contrast-enhanced computed tomography image in a soft tissue window shows a large subperiosteal abscess (top arrows) and a large epidural abscess (bottom arrows). B, An axial image in a bone window shows erosion of the frontal bone consistent with osteomyelitis (arrows).
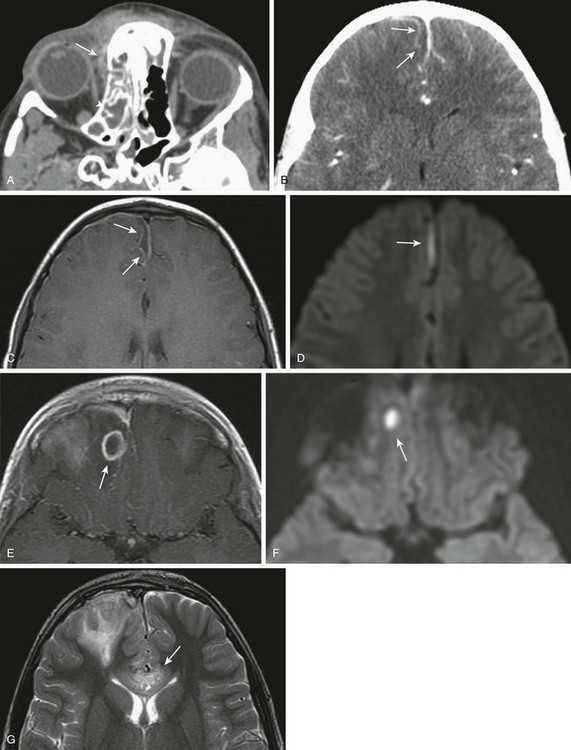
e-Figure 8-19 Acute bacterial sinusitis with intracranial complications in an 8-year-old girl.
A, Axial contrast-enhanced computed tomography (CT) shows a small right orbital subperiosteal abscess (long arrow) and right ethmoid and sphenoid sinusitis (arrowhead). B, An axial contrast-enhanced CT image shows a small subdural empyema along the interhemispheric fissure. C, An axial T1-weighted image with contrast confirms small parasagittal subdural empyema with corresponding decreased diffusivity (D). E, The same patient 4 days later with a ring-enhancing lesion consistent with a right frontal lobe abscess with corresponding decreased diffusivity (F). G, Further progression of cerebritis with involvement of the corpus callosum (arrow).
Imaging: Imaging enables diagnosis and accurate presurgical planning when necessary. For the initial evaluation, contrast-enhanced CT is the imaging modality of choice and should include both the paranasal sinuses and brain. Noncontrast CT should not be performed because it increases the radiation dose without providing any additional diagnostic information. CT can demonstrate fluid collections with rim enhancement consistent with subperiosteal abscesses (see Fig. 8-17) and bone erosion of osteomyelitis as seen with Pott puffy tumor (extracranial subperiosteal abscess from frontal sinusitis) (Fig. 8-20, A and B).38,39
MRI is indicated to further assess known or suspected intracranial extension of disease and should include imaging of the brain, sinuses, and orbits. MRI protocols for the brain should include diffusion and contrast-enhanced sequences and should evaluate for venous thromboses. MRI protocols for the paranasal sinuses and orbits should include high-resolution axial and coronal images before and after administration of gadolinium. MRI is more sensitive than CT in the assessment of leptomeningeal enhancement, small extraaxial fluid collections, and parenchymal abnormalities such as cerebritis and cerebral abscess (see e-Fig. 8-19, E-G). MRI also can detect early stages of osteomyelitis by demonstrating bone marrow edema with associated contrast enhancement. Diffusion-weighted imaging will show restricted diffusion of fluid collections with purulent contents (see Fig. 8-11, A-E).
Subperiosteal abscesses will appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging with peripheral contrast enhancement. Signs of cavernous sinus thrombosis include enlargement or heterogeneous enhancement of the cavernous sinus with associated enlargement or thrombosis of the superior ophthalmic vein (Fig. 8-21, A-E). Subdural and epidural empyemas appear as T1-hypointense and T2-hyperintense fluid collections with adjacent dural enhancement and central decreased diffusivity as a result of the presence of purulent material (see e-Fig. 8-19, B-D). Cerebritis appears as a poorly defined area of T2 prolongation without ring enhancement, whereas a cerebral abscess will present as a ring-enhancing lesion with central decreased diffusivity and perilesional edema (see e-Fig. 8-19, E and F).
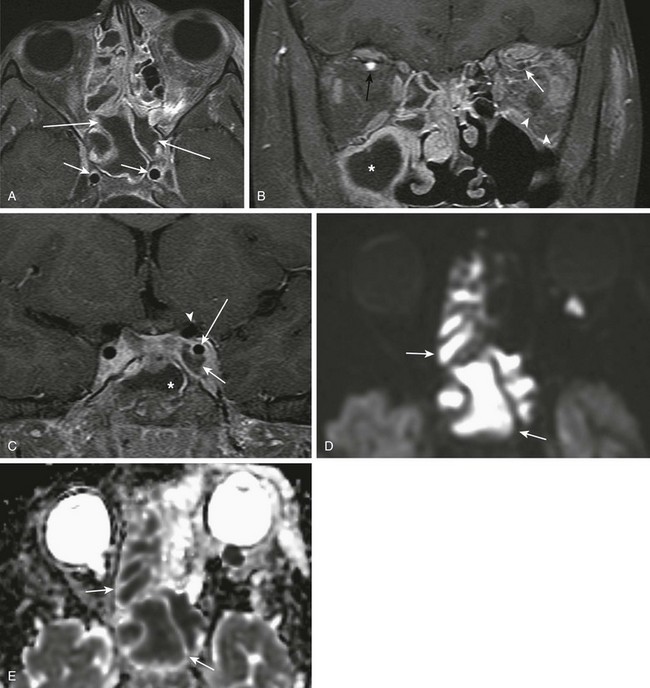
Figure 8-21 Sinusitis with a left periorbital abscess and cavernous sinus thrombosis in a 10-year-old boy.
A, A postcontrast axial T1-weighted magnetic resonance image with fat saturation shows hypointense fluid within the ethmoid and sphenoid sinuses with circumferential mucosal enhancement (long arrows) and normal flow voids of the cavernous internal carotid artery (ICA) segments (short arrows). B, A coronal T1-weighted image with fat saturation shows a thrombus in the left superior ophthalmic vein (white arrow), normal enhancement of the right superior ophthalmic vein (black arrow), and inflammatory changes in the retro-orbital fat (arrowheads). C, A coronal T1-weighted image with fat saturation shows a thrombus in the left cavernous sinus (bottom arrow) and normal flow void of the left ICA cavernous segment (long arrow) and left ICA supraclinoid segment (arrowhead). A fluid-filled sphenoid sinus is shown (asterisk). Corresponding restricted diffusion on diffusion-weighted imaging (D) and apparent diffusion coefficient maps (E) consistent with purulent material.
Treatment: Treatment includes combinations of systemic intravenous antibiotics and surgical interventions such as drainage of the sinuses, functional endoscopic sinus surgery, and craniotomy. Because of the high incidence of seizures associated with intracranial abscess, anticonvulsant therapy is recommended as prophylaxis in patients with intracranial complications.36
Sinonasal Disease in Persons with Cystic Fibrosis
CF is caused by a genetic defect in the long arm of chromosome 7, which affects the protein that regulates transmembrane passage of chlorine ion and causes dysfunction of multiple organs and systems.40 High concentrations of chlorine, mucus thickness, and reduction of mucociliary clearance predisposes to inflammation and chronic infection of the respiratory tract. Nearly 100% of persons with CF present clinically with nasal obstruction and chronic rhinosinusitis (Fig. 8-22, A and B).41 The most common organisms colonizing the sinonasal mucosa in order of frequency are Pseudomonas aeruginosa, S. aureus, and Streptococcus viridans.42
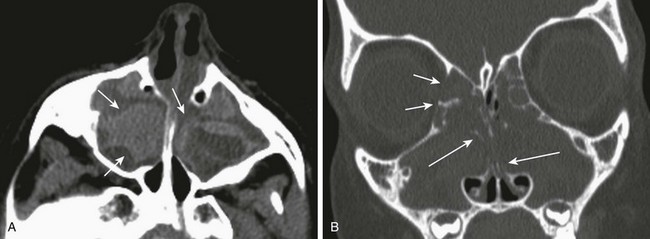
Figure 8-22 Cystic fibrosis in an 8-year-old child.
A, High attenuation secretions in the maxillary sinuses shown on axial computed tomography (CT) (arrows). B, Polyps causing expansion of the sinuses (short arrows) and nasal cavity with demineralization of septations shown on a coronal CT image (long arrows).
Imaging: CT is the imaging modality of choice for evaluating sinus disease in patients with CF and will show abnormalities in nearly all of these persons. Findings include mucosal thickening and sinonasal polyposis. The incidence of nasal polyposis in children with CF varies between 10% and 86%.43–45 High-attenuation material often is present in the sinus cavities, consistent with inspissated secretions (see Fig. 8-22, A). However, superimposed fungal infection has a similar appearance and cannot be excluded on the basis of imaging. Bony sclerosis and thickening of the sinuses can be seen in older patients. Frontal sinus hypoplasia or aplasia is a common finding and may be a result of poor aeration from chronic obstruction. The severity of the imaging findings does not necessarily correlate with the severity of symptoms.41
Treatment: Medical and surgical treatment depends on symptoms and the overall condition of the patient, including pulmonary status and infection frequency. Not all patients will require surgery.46 Imaging findings of CF cannot be differentiated from other causes of chronic sinusitis such as allergic sinusitis with polyposis or fungal allergic sinusitis.
Allergic Sinusitis
It is estimated that allergic rhinosinusitis affects about 10% of the population. In the United States, the most common form is seasonal pollinosis, resulting from an immunoglobulin E reagin–antibody reaction (type I immunologic disorder), which manifests clinically with sneezing, nasal obstruction, and rhinorrhea. This condition can result in hypertrophic, thickened, and redundant mucosa, known as hypertrophic polypoid mucosa, which is prone to bacterial infection. Allergic sinusitis often coexists with chronic asthma, and 30% of patients will present with polyps (e-Fig. 8-23, A and B). Conversely, up to 80% of patients with polyps have asthma.47,48 The association of allergic asthma, allergy to aspirin, and aggressive polyposis is known as the Samter triad (e-Fig. 8-23, A and B). Polyps in this condition can be destructive and invade the anterior cranial fossa.2
Fungal Rhinosinusitis Disorders
Fungal infections can cause both acute and chronic rhinosinusitis. These infections have been classified into four clinicopathologic types that can present as either tissue-invasive disorders (acute invasive fulminant disease and chronic invasive infection) or noninvasive disorders (noninvasive mycotic colonization [“fungus ball” or mycetoma] and allergic fungal [mycotic] sinusitis).49 All of these entities are rare in children.
Acute invasive fulminant disease occurs in persons who are immunosuppressed and clinically presents as pale mucosa that can progress to gangrene. Spread through neuronal routes can cause vascular invasion with intracranial spread and parenchymal infarcts. Mucormycosis is a disease caused by several types of fungi and can have an acute and fulminant course. In persons with diabetes, mucormycosis can present more as a chronic tissue-invasive infection and frequently causes periorbital invasion (orbital apex syndrome).49
A fungal ball or “mycetoma” consists of fungal hyphae compressed into a thick exudate within a sinus lumen.49 It is a benign fungal colonization and may occur in patients with previous sinus surgery, oral-sinus fistula, a history of chemotherapy or radiotherapy for cancer, or in those without any known predisposing factor.
Allergic fungal rhinosinusitis should be considered in all adults with chronic sinusitis, but it is rarely seen in young children. Clinical presentation may include a history of allergic sinusitis, chronic rhinosinusitis, nasal polyps, allergic asthma, and immunosuppression, and it can present with proptosis from an inflammatory mass.35,50 The diagnosis is primarily histopathologic. At surgery a characteristic inspissated sinonasal inflammatory exudate called allergic mucin is encountered and must be positive for fungal hyphae on fungal staining or cultures. No evidence for mucosal fungal invasion should be present.50
Imaging: Radiographically, fungal disease is difficult, if not impossible, to distinguish from bacterial infection. Radiographic signs include sinonasal polyposis with a heterogenous soft tissue inflammatory mass with remodeling or destruction of bone and increased densities in the sinuses, which can represent calcifications. Allergic mucin is largely responsible for the sinus CT hyperattenuation.50 MRI findings can suggest fungal infection because, in contrast to the high signal intensity on T2-weighted images seen with acute bacterial disease, the presence of calcium, air, or ferromagnetic elements in the fungus causes decreased signal intensity on T1-weighted and T2-weighted images. However, inspissated secretions as seen in patients with CF can have a similar appearance.
Benign Masses in the Sinuses
Cysts and polyps are frequent complications of inflammatory rhinosinusitis. A mucous retention cyst results from the obstruction of a submucosal mucinous gland, and the cyst wall is the duct epithelium. These cysts can occur in any sinus but are more frequently found in the inferior aspect of the maxillary sinus. A serous retention cyst results from the accumulation of serous fluid in the submucosal layer of the sinus mucosal lining.2 These cysts are not inflammatory lesions and appear as dome-shaped, well-defined, homogeneous, nonenhancing structures on CT; they are hypointense on T1-weighted MR images and hyperintense on T2-weighted images (Fig. 8-24, A). Cysts also can be found incidentally in 10% to 30% of patients.51
An antrochoanal polyp (sinonasal solitary polyp) is a maxillary sinus polyp that fills the sinus, expands the sinus ostium, and prolapses through it into the posterior choana, presenting as a nasal or nasopharyngeal polyp (Fig. 8-24, B and C). Although they are rare, sphenochoanal and ethmochoanal polyps also can occur. In the general population these polyps represent 4% to 6% of all nasal polyps; however, in children the incidence increases to 28% to 33%.52 Antrochoanal polyps differ from inflammatory polyps; they tend to be fibrotic with minimal inflammation and are composed of a cystic portion filling the maxillary sinus and a solid dumbbell-shaped portion that emerges into the nasal cavity through the maxillary infundibulum or through an accessory ostium of the maxillary sinus. These polyps are usually unilateral solitary lesions; however, bilateral antrochoanal polyps can be seen in 20% to 25% of cases. The clinical presentation is nasal obstruction and discharge, mouth breathing, snoring, and sleep apnea.
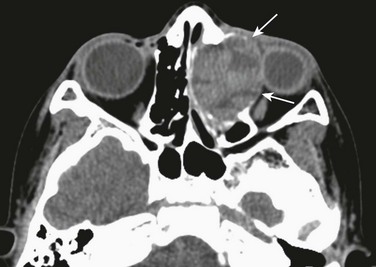
Mucoceles, which are most common in the frontal (60%) and ethmoid (30%) sinuses and result from obstruction or sequestration of a portion of the sinus, are cystic lesions lined with respiratory epithelium and filled with mucoid secretions.53 Mucoceles are rare in the sphenoid54 and maxillary sinuses. The association of mucoceles and CF is frequent.55 Clinical symptoms result from the mass effect of the lesion and depend on location; these symptoms may include proptosis, headache, cranial nerve impingement, or nasal obstruction (e-Fig. 8-25, A-D).
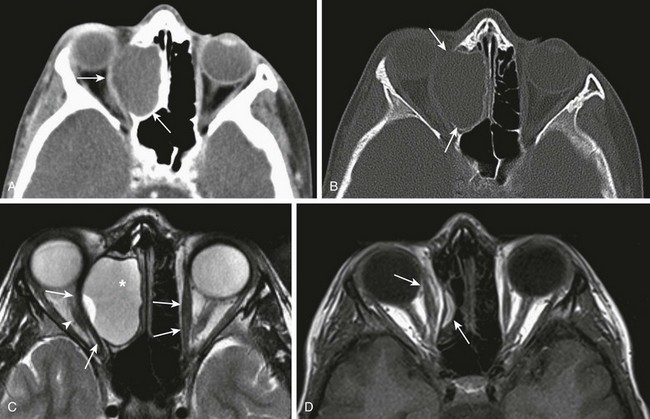
e-Figure 8-25 An ethmoidal mucocele in a 13-year-old.
A, An axial computed tomography (CT) image shows a well-circumscribed, nonenhancing, hypodense, expansile mass centered in the right ethmoid sinus with significant effect of the mass on the right orbit, causing proptosis and lateral deviation of the medial rectus muscle (arrows). B, An axial CT image shows bone remodeling and septal destruction (arrows). C, An axial T2-weighted magnetic resonance image shows a cystic, hyperintense, expansile lesion (asterisk) and lateral displacement of the right medial rectus muscle compared with the left (arrows). The optic nerve is laterally displaced (arrowhead). D, After endoscopic marsupialization, resolution of the ethmoidal expansion and proptosis is seen. Note the normal position of the medial rectus muscle (arrows).
Neoplasms of the Sinonasal Cavity
Benign neoplasms and tumorlike conditions of the sinonasal cavity in children include fibro-osseous lesions and tumors (e.g., fibrous dysplasia and ossifying fibroma), extramedullary hematopoiesis in anemias (e.g., sickle cell anemia and thalassemia), bony tumors (e.g., giant cell tumor and aneurysmal bone cyst) (e-Fig. 8-26), juvenile nasopharyngeal angiofibroma (Fig. 8-27, A-E) and odontogenic cysts or tumors.
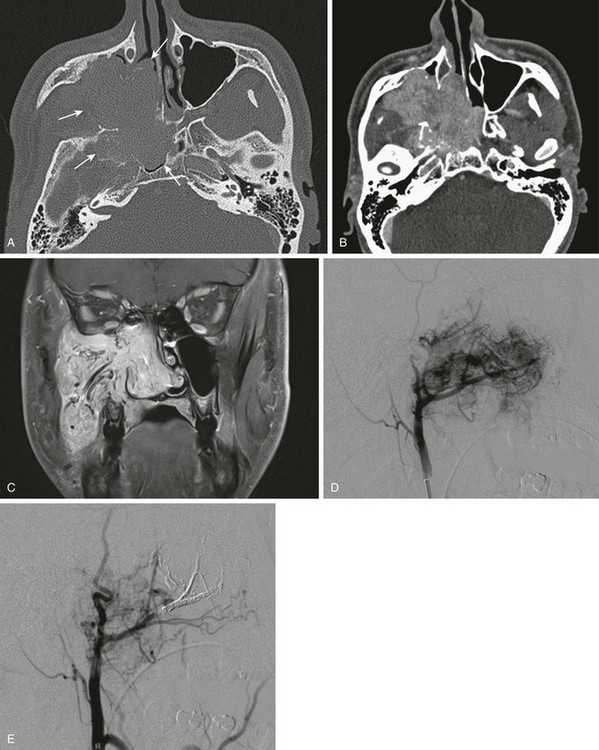
Figure 8-27 Juvenile nasopharyngeal angiofibroma in a 15-year-old boy.
A, An axial contrast-enhanced computed tomography (CT) image in a bone window shows a large nasopharyngeal soft tissue mass with extension through the pterygopalatine fossa and extensive bony destruction. B, An axial contrast-enhanced CT image in a soft tissue window shows a large, enhancing, lobulated mass. C, A coronal T1-weighted postcontrast magnetic resonance image shows an avidly enhancing mass with intrinsic prominent flow voids. D, Conventional angiography shows intense capillary blush fed by large feeding external carotid artery vessels. E, Preoperative, postembolization angiography.
Malignant neoplasms of the sinonasal cavity in children include rhabdomyosarcoma (e-Fig. 8-28, A-C), lymphoma/leukemia (e-Fig. 8-29, A and B), esthesioneuroblastoma, nasopharyngeal adenocarcinoma, and metastatic disease (neuroblastoma). These neoplasms will be discussed in greater detail in Chapter 16.
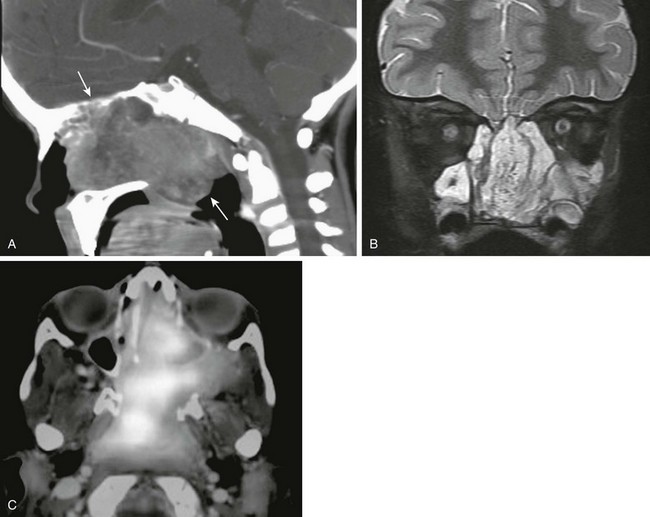
e-Figure 8-28 A nasopharyngeal rhabdomyosarcoma in a 4-year-old girl.
A, A sagittal contrast-enhanced computed tomography image shows a large heterogeneous soft tissue mass in the nasopharynx with bony destruction. B, A coronal T2-weighted magnetic resonance image with fat saturation shows a large, nasopharyngeal, heterogeneous mass. C, An axial fluorodeoxyglucose–positron emission tomography image shows a fluorodeoxyglucose-avid mass.
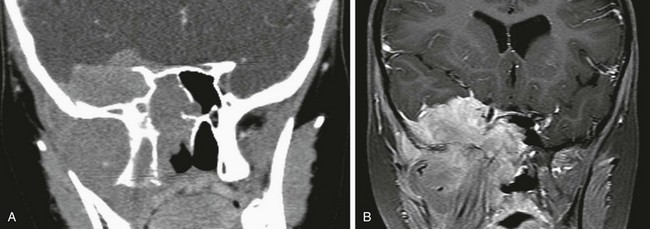
e-Figure 8-29 Acute lymphocytic leukemia in a 9-year-old girl.
A, A coronal contrast-enhanced computed tomography image shows a large enhancing soft tissue mass centered in the right infratemporal fossa with extension to the skull base, middle cranial fossa, and sphenoid sinus, with bony remodeling and destruction. B, A coronal T1-weighted postcontrast magnetic resonance image shows a large, enhancing, lobulated mass.
Fractures of Facial Bones
Plain radiographs may evaluate simple fractures, but CT is the preferred method to evaluate more complex fractures and often is used in preoperative planning.56 High-resolution ultrasonography also has proven to be a reliable diagnostic tool for the evaluation of nasal fractures, especially in low-grade nasal fractures.57,58
Karmazyn, B, Coley, BD, Robertson, ME, et al. Sinusitis child. American College of Radiology (ACR) appropriatness criteria http://www.acr.org/~/media/485AEEC108E941C6B5551A8D21017EED.pdf, 2012. Available at
Mafee, MF, Tran, BH, Chapa, AR. Imaging of rhinosinusitis and its complications: plain film, CT, and MRI. Clin Rev Allergy Immunol. 2006;30(3):165–186.
Steele, RW. Chronic sinusitis in children. Clin Pediatr (Phila). 2005;44(6):465–471.
Steele, RW. Rhinosinusitis in children. Curr Allergy Asthma Rep. 2006;6(6):508–512.
Ummat, S, Riding, M, Kirkpatrick, D. Development of the ostiomeatal unit in childhood: a radiological study. J Otolaryngol. 1992;21(5):307–314.
References
1. Antunes, MB, Gudis, DA, Cohen, NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29(4):631–643.
2. Som C, ed. Head and neck imaging, 5th ed, St Louis: Elsevier Mosby, 2011.
3. Wang, R, Jiang, S, Gu, R. The embryonic development of the ostiomeatal complex from 8 to 40 weeks [in Chinese]. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1994;29(3):131–133.
4. Adibelli, ZH, Songu, M, Adibelli, H. Paranasal sinus development in children: a magnetic resonance imaging analysis. Am J Rhinol Allergy. 2011;25(1):30–35.
5. Weiglein, A, Anderhuber, W, Wolf, G. Radiologic anatomy of the paranasal sinuses in the child. Surg Radiol Anat. 1992;14(4):335–339.
6. Shah, RK, Dhingra, JK, Carter, BL, et al. Paranasal sinus development: a radiographic study. Laryngoscope. 2003;113(2):205–209.
7. Medina, J, Hernandez, H, Tom, LW, et al. Development of the paranasal sinuses in children. Am J Rhinol. 1997;11(3):203–209.
8. Nayak, S. Radiologic anatomy of the paranasal air sinuses. Semin Ultrasound CT MR. 1999;20(6):354–378.
9. Szolar, D, Preidler, K, Ranner, G, et al. Magnetic resonance assessment of age-related development of the sphenoid sinus. Br J Radiol. 1994;67(797):431–435.
10. Aoki, S, Dillon, WP, Barkovich, AJ, et al. Marrow conversion before pneumatization of the sphenoid sinus: assessment with MR imaging. Radiology. 1989;172(2):373–375.
11. Barghouth, G, Prior, JO, Lepori, D, et al. Paranasal sinuses in children: size evaluation of maxillary, sphenoid, and frontal sinuses by magnetic resonance imaging and proposal of volume index percentile curves. Eur Radiol. 2002;12(6):1451–1458.
12. Wolf, G, Anderhuber, W, Kuhn, F. Development of the paranasal sinuses in children: implications for paranasal sinus surgery. Ann Otol Rhinol Laryngol. 1993;102(9):705–711.
13. Vaid, S, Vaid, N, Rawat, S, et al. An imaging checklist for pre-FESS CT: framing a surgically relevant report. Clin Radiol. 2011;66(5):459–470.
14. Reiss, M, Reiss, G. Height of right and left ethmoid roofs: aspects of laterality in 644 patients. Int J Otolaryngol. 2011;2011:508907.
15. Lebowitz, RA, Terk, A, Jacobs, JB, et al. Asymmetry of the ethmoid roof: analysis using coronal computed tomography. Laryngoscope. 2001;111(12):2122–2124.
16. Alberti, PW. Applied surgical anatomy of the maxillary sinus. Otolaryngol Clin North Am. 1976;9(1):3–20.
17. McAlister, WH, Lusk, R, Muntz, HR. Comparison of plain radiographs and coronal CT scans in infants and children with recurrent sinusitis. AJR Am J Roentgenol. 1989;153(6):1259–1264.
18. Konen, E, Faibel, M, Kleinbaum, Y, et al. The value of the occipitomental (Waters’) view in diagnosis of sinusitis: a comparative study with computed tomography. Clin Radiol. 2000;55(11):856–860.
19. Triulzi, F, Zirpoli, S. Imaging techniques in the diagnosis and management of rhinosinusitis in children. Pediatr Allergy Immunol. 2007;18(suppl 18):46–49.
20. Karmazyn, B, Coley, BD, Dempsey-Robertson, ME, et al. Sinusitis child. American College of Radiology (ACR) appropriatness criteria http://www.acr.org/~/media/485AEEC108E941C6B5551A8D21017EED.pdf, 2012. [Available at].
21. American Academy of Pediatrics Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108(3):798–808.
22. Paterson, A, Frush, DP. Dose reduction in paediatric MDCT: general principles. Clin Radiol. 2007;62(6):507–517.
23. Shah, NB, Platt, SL. ALARA: is there a cause for alarm? Reducing radiation risks from computed tomography scanning in children. Curr Opin Pediatr. 2008;20(3):243–247.
24. Goske, MJ, Applegate, KE, Boylan, J, et al. The ‘Image Gently’ campaign: increasing CT radiation dose awareness through a national education and awareness program. Pediatr Radiol. 2008;38(3):265–269.
25. Mulkens, TH, Broers, C, Fieuws, S, et al. Comparison of effective doses for low-dose MDCT and radiographic examination of sinuses in children. AJR Am J Roentgenol. 2005;184(5):1611–1618.
26. Ummat, S, Riding, M, Kirkpatrick, D. Development of the ostiomeatal unit in childhood: a radiological study. J Otolaryngol. 1992;21(5):307–314.
27. Wake, M, Takeno, S, Hawke, M. The uncinate process: a histological and morphological study. Laryngoscope. 1994;104(3 pt 1):364–369.
28. Brown, OE, Pownell, P, Manning, SC. Choanal atresia: a new anatomic classification and clinical management applications. Laryngoscope. 1996;106(1 pt 1):97–101.
29. Slovis, TL, Renfro, B, Watts, FB, et al. Choanal atresia: precise CT evaluation. Radiology. 1985;155(2):345–348.
30. Brown, OE, Myer, CM, 3rd., Manning, SC. Congenital nasal pyriform aperture stenosis. Laryngoscope. 1989;99(1):86–91.
31. Visvanathan, V, Wynne, DM. Congenital nasal pyriform aperture stenosis: a report of 10 cases and literature review. Int J Pediatr Otorhinolaryngol. 2012;76(1):28–30.
32. Van Den Abbeele, T, Triglia, JM, Francois, M, et al. Congenital nasal pyriform aperture stenosis: diagnosis and management of 20 cases. Ann Otol Rhinol Laryngol. 2001;110(1):70–75.
33. Wong, RK, VanderVeen, DK. Presentation and management of congenital dacryocystocele. Pediatrics. 2008;122(5):e1108–e1112.
34. Steele, RW. Rhinosinusitis in children. Curr Allergy Asthma Rep. 2006;6(6):508–512.
35. Steele, RW. Chronic sinusitis in children. Clin Pediatr (Phila). 2005;44(6):465–471.
36. Hicks, CW, Weber, JG, Reid, JR, et al. Identifying and managing intracranial complications of sinusitis in children: a retrospective series. Pediatr Infect Dis J. 2011;30(3):222–226.
37. Perez Barreto, M, Sahai, S, Ameriso, S, et al. Sinusitis and carotid artery stroke. Ann Otol Rhinol Laryngol. 2000;109(2):227–230.
38. Tsai, BY, Lin, KL, Lin, TY, et al. Pott’s puffy tumor in children. Childs Nerv Syst. 2010;26(1):53–60.
39. Blumfield, E, Misra, M. Pott’s puffy tumor, intracranial, and orbital complications as the initial presentation of sinusitis in healthy adolescents, a case series. Emerg Radiol. 2011;18(3):203–210.
40. Rommens, JM, Iannuzzi, MC, Kerem, B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059–1065.
41. Sakano, E, Ribeiro, AF, Barth, L, et al. Nasal and paranasal sinus endoscopy, computed tomography and microbiology of upper airways and the correlations with genotype and severity of cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2007;71(1):41–50.
42. Godoy, JM, Godoy, AN, Ribalta, G, et al. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg. 2011;145(4):673–676.
43. Weber, SA, Ferrari, GF. Incidence and evolution of nasal polyps in children and adolescents with cystic fibrosis. Braz J Otorhinolaryngol. 2008;74(1):16–20.
44. Yung, MW, Gould, J, Upton, GJ. Nasal polyposis in children with cystic fibrosis: a long-term follow-up study. Ann Otol Rhinol Laryngol. 2002;111(12 pt 1):1081–1086.
45. Kovell, LC, Wang, J, Ishman, SL, et al. Cystic fibrosis and sinusitis in children: outcomes and socioeconomic status. Otolaryngol Head Neck Surg. 2011;145(1):146–153.
46. Rickert, S, Banuchi, VE, Germana, JD, et al. Cystic fibrosis and endoscopic sinus surgery: relationship between nasal polyposis and likelihood of revision endoscopic sinus surgery in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2010;136(10):988–992.
47. Marseglia, GL, Merli, P, Caimmi, D, et al. Nasal disease and asthma. Int J Immunopathol Pharmacol. 2011;24(suppl 4):7–12.
48. Triglia, JM, Nicollas, R. Nasal and sinus polyposis in children. Laryngoscope. 1997;107(7):963–966.
49. deShazo, RD, Chapin, K, Swain, RE. Fungal sinusitis. N Engl J Med. 1997;337(4):254–259.
50. Schubert, MS. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol. 2009;47(suppl 1):S324–S330.
51. Kanagalingam, J, Bhatia, K, Georgalas, C, et al. Maxillary mucosal cyst is not a manifestation of rhinosinusitis: results of a prospective three-dimensional CT study of ophthalmic patients. Laryngoscope. 2009;119(1):8–12.
52. Chen, JM, Schloss, MD, Azouz, ME. Antro-choanal polyp: a 10-year retrospective study in the pediatric population with a review of the literature. J Otolaryngol. 1989;18(4):168–172.
53. Lund, VJ, Milroy, CM. Fronto-ethmoidal mucocoeles: a histopathological analysis. J Laryngol Otol. 1991;105(11):921–923.
54. Lui, YW, Dasari, SB, Young, RJ. Sphenoid masses in children: radiologic differential diagnosis with pathologic correlation. AJNR Am J Neuroradiol. 2011;32(4):617–626.
55. Nicollas, R, Facon, F, Sudre-Levillain, I, et al. Pediatric paranasal sinus mucoceles: etiologic factors, management and outcome. Int J Pediatr Otorhinolaryngol. 2006;70(5):905–908.
56. Kim, BH, Seo, HS, Kim, AY, et al. The diagnostic value of the sagittal multiplanar reconstruction CT images for nasal bone fractures. Clin Radiol. 2010;65(4):308–314.
57. Lee, MH, Cha, JG, Hong, HS, et al. Comparison of high-resolution ultrasonography and computed tomography in the diagnosis of nasal fractures. J Ultrasound Med. 2009;28(6):717–723.
58. Hong, HS, Cha, JG, Paik, SH, et al. High-resolution sonography for nasal fracture in children. AJR Am J Roentgenol. 2007;188(1):W86–W92.