Chapter 2 Normal or Innocent Murmurs
Seven types of normal systolic murmurs and three types of normal continuous murmurs are known (Box 2-1).1 Normal systolic murmurs include the vibratory murmur, the main pulmonary artery murmur, the branch pulmonary artery murmur of the neonate, the supraclavicular systolic murmur, the systolic mammary souffle, the aortic systolic murmur of older adults, and the cardiorespiratory systolic murmur. All normal systolic murmurs are midsystolic, except the mammary souffle, and are not loudest at the right base.2 Normal continuous murmurs include the venous hum, the continuous mammary souffle, and the continuous cephalic (cranial) murmur (see Box 2-1).
Normal murmurs are never solely diastolic, with one exception—the transient left basal holodiastolic or middiastolic ductus arteriosus murmur, sometimes heard during the first 3 or 4 days of life.3 A valve-like structure at the pulmonary arterial end of the ductus (see Chapter 20) is responsible for selective diastolic flow.3,4
Normal systolic murmurs
Vibratory Systolic Murmur
The normal vibratory midsystolic murmur was described by George F. Still in 1909 (Figures 2-1 and 2-2).5 Still wrote, “It is heard usually just below the level of the nipple, and about halfway between the left margin of the sternum and the vertical nipple line. … It’s characteristic feature is a twanging sound very like that made by twanging a piece of tense string. … Whatever may be it’s origin, I think it is clearly functional, that is to say, not due to any organic disease either congenital or acquired.”
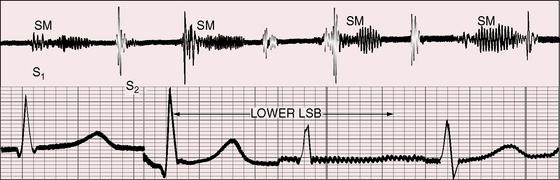
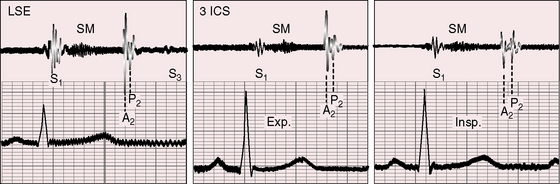
Still’s murmur is seldom heard in infants6 but is prevalent after age 3 years, with diminishing frequency toward adolescence.7,8–10 The murmur ranges from grade 1 to 3/6 and is loudest between the apex and lower left sternal edge in the supine position.9,11–14 During exercise, excitement, or fever, the murmur intensifies (see Figure 2-1).9 The quality is distinctive:9,15 vibratory or buzzing with a uniform, medium, pure frequency (70 to 130 cycles per second)16 that requires the stethoscopic bell for best assessment.11 The closest acoustic analogy is Still’s twanging of a taut rubber band or string (see previous quotation).17 The murmur begins shortly after the first heart sound and is typically confined to the first half of systole with a relatively long gap between the end of the murmur and the second heart sound (see Figures 2-1 and 2-2).
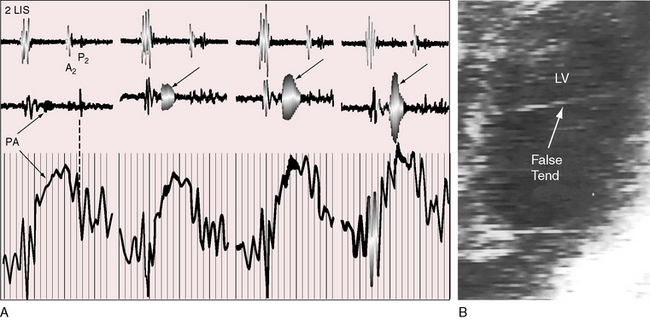
The mechanism of Still’s murmur remains to be established, but theories take into account the distinctive frequency composition and configuration, location on the chest wall, and incidence according to age. The pure medium frequency implies that a cardiac structure is set into periodic vibration during ventricular systole. Origin in the right side of the heart has been assigned to the pulmonary valve itself when “trigonoidation” of the leaflets results in periodic vibrations of the base of the cusps.10 A catheter across the pulmonary valve can tense the cusps and generate a transient pure-frequency midsystolic murmur (Figure 2-3A). The relatively low right ventricular ejection pressure and velocity are thought to cause the attachments of the pulmonary cusps to vibrate at a low to medium frequency. A murmur produced by a vibrating semilunar valve at its arterial attachment tends to be transmitted into the cavity of the concordant ventricle,2,18,19 which could account for the thoracic location of Still’s murmur between the apex and lower left sternal edge (i.e., topographically over the right ventricle). In children with Still’s murmur, Doppler echocardiocardiography has been used to identify systolic vibrations in the aortic valve and higher maximal acceleration of flow in the left ventricular outflow tract.14,20 However, a murmur that originates in a vibrating aortic valve is transmitted into the left ventricular cavity and heard best over the left ventricular impulse. Midsystolic murmurs in adults have been ascribed to high intraventricular velocities generated by vigorous left ventricular contraction associated with an increase in left ventricular mass.21 Origin of Still’s murmur has also been assigned to the left ventricular cavity, a location that is in accord with delayed response to the Valsalva’s maneuver.22 Left ventricular bands or false tendons (Figure 2-3B) are thought to vibrate periodically during ventricular systole and transmit their vibrations to the chest wall.23–25 A high percentage of patients with Still’s murmur reportedly have left ventricular bands, especially in the outflow tract.23–26 However, the prevalence rate of Still’s murmur declines from childhood to adolescence,9,10 whereas the prevalence rate of left ventricular bands is the same in children, adolescents, and adults.23,24,27 The incidence rate of Still’s murmur is thought to exceed the incidence rate of left ventricular bands,14 although incidence rate depends largely on the avidity with which bands are sought with echocardiography.
Pulmonary Artery Systolic Murmur
The normal systolic murmur in the main pulmonary artery is most prevalent in children, adolescents, and young adults.9,11,28 The murmur is midsystolic with maximal intensity in the second left intercostal space next to the sternum (Figure 2-4) and ranges from bare audibility to grade 3/6 in response to exercise, fever, or excitement. The frequency composition is medium pitched and impure, best heard in the supine position with the stethoscopic diaphragm or moderate pressure of the bell during full held exhalation.9,11,29 The murmur represents normal ejection vibrations that reach the threshold of audibility from within the main pulmonary artery during right ventricular systole. The chest wall location is appropriate for origin in the pulmonary trunk, and intracardiac phonocardiograms record midsystolic murmurs within the pulmonary trunk in healthy young subjects.29
These murmurs are commonly heard during pregnancy and in subjects with anemia or hyperthyroidism. Loss of thoracic kyphosis increases proximity of the pulmonary trunk to the chest wall and increases the incidence rate of pulmonary systolic murmurs in the second left interspace.30
Branch Pulmonary Artery Systolic Murmur
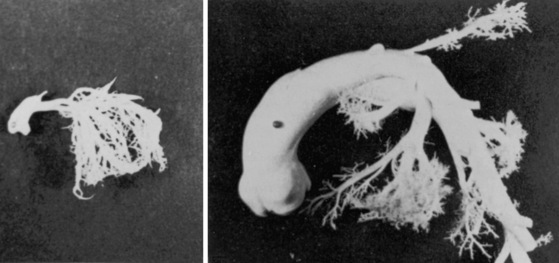
Branch pulmonary artery systolic murmurs are occasionally heard in healthy neonates, especially premature neonates.28,31–33 These murmurs are typically grade 1 to 2/6 and are medium pitched and impure but most importantly, are distributed to the left and right anterior chest, axillae, and back. The similarity of frequency composition to breath sounds, the rapid respiratory rate of infants, and the widespread thoracic locations of pulmonary artery systolic murmurs cause these murmurs to be overlooked. Audibility is improved if respiration is temporarily arrested with pinching the nostrils while the infant sucks a pacifier. Auscultation is best carried out with examination of the infant in both supine and prone positions and with use of the stethoscopic diaphragm applied to the right and left anterior chest, back, and axillae. The murmurs are typically confined to neonates, are usually absent at the first well-baby examination, and seldom persist beyond 3 to 6 months of age.13,28,34 The transient branch pulmonary artery systolic murmur in healthy neonates is indistinguishable from the peripheral murmur of fixed stenosis of the pulmonary artery and its branches (see Chapter 10). The analogy sheds light on the mechanism of production.28,32,33 The pulmonary trunk in the fetus is a relatively dilated domed structure because it receives the output of the high-pressure right ventricle. Proximal right and left pulmonary arteries arise from the pulmonary trunk as comparatively small lateral branches that receive a paucity of intrauterine blood flow. When the lungs expand at birth, the difference in size between the pulmonary trunk and its right and left branches transiently persists, especially in premature infants (Figure 2-5).34 In addition to the disparity in size, the branches arise at relatively sharp angles from the inferior and posterior walls of the pulmonary trunk. These anatomic arrangements account for both the turbulence and the physiologic drop in systolic pressure from pulmonary trunk to proximal branches and for the branch pulmonary artery systolic murmur.28,33
Supraclavicular Systolic Murmur
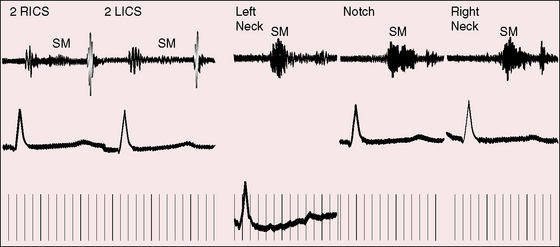
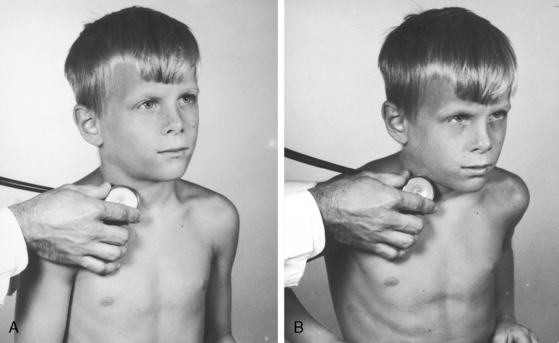
Normal supraclavicular systolic arterial murmurs are typically heard in children and young adults, are always maximal above the clavicles, tend to be louder on the right, are generally bilateral, and are prominent in the suprasternal notch (Figure 2-6).2,35 The weight of evidence assigns supraclavicular systolic murmurs to the aortic origins of major brachiocephalic arteries, especially the subclavians.36 Intensity can reach grade 4/6, may generate a thrill, and may be sufficient for radiation below the clavicles but with distinct attenuation (see Figure 2-6). The configuration of the supraclavicular systolic murmur is crescendo-decrescendo, the onset is abrupt, the duration is brief, and the timing is maximal in the first half or two thirds of systole (see Figure 2-6). The frequency composition is uneven, but the murmur is seldom noisy even when loud. Partial compression of the subclavian artery intensifies the murmur, whereas compression sufficient to obliterate the ipsilateral radial pulse has the opposite effect. Auscultation is most effectively carried out when the patient is sitting upright and looking straight ahead with shoulders relaxed and forearms and hands on the lap (Figure 2-7A).2,37 The stethoscopic bell is applied above the medial aspect of the right clavicle. The shoulders are then hyperextended, with elbows brought sharply behind the back until shoulder girdle muscles are taut (Figure 2-7B). When this maneuver is done smoothly but rapidly, the supraclavicular murmur diminishes considerably or disappears altogether.37
Systolic Mammary Souffle
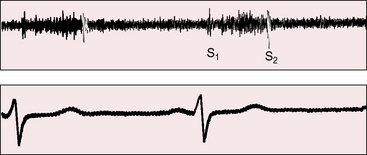
The souffle either is confined to systole or is louder in systole even when continuous (see subsequent discussion). As the name implies, the murmur is heard over the breasts late in pregnancy but especially postpartum in lactating women (Figure 2-8). The systolic component was recognized by van den Bergh in 1908.38 The murmur begins distinctly after the first heart sound because sufficient time must elapse from left ventricular ejection to arrival at the artery of origin. The length of the murmur ranges from short (midsystolic), to long (up to the second heart sound), to beyond the second heart sound into diastole (continuous; see subsequent discussion).
Aortic Systolic Murmur in Older Adults
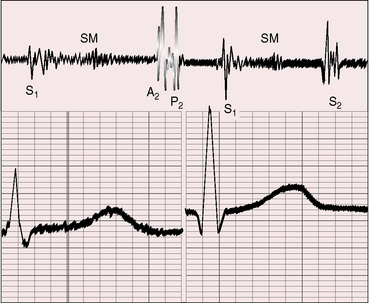
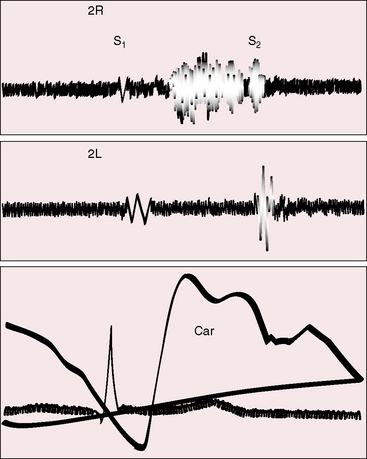
The most common form of normal midsystolic murmur in older adults is caused by fibrous or fibrocalcific thickening of the bases of inherently normal aortic cusps as they insert into the sinuses of Valsalva (Figure 2-9).39,40 As long as the fibrous or fibrocalcific thickening is confined to the base of the leaflets, the cusps move well, so no functional deficit is seen. These structural alterations of inherently normal trileaflet aortic valves affect 20% to 25% of adults over age 65 years.2,39–44 The fibrous, ridge-like thickenings that are initially confined to the base of the aortic cusps may subsequently extend to the free edges as fibrocalcific changes without commissural fusion. Because the initial morphologic changes do not impair cusp mobility and therefore do not cause obstruction, the accompanying murmur has been called the aortic sclerotic systolic murmur of older age. Intraarterial phonocardiography has identified midsystolic murmurs above the aortic valve before audibility on the chest wall occurs in clinically healthy adults over age 40 years.44 The fully developed aortic sclerotic murmur has two mechanisms and represents a combination of two murmurs. The murmur in the second right intercostal space is midsystolic, impure, and grade 2 or 3/6 and originates within the aortic root (mixed-frequency ejection vibrations of nonlaminar flow). The murmur heard over the left ventricular impulse is midsystolic, high-frequency musical, pure, and grade 2 to 3/6 and originates from periodic vibrations of the stiffened bases of aortic cusps at the sinus of Valsalva attachments (see Figure 2-9).2,18,19,39 These periodic vibrations have been recorded from aortic valve leaflets with M mode echocardiography.2 Intracardiac phonocardiography is used to detect the musical high-frequency murmur within the left ventricular cavity and the harsh impure right basal murmur within the aortic root (Gallavardin dissociation; see Figure 2-9).2 What begins as an aortic sclerotic murmur can culminate in severe calcific aortic stenosis. In adults with increased anteroposterior chest dimensions, the precordial murmur of calcific aortic stenosis can be deceptively soft and is best heard in the suprasternal notch.
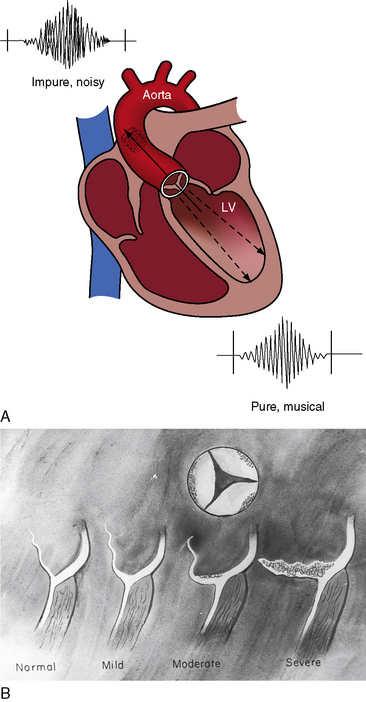
Figure 2-9 A, Illustration of Gallavardin dissociation of the two murmurs associated with fibrocalcific aortic stenosis of an inherently normal trileaflet aortic valve.68 The impure midsystolic murmur at the right base originates within the aortic root because of turbulence caused by the high-velocity jet. The pure musical midsystolic murmur at the apex originates from periodic high-frequency vibrations of the fibrocalcific aortic leaflets and radiates into the left ventricle cavity (LV). B, Schematic illustrations that begin with the normal attachment of an aortic cusp to its sinus of Valsalva (left). The initial alteration is a ridge-like fibrous thickening at the base of the aortic cusp as it inserts into its sinus. Calcium is subsequently deposited on the aortic surface of the leaflet (moderate) and ultimately extends to the free edge of the leaflet (severe), converting an inherently normal trileaflet valve into calcific aortic stenosis.
There is reason to believe that the essential morphologic substrate for the development of aortic sclerosis on an inherently normal trileaflet aortic valve is cuspal inequality—a congenital variation of normal—as originally proposed by Roberts.45 The ideal aortic valve is equipped with cusps of identical size so that closing forces are equally distributed on the aortic surfaces of the three leaflets. Congenital variations in aortic leaflet size—cuspal inequality—is the rule rather than the exception and results in unequal distribution of tension during valve closure, the long-term effects of which are thought to culminate in the morphologic changes of aortic sclerosis/stenosis.46 At its inception, the process is functionally benign, although there is an association between aortic valve sclerosis and cardiovascular mortality and morbidity.41,47 Echocardiography can be used to detect focal areas of increased echogenicity and thickening at aortic cusp attachments to the sinuses of Valsalva without restriction of leaflet motion.41 The auscultatory diagnosis of aortic sclerosis (see previous discussion) requires nothing more than proper use of a stethoscope.
Functionally Normal Bicuspid Aortic Valve
More than 400 years ago, Leonardo da Vinci sketched and described the bicuspid aortic valve.48 He wrote, “Diagrams to illustrate…the triangular shape of the aortic aperture. In figure 2-2 the customary three cusps are illustrated. In figure 2-3, only two cusps fill the orifice.”48 Functionally normal refers to a bicuspid aortic valve that has the trivial gradient and the trivial regurgitation inherent in a mechanism equipped with two rather than three leaflets. A bicuspid aortic valve at birth is either functionally normal or intrinsically stenotic; it can become “thick and unyielding” or conversely can become incompetent so that “blood which has entered the aorta is allowed to regurgitate into the ventricle,”49 as Thomas Peacock observed in 1858. It was William Osler in 1866 who called attention to the susceptibility of bicuspid aortic valves to infective endocarditis.50
Because of potential hazards, it is important to distinguish the murmur of a functionally normal congenitally bicuspid aortic valve from normal systolic murmurs that prevail in the same age group. Normal systolic murmurs in the young, with the exception of a systolic mammary souffle, are not heard maximally at the right base. A midsystolic murmur that is most prominent in the second right intercostal space in children or young adults arouses suspicion of a functionally normal bicuspid aortic, especially in males (relative male prevalence rate, 70% to 75%).45 Auscultation should then seek the confirmatory evidence of an aortic ejection sound that is most prominent at the apex where it is readily mistaken for the second component of a split first heart sound.2,51 The first component of a normally split first heart sound is louder at the apex, and the second component is louder at the lower left sternal border.2 Additional evidence of a functionally normal bicuspid aortic valve is the soft high-frequency early diastolic murmur of bicuspid aortic regurgitation that adds materially to the diagnosis. The murmur is heard best when firm pressure of the stethoscopic diaphragm is applied at the mid left sternal edge during held exhalation as the patient sits and leans forward. Isometric exercise (clenched fists), squatting, and Valsalva’s maneuver are used to improve audibility.2,52
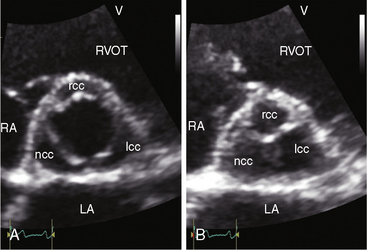
The combination of a midsystolic murmur at the right base, an aortic ejection sound at the apex, and a soft high-frequency early diastolic murmur at the left sternal border in a young male is used to establish the diagnosis of bicuspid aortic valve. When the ejection sound is equivocal and the aortic regurgitation murmur is absent, clinical suspicion rests on the right basal midsystolic murmur alone. The echocardiogram then plays a pivotal role. The normal trileaflet aortic valve in the short axis resembles the letter Y during diastole (Figure 2-10A) and resembles an inverted triangle during systole.53 A congenitally bicuspid aortic valve appears as a single linear band in diastole (Figure 2-10B) and appears as a fish mouth in systole. Color flow imaging is used to detect mild inaudible aortic regurgitation.
Cardiorespiratory Murmur
In 1915, Richard Cabot wrote, “Such murmurs may be heard under the left clavicle or below the angle of the left scapula, as well as near the apex of the heart—less often in other parts of the chest. … Cardiorespiratory murmurs may be either systolic or diastolic, but in the vast majority of cases are systolic. The area over which they are audible is usually a very limited one. They are generally affected by a position and by respiration, and are heard most distinctly if not exclusively during inspiration, especially at the end of that act.”54
Cardiorespiratory murmurs were known to Laënnec, but James Hope’s description is the most colorful. Hope described his examination of two university students: “Both wore very tight waistcoats, preventing the expansion of the lower ribs. During this state of breathing, a bellows murmur… existed in both. In both, the murmur ceased entirely when, unbuttoning their waistcoats and waistbands of their trousers, they breathed with the lungs naturally inflated. By altering the circumstances, the murmur could be created or removed at pleasure. I presume therefore that it proceeded from a cause exterior to the heart.”55
The mechanism responsible for the cardiorespiratory murmur is unclear, but its benign nature as well as its location, timing, and relation to respiration remain as Cabot originally described.54
Normal continuous murmurs
Venous Hum
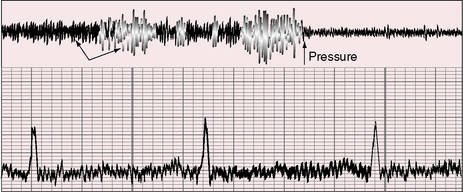
The venous hum was described by Potain56 in 1867 and is the most common type of normal continuous murmur. It is universal in children and occurs in healthy young adults,57,58 even in the absence of thyrotoxicosis, anemia, or pregnancy.2 Maximal intensity is in the supraclavicular fossa just lateral to the sternocleidomastoid muscle. The hum may radiate widely and is often bilateral but is usually more prominent on the right (Figure 2-11). A loud venous hum, especially in children, may radiate below the clavicles and be mistaken for a patent ductus arteriosus. Abolition of the hum with digital compression prevents this error (Figures 2-11 and 2-12). Intensity varies from faint to grade 6/6, and occasionally, patients are subjectively and unpleasantly aware of a loud hum that is sensed as audible pulsatile tinnitus.59–61
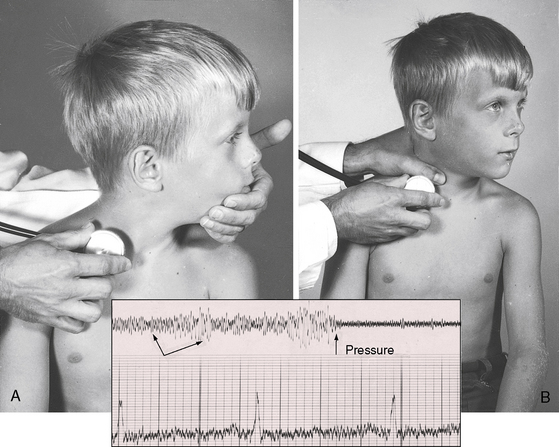
A venous hum is best elicited with the patient sitting upright. The stethoscope is held in the right hand of the examiner and the bell is applied to the medial aspect of the supraclavicular fossa (Figure 2-12A) while the examiner’s left hand grasps the patient’s chin from behind and pulls it tautly to the left and upward (see Figure 2-12A).2 Occasionally, the hum develops or increases when the chin is simply tilted upward, and a prominent hum is sometimes audible without neck maneuvers and irrespective of position. In a child who is either sitting or supine, a venous hum may appear when the patient’s head is voluntarily turned to the left or tilted upward. The hum may appear when the child looks up at the examiner and may disappear when the child looks down at the stethoscope. The hum is reduced or abolished with digital compression of the ipsilateral internal jugular vein (Figure 2-12B), with removal of the stretch on the neck as the head is returned to a neutral position, with the Valsalva’s maneuver, and with recumbency. The simplest procedure for abolishing the hum is compression of the deep jugular vein with the thumb of the free hand (see Figure 2-12B). Compression causes instantaneous obliteration of the hum (see Figure 2-11), which suddenly and transiently intensifies as pressure is released.2,58
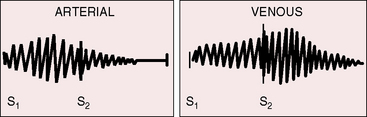
The term hum does not necessarily characterize the quality of these cervical venous murmurs, which can be rough and noisy and are occasionally accompanied by a high-pitched whine.58 The hum is truly continuous (see Figure 2-11) although typically louder in diastole, as is the case with venous continuous murmurs in general (Figure 2-13). The mechanism responsible for the venous hum is unclear. Laminar flow in the internal jugular vein may be disturbed by deformation at the level of the transverse process of the atlas during head rotation.62
Continuous Mammary Souffle
A second but far less common benign continuous murmur occurs during late pregnancy and early postpartum in healthy lactating women. A consensus supports the view that the mammary souffle is arterial in origin,63–65 an opinion originally held by both van den Bergh38 and Jones.66 Origin in superficial veins of the breast has less credibility.67 The delay in onset, accentuation in systole, relatively high frequency, and persistence during Valsalva’s maneuver are in accord with arterial origin.
The continuous mammary souffle was included in Jones’ 1951 book Heart Disease in Pregnancy.66 Soufflare is Latin, meaning to blow. Maximal intensity of the souffle can be anywhere over either breast, but the souffle tends to be louder in the second or third right or left intercostal space, and occasionally the souffle is bilateral.65 A distinct gap between the first heart sound and the onset of the murmur represents the interval between ejection of blood from the left ventricle and arrival of blood at the artery that gives rise to the souffle.63–65 The murmur is typically louder in systole, as is usually the case with continuous murmurs of arterial origin, with the diastolic portion often fading completely before the subsequent first heart sound (Figures 2-13 and 2-14). The pitch may be relatively high, but the murmur is not musical.65 Audibility is best with the patient supine, and the murmur may vanish in the upright position.65 Light pressure with the stethoscope tends to augment the murmur and bring out its continuous features.64 Firm pressure with the stethoscope or digital pressure adjacent to the site of auscultation can abolish the murmur completely.10,64,65 Intensity may vary from beat to beat, from hour to hour, and from day to day.65 Valsalva’s maneuver does not affect the intensity.64
The location of a continuous mammary souffle may arouse suspicion of patent ductus arteriosus or of an arteriovenous fistula. However, the typical ductus murmur peaks at the second heart sound, whereas the mammary souffle peaks much earlier (see Figure 2-14); and obliteration with local compression excludes patent ductus. An arteriovenous fistula may generate a continuous murmur that is maximal in systole and attenuates with pressure, but the cycle-to-cycle or day-to-day variation of the mammary souffle and its invariable disappearance after termination of lactation resolve the issue.
Cephalic (Cranial) Continuous Murmur
Low-intensity cephalic murmurs, which are usually continuous and less often systolic, are occasionally detected over the cranium of normal children under 4 years of age, especially in association with a febrile illness.13 These murmurs tend to be most prominent over the anterior fontanel, the temporal regions, or the orbits and are best heard with the diaphragm of the stethoscope. Because auscultation is not routinely applied to the head, cephalic murmurs are overlooked. Their mechanism is unknown.
1 Pelech A.N. The physiology of cardiac auscultation. Pediatr Clin North Am. 2004;51:1515-1535.
2 Perloff J.K., editor. Physical examination of the heart and circulation, 4th ed, Shelton, Connecticut: People’s Medical Publishing House, 2009.
3 Papadopoulos G.S., Folger G.M.Jr. Transient solitary diastolic murmurs in the newborn. Clin Pediatr (Phila). 1983;22:548-550.
4 Keith T.R., Sagarminaga J. Spontaneously disappearing murmur of patent ductus arteriosus. A case report. Circulation. 1961;24:1235-1238.
5 Still G.F. Common disorders and diseases of childhood. London: Frowde, Hodder & Stoughton; 1909.
6 Braudo M., Rowe R.D. Auscultation of the heart: early neonatal period. Am J Dis Child. 1961;101:575-586.
7 Advani N., Menahem S., Wilkinson J.L. The diagnosis of innocent murmurs in childhood. Cardiol Young. 2000;10:340-342.
8 De Monchy C., Van Der Hoeven G.M., Beneken J.E. Studies on innocent praecordial vibratory murmurs in children. 3. Follow-up study of children with an innocent praecordial vibratory murmur. Br Heart J. 1973;35:685-690.
9 Fogel D.H. The innocent systolic murmur in children: a clinical study of its incidence and characteristics. Am Heart J. 1960;59:844-855.
10 Humphries J.O., McKusick V.A. The differentiation of organic and “innocent” systolic murmurs. Prog Cardiovasc Dis. 1962;5:152-171.
11 Castle R.F., Craige E. Auscultation of the heart in infants and children. Pediatrics. 1960;26:511-561.
12 Lessof M., Brigden W. Systolic murmurs in healthy children and in children with rheumatic fever. Lancet. 1957;273:673-674.
13 Rosenthal A. How to distinguish between innocent and pathologic murmurs in childhood. Pediatr Clin North Am. 1984;31:1229-1240.
14 Van Oort A., Hopman J., De Boo T., Van Der Werf T., Rohmer J., Daniels O. The vibratory innocent heart murmur in schoolchildren: a case-control Doppler echocardiographic study. Pediatr Cardiol. 1994;15:275-281.
15 Goldblatt E. Diseases of the heart and blood-vessels. Innocent systolic murmurs in childhood. Br Med J. 1966;2:95-98.
16 Harris T.N., Lisker L., Needleman H.L., Saltzman H.A. Spectrographic comparison of ranges of vibration frequency among some innocent cardiac murmurs in childhood and some murmurs of valvular insufficiency. Pediatrics. 1957;19:57-67.
17 Guntheroth W.G. Innocent murmurs: a suspect diagnosis in non-pregnant adults. Am J Cardiol. 2009;104:735-737.
18 Perloff J.K. Clinical recognition of aortic stenosis: the physical signs and differential diagnosis of the various forms of obstruction to left ventricular outflow. Prog Cardiovasc Dis. 1968;10:323-352.
19 Roberts W.C., Perloff J.K., Costantino T. Severe valvular aortic stenosis in patients over 65 years of age. A clinicopathologic study. Am J Cardiol. 1971;27:497-506.
20 Klewer S.E., Donnerstein R.L., Goldberg S.J. Still’s-like innocent murmur can be produced by increasing aortic velocity to a threshold value. Am J Cardiol. 1991;68:810-812.
21 Spooner P.H., Perry M.P., Brandenburg R.O., Pennock G.D. Increased intraventricular velocities: an unrecognized cause of systolic murmur in adults. J Am Coll Cardiol. 1998;32:1589-1595.
22 Barlow J.B., Bosman C.K. The origin of the innocent vibratory systolic murmur. S Afr J Med Sci. 1965;30:96.
23 Darazs B., Hesdorffer C.S., Butterworth A.M., Ziady F. The possible etiology of the vibratory systolic murmur. Clin Cardiol. 1987;10:341-346.
24 Malouf J., Gharzuddine W., Kutayli F. A reappraisal of the prevalence and clinical importance of left ventricular false tendons in children and adults. Br Heart J. 1986;55:587-591.
25 Perry L.W., Ruckman R.N., Shapiro S.R., Kuehl K.S., Galioto F.M.Jr, Scott L.P.3rd. Left ventricular false tendons in children: prevalence as detected by 2-dimensional echocardiography and clinical significance. Am J Cardiol. 1983;52:1264-1266.
26 Gerlis L.M., Wright H.M., Wilson N., Erzengin F., Dickinson D.F. Left ventricular bands. A normal anatomical feature. Br Heart J. 1984;52:641-647.
27 Gardiner H.M., Joffe H.S. Genesis of Still’s murmurs: a controlled Doppler echocardiographic study. Br Heart J. 1991;66:217-220.
28 Rodriguez R.J., Riggs T.W. Physiologic peripheral pulmonic stenosis in infancy. Am J Cardiol. 1990;66:1478-1481.
29 Lewis D.H., Ertugrul A., Deitz G.W., Wallace J.D., Brown J.R.Jr, Moghadam A.N. Intracardiac phonocardiography in the diagnosis of congenital heart disease. Pediatrics. 1959;23:837-853.
30 Deleon A.C.Jr, Perloff J.K., Twigg H., Majd M. The straight back syndrome: clinical cardiovascular manifestations. Circulation. 1965;32:193-203.
31 Arlettaz R., Archer N., Wilkinson A.R. Closure of the ductus arteriosus and development of pulmonary branch stenosis in babies of less than 32 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;85:F197-F200.
32 Barrillon A., Havy G., Scebat L., Baragan J., Gerbaux A. Congenital pressure gradients between main pulmonary artery and its primary branches. Br Heart J. 1974;36:669-675.
33 Danilowicz D.A., Rudolph A.M., Hoffman J.I., Heymann M. Physiologic pressure differences between main and branch pulmonary arteries in infants. Circulation. 1972;45:410-419.
34 Arlettaz R., Archer N., Wilkinson A.R. Natural history of innocent heart murmurs in newborn babies: controlled echocardiographic study. Br Med J. 1998;78:F166.
35 Stapleton J.F., El-Hajj M.M. Heart murmurs simulated by arterial bruits in the neck. Am Heart J. 1961;61:178-183.
36 Kawabori I., Stevenson J.G., Dooley T.K., Phillips D.J., Sylvester C.M., Guntheroth W.G. The significance of carotid bruits in children: transmitted murmur or vascular origin, studies by pulsed Doppler ultrasound. Am Heart J. 1979;98:160-167.
37 Nelson W.P., Hall R.J. The innocent supraclavicular arterial bruit—utility of shoulder maneuvers in its recognition. N Engl J Med. 1968;278:778.
38 Van Den Bergh AaH. Een Schijnbaar Hartgeruisch. Ned Tijdschr Geneeskd. 1908;52:1104.
39 Bruns D.L., Van Der Hauwaert L.G. The aortic systolic murmur developing with increasing age. Br Heart J. 1958;20:370-378.
40 Pomerance A. Cardiac pathology and systolic murmurs in the elderly. Br Heart J. 1968;30:687-689.
41 Otto C.M., Lind B.K., Kitzman D.W., Gersh B.J., Siscovick D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142-147.
42 Howard T.H. Cardiac murmurs in old age: a clinico-pathological study. J Am Geriatr Soc. 1967;15:509.
43 Perez G.L., Jacob M., Bhat P.K., Rao D.B., Luisada A.A. Incidence of murmurs in the aging heart. J Am Geriatr Soc. 1976;24:29-31.
44 Stein P.D., Sabbah H.N. Aortic origin of innocent murmurs. Am J Cardiol. 1977;39:665-671.
45 Roberts W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72-83.
46 Vollebergh F.E., Becker A.E. Minor congenital variations of cusp size in tricuspid aortic valves. Possible link with isolated aortic stenosis. Br Heart J. 1977;39:1006-1011.
47 Nightingale A.K., Horowitz J.D. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 2005;91:1389-1393.
48 O’Malley C.D., Saunders J.B. Leonardo on the human body. New York: Dover Publications; 1983.
49 Peacock T.B. On malformations of the human heart. London: John Churchill; 1858.
50 Osler W. The bicuspid condition of the aortic valve. Trans Assoc Am Physicians. 1886;2:185.
51 Leech G., Mills P., Leatham A. The diagnosis of a non-stenotic bicuspid aortic valve. Br Heart J. 1978;40:941-950.
52 Nishimura R.A., Tajik A.J. The Valsalva maneuver-3 centuries later. Mayo Clin Proc. 2004;79:577-578.
53 Child J.S. Transthoracic and transesophageal echocardiographic imaging: anatomic and hemodynamic assessment. In: Perloff J.K., Child J.S., editors. Congenital heart disease in adults. Phildelphia: W.B. Saunders Company, 1998.
54 Cabot R.C. Physical diagnosis. New York: William Wood and Company; 1915.
55 Hope J. A treatise on the diseases of the heart and great vessels. London: William Kidd; 1839.
56 Potain S.C. Des mouvements et des bruits qui se passent dans les veines jugulaires. Bull Mem Soc Med Hop Paris. 1867;4:3.
57 Hardison J.E. Cervical venous hum. A clue to the diagnosis of intracranial arteriovenous malformations. N Engl J Med. 1968;278:587-590.
58 Jones F.L.Jr. Frequency, characteristics and importance of the cervical venous hum in adults. N Engl J Med. 1962;267:658-660.
59 Cary F.H. Symptomatic venous hum. Report of a case. N Engl J Med. 1961;264:869-870.
60 Chandler J.R. Diagnosis and cure of venous hum tinnitus. Laryngoscope. 1983;93:892-895.
61 Rothstein J., Hilger P.A., Boies L.R.Jr. Venous hum as a cause of reversible factitious sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1985;94:267-268.
62 Cutforth R., Wiseman J., Sutherland R.D. The genesis of the cervical venous hum. Am Heart J. 1970;80:488-492.
63 Grant R.P. A precordial systolic murmur of extracardiac origin during pregnancy. Am Heart J. 1965;52:944.
64 Scott J.T., Murphy E.A. Mammary souffle of pregnancy: report of two cases simulating patent ductus arteriosus. Circulation. 1958;18:1038-1043.
65 Tabatznik B., Randall T.W., Hersch C. The mammary souffle of pregnancy and lactation. Circulation. 1960;22:1069-1073.
66 Jones A.M. Heart disease in pregnancy. London: Harvey & Blythe; 1951.
67 Hurst J.W., Staton J., Hubbard D. Precordial murmurs during pregnancy and lactation. N Engl J Med. 1958;259:515-517.
68 Gallavardin L., Ravault P. Le souffle de retrecissement aortique peut changer de timbre et devenir musical dans sa propagation apexiene. Lyon Med. 1925;135:523.