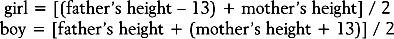Chapter 11 Normal Puberty and Pubertal Disorders
NORMAL PUBERTY
Hypothalamic-Pituitary-Ovarian Axis
The HPO axis lies dormant before puberty, although well-developed and functional during fetal development. In the higher cortical centers, from the arcuate nucleus of the hypothalamus, gonadotropin-releasing hormone (GnRH) is synthesized and released.1 This decapeptide is secreted in a pulsatile fashion and has a fleeting half-life of 2 to 4 minutes. Through its effect on the anterior pituitary, GnRH regulates the synthesis, storage, and release of the pituitary gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
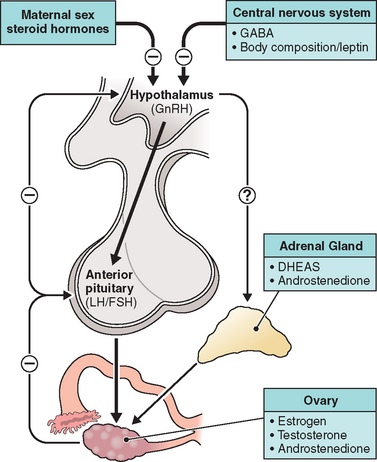
These hormone levels approach those of an adult in the fetal circulation by midgestation. However, with increasing maternal steroid hormone production closer to term, gonadotropin levels decline. Shortly after delivery, as the maternal source of estrogen is withdrawn, gonadotropin levels are noted to increase as a result of the release from the negative feedback circuit.2 The HPO feedback system is illustrated in Figure 11-1.
It is thought that the two primary inhibitory influences on the pulsatile release of GnRH and the downregulation of the HPO axis during childhood are the (1) intrinsic central nervous system (CNS) inhibition via γ-aminobutyric acid and the (2) negative feedback system driven by ovarian steroid hormones.3,4 With continued maturation of the CNS after birth, a more profound internal inhibitory effect can be noted in reference to GnRH-secreting neurons. In premature infants with less developed neuronal pathways, pituitary gonadotropins are higher than in term counterparts, presumably due to a weaker inhibitory influence.5 The presence of a nonsteroidal regulator of these pathways is further substantiated by the ability of patients with gonadal agenesis to secrete moderate levels of gonadotropins in response to GnRH.6 Other potential executors of the CNS regulation of steroid hormone production have been assessed, but none have shown a definitive effect.
Onset of Puberty
The events that then lead to the onset of puberty are fairly well understood; however, the signal for the CNS to release its inhibitory influence on the hypothalamus remains a mystery. Pulsatile secretion of GnRH from the arcuate nucleus of the hypothalamus leads to gonadarche, documented by profound increases in sex steroid hormone production.1 Early pubertal changes are temporally associated with an increase in GnRH pulse frequency, primarily during the sleep cycle.7 As menarche approaches, GnRH pulses increase in amplitude and can be detected throughout the day, similar to those in an adult.8,9 Ovulatory cycles occur with the arrest of the inhibitory effect of the CNS, and regulation of the HPO axis is then managed primarily by circulating steroid hormones via functional feedback circuits.
LH and FSH are also released in a pulsatile fashion, which increases with the preceding increase in GnRH activity. In prepubertal girls, FSH responses to GnRH action are marked, whereas the response seen by LH is quite low (LH/FSH ratio < 1). Conversely, during puberty, a predominant LH response is seen and the LH/FSH ratio reverses. LH pulsatility is always dependent on GnRH secretion regardless of age, whereas there is a diminished FSH response to GnRH with the increase in ovarian activity that occurs during puberty.10 This may help explain the dichotomous maturation of the LH/FSH response.
Both genetic and environmental pressures may play a role with the initiation of pubertal development. It has been suggested that appropriate weight gain and percentage of body fat are required for these events to occur.11 This postulate is based on data from adolescent females who suffer from chronic illness, malnutrition, or have low body mass indices due to extreme athletic pursuits. These young girls frequently have delays in sexual maturation and often present with primary amenorrhea resulting from hypothalamic hypogonadism. Normal menstrual cycles resume with reversal of their nutritional status.12 Whether the increase in body fat occurs as a result of these hormonal changes or whether it is a prerequisite for activation of the HPO axis was challenged by investigators who followed healthy females throughout puberty and found that body composition did not change prior to, but rather along with, the increase in GnRH secretion.13
Leptin, an adipocyte-derived hormone, has received much attention in recent years. Plasma concentrations correlate well with body composition and have been shown to rise throughout puberty in female patients.14 Accordingly, several investigators have attempted to establish a causal relationship between leptin and the activation of the HPO axis. specific leptin deficiencies have been shown to prevent sexual maturation, which can then be triggered by restoring normal levels.15 Nevertheless, the role of leptin in pubertal development has not been clearly elucidated.
Another molecule that may play a role in the reversal of HPO downregulation is neuropeptide Y (NPY). Circulating levels are regulated by steroid hormones as well as nutritional status, with a net influence on gonadotropin synthesis through an alteration in GnRH pulsatility and pituitary response to GnRH.16 Increased levels of NPY have been documented in females with eating disorders such as anorexia nervosa and bulimia,17 indicating another possible correlation with percentage of body fat and reproductive potential.
Characteristics of Sexual Development
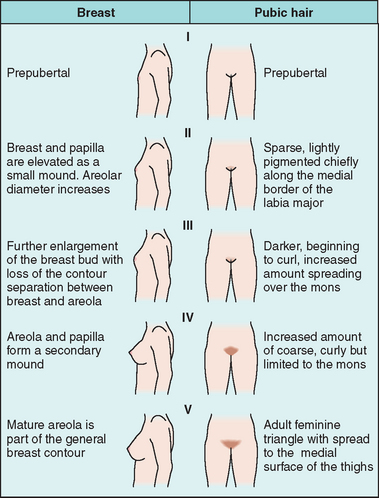
The series of events that occur during puberty have been well-defined. These predictable events, which are currently utilized as the standard for sexual development and somatic growth, were initially described by Tanner and Marshall more than 30 years ago (Fig. 11-2).18 Tanner stages describe characteristic (1) breast development, (2) pubic hair distribution, and (3) growth and maturation of genitalia by dividing each into five groups. For each characteristic, Stage I describes the prepubertal state and Stage V represents adult development. These guidelines are traditionally used to determine if an adolescent female is developing in a typical fashion.
Thelarche
The first sign of development in the majority of white females is breast budding. According to Tanner and Marshall, this initial event occurs between ages 8 and 13 in most, with a mean of 10.6 years. The transition period from Stage II to Stage V breast development may last 4.2 years.18
Growth Spurt
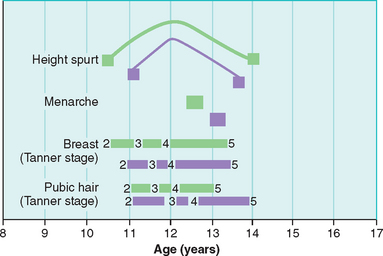
The growth spurt (peak growth velocity), during which adolescents achieve approximately 20% of their adult final height, occurs with the onset of puberty (Fig. 11-3).19 Peak growth velocity (2 to 3 cm/year) precedes menarche and typically occurs earlier in girls than in boys. Rapid growth of the extremities occurs first, followed by a gradual lengthening within the vertebral column. The timing of the growth spurt varies according to ethnicity and environmental factors that may influence the onset of puberty. Growth charts are useful in predicting final height achievement.
There are conflicting reports in reference to final height and timing of the onset of puberty. Patients with a later onset had smaller gains throughout puberty; however, they were initially taller than those who had earlier onset. Final adult height among these groups was comparable.20,21 Growth hormone through the actions of insulin-like growth factor I (IGF-I) is likely the principal factor responsible for somatic growth and final height. As low levels of sex steroid hormones rise early in puberty, there is a concomitant increase in the pulsatile secretion of growth hormone. As such, the initial growth spurt typically occurs before advanced secondary sexual characteristics appear. Higher levels of estrogens suppress the action of IGF-I, and so the same factors that promote growth can also impact final height by inducing epiphyseal closure.22
Menarche
According to Tanner, girls in the United Kingdom in 1969 had their first menses at the average age of 13.5 years, with a range of 9 to 16 years.18 The mean age of menarche for a white adolescent in the United States is approximately 12.7 years. At the time of menarche, most have achieved Tanner Stage IV breast development,18 and the interval from initial breast development to menarche is 2.3 years.18
There seems to have been a decline in the average age of menarche in the first half of the 20th century, in part due to the improvement in general health and nutrition.23 Nonetheless, few reports have documented any further changes since the mid-20th century.
There is good evidence that African-American girls have an earlier onset of puberty compared to white girls.24,25 This was well demonstrated by the Pediatric Research in Office Settings (PROS) study published by Herman-Giddens in 1999.24 This multicenter, cross-sectional study evaluated over 17,000 female patients between ages 3 and 12.24 On average, African-American females show early signs of puberty up to 1.5 years earlier than their white counterparts. By age 7, 27.2% of African-American girls and 6.7% of white girls showed breast or pubic hair development. Menarche was achieved almost a year earlier. The mean age for onset of breast development was 8.87 years in African-American girls and 9.96 years in white girls. At each consecutive stage of development, African Americans were more advanced per year than white girls. Girls of other ethnic backgrounds may also demonstrate characteristic differences in onset of pubertal maturation. However, only white and African-American girls were included in this study.
The PROS was the first large publication to address current and demographically relevant standards for assessing normal and abnormal onset of puberty. Updated guidelines have since been proposed and recommend a formal evaluation for precocious puberty be initiated in African-American girls who present before age 6 and white girls who present before age 7. Although this provocative investigation has drawn much criticism, it does invite us to reconsider the current standards (see Fig. 11-3).
History and Physical Examination
History
At times, it may be necessary to excuse the parent to address specific issues that the adolescent may feel uncomfortable discussing with the parent present. This may include issues dealing with sexual health and contraception. The clinician should be aware of specific state laws regarding consent and reporting. All states allow for the treatment of minors without parental consent, specifically for sexually transmitted diseases and routine obstetrical care. A trusting relationship often requires several visits, and confidentiality must be assured from the beginning. Comments should not be judgmental on the part of the healthcare provider.
Psychosocial Concerns and Sexuality
Although cognitive function may not be impaired with the great changes that occur during puberty, the social concerns that commonly afflict these young teens may influence overall functioning. Appearance plays a crucial role in social acceptance among peers and may be affected by the development of acne vulgaris, a common occurrence during puberty. Excessive acne should prompt the physician to rule out endocrinologic abnormalities such as nonclassic congenital adrenal hyperplasia or other disorders related to androgen excess. An adolescent female who has earlier but normal onset of puberty may be taller than her peers. As a result, she may be taunted by her classmates and feel isolated from her peers. Episodes of depression may be twice as common in girls as in boys after the onset of puberty.26
PRECOCIOUS PUBERTY
Effects of Precocious Puberty on Adult Height
Low levels of estrogens have been shown to promote bone growth, as is manifest by rapid growth velocity during the growth spurt. Conversely, high levels promote closure of the epiphyseal plates. Girls who present early in the course of central precocious puberty are generally taller than their age-respective cohorts due to increased levels of steroids and the actions of IGF-I. This growth is premature and limited, so that the final height in untreated patients will likely be less than 155 cm.27 As a result, by the time most adolescents achieve menarche, they have likely reached their final height. Notwithstanding the apparent risk for short stature, a significant number of untreated patients with idiopathic disease will likely attain relatively normal adult height, greater than the 3rd percentile.27
When to Initiate an Assessment
One challenge each clinician faces is when to initiate the assessment of a child suspected of having precocious puberty. Historical accounts from the 19th century report a relative later age of onset of menstruation (16 to 17 years), presumably due to malnutrition. The definition of precocious puberty has since remained stable, such that any female who presented before age 8 was observed, if not evaluated, for progression of sexual growth.18 As referred to earlier, the traditional definition was challenged by Herman-Giddens, who strongly suggested that normal pubertal development may begin as early as age 6.24
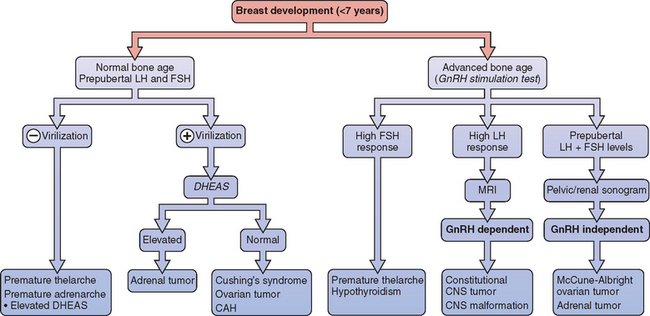
Isosexual precocity is characterized by early onset of otherwise normal physiologic development. This heterogeneous disorder can be categorized into central (GnRH-dependent), peripheral (GnRH-independent), or incomplete precocious puberty. Heterosexual precocity is evidenced by excessive androgen production and virilization of a female child. Causes of precocious puberty are listed in Table 11-1.
Central Precocious Puberty
Central precocious puberty is more frequently noted among girls, with an incidence of 1:5,000 to 1:10,000.28 It results from premature activation of the hypothalamic GnRH neurons. Approximately 70% to 90% of such cases are idiopathic in nature; however, other potential etiologies must first be considered.29,30 Recent evidence suggests an inheritable risk with an autosomal dominant mode of transmission for certain cases of central precocious puberty.31
Known organic causes of central precocious puberty, however, include a multitude of CNS lesions. These include benign hamartomas, space-occupying lesions that can involve the hypothalamus and result in early precocious development, generally before age 4.29,30 Other differential diagnoses of central precocious puberty include congenital hydrocephalus, neural tube defects, CNS irradiation, encephalitis, and neurofibromatosis type I.32
Physical Examination
Height and weight should be charted on a linear growth curve and followed until final height is achieved. Predicted final height has traditionally been based on the methodology described by Bayley and Pinneau.33 Target height (cm) considers genetic potential and is calculated from the averages of the height of the child’s parents:
Imaging Studies
Imaging studies play a key role in the evaluation of children with precocious puberty because a rapid increase in growth and bone age are typically seen in children with rapidly increasing levels of sex steroid hormones. Linear growth and skeletal maturation are often a more accurate assessment of pubertal development than progression of secondary sexual characteristics.
Bone age is typically evaluated by radiographic plain films of the left hand and wrist. This is a simple and noninvasive test that is well tolerated by most children. Bone age advance over chronological age is diagnostic for precocious puberty, and a disparity of greater than 2 years is more suspicious for a progressive disorder.34 Given the higher prevalence of CNS abnormalities, neuroimaging is always indicated to rule out space-occupying lesions, malignant neoplasms, and other CNS anomalies even in the absence of neurologic complaints. A diagnostic approach to precocious puberty is given in Figure 11-4.
Treatment
Psychological Issues of Premature Puberty
An important goal of treatment is to reverse sexual maturation, thereby limiting psychosocial repercussions and early reproductive potential. Girls who experience early puberty are at risk of persistent difficulty during adolescence.35 It is important to remember that these patients are young and naíve, and lack the psychosocial maturity often expected of them given their appearance. It is also reasonable to assume that due to early physical development, sexual activity and the potential for abuse may occur at an earlier age. It is assumed that these reasons explain why girls with precocious puberty have been found to have more emotional problems, increased antisocial behavior, and are at risk for both dropping out of school and early pregnancy. For this reason, the parents of these girls should be made aware of these potential problems so that appropriate counseling and preventive efforts can be made.36
Hypothalamic Suppression
Initial attempts to achieve such a degree of hypothalamic suppression included the use of progestins; however, these were unsuccessful at limiting progressive changes and their use has since been abandoned.37 The use of GnRH analogs to treat central precocious puberty was popularized more than 20 years ago and has become the gold standard of therapy. The most commonly used preparations in the United States are leuprolide, nafarelin, and goserelin. Children with precocious puberty generally require higher doses to achieve suppression, which can be monitored with serum estradiol levels and GnRH stimulation tests. To improve compliance, subcutaneous formulations are currently used. Monthly intramuscular injections are also available.
Early treatment protocols using long-acting GnRH agonists reported significant regression of secondary sexual characteristics and overall improvement in final height compared to nonrandomized controls.38 The difficulty with interpreting these results came from the lack of well-designed randomized trials and a limited ability to accurately predict adult height. In the absence of a more effective system, the methodology described by Bayley and Pinneau more than 50 years ago has been maintained throughout the literature despite the potential for overestimating predicted final height.39 Furthermore, children with suspected idiopathic precocious puberty were grouped together, and the investigators did not delineate which of the patients were more at risk for significantly shortened stature. This, in part, is due to our inability to properly identify such patients.
The variable presentation of central precocious puberty creates a dilemma for investigators who would otherwise design a randomized trial. Several months of simple observation are often required before progression can be documented. A few randomized series have been published that addressed the effect of GnRH agonists on final height in girls who presented with early or slowly progressive puberty.40,41 They confirmed results from previous observational and nonrandomized reports, which documented very little effect of hypothalamic suppression on improving final height in patients presenting at a later age. Furthermore, children presenting with either “early puberty” or advanced “slowly progressive puberty” were likely to achieve reasonable adult height without hypothalamic suppression. One theory that may help explain impaired growth during GnRH analog therapy in this group is early growth plate senescence related to estrogen exposure before onset of treatment.42 And so it may be this rate-limiting step, patients presenting beyond the window of opportunity, which limits final height. A summary of predicted and final height in patients treated for CPP is provided in Table 11-2.
Table 11-2 Comparison of Predicted, Target, and Final Height in Patients with Central Precocious Puberty
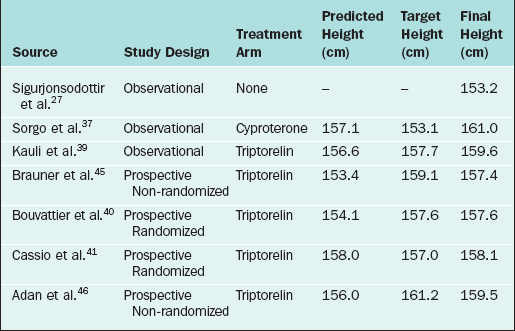
Significant consideration should then be given to promptly initiating therapy in girls presenting early with advanced bone age, because they will likely benefit most from GnRH agonist therapy.43–46 The question still remains as to what criteria should be used to determine maximum therapeutic benefit. Adan and colleagues suggested the following as risk factors for decreased stature and appropriate indications for therapy, especially at an earlier age of onset: (1) predicted adult height below 155 cm (may include those with a predicted height over 155 cm if the LH/FSH ratio is consistent with central precocious puberty) and (2) bone age advanced over chronological age by more than 2 years.46 Hormonal monitoring of therapy can be performed with the GnRH stimulation test at 3, 6, and 12 months after initiation, with annual follow-up thereafter.
Recombinant Growth Hormone
Some children with precocious puberty will have early closure of their epiphyseal plates despite the use of GnRH analogs. As a result, these girls will grow up to be short adults without further intervention. Since the 1980s, recombinant human growth hormone (somatropin) has been available to treat patients with growth hormone deficiency and other conditions associated with poor growth. The use of growth hormone as an adjunct to GnRH agonists in girls with precocious puberty has been evaluated by several observational and randomized series and has been found to improve final height prognosis.47 Early initiation of therapy in patients with central precocious puberty may further enhance the response.
GNRH-INDEPENDENT PRECOCIOUS PUBERTY
Autonomous Ovarian Estrogen Production
Ovarian tumors are uncommon but important childhood neoplasms that present with precocious puberty in approximately 10% of cases.48 Granulosa cell tumors are the most common estrogen-producing neoplasms detected. However, other tumors, such as thecal cell tumors, gonadoblastomas, teratomas, cystadenomas, and ovarian cancers, may be responsible. Intra-abdominal masses are often palpable, but sonography or magnetic resonance imaging (MRI) may help characterize the tumor, and surgical exploration is generally warranted.
Laboratory criteria that help distinguish these processes from a central source include low baseline gonadotropin levels and a prepubertal response to the GnRH stimulation test. As with central precocious puberty, estradiol levels will be high and bone age advanced (see Fig. 11-4). Treatment is based on surgical extirpation of the source, which results in regression of pubertal changes.
McCune-Albright Syndrome
Children with this rare disorder also have fibrous dysplasia in their bones, which leads to fractures, deformities, and X-ray abnormalities. Facial bone deformities may cause cosmetic problems. In addition, these children have café-au-lait spots, which are light tan birth marks. McCune-Albright syndrome is often associated with several other endocrinopathies, including hyperthyroidism, acromegaly, pituitary adenomas, and adrenal hyperplasia.49
McCune-Albright syndrome results in mosaicism, such that the abnormal gene is present in only a fraction of the patient’s cells. The timing of the mutation determines which organ systems are affected and the severity of the presentation, thus explaining the heterogeneity of these syndromes. In a recent, large collaborative study, investigators were able to identify the Gsα mutation in 43% of patients presenting with at least one sign of McCune-Albright syndrome and in 33% of those presenting with isolated signs of gonadotropin-independent precocious puberty.50
Treatment
In contrast to central precocious puberty, girls with McCune-Albright syndrome exhibit a lack of GnRH pulsatility, low gonadotropin levels, and estradiol production from autonomous follicle development. Accordingly, diagnostic GnRH administration results in a prepubertal LH/FSH response (see Fig. 11-4). As would be expected, therapy with GnRH agonists is rarely effective. Hence, treatment protocols for McCune-Albright syndrome are aimed at inhibiting peripheral estradiol production with aromatase inhibitors or blocking the effects at the receptor level with selective estrogen receptor modulators (SERMs).
Aromatase inhibitors offer several theoretical benefits for the treatment of McCune-Albright syndrome. Unfortunately, results from studies evaluating testolactone have been inconclusive.51–53 Testolactone is a synthetic antineoplastic agent that also inhibits aromatase activity, thereby lowering estrogen levels. Testolactone may induce regression of breast development and menstruation in the short term; however, the beneficial effect wanes and frequent dosing intervals are often required. Other formulations may someday prove more useful.
It has been suggested that continuous estrogen exposure from a peripheral source may secondarily induce the HPO axis, such that a central component may occur simultaneously.54 These findings may help to explain the lack of therapeutic benefit of aromatase inhibitors in certain patients with McCune-Albright syndrome. Evaluation and management of these complicated patients should then be based on algorithms used for central precocious puberty.
The SERM tamoxifen was studied in a prospective, multicenter trial for the 12-month treatment of 25 girls with McCune-Albright syndrome. This treatment decreased the incidence of vaginal bleeding, and also decreased bone growth velocity and bone maturation.55
Other Causes
Other causes of GnRH-independent precocious puberty include adrenal disorders such as congenital adrenal hyperplasia (see Table 11-1). Patients present with heterosexual development, and androgen levels, including 17OH-progesterone, will be elevated. Corticosteroid therapy is the treatment of choice. Primary hypothyroidism, albeit uncommon, may result in ovarian cyst development, resulting in high estradiol levels. This has been hypothesized to be the result of cross-reactivity of high levels of thyroid-stimulating hormone (TSH) with FSH at the level of the ovarian receptors.56
PREMATURE ADRENARCHE
Adult pubic hair growth before age 6 may result from an abnormal adrenal secretory response that promotes androgen production (17-hydroxypregnenolone, DHEA, and DHEA-S). Like premature thelarche, the diagnosis can only be made after longitudinal evaluation, in the absence of other signs of sexual development. Although mild increases in bone age may occur, no treatment is necessary because these children will likely achieve normal adult height.57
Premature Adrenarche and Polycystic Ovary Syndrome
There is evidence to suggest that girls with premature adrenarche may be at risk for developing polycystic ovary syndrome (PCOS). Known outcomes of this syndrome include obesity, dyslipidemia, hyperinsulinemia, hirsutism, and infertility. A recent randomized trial evaluating the effectiveness of metformin therapy in adolescents with a history of precocious pubarche and low birth weight noted significant reversal in dyslipidemia, excess truncal fat, increased levels of IGF-I, and insulin insensitivity compared to controls. Early therapy aimed at improving insulin resistance and reducing percentage of body fat may improve cardiovascular risk later in life.58
DELAYED PUBERTY AND PRIMARY AMENORRHEA
Primary Amenorrhea with Otherwise Normal Sexual Development
Genetically normal patients with a normally functioning HPO axis who present with primary amenorrhea typically have anomalies of the genital outflow tract, such as imperforate hymen or vaginal septum (Table 11-3). The classic presentation is cyclic abdominal pain with a tender midline pelvic mass on rectal examination (see Chapters 12, 14, and 16).
Data from Timmreck LS, Reindollar RH. Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am 30:287-302, 2003
Androgen insensitivity syndrome is another common cause of primary amenorrhea. Previously termed testicular feminization, this condition is the result of an abnormal androgen receptor. This maternal X-linked recessive disease occurs in individuals with XY genotypes and normal but partially or completely undescended testicles that produce testosterone (see Chapter 16). Because of the abnormal androgen receptors, high levels of circulating testosterone result in appropriately timed puberty in females who appear phenotypically normal. A harbinger is sparse or absent pubic hair.
Evaluation
Both physical and psychosocial concerns of these patients and their parents must be considered.
Treatment of Delayed Puberty
Specific Therapy
Therapy for hypogonadotropic disorders is focused at treating the primary etiology whenever possible. If an intracranial lesion compresses the pituitary stalk, surgical therapy is indicated. If prolactinoma is the cause, bromocriptine or cabergoline becomes the first line of therapy. Medical therapy generally restores menses and fertility, and although surgical treatment may portend good results initially, there is a high incidence of recurring hyperprolactinemia. Surgery may therefore be postponed unless the condition is refractory to medical management.
Estrogen Therapy
Hormone replacement for treatment of delayed puberty is begun with low-dose estrogens, typically 0.3 mg conjugated estrogens for 6 to 12 months. The main goal is to induce normal breast development, because too high of an estrogen dose can result in the development of tuberous breasts.59 Subsequent goals include regulation of menses and maintenance of bone mass. This can be achieved by increasing the dosage of estrogen slowly after the first year until menstruation occurs. Progestin therapy (e.g., medroxyprogesterone acetate [5–10 mg]) is initiated approximately 3 months after the increase in dosage of estrogen, typically when breakthrough bleeding occurs. The most common formulation includes continuous estrogen therapy with sequential progestins given orally in the latter part of the cycle to create regular menses. Alternative forms include transdermal estrogen replacement and micronized progestins that have less negative impact on lipid profiles. Gonadotropins have also been used to induce ovulation, but are costly and more difficult to administer, especially in an adolescent patient population.
CONCLUSIONS
1 Emans SJ, Laufer MR, Goldstein DP, editors. Pediatric and Adolescent Gynecology: The Physiology of Puberty. Baltimore: Lippincott Williams & Wilkins; 1998:109-140.
2 Kaplan SL, Grumbach MM, Aubert ML. The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: Maturation of central nervous system regulation of anterior pituitary function. Recent Prog Hor Res. 1976;32:161.
3 Grumbach MM, Kaplan SL. The neuroendocrinology of human puberty: An ontogenetic perspective. In: Grumbach MM, Kaplan SL, Sizoneko PC, Aubert ML, editors. Control of the Onset of Puberty. Baltimore: Williams & Wilkins; 1990:1-68.
4 Mitsushima D, Hei DL, Terasawa E. γ-Aminobutyric acid is an inhibitory neurotransmitter restricting release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395-399.
5 Tapanainen J, Koivisto M, Vijko R, et al. Enhanced activity of the pituitary-gonadal axis in premature human infants. J Clin Endocrinol Metab. 1981;52:235-238.
6 Roth JC, Kelch RP, Kaplan SL, et al. FSH and LH response to luteinizing hormone-releasing factor in prepubertal and pubertal children, adult males and patients with hypogonadotropic and hypergonadotropic hypogonadism. J Clin Endocrinol Metab. 1973;37:680.
7 Boyar R, Finkelstein J, Roffwarg H, et al. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. NEJM. 1972;287:582.
8 Landy H, Boepple PA, Mansfield MJ, et al. Sleep modulation of neuroendocrine function: Developmental changes in gonadotropin-releasing hormone secretion during sexual maturation. Pediatr Res. 1990;28:213-217.
9 Yen SS, Apter D, Butzow T, et al. Gonadotropin releasing hormone pulse generator activity before and during sexual maturation in girls: New insights. Hum Reprod. 1993;8(Suppl 2):66-71.
10 Apter D, Bhtzow TL, Laughlin GA, et al. Gonadotropin releasing hormone pulse generator activity before and during sexual maturation in girls: New insights. Hum Reprod. 1993;8:66.
11 Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169:397.
12 Warren MP. The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab. 1980;50:1150.
13 Penny R, Goldstein IP, Frasier SD. Gonadotropin secretion and body composition. Pediatrics. 1978;61:294.
14 Apter D. Leptin and puberty. Curr Opin Endocrinol Diabet. 2000;7:57-64.
15 Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. NEJM. 1999;341:879-884.
16 Sahu A, Phelps CP, White JD, et al. Steroidal regulation of hypothalamic neuropeptide Y release and gene expression. Endocrinology. 1992;130:3331.
17 Kaye WH, Berrettini W, Gwirtsman H, et al. Altered cerebrospinal fluid of neuropeptide Y and peptide YY immunoreactivity in anorexia and bulimia nervosa. Arch Gen Psychiatry. 1990;47:548.
18 Marshall W, Tanner J. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291.
19 Abbassi V. Growth and normal puberty. Pediatrics. 1998;102:507.
20 Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: Implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics. 1999;104:936-941.
21 He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty and final height. Pediatr Res. 2001;49:244-251.
22 Mansfield MJ, Rudlin CR, Crigler JFJr, et al. Changes in growth and serum growth hormone and plasma somatomedin-C levels during suppression of gonadal sex steroid secretion in girls with precocious puberty. J Clin Endocrinol Metab. 1988;66:3.
23 Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. NEJM. 1982;306:1033.
24 Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505-512.
25 MacMahon B. National Health Examination Survey: Age at menarche. National Center for Health Statistics. DHEW publication (HRA). 1973;12:74-1615.
26 Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145.
27 Sigurjonsdottir TJ, Hayles AB. Precocious puberty. A report of 96 cases. Am J Dis Child. 1968;115:304-321.
28 Cutler GB. Precocious puberty. In: Hurst JW, editor. Medicine for the Practicing Physician. 2nd ed. Woburn, Mass.: Butterworth; 1988:526-530.
29 Cisternimo M, Arrigo T, Pasquino AM, et al. Etiology and age incidence of precocious puberty in girls: A multicentric study. J Pediatr Endocrinol. 2000;13(Suppl I):695-701.
30 Chalumeau M, Chemaitilly W, Trivin C, et al. Central precocious puberty in girls: An evidence-based diagnosis tree to predict central nervous system abnormalities. Pediatrics. 2002;109:61.
31 de Vries L, Kauschansky A, Shohat M, et al. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89:198-200.
32 Partsch CJ, Sippell WG. Treatment of central precocious puberty. Best Pract Res Clin Endocrinol Metab. 2002;16:165-189.
33 Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40:423-441.
34 Fontoura M, Brauner R, Prevot C, et al. Precocious puberty in girls: Early diagnosis of a slowly progressing variant. Arch Dis Child. 1989;64:1170-1176.
35 Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? J Am Acad Child Adolesc Psychiatry. 2004;43:6718-6726.
36 Waylen A, Wolke D. Sex ‘n’ drugs ‘n’ rock ‘n’ roll: The meaning and social consequences of pubertal timing. Eur J Endocrinol. 2004;151(Suppl 3):U151-U159.
37 Sorgo W, Kiraly E, Homoki J, et al. The effects of cyproterone acetate on statural growth in children with precocious puberty. Acta Endocrinol. 1987;115:44-56.
38 Comite F, Cutler GBJr, Rivier J, et al. Short-term treatment of idiopathic precocious puberty with a long-acting analogue of lutenizing hormone-releasing hormone. NEJM. 1981;305:1546-1550.
39 Kauli R, Galatzer A, Kornreich L, et al. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. A comparative study with re-evaluation of predictions by the Bayley-Pinneau method. Horm Res. 1997;47:54-61.
40 Bouvattier C, Coste J, Rodrigue D, et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: A randomized long-term pilot study. J Clin Endocrinol Metab. 1999;84:3378-3575.
41 Cassio A, Cacciari E, Balsamo A, et al. Randomised trial of LHRH analogue treatment on final height in girls with onset of puberty aged 7.7–8.5 years. Arch Dis Child. 1999;81:329-332.
42 Weise M, Armando F, Barnes KM, et al. Determinants of growth during gonadotropin-releasing hormone analog therapy for precocious puberty. J Clin Endocrinol Metab. 2004;89:103-107.
43 Oerter KE, Manasco P, Barnes KM, et al. Adult height in precocious puberty after long-term treatment with deslorelin. J Clin Endocrinol Metab. 1991;73:1235-1240.
44 Kauli R, Galatzer A, Kornreich L, et al. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. A comparative study with re-evaluation of predictions by Bayley-Pinneau method. Horm Res. 1997;47:54-61.
45 Brauner R, Adan L, Malandry F, et al. Adult height in girls with idiopathic true precocious puberty. J Clin Encodrinol Metab. 1994;79:415-420.
46 Adan L, Chemaitilly W, Trivin C, et al. Factors predicting adult height in girls with idiopathic central precocious puberty: Implications for treatment. Clin Endocrinol. 2002;56:297-302.
47 Khadilkar VV, Khadilkar VV, Maskati GB. Growth hormone and GnRHα combination therapy in the management of precocious puberty. Indian Pediatr. 2005;42:157-160.
48 Schultz KA, Sencer SF, Messinger Y, et al. Pediatric ovarian tumors: A review of 67 cases. Pediatr Blood Cancer. 2005;44:2167-2173.
49 Lumbroso S, Paris F, Sultan C. McCune-Albright syndrome: Molecular genetics. J Pediatr Endocrinol Metab. 2002;15(Suppl 3):875-882.
50 Lumbroso S, Paris F, Sultan C. Activating Gsα mutations: Analysis of 113 patients with signs of McCune-Albright syndrome—a European Collaboarative Study. J Clin Endocrinol Metab. 2004;89:2107-2113.
51 Roth C, Freiberg C, Zappel H, Albers N. Effective aromatase inhibition by anastrozole in a patient with gonadotropin-independent precocious puberty in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2002;3(Suppl 15):945-948.
52 Nunez SB, Calis K, Cutler GBJr, et al. Lack of efficacy of fadrozole in treating precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 2003;88:5730-5733.
53 Feuillan PP, Jones J, Cutler GBJr. Long-term testolactone therapy for precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 1993;77:647-651.
54 Abnormal puberty and growth problems. In: Speroff L, Glass RH, Kase NG, editors. Clinical Gynecologic Endocrinology and Infertility. 6th ed. Baltimore: Lippincott Williams & Wilkins; 1999:381-420.
55 Eugster EA, Rubin SD, Reiter EO, et al. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: A multicenter trial. J Pediatr. 2003;143:9-10.
56 Anasti JN, Flack MR, Froehlich J, et al. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab. 1995;80:276.
57 Ibáñez L, Virdis R, Potau N, et al. Natural history of premature pubarche: An auxological study. J Clin Endocrinol Metab. 1992;74:254.
58 Ibáñez L, Ferrer A, Ong K, et al. Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovarian syndrome. J Pediatr. 2004;144:23-29.
59 Griffin JE. Hormonal replacement therapy at the time of expected puberty in patients with gonadal failure. Endocrinologist. 2003;13:3211-3213.