Chapter 6 Normal Fertilization and Implantation
HISTORICAL PROSPECTIVE
Before the initiation of modern reproductive and developmental biology in the 17th century, the theory of “seeds” belonging to the pluralistic current of the Pythagorean School led by Anaxagoras of Clazomenae and Empedocles of Akragas was the most believed (5th century B.C.).1 In the field of human reproduction, pluralism means that a fetus results from the mixing of two parental seeds. Hippocrates (c. 460–370 B.C.) believed that “seeds” are produced in all parts of the body, each containing both the masculine and feminine principle; when transmitted to offspring at the time of conception, they cause certain parts of the offspring to resemble their parents. A century later, Aristotle (384–322 B.C.) rejected Hippocrates’ theory. For him, only the male’s seed contributes to form the fetus; the female’s role in reproduction is to contribute menstrual blood. He had noticed that offspring sometimes resemble their grandparents rather than their parents. It was difficult to understand how seed of tissue and blood could be maintained unnoticed in the parents only to be revealed again in the children. Aristotle proposed that male semen was a mix of ingredients that was not blended perfectly so that materials from previous generations could sometimes get through. Most of Aristotle’s ideas were presented in his treatise, On Generation of Animals. This was the very first complete embryologic work ever written. Moreover, he was the first to illustrate his treatises, which significantly helped to clarify his writings.
Galen (130–201 A.D.), who is considered the greatest Greek physician after Hippocrates and founder of experimental physiology, supported Hippocrates’ theory that the seeds of both man and woman contribute to reproduction, but that each contains only one principle.1 In the 17th century, several outstanding findings fueled new scientific developments in modern reproductive biology. William Harvey (1578–1657) was the first to suggest that humans and other mammals reproduced via the fertilization of an oocyte by sperm. However, many authors see Regnier de Graaf (1641–1673) as the founder of modern reproductive biology.2 De Graaf is the one who discovered (1672) the source of oocytes in the testes of women, which we now call ovaries. Five years later the initial discovery of spermatozoa was made by a medical student named John Ham, who told Anton van Leeuwenhoek of the presence of what was termed animalcules in human seminal fluid, thought to have arisen from putrefaction. Leeuwenhoek (1632–1723) was the first to make detailed and accurate identification of sperm as a normal constituent of semen.3 Van Leeuwenhoek proposed that fertilization occurs when the sperm enters the oocyte, but this could not actually be observed for another 100 years because of the quality of microscopes available.
Another discovery that revolutionized scientific thinking was by the Italian priest and physiologist Lazaro Spallanzani in 1779. Until that time, our understanding of reproduction was based on our knowledge of how plants grow. It was believed that the embryo was the “product of male seed, nurtured in the soil of the female.” Spallanzani’s experiment established for the first time that for an embryo to develop there must be actual physical contact between the oocyte and the sperm. Spallanzani successfully inseminated frogs, fish, and dogs. The first successful artificial insemination of a woman was recorded just 11 years after Spallanzani’s experiment. In 1790, the renowned Scottish anatomist and surgeon, Dr. John Hunter, reported successful insemination of the wife of a linen draper using her husband’s sperm. These discoveries led to the emergence of modern reproductive technologies, by which the world’s first IVF baby was born just before midnight on 25 July 1978 as a result of continued efforts of Drs. Edwards and Steptoe.
SPERM TRANSPORT IN THE MALE REPRODUCTIVE TRACT
Spermatogenesis refers to the complex process of transformation of germline stem cells into sperm cells within the seminiferous tubules of the testes. Spermatogenesis is regulated through endocrine interactions between the pituitary gland and Sertoli cells. This endocrine system is referred to as the hypothalamic-pituitary-gonadal axis and involves a series of signaling mechanisms. Two hormones, follicle-stimulating hormone (FSH) secreted by the pituitary, and androgens (i.e., testosterone) produced by the Leydig (interstitial) cells in the testis, control Sertoli cell functions. Once formed within the seminiferous tubules, the immotile spermatozoa are released into the luminal fluid and transported to the epididymis, where they gain the ability to move and fertilize the oocyte.4 The testicular spermatozoa are transported passively to the rete testis, which is a branched reservoir of the openings of the seminiferous tubules. From the rete testis, the spermatozoa are transported to the epididymis via the efferent ductules.5 In mammals, the transit of spermatozoa through the epididymis usually takes 10 to 13 days; in humans the estimated transit time is 2 to 6 days.6 The epididymal segment where most spermatozoa attain their full fertilizing capacity appears to be the proximal cauda. The spermatozoa from that region are capable of moving progressively, which is characteristic of spermatozoa preceding fertilization, and bind to zona-free hamster ova in vitro at a higher percentage than spermatozoa obtained from more proximal locations.7
To attain the capacity to fertilize an oocyte, sperm undergo many maturational changes during its transit in the epididymal duct.4 These include, for instance, changes in plasma membrane lipids, proteins, and glycosylation; alterations in the outer acrosomal membrane; gross morphologic changes in acrosome in some species; and cross-linking of nuclear protamines and proteins of the outer dense fiber and fibrous sheath. The cauda epididymidis (and proximal ductus deferens) are the regions where spermatozoa are stored before ejaculation.7 When ejaculation occurs, the stored spermatozoa with the surrounding fluid are mixed with the alkaline secretions of the male accessory sex glands and deposited in the vagina.
SPERM TRANSPORT IN THE FEMALE REPRODUCTIVE TRACT
Spermatozoa are actively transported from the vagina via the cervical canal and the uterine cavity to the ampulla of the oviducts, where fertilization occurs. In the human vagina, the ejaculated semen is deposited near the external cervical opening, where the environment is very acidic due to lactic acid and thus is hostile to spermatozoa.8 The alkaline pH of the ejaculate protects spermatozoa in this acidic environment.9 This protection is, however, temporary, and most spermatozoa only remain motile in the vagina for a few hours. Thus, the human vagina does not serve as an effective reservoir for spermatozoa and the role of the vagina in sperm transport is transitory at best. There is significant sperm loss in the vagina, and the proportion of ejaculated sperm that enter the cervical mucus in vivo is not known. The spermatozoa are transported into the cervical canal by pressure alterations in the vagina due to the female orgasm, assisted by the normal motility of sperm. Migration of spermatozoa through the cervical canal is thought to be dependent at least in part on sperm concentration, motility, and morphology and is modulated by the physiochemical characteristics of cervical mucus. The interactions between sperm and mucus and the motility of spermatozoa during transport are important; one cause of infertility is presumably impaired sperm movement through the cervical mucus.9 The change in the composition of cervical mucus at midcycle also affects the passage of sperm.10 In ovulatory mucus, sperm penetration and forward progression occur in an efficient and directional manner. In this phase of the cycle, mucin molecules are arranged in a parallel position, directing the spermatozoa into the uterus and also to the cervical crypts where they are stored.11
It is not clear whether the human female reproductive tract has the capacity to establish sperm reservoirs as do the resproductive tracts of other mammals. However, it has been shown that the release of spermatozoa from human cervical crypts may continue for several days.4 In a small percentage of women displaying no sperm in the cervical mucus, however, motile sperm can be recovered from the uterine cavity.12 The uterus seems to be a conduit to sperm transport. The transport of spermatozoa from the cervix to the uterotubal junction is mainly attributable to the myometrial contractility, ciliary movements on the surface of the endometrial cells, and intrinsic sperm motility, although the latter has been shown not to be critical.4 The human endometrium prepares for ovulation by secreting a unique kind of fluid into the uterine lumen. The fluid has a different protein pattern, ionic composition, and volume than at other stages of the cycle.13 This fluid serves to suspend spermatozoa and to keep them viable during the transport process and to remove the coating from the sperm surface as one facet of capacitation. It also contains macrophages that remove dead and nonviable spermatozoa, which in mice is probably the most important physiologic mechanism for the disposal of sperm from the uterus.14 The passage of sperm from the uterus to the fallopian tube is apparently modulated by the uterotubal junction. This segment prevents the entry of nonmotile cells and therefore acts as a selective barrier. The isthmus functions as a sperm reservoir and only a few sperm pass along the fallopian tube to the site of fertilization at any given time.11 The ampulla of the oviduct is the site of fertilization, and motile spermatozoa can be found there up to 85 hours after intercourse. The transport of spermatozoa through the oviducts is a combination of sperm motility, fluid flow, and the contractive movements of the oviduct walls.8
Sperm Capacitation
Immediately after deposition in the female genital tract, besides active motility, the sperm are not able to fertilize the oocyte. The physiologic changes that occur to sperm during their transport in the female reproductive tract are collectively referred as capacitation. Capacitation, first described in 1951 (independently by Chang in the United States and Austin in Australia), was later shown to be a requirement for fertilization. It seems that the initiation and completion of capacitation in human spermatozoa takes place in the cervix.4,9 The molecular events that initiate capacitation include removal of cholesterol from the sperm plasma membrane, increase in membrane fluidity, ion influxes resulting in alteration of sperm membrane potential, hyperpolarization of sperm membrane, increase in tyrosine phosphorylation, and changes in the adenylate cyclase-cAMP system, nucleus, and acrosome.4,15 In humans, capacitation can be mimicked in vitro in defined culture media, the composition of which is based on the electrolyte concentration of oviductal fluid. In most cases capacitation media contain energy substrates such as pyruvate, lactate, and glucose; a cholesterol receptor (such as serum albumin); NaHCO3, calcium, potassium, and physiologic sodium concentrations.
Hyperactivation and Acrosome Reaction
Capacitation, as a molecular event, occurs both in the sperm head (i.e., the acrosome reaction) and in the tail (i.e., changes in the sperm motility pattern designated as sperm hyperactivation). Sperm hyperactivation occurs before the acrosome reaction.4 The vigorous pattern of movement observed in hyperactivation is a result of physiologic changes in spermatozoa. Compared to sperm in seminal plasma, the speed, velocity, rate of flagellar beating or beat frequency, and mean beat width (lateral head displacement) are increased in capacitated sperm. This pattern of movement enables the spermatozoa to swim in the viscous oviduct fluid and to overcome the physical resistance of the three vestments—cumulus oophorus, corona radiata, and the zona pellucida—which surround the oocyte.16,17 Hyperactivation is a prognostic indicator for IVF; low levels are associated with male infertility and decreased binding to the zona pellucida.
The endpoint of capacitation is the acrosome reaction, which occurs when spermatozoa come into close contact with the oocyte in the ampullae of the oviduct. The acrosome reaction is an exocytotic event and enables spermatozoa to penetrate through the zona pellucida and fuse with the oocyte plasma membrane. Many artificial stimuli are reported to trigger the acrosome reaction, either by driving extracellular Ca2+ into the sperm cell (Ca2+ ionophores) or by acting through intracellular second messengers that are involved in the cascade leading to acrosomal exocytosis. The anterior region of the sperm head is covered by the acrosome, containing several hydrolytic enzymes, including proteases, phosphatases, arylsulfatases, and phospholipases. Hyaluronidase and acrosin have been the most extensively studied of these enzymes. The membranes surrounding the nucleus of spermatozoa are the nuclear membrane, the inner acrosomal membrane, the outer acrosomal membrane, and the plasma membrane. In this reaction, the plasma membrane and the outer acrosomal membrane fuse, enabling release of the acrosomal contents important for the events preceding fertilization.4 The acrosome reaction may facilitate recognition, adhesion, and fusion with oocytes by at least three different mechanisms: externalization of ligand proteins (e.g., CD46 on the inner acrosomal membrane); protein migration through the fluid membrane to reach binding sites, such as that for PH-20; or conformational changes of preexisting membrane proteins.18
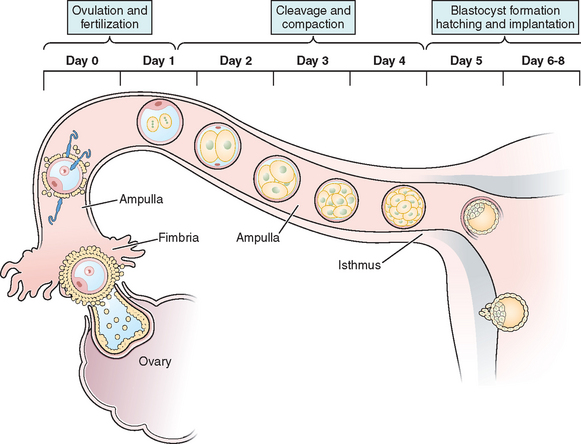
OOCYTE TRANSPORT
The transport of oocytes from the ovarian follicles to the site of implantation has been long recognized as a fundamental step in the reproductive process in the female.19 The fallopian tubes provide the path and means of transport for the ovum from the ovary to the uterus; the duration of ovum transport is the time from its discharge from the follicle until it reaches the normal site of implantation.20 At midmenstrual cycle, approximately day 14 of an idealized 28-day cycle, the surge of pituitary luteinizing hormone (LH) results in the maturation of the oocyte by resumption of meiosis and completion of the first meiotic division. The oocyte nucleus or germinal vesicle undergoes a series of changes that involve germinal vesicle breakdown. The oocyte then enters into the second meiotic division and arrests in the second metaphase or first polar body stage. Meiosis will proceed no further unless the oocyte is fertilized. Meiotic maturation is a vital event in ovulation because it is obligatory for normal fertilization. During the process of meiotic maturation, the cumulus granulosa cells undergo mucification followed by expansion.21 The preovulatory surge of FSH appears to initiate the process of cumulus expansion. The onset of mucification is marked by a dramatic increase in the secretion of mucopolysaccharides into the extracellular spaces. This leads to the dispersal of the cumulus cells and causes the oocyte–cumulus complex to expand tremendously.
After ovulation and discharge of the follicular fluid, the oocyte–cumulus complex, consisting of an oocyte surrounded by a zona pellucida, noncellular porous layers of glycoprotein secreted by the oocyte, and granulosa cells (cumulus oophorus), is ovulated from ovarian follicles into the peritoneal cavity and is picked up by the infundibulum of the oviduct.8 The infundibulum is a highly specialized, funnel-shaped portion of oviduct (up to 10 mm in diameter) that bears long, fingerlike extensions of the mucosa (i.e., fimbriae), which sweep over the surface of the ovary; the oocyte–cumulus complex is transported by means of ciliary action along the surface of the fimbriae toward the opening of the oviduct. Cilia that cover the exterior surface of the infundibulum beat in the direction of the ostium and are important in moving the oocyte–cumulus complex into the oviduct. Oocyte pickup is not dependent on a suction effect secondary to muscle contractions, and ligation of the tube just proximal to the fimbriae in the rabbit does not interfere with pickup.22 The mucosal layer of the fallopian tube is lined with a single layer of columnar epithelium that undergoes cyclic changes in response to hormonal changes of the menstrual cycle. There are two distinct types of oviductal epithelium—ciliated and nonciliated secretory epithelium—and the ratio of these two fluctuate in response to ovarian steroids. There are fewer ciliated cells in the isthmus than in the ampullary portion of the tube, whereas they are most prominent in the fimbriated infundibulum. Ciliary activity is responsible for the pickup of oocytes by the fimbrial ostium and for movement through the ampulla, as well as the distribution of the tubal fluid, which supports gamete maturation and fertilization and facilitates gamete and embryo transport. Under the influence of the estrogens of the follicular phase of the menstrual cycle, the cilia of the isthmus beat in the direction of the ovary. At the same time, the cilia of the fimbria and the cilia of the ampulla next to the fimbria beat in the direction of the uterus. In this fashion, the ovulated oocyte will be sequestered within the middle of the ampulla. If sperm enter the uterus at this time, they will be directed into the oviduct and propelled to the awaiting oocyte.
It is within the ampulla that fertilization takes place; in humans the oocyte spends about 90% of its stay in the ampulla. After surgical reversal of tubal ligation where a large part of the ampulla was removed, excellent pregnancy rates result along with mildly increased rates of ectopic pregnancies. Pregnancy is possible after reversal of tubal ligation where complete excision of the entire ampula or fimbria was performed, but pregnancy rates are poor. After ovulation and in the presence of an increasing concentration of progesterone, the cilia and myosalpingeal contractions all pulse from the ovary toward the uterus.23 In most species, transport of the fertilized oocyte through the tube requires approximately 3 days.19 Tubal fluid is rich in mucoproteins, electrolytes, and enzymes. This fluid is abundant in midcycle when gametes or embryos are present and may play an important role during fertilization and early cleavage. Fluid in the tubes is believed to be formed by selective transudation from the blood and active secretion from the epithelial lining.
SUCCESSIVE FERTILIZATION STEPS
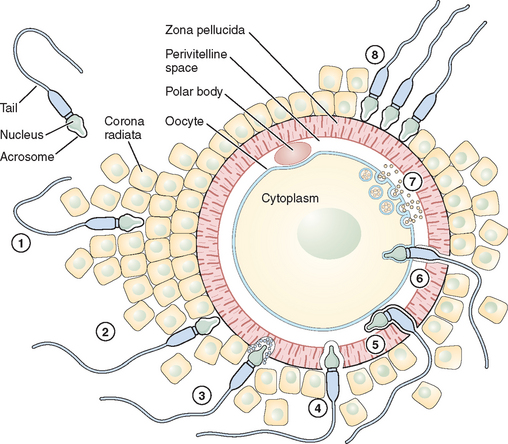
Fertilization is a series of steps that require immense coordination and communication between the two principal participants, the oocyte and sperm. Each gamete must undergo a series of changes before the final event of union can occur (Figs. 6-1 and 6-2).
Adhesion of Spermatozoa to Oocyte
Sperm Penetration of the Cumulus Oophorus
The cumulus oophorus is a specialized layer of granulosa cells that surrounds the ovulated mammalian oocyte. During oocyte maturation, the cumulus cell mass undergoes expansion and mucification, resulting in a well-expanded mass with an extensive amount of extracellular matrix material binding the cells together. The cumulus cells and their matrix are probably involved in several reproductive processes, including pickup of the oocyte–cumulus complex by the oviduct and increasing the chances of an encounter with one of the few sperm that have reached the ampulla of the tube.24 The extracellular matrix consists of a variety of glycoproteins and proteoglycans, with hyaluronic acid being a major component. The mechanisms of sperm penetration through the cumulus mass may involve both mechanical and chemical forces. Hyperactivated sperm and membrane-bound hyaluronidase are necessary, and perhaps sufficient, to digest a path through the extracellular matrix of the cumulus cells. The role of hyaluronidase in cumulus mass penetration is controversial because sperm remain largely acrosome-intact during in vitro penetration of the cumulus; sperm with no hyaluronidase can penetrate the cumulus mass and reach the zona pellucida.25 The role of the cumulus cell mass in fertilization is not fully understood. In human fertilization, however, a body of evidence has shown that the presence of the cumulus oophorus is beneficial for fertilization, partly by stimulation of proacrosin conversion to acrosin and initiation of the acrosome reaction. However, removal of the cumulus does not prevent sperm penetration and fertilization.
Sperm Interaction with the Zona Pellucida
Eventually after a long journey and penetration of the cumulus mass, the sperm encounters the zona pellucida, the cell type-specific extracellular matrix or coat of the oocyte where species-specific gamete recognition is believed to occur. The mammalian zona pellucida is composed of the transcripts of three highly conserved gene families named ZPA, ZPB, and ZPC. The mature products of the genes based on their deduced amino acid sequence are classified as ZP1, ZP2, and ZP3. They are synthesized during oogenesis as a product of the oocyte itself and exhibit heterogeneity because of extensive post-translational modification, including glycosylation.26 Determining the function of individual ZP proteins in humans has been difficult because of the limited availability of native human ZP, and much of the research on the zona pellucida and sperm recognition of the zona pellucida has been carried out using the mouse as a model system. In this species, the ZP3 protein serves as a sperm receptor molecule, mediating binding of the sperm to the intact zona pellucida. The currently favored model is that O-linked oligosaccharide chains on ZP3 act as a binding epitope for sperm. ZP3 is the primary ligand for sperm, whereas ZP2 in the mouse acts as a secondary sperm receptor that binds acrosome-reacted sperm and ZP1 is considered as a scaffold-like protein that appears to cross-link the ZP2 and ZP3 proteins.27 The complexity of the sperm membrane architecture, along with the difficulty of isolating and purifying it, have made the identification of the spermatozoon counterpart of the oocyte coat ligand a difficult task.
Sperm proteins thought to participate in zona binding have been identified by a range of approaches, including inhibitory monoclonal antibodies. Some of these sperm proteins have been specifically implicated as primary sperm receptors for ZP3, whereas others are thought to function as generalized adhesive proteins for the zona matrix. Thus far, among the sperm proteins thought to bind ZP3 oligosaccharides in particular, the sperm surface enzyme β1,4-galactosyltransferase-I (GalT) and two sperm membrane proteins called sp56 and sp95 are the main candidates; GalT satisfies virtually all the criteria expected of a ZP3 receptor.25,28 It presumably recognizes and binds specifically to N-acetylglucosamine residues on ZP3, an enzyme–substrate reaction in which ZP3 serves as substrate. The location of GalT, on the plasma membrane covering the dorsal aspects of the anterior region of the sperm head, is consistent with the inability of acrosome-reacted sperm to bind the zona pellucida because the plasma membrane bearing GalT is lost during the acrosome reaction.29 The binding of ZP3 to sperm activates a range of intracellular signal cascades that culminate in fusion of the plasma membrane and underlying outer acrosomal membrane (i.e., the acrosome reaction).30 The ability of ZP3 to act as an acrosome reaction inducer depends on its polypeptide chain, whereas its ability to act as a sperm receptor rests entirely on its oligosaccharide side chains. ZP3-induced exocytosis of the acrosomal contents proceeds through two sperm-signaling pathways. In the first, ZP3 binding to GalT and other potential receptors results in activation of a heterotrimeric GTP-binding protein and phospholipase C, thus elevating the concentration of cytoplasmic calcium. In the second pathway, ZP3 binding to the same receptor(s) stimulates a transient influx of calcium through T-type channels. In a later phase of the signaling, these initial ZP3-induced events produce additional calcium entry through transient receptor potential proteins, candidate subunits of the ion channels, resulting in a sustained increase in cytoplasmic calcium concentration that triggers exocytosis.24,31
Sperm–Oocyte Membrane Fusion
After sperm bind to the zona pellucida and the acrosome reacts, they penetrate the zona and enter the perivitelline space, the extracellular region between the zona and the oocyte plasma membrane where the final adhesion in the fertilization process occurs (i.e., adhesion of the sperm plasma membrane to the oocyte plasma membrane). Spermatozoa entering the perivitelline space approach the oocyte surface at an angle that lies somewhere between the vertical and the horizontal. Mammalian oocytes are spherical cells covered with microvilli before fertilization. The sperm head is like a flat dish, and the thickness of the head (∼0.2 μm) is a little less than the average distance between oocyte microvilli.32 Spermatozoa, therefore, just pass between the microvilli and make contact with the oocyte close to the cortex through the equatorial segment of the head. In the search for sperm surface proteins that function in this process, most attention has recently been given to the cysteine-rich secretory protein 1 (Crisp1) and members of the ADAM family of metallopeptidase domains (fertilin α [ADAM1], fertilin β [ADAM2], and cyritestin [ADAM3]) that function as cell adhesion molecules. The ADAM family molecules have an adhesion module, the disintegrin domain, which leads directly to the idea that oocytes have an appropriate plasma membrane adhesion partner (i.e., an integrin).33 To better assess the requirement for specific members of the ADAM family in binding (and possibly fusion), knockout mice were constructed that were null for fertilin β, cyritestin, or both (double knockout). Sperm from each of these mouse lines showed dramatically (90%) reduced binding to zona-free oocytes.34,35 Because other sperm proteins may be lost in these knockouts, this reduction in total sperm binding could possibly be caused by loss of an unidentified protein. Initial attachment of the sperm to the oocyte membrane are reversible and appear to require sperm motility. Sperm tail movement decreases or stops within a few seconds of sperm–oocyte fusion. Electron microscopy shows that the inner acrosomal membrane is later engulfed by the oocyte through a process that appears similar to phagocytosis.33 The sperm tail is also eventually incorporated into the oocyte. The adhesion of sperm to oocyte is frequently cited as likely to be analogous to the mechanism by which leukocytes interact with endothelial cells in a stepwise fashion; sperm–oocyte fusion shares common features with another classical, well-known membrane fusion event between cells and virus particles. Fusion of the sperm and oocyte membrane is followed by the cortical reaction and metabolic activation of the oocyte.
Oocyte Activation
As a consequence of successful sperm–oocyte fusion, the oocyte undergoes a series of well-defined morphologic and biochemical endpoints, some of which occur within seconds or minutes of sperm–oocyte plasma membrane interaction and some that occur over the course of several hours.4 These modifications can collectively be termed oocyte activation. One of the earliest events of oocyte activation is an increase in the level of intracellular free calcium in a periodic and oscillatory pattern known as calcium signal. It is clear that this calcium signal induces the release of meiosis, cortical granule exocytosis, zygote formation, and first cell mitosis. The oocyte possesses receptors for sperm glycoproteins in its membrane, and binding the sperm to the receptor alters the receptor so that it might trigger the G-protein cascade or activate tyrosine kinase pathways that then lead to activation of phospholipase C and the production of inositol triphosphate, which mobilizes internal calcium. Alternatively, the sperm may activate the oocyte by direct injection into the oocyte cytoplasm of a sperm-derived protein or factor, called oscillin, which may be the signaling agent for the critical calcium oscillation. This theory helps to explain the clinical success of intracytoplasmic sperm injection (ICSI), in which sperm is introduced directly into the human oocyte cytoplasm and bypasses the activation of the oocyte through binding to a receptor.36 Regardless of the regulatory mechanism, the end results are the resumption and completion of the second meiotic division and ultimately the formation of the female pronuclei.
Cortical Reaction and Block to Polyspermy
It is essential that only one spermatozoon fuses and enters the oocyte; therefore, establishment of the block to polyspermy is one of the most critical sequelae of oocyte activation. Entry of more than one sperm into the oocyte cytoplasm at fertilization represents a pathologic condition and leads to the breakdown of development. Under in vivo conditions, sperm–oocyte interaction is regulated in part by the fact that relatively few sperm reach the site of fertilization in the fallopian tube. However, the oocyte must possess mechanisms that can rapidly and precisely eliminate the likelihood of penetration by supernumerary sperm. Fusion of the sperm with the oocyte triggers a release of calcium ions from calmodulin, which results in depolarization of the oocyte plasma membrane, by which fusion of another sperm is prevented.29 The rapid depolarization of the oocyte plasma membrane during fertilization provides an early (fast) block to polyspermy that is only transient and returns to normal within a few minutes. The initiation of the block to penetration of the zona by other sperm is mediated by the cortical reaction, an exocytotic discharge of the oocyte’s cortical granules that are found just below the oocyte surface and is also apparently triggered by the increase in calcium. Cortical granules contain a variety of hydrolytic enzymes, and these enzymes presumably result in the hardening of the zona pellucida by cross-linking of structural proteins and inactivation of ligands for sperm receptors.37 This process, which is collectively known as the zona reaction, results in the lost of zona ability to bind sperm. The loss of sperm-binding activity is due, at least in part, to the release of β-N-acetylhexosaminidase from the oocyte cortical granules, which destroys the GalT-binding sites on ZP3.38
Male Pronucleus Formation and Genomic Union
Fertilization requires accurate cytoplasmic events mediated by the centrosome, which is the cell’s microtubule organizing center.39 The centrosome is a complex organelle composed of many different proteins such as γ-tubulin. Shortly after sperm penetration, maternal γ-tubulin is drawn to the sperm centrosome to assist in the formation of the sperm aster, which is found adjacent and affixed to the sperm nucleus. The continuing elongation of the sperm astral microtubules throughout the cytoplasm allows them to come into contact with the female pronucleus, which is then translocated toward the male pronucleus resulting in pronuclear migration and apposition. Defects in centrosome function during microtubule elongation may result in the failure of normal fertilization. The transformation of the sperm centrosome into a functional zygotic centrosome capable of aster formation suggests that centrosome inheritance in humans is paternal in origin.40 Remodeling of the sperm chromatin into a male pronucleus is guided by the oocyte-produced reducing peptide glutathione and a number of molecules required for the reconstitution of the functional nuclear envelope and nuclear skeleton. After migration and union of the pronuclei, the centrosome duplicates and splits, with each centrosome serving as one pole for the first mitotic spindle. Once the two pronuclei are closely apposed, the nuclear envelope dissolves. The DNA undergoes replication and the chromosomes from each pronuclei are now paired, aligned on the newly formed mitotic spindle, and ready for initiation of first mitotic division through a new cascade of cell cycle-mediating events. Although some of the sperm structures are transformed into zygotic components, the elimination of others is vital to the early stages of embryonic development. The accessory structures of the sperm axoneme, including the fibrous sheath, microtubule doublets, outer dense fibers, and the striated columns of the connecting piece, are discarded in an orderly fashion. During ICSI, which bypasses multiple steps of natural fertilization by introducing an intact spermatozoon into oocyte cytoplasm, the sperm accessory structures that would normally be eliminated before or during the entry of sperm into the oocyte cytoplasm persist and may interfere with early embryonic development, thus decreasing the success rate of assisted reproductive technologies (ART) and possibly causing severe embryonic anomalies.41
IMPLANTATION
The molecular events of embryonic attachment to the endometrial epithelium and subsequent invasion into the stroma have long been of interest for reproductive scientists and clinicians. In most successful human pregnancies, the conceptus implants 7 to 10 days after ovulation. A successful pregnancy in the human requires a receptive endometrium, a functionally normal embryo at the blastocyst stage, a dialogue between the maternal and embryonic tissues, transformation of the endometrium to deciduas, and finally formation of the definitive placenta. Hatching of the blastocyst from the zona pellucida is a key event in mammalian development. The zona pellucida initially prevents the blastocyst from adhering to the oviduct wall, in theory preventing a tubal or ectopic pregnancy. Because the embryo is covered by the zona pellucida until immediately before implantation, all embryo–maternal signaling has to pass the zona and be detectable within it. When the embryo reaches the uterus it must hatch from the zona pellucida; then it can adhere to the uterine wall to establish a pregnancy. On differentiation at the early blastocyst stage, it would appear that trophectoderm is responsible for secreting the zona lysine required for hatching. A trypsinlike protease, termed strypsin, released by the trophectoderm has been proposed to be the zona lysine. On the other hand, impaired uterine receptivity is one of the major reasons for the failed implantation and failure of ART.42,43 The phenomena of implantation and trophoblast invasion are currently considered as the major limiting factor for the establishment of pregnancy.44
Role of Ovarian Hormones
In animals, ovarian hormones regulate the development of endometrial receptivity. To prepare for implantation, the endometrium undergoes a precise developmental progression, starting at the proliferative phase under the control of follicular phase estradiol. Estrogen triggers the expression of a unique set of genes in the preimplantation endometrium that in turn control implantation. Expression of the estrogen receptor amplifies the estrogen effects required for uterine cell proliferation and/or differentiation during implantation. Following ovulation, the endometrium is transformed into a specialized secretory structure under the direction of progesterone.45 The cellular actions of progesterone are mediated through intracellular progesterone receptors, which are well-studied gene regulators. It is postulated that hormone-occupied progesterone receptors trigger the expression of specific gene networks in different cell types within the uterus, and the products of these genes mediate the hormonal effects during early pregnancy.
Embryonic Contribution to Implantation
In addition to hormonal regulation, increasing evidence demonstrates that embryonic regulation also has a significant impact by inducing reciprocal embryo–uterine interactions that change throughout the implantation process. The preimplantation embryo produces several factors during its development to signal its presence to the maternal organism. The appropriate interaction between the preimplantation embryo and maternal endometrium is at least partly controlled by paracrine cytokines.46,47 Cytokines and growth factors and their corresponding receptors have, on the mRNA level, been detected in blastomeres and in preimplantation embryos from different species as well as in the human endometrium throughout the menstrual cycle. Most of our knowledge of the physiology of implantation has come from animal studies; for obvious ethical reasons, it is not possible to study the process of implantation in humans in detail. However, we focus on biochemical and molecular events that are relevant to humans.
APOSITION AND ADHESION
Endometrial Receptivity and the Window of Implantation
Endometrial receptivity can be defined as the capacity of the uterine mucosa to facilitate successful embryonic implantation. The human endometrium is receptive to blastocyst implantation only during a very short and precise period in the luteal phase. It is the so-called nidation or implantation window, which can be defined as the period of maximum uterine receptivity for implantation.48 In the implantation window, changes occur in endometrial epithelial morphology characterized by the appearance of membrane projections called pinopodes. The time of maximal endometrial receptivity is thought to happen on cycle days 20 to 24 and is manifested by the expression of peptides and proteins that can serve as biomarkers of uterine receptivity.45 Additional information derives from ART cycles in which the window of endometrial receptivity was tested by performing embryo transfer at different times after luteinizing hormone (LH) peak. It has been recently shown that ongoing pregnancy is significantly higher for embryos that implant between cycle days 22 and 24 (postovulation days 8 to 10) than those embryos that are implanted 11 days or beyond after ovulation. Also, the risk of early pregnancy loss increases with later implantation, from 13% among conceptuses that implanted by the day 9 to 52% on day 11, and to 82% after day 11.49
Pinopodes
During the receptive phase, the apical plasma membranes of the epithelial cells lining the uterine cavity lose their microvilli and develop large and smooth apical projections called pinopodes. Pinopodes are progesterone-dependent organelles, appearing as apical cellular protrusions that become visible between days 20 and 21 of the natural menstrual cycle, as shown by scanning electron microscopy in sequential endometrial biopsies.50 The cellular and molecular functions of pinopodes in humans are still unclear; however, tracer experiments in the rat and mouse have shown that these structures perform pinocytosis. They may be also prevent the cilia from sweeping off the blastocyst and may promote withdrawal of uterine fluid, facilitating adhesion of the blastocyst to molecules of the pinopodes, drawing the uterus closely around the embryo.51,52 The disappearance of pinopodes was shown to occur at the time of downregulation of the progesterone receptors.53 Pinopodes last for less than 2 days in all cases, and the timing of their formation depends both on the hormone treatment applied and the patient’s individual response in an IVF cycle. On average, they form on day 20 to 21 in a natural cycle, day 19 to 20 in controlled ovarian stimulation, and day 21 to 22 in a hormone-controlled cycle. Such short duration and discrete timing of the window of receptivity could significantly affect the outcome of ART. Pinopode appearance, loss of steroid receptors, and maximal expression of αvβ3 integrin, osteopontin, and leukemia inhibitory factor (LIF) and receptor have been demonstrated in the same biopsy, showing a consistent association of pinopode appearance and other receptivity changes.54
Adhesion Molecules
Integrins
Attachment of the embryo may involve temporary adhesion between exposed surface receptors and ligands on the embryonic and endometrial epithelium. The role of integrins in the processes of adhesion and migration makes them attractive potential participants in the complex events of embryo implantation and placentation. Integrins are glycoproteins that serve as cell-surface receptors for the extracellular matrix and connect extracellular cell adhesion proteins to cytoskeletal components. Certain integrin subunits appear to be regulated within the cyclic endometrium,55 and specific alterations were shown in the preimplantation period, suggesting a possible role for α4, β3, and αvβ3 subunits in the establishment of endometrial receptivity. The apical surface of the luminal epithelium expresses the αvβ3 integrin, which localizes on the pinopodes. The loss of αvβ3 is highly associated with endometriosis, retarded endometrial development, and infertility.45,55 The αvβ3 integrin recognizes extracellular matrix ligands that contain the 3-amino acid sequence that has been implicated in trophoblast attachment and outgrowth, and blockade of either the integrin or its ligand-binding sequence reduces implantation in the mouse.56,57 Osteopontin is an endometrial glycoprotein that is recognized by αvβ3 integrin and is present in glandular and luminal epithelium and appears approximately 7days after ovulation on pinopodes.45
Trophinin
Trophinin is an intrinsic membrane protein that is important in trophoblast cell adhesion; bystin and tastin are cytoplasmic proteins that associate with trophinin by presumably forming active adhesion machinery.58 Both trophoblasts and endometrial epithelial cells express trophinin, which mediates apical cell adhesion through homophilic trophinin-trophinin binding. The expression patterns of these molecules are suggestive of their involvement in the initial blastocyst attachment to the uterus as well as in subsequent placental development.
Mucins
Mucins are glycosylated molecules found on a great number of tissues, including the endometrial epithelial cells. Large-molecular-weight mucin glycoproteins, including MUC1 through MUC7 and the sialomucin ASGP are present at the apical surface of the uterine epithelium and are secreted into gland lumens. These mucins appear to protect the mucosal surface from infection and the degrading action of enzymes. MUC1, the prominent component, may also play a role in forming a protective barrier in the upper genital tract, preventing infections, and modulating sperm access, but the actual role of MUC1 in human implantation remains to be determined.59 MUC1 has been shown experimentally to inhibit cell-to-cell interactions by steric hindrance of binding interactions mediated by receptors, including integrins. These mucins represent a barrier to embryo attachment and their expression persists in the human uterus during the proposed receptive phase. Pinopodes may function to elevate the implantation surface toward the embryo, free of antiadhesion MUC1. It is also possible that mucin loss is localized to the implantation sites in humans.59,60
Cytokines and Growth Factors
The endometrium of most species is now recognized as an important site of production of cytokines and their receptors. The cellular origin of the cytokines varies, but many predominate in the uterine glandular or luminal epithelium or in the decidualized stromal cells.61 Cytokines and growth factors are cell-derived polypeptides and proteins that have the capacity to bind to specific cell-surface receptors and may act as potent intercellular signals, regulating functions of endometrial cells. They regulate cell proliferation, differentiation, and apoptosis by autocrine, paracrine, and endocrine mechanisms.62,63 Cytokines produced by the uterine mucosa and the embryo may play a role in maternal–embryonic interaction, enhancing endometrial receptivity by controlling the expression of adhesion and antiadhesion proteins.64
Interleukins
There is evidence that the interleukin-1 (IL-1) system is important to endometrial and embryonic cross-talk during human implantation.65 The IL-1 receptor is expressed in the endometrium of various species; antagonizing the biologic effects of IL-1 leads to implantation failure in mice. This has been shown to be due to an endometrial rather than an embryonic effect. IL-1 has a possible influence on other systems involved in embryonic implantation, including invasion and angiogenesis, therefore suggesting a role for this cytokine family during early embryonic development.66 Members of the IL-6 family of cytokines include LIF, IL-6, IL-11, cardiotrophin, ciliary neurotropic growth factor, oncostatin M, and the recently discovered cardiotropin-like cytokine novel neurotrophin-1. Gene targeting in mice provided the first indication of a role for the IL-6 family of cytokines in implantation with the generation of mice with a null mutation of the gene encoding LIF. LIF-null female mice were infertile because of failure of blastocyst implantation. More recently, IL-11 signaling has been shown to be required for the uterine decidualization response.67
Vascular Endothelial Growth Factor (VEGF)
VEGF has been shown to increase the proliferative ability of vascular endothelial cells in vitro by acting as a highly specific mitogen for these cell types. After the embryo invades the maternal endometrium, its development is characterized by a dramatic growth of blood vessels coincident with decidualization, development of vascular membranes, and placenta formation.66 Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) is highly expressed in the rat uterus around the time of implantation and modulates the proliferation of rat uterine cells and their production of prostacyclin (PGI2) during the peri-implantation period. Furthermore, IGFBP-rP1 significantly stimulated PGI2 synthesis and cyclo-oxygenase-2 mRNA expression in myometrial cells, both of which are essential molecules for successful implantation.68 Known biologic effects of the epidermal growth factor (EGF), VEGF, and fibroblast growth factor in the oviduct and endometrium during the estrous cycle and early pregnancy in pigs are related to cellular differentiation and angiogenesis. This suggests their involvement in the transformation of the endometrium into a decidua, subsequently leading to successful establishment of pregnancy.69
Prostaglandins
Prostaglandins, either maternally or embryo derived, have been thought to be involved in the initial phase of implantation. The principal role of prostaglandins may be to create a mild inflammatory reaction and increase vascular permeability in the endometrium during implantation.70 Cyclo-oxygenase (COX) is the rate-limiting enzyme in the synthesis of prostaglandins and exists in two isoforms: COX-1 and COX-2. COX-1-deficient mice are fertile; mice lacking COX-2 are infertile due to both anovulation and impaired implantation.71 The candidate prostaglandins that participate in these processes and their mechanism of action remain undefined. Using COX-2-deficient mice, it has been demonstrated that COX-2-derived prostacyclin is the primary prostaglandin that is essential for implantation and decidualization.72 Immunostaining for COX-1 found it to be present mainly in the glandular and luminal epithelium; COX-2 was localized to the luminal epithelium and perivascular cells. Treatment with mifepristone significantly reduced the expression of COX-1 in glandular epithelium and COX-2 in luminal epithelium and has been shown to reduce immunostaining for 15-hydroxyprostaglandin dehydrogenase within endometrial glands.73
Human Chorionic Gonadotropin
Recent evidence suggests that human chorionic gonadotropin (hCG), in addition to its well-known endocrine effects on the corpus luteum, may act as a growth and differentiation factor during pregnancy. According to experimental results, its mode of action may be divided into three sequential phases. During the first phase, which begins at the blastocyst stage and lasts until hCG is seen in the serum, hCG acts preferentially in a juxtacrine manner. Administration of hCG may provoke profound effects on paracrine parameters of differentiation and implantation. VEGF, which is important for neoangiogenesis, is stimulated by hCG, suggesting a role for hCG in the control of endometrial vascularization and placentation. The second, endocrine, phase of hCG action is marked by the appearance of hCG in the maternal serum. Rising systemic hCG levels cause a very rapid elevation of serum progesterone, reflecting the rescue of the corpus luteum. Other endocrine functions of hCG include its intrinsic thyrotropic activity as well as modulation of fetal testicular, ovarian, and adrenal function. The third phase may be characterized by the expression of full-length hCG/LH receptors on the trophoblasts themselves. hCG seems to have a variety of local and systemic functions in and outside the embryo–endometrial microenvironment.74
HOX Genes
HOX genes are highly evolutionarily conserved and act as regulators of embryonic morphogenesis and differentiation. Mammalian species have at least 39 HOX genes arranged in four clusters, termed HOXA, HOXB, HOXC, and HOXD.75 specific HOXA genes are important in the development of the müllerian tract. HOXA-10 and HOXA-11 expression rises during the menstrual cycle, increasing dramatically in the midluteal phase, the time of implantation. Recently, studies using targeted mutagenesis have revealed that mice homozygous for HOXA-10 deficiency show implantation failure and embryonic resorption in the early postimplantation period.76 The regulation of a HOX gene by sex steroids may provide a mechanism to allow differential HOX gene expression in the reproductive tract. Estrogen and progesterone each upregulate HOX-10 expression. The sex steroid regulation pattern of expression is consistent with HOX genes having a role in implantation. HOX genes are necessary for implantation; they activate the endometrial downstream target genes in each menstrual cycle that are necessary for implantation.75
INVASION AND PLACENTATION
Endometrial Decidualization
To protect the mother from the attack of invasive trophoblasts migrating toward the uterine spiral arteries, and in response to ovarian hormones, the endometrial stroma transforms itself into a dense cellular matrix known as the decidua.77 During the process of decidualization, the fibroblast-like mesenchymal cells in the uterine stroma differentiate to epitheloid-like cells. This morphologic change in humans is initiated in the luteal phase under the influence of estradiol, progesterone, and relaxin. The other changes include appearance within the tissue of a specialized lymphocyte subset characterized by an abundant expression of CD56. These cells have been defined in both women and rodents as members of the natural killer (NK) cell lineage and are called uterine (u) NK cells.78 In women, the cells are also called decidual CD56bright cells; a term previously used in mice was granulated metrial gland cells.79 These CD56-positive cells comprise over 90% of the leukocyte population at the time of implantation. The precise function of CD56 cells in the developing decidua is unknown, although they have been proposed to be important in implantation and pregnancy maintenance.80 The mechanism is unclear; however, recognition by NK cells and their receptors and receptor inhibitors of HLA class I molecules, specifically HLA-G on trophoblasts, has been proposed to protect the implanting blastocyst from NK cell lysis.81 Dysregulation of these potentially important events may be involved in either failed implantation or pregnancy failure. Decidual cytokine levels have been characterized primarily at the mRNA level. Among the many genes upregulated during the implantation window, the most prominent include IL-1, CSF, LIF, EGF, and TBF-β.61,64 The secretion of these factors can be modified by gonadal steroids and the blastocyst itself. The blastocyst expresses receptors for these same factors, providing a communicative link from maternal tissue to embryo.
The sequence of biochemical and molecular events associated with decidualization is not completely understood. In the baboon, the sequential changes during this period in vivo are characterized by the downregulation of β-smooth actin followed by induction of COX-2 at the implantation site and the expression of insulin growth factor binding protein-1 (IGFBP-1). IGFBP-1 is the predominant protein in decidualized cells and is considered to be a biochemical marker of decidualization. In addition IL-1β, as a possible conceptus-mediated factor, can induce IGFBP-1 expression in the presence of hormones after 3 days of incubation. Current data suggest that IL-1β can activate multiple signaling pathways that either positively (no exogenous cAMP) or negatively (in presence of exogenous cAMP) regulate IGFBP-1 gene expression and decidualization in vitro. Signaling pathways activated by IL-1β following 10 minutes of stimulation result in the phosphorylation of mitogen-activated protein kinase (MAPK, specifically p38 MAPK) and also lead to NF-κB activation. The expression of COX-2 and matrix metalloproteinase-3 (MMP-3) genes follows after 4 to 6 hours. The steroid hormones, particularly progesterone, which are critical for IGFBP-1 expression, modulate the activity of IL-1β by downregulating MMP-3 activity. IL-1β-induced MMP-3 may upregulate IGFBP-1 by initiation of cytoskeletal reorganization through degradation of extracellular matrix.82
Trophoblast Invasion
During the invasive phase of implantation, several events occur, including cytotrophoblast adhesion to the extracellular matrix via cell adhesion molecules, local extracellular matrix proteolysis by MMPs, cellular migration, and inhibition of these processes.63 It is difficult to obtain human material in early gestation; therefore, much of our understanding of the earliest phases of trophoblast invasion has been extrapolated from monkey material.83 Examination of monkey implantation sites has revealed that trophoblasts begin to migrate down into the maternal spiral arteries as early as 10 days after fertilization, and at 14 days, many of the spiral arteries beneath the conceptus are totally occluded.84 Cytotrophoblastic cells are derived from the trophectodermal cells of the blastocyst and represent a heterogeneous population during early pregnancy. Cytotrophoblastic cells follow one of two existing differentiation pathways: Villous cytotrophoblastic cells (vCTBs) form a monolayer of polarized epithelial stem cells that proliferate and fuse to form syncytiotrophoblasts covering the entire surface of the vCTBs. Cytotrophoblastic cells can also break through the syncytium at selected sites (anchoring villi) to form multilayered columns of nonpolarized cytotrophoblastic cells. These motile and highly invasive extravillous cytotrophoblastic cells (evCTBs) are found as cytokeratin-positive cells in the decidua, the intima of the uterine spiral arteries, and the proximal third of the myometrium.85
Trophoblast invasion, like tumor invasion, is due to the active secretion of proteolytic enzymes capable of digesting the different extracellular matrix of the host’s tissues. Serine proteases, cathepsins, and metalloproteinases have been implicated in invasive processes.86 MMPs, also called matrixins, form a family of at least 17 human zinc-dependent endopeptidases collectively capable of degrading essentially all components of the extracellular matrix. According to their substrate specificity and structure, members of the MMP gene family can be classified into four subgroups: gelatinases (digest type IV collagen, the major constituent of basement membranes and denatured collagen), collagenases (digest types I, II, III, VII, and X collagens), stromelysins (digest type IV, V, and VII collagens as well as laminin, fibronectin, elastin, proteoglycans, and gelatin), and membrane-type MMPs. The substrate of the membrane metalloproteinases (MMP-14, MMP-15, MMP-16) is essentially proMMP-2, and these enzymes allow activation of proMMP-2 at the cell surface on the invasive front.87 They are thus appropriately designed for digesting the collagens of the extracellular matrix of the interstitium. Several enzymes are capable of activating the promatrixins, the most well-known being plasmin. The activity of MMPs in the extracellular space is specifically inhibited by tissue inhibitor of metalloproteinases (TIMP), which binds to the highly conserved zinc-binding site of active MMPs at molar equivalence.85 TIMP and broad-spectrum protease inhibitors (such as α2-macroglobulin) of primarily decidual and cytotrophoblast origin are important in limiting cytotrophoblast invasion and in endometrial stromal receptivity.63 Further penetration and survival depend on factors that are capable of suppressing the maternal immune response to paternal antigens. During implantation, the uterine decidua is invaded by extravillous trophoblast cells that express an unusual combination of HLA class I molecules HLA-C, HLA-E, and HLA-G. The decidua is infiltrated by a population of NK cells that are particularly numerous in the decidua basalis at the implantation site, where they come into close contact with invading extravillous trophoblast cells. It is believed that interaction between these NK cells and extravillous trophoblast cells provides the controlling influence for implantation. On the other hand the increased activity of NK cells has been related to an increased risk of recurrent miscarriage. It seems that the endometrial-derived glycoprotein with immunosuppressive properties, glycodelin-A, may locally inhibit NK cell activity and contribute to protection of the embryonic semi-allograft. Glycodelin-A is the major progesterone-regulated glycoprotein secreted into the uterine luminal cavity by secretory/decidualized endometrial glands.88
Angiogenesis and Vasculogenesis
After the embryo invades the maternal endometrium, its development is characterized by a dramatic growth of blood vessels coincident with decidualization, development of vascular membranes, and placenta formation. These active processes involve both angiogenesis, the growth of blood vessels by sprouting from a preexisting endothelium, and vasculogenesis, the in situ formation of primordial vessels from hemangioblasts.89 Human placenta is a rich source of angiogenic substances, and these may play an important role in the regulation of placental vessel formation as well as in maternal vascular adaptation to pregnancy. VEGF expression has been described in villous trophoblasts and fetal macrophages within villous stroma. VEGF increase the proliferative ability of the vascular endothelial cells in vitro by acting as a highly specific mitogen for this cell type. It induces angiogenesis and increases the permeability of blood vessels.90
Another member of the VEGF family, placental growth factor, has been also detected in the villous trophoblast and in the tunica media of larger stem vessels.91 Basic fibroblast growth factor is expressed in the villous trophoblast and can be detected in the conditioned medium of villous tissue explants.92 Proliferin and proliferin-related protein, two prolactin-related peptides expressed by trophoblast giant cells, have been identified as potent regulators of angiogenesis in the murine placenta, although the role of these placental proteins has yet to be evaluated in humans.93 Another recently described angiogenic factor, leptin, is expressed in the placenta and serum during pregnancy and may play a role in vascular development during pregnancy.94
CLINICAL RELEVANCE
Clinical evidence indicates the existence in humans of a narrow window of uterine receptivity, which opens during the midluteal phase. At the same time, formation of pinopodes on the apical membrane of the endometrial epithelial cells occurs. Detection of pinopodes may be extremely useful for the assessment of receptivity in women with history of multiple implantation failure.95 Also it is important to note that in normal fertile women, pinopodes are present on day 6 to 8 postovulation, whereas pinopode formation is observed 1 to 2 days earlier in patients undergoing controlled ovarian hyperstimulation. In addition to the individual variations in the timing of pinopode formation, the number of pinopodes also varies between patients, some showing plentiful and others only sparse pinopodes. More interestingly, the number of pinopodes correlated strongly with implantation success after embryo transfer in a subsequent cycle.54
The contemporary approach to ovarian stimulation for IVF treatment results in supraphysiologic concentrations of steroids during the follicular and luteal phases of the menstrual cycle. These sex steroids act directly and indirectly to mature the endometrium, influencing receptivity for implantation. Altered endometrial development has been demonstrated with most of the protocols used for ovarian stimulation.96 A series of studies shows that high estradiol concentrations on the day of hCG administration are detrimental to uterine receptivity.97 Corpus luteum function is distinctly abnormal in IVF cycles, and therefore luteal support is widely used. Since the introduction of gonadotropin-releasing hormone agonists, it has been observed that pregnancy rates increased significantly with luteal-phase supplementation.98 The development of an endometrium receptive to embryo implantation is a complex process that may be altered by inappropriate exposure to sex steroids in terms of timing, duration, and magnitude.99
Embryo transfer has received little clinical attention and has been, until recently, the most inefficient step in IVF. Factors such as contamination of the catheter tip with cervical bacteria, stimulation of uterine contractions during the procedure, the type of catheter, ultrasound guidance during the transfer, and the position of the embryos in the uterine cavity appear to influence implantation rates. Easy and atraumatic transfer is essential for successful implantation; the embryos need to be placed in the middle of the cavity, away from the fundus. Standardization of the transcervical intrauterine transfer of embryos in a large randomized study is needed before definitive conclusions can be drawn.100
There is an evident decline of female fertility with age. This decline is mainly due to increased risk of pregnancy termination either after conception or after embryo implantation. The observation that very good clinical results could be obtained in aged female patients using oocytes donated by younger women has led to the conclusion that the reproductive aging of the women is solely due to a decline of oocyte quality rather than uterine aging and lack of receptivity. Despite some macroscopic possible causes that may play a role in the reduction of age-related endometrial receptivity, many endometrial factors possibly related to its receptivity need to be further studied, especially in older women.101
1 Alexandre H. A history of mammalian embryological research. Int J Dev Biol. 2001;45:457-467.
2 Setchell BP. The contributions of Regnier de Graaf to reproductive biology. Eur J Obstet Gynecol Reprod Biol. 1974;4:1-13.
3 Tan SY. Anton van Leeuwenhoek (1632–1723) father of microscopes. Singapore Med J. 2003;44:557-558.
4 Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1994:189-317.
5 Saitoh K, Terada T, Hatakeyama S. A morphological study of the efferent ducts of the human epididymis. Int J Androl. 1990;13:369-376.
6 Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol. 1980;124:211-215.
7 Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl. 1995;16:292-298.
8 Harper JK. Gamete and zygote transport. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1994:123-185.
9 Speroff L, Glass RH, Kase NG. Clinical and Gynecologic Endocrinology and Infertility. Baltimore: Williams & Wilkins, 1994;231-250.
10 Barratt CLR, Cooke ID. Sperm transport in the human female reproductive tract—a dynamic interaction. Int J Androl. 1991;14:394-411.
11 Mastroianni JR, Zausner-Guelman B, Go KJ. Sperm transport in the female reproductive tract. In: Behrman SJ, Kistner RW, Patton GW, editors. Progress in Infertility. Boston/Toronto: Little-Brown; 1988:663-672.
12 Grant A. Cervical hostility; incidence, diagnosis, and prognosis. Fertil Steril. 1958;9:321-333.
13 Casslén B, Nilsson B. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea. Am J Obstet Gynecol. 1984;150:877-881.
14 Austin CR. Anomalies of fertilization leading to triploidy. J Cell Comp Physiol. 1960;56(Suppl 1):1-15.
15 Fraser LR. Cellular biology of capacitation and the acrosome reaction. Hum Reprod. 1995;10(Suppl):22-30.
16 Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod. 1995;53:1280-1285.
17 Suarez SS. Hyperactivated motility of sperm. J Androl. 1996;17:331-335.
18 Fenichel P, Durand-Clement M. Role of integrins during fertilization in mammals. Hum Reprod. 1998;13(Suppl 4):31-46.
19 Croxatto HB, Ortiz ME. Egg transport in the fallopian tube. Gynecol Invest. 1975;6:215-225.
20 Croxatto HB, Ortiz ME, Diaz S, et al. Studies on the duration of egg transport by the human oviduct. II. Ovum location at various intervals following luteinizing hormone peak. Am J Obstet Gynecol. 1978;132:629-634.
21 Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140:307-317.
22 Clewe TH, Mastroianni L. Mechanisms of ovum pickup. I. Functional capacity of rabbit oviducts ligated near the fimbria. Fertil Steril. 1958;9:13-17.
23 Vernon MW. Female reproductive physiology. In: Andrology and Embryology review course. St. Louis: American Association of Bioanalysis; 2003:132.
24 Talbot P, Shur BD, Myles DG. Cell adhesion and fertilization: Steps in oocyte transport, sperm–zona pellucida interactions, and sperm–egg fusion. Biol Reprod. 2003;68:1-9.
25 Talbot P, DiCarlantonio G, Zao P, et al. Motile cells lacking hyaluronidase can penetrate the hamster oocyte–cumulus complex. Dev Biol. 1985;108:387-398.
26 Prasad SV, Skinner SM, Carino C, et al. Structure and function of the proteins of the mammalian zona pellucida. Cells Tissues Organs. 2000;166:148-164.
27 Prasad SV, Dunbar BS. Human sperm–oocyte recognition and infertility. Semin Reprod Med. 2000;18:141-149.
28 Rosati F, Capone A, Giovampaola CD, et al. Sperm–egg interaction at fertilization: Glycans as recognition signals. Int J Dev Biol. 2000;44:609-618.
29 Dietl JA, Rauth G. Molecular aspects of mammalian fertilization. Hum Reprod. 1989;4:869-875.
30 Bleil JD, Wassarman PM. Sperm–egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317-324.
31 Jungnickel MK, Marrero H, Birnbaumer L, et al. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499-502.
32 Green DP. Mammalian fertilization as a biological machine: A working model for adhesion and fusion of sperm and oocyte. Hum Reprod. 1993;8:91-96.
33 Evans JP. Fertilin β and other ADAMs as integrin ligands: Insights into cell adhesion and fertilization. Bioessays. 2001;23:628-639.
34 Cho C, Bunch DO, Faure JE, et al. Fertilization defects in sperm from mice lacking fertilin β. Science. 1998;281:1857-1859.
35 Nishimura H, Cho C, Branciforte DR, et al. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Dev Biol. 2001;233:204-213.
36 Hewitson L, Simerly C, Schatten G. Fertilization. In: Fauser BCJM, editor. Reproductive Medicine. New York: Parthenon Publishing; 2003:401-419.
37 Horvath PM, Kellom T, Caulfield J, Boldt J. Mechanistic studies of the plasma membrane block to polyspermy in mouse eggs. Mol Reprod Dev. 1993;34:65-72.
38 Miller DJ, Gong X, Decker G, Shur BD. Egg cortical granule N-acetylglucosaminidase is required for the mouse zona block to polyspermy. J Cell Biol. 1993;123:1431-1440.
39 Hewitson L, Simerly C, Dominko T, Schatten G. Cellular and molecular events after in vitro fertilization and intracytoplasmic sperm injection. Theriogenology. 2000;53:95-104.
40 Simerly C, Wu GJ, Zoran S, et al. The paternal inheritance of the centrosome, the cell’s microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47-52.
41 Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: Fertilization after sperm–egg fusion. Int Rev Cytol. 2000;195:1-65.
42 Edwards RG. Human uterine endocrinology, and the implantation window. Ann NY Acad Sci. 1988;541:445-454.
43 Edwards RG. Clinical approaches to increasing uterine receptivity during human implantation. Hum Reprod. 1995;10(Suppl 2):60-66.
44 Herrler A, Von Rango U, Beier HM. Embryo–maternal signalling: How the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online. 2003;6:244-256.
45 Lessey BA. The role of the endometrium during embryo implantation. Hum Reprod. 2000;15(Suppl 6):39-50.
46 Giudice LC. Endometrial growth factors and proteins. Semin Reprod Endocrinol. 1995;13:93-101.
47 Kauma SW. Cytokines in implantation. J Reprod Fertil Suppl. 2000;55:31-42.
48 Duc-Goiran P, Mignot TM, Bourgeois C, Ferré F. Embryo–maternal interactions at the implantation site: A delicate equilibrium. Eur J Obstet Gynecol Reprod Biol. 1999;83:85-100.
49 Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. NEJM. 1999;340:1796-1799.
50 Nikas G, Drakakis P, Loutradis D, et al. Uterine pinopodes as markers of “nidation window” in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod. 1995;10:1208-1213.
51 Psychoyos A, Nikas G. Uterine pinopodes as markers of uterine receptivity. Assist Reprod Rev. 1994;4:26-32.
52 Stavréus-Evers A, Masironi B, Landgren BM, et al. Immunohistochemical localization of glutaredoxin and thioredoxin in human endometrium: A possible association with pinopodes. Mol Hum Reprod. 2002;8:546-551.
53 Stavreus-Evers A, Nikas G, Sahlin L, et al. Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil Steril. 2001;7:782-791.
54 Nikas G, Aghajanova L. Endometrial pinopodes: Some more understanding on human implantation? Reprod Biomed Online. 2002;4(Suppl 3):18-23.
55 Lessey BA, Damjanovich L, Coutifaris C, et al. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188-195.
56 Illera MJ, Cullinan E, Gui Y, et al. Blockade of the αvβ3 integrin adversely affects implantation in the mouse. Biol Reprod. 2000;62:1285-1290.
57 Yelian FD, Yang Y, Hirata JD, et al. Molecular interactions between fibronectin and integrins during mouse blastocyst outgrowth. Mol Reprod Dev. 1995;414:435-448.
58 Fukuda MN, Nozawa S. Trophinin, tastin, and bystin: A complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol. 1999;17:229-234.
59 Aplin JD. MUC-1 glycosylation in endometrium: Possible roles of the apical glycocalyx at implantation. Hum Reprod. 1999;14(Suppl 2):17-25.
60 Carson DD, DeSouza MM, Regisford EG. Mucin and proteoglycan functions in embryo implantation. Bioessays. 1998;20:577-583.
61 Salamonsen LA, Dimitriadis E, Robb L. Cytokines in implantation. Semin Reprod Med. 2000;18:299-310.
62 Beier HM, Beier-Hellwig K. Molecular and cellular aspects of endometrial receptivity. Hum Reprod Update. 1998;4:448-458.
63 Giudice LC. Potential biochemical markers of uterine receptivity. Hum Reprod. 1999;14(Suppl 2):3-16.
64 Simón C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:815-826.
65 Simón C, Mercader A, Gimeno MJ, Pellicer A. The interleukin-1 system and human implantation. Am J Reprod Immunol. 1997;37:64-72.
66 Krussel JS, Bielfeld P, Polan ML, Simon C. Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S2-S9.
67 Robb L, Dimitriadis E, Li R, Salamonsen LA. Leukemia inhibitory factor and interleukin-11: Cytokines with key roles in implantation. J Reprod Immunol. 2002;57:129-141.
68 Dominguez F, Avila S, Cervero A, et al. A combined approach for gene discovery identifies insulin-like growth factor-binding protein-related protein 1 as a new gene implicated in human endometrial receptivity. J Clin Endocrinol Metab. 2003;88:1849-1857.
69 Wollenhaupt K, Welter H, Einspanier R, et al. Expression of epidermal growth factor receptor (EGF-R), vascular endothelial growth factor receptor (VEGF-R) and fibroblast growth factor receptor (FGF-R) systems in porcine oviduct and endometrium during the time of implantation. J Reprod Dev. 2004;50:269-278.
70 Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107-122.
71 Lim H, Paria BC, Das SK, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197-208.
72 Lim H, Gupta RA, Ma WG, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Dev. 1999;13:1561-1574.
73 Cameron ST, Critchley HO, Buckley CH, et al. Effect of two antiprogestins (mifepristone and onapristone) on endometrial factors of potential importance for implantation. Fertil Steril. 1997;67:1046-1053.
74 Licht P, Russu V, Wildt L. On the role of human chorionic gonadotropin (hCG) in the embryo-endometrial microenvironment: Implications for differentiation and implantation. Semin Reprod Med. 2001;19:37-47.
75 Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6:75-79.
76 Benson GV, Lim H, Paria BC, et al. Mechanisms of reduced fertility in Hoxa-10 mutant mice: Uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687-2696.
77 Kearns M, Lala PK. Life history of decidual cells: A review. Am J Reprod Immunol. 1983;3:78-82.
78 King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28-36.
79 Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1-115.
80 Loke YW, King A. Decidual natural killer cell interaction with trophoblast: Cytolysis or cytokine production? Biochem Soc Trans. 2000;28:196-198.
81 LeBouteiller P, Blaschitz A. The functionality of ALA-G is emerging. Immunol Rev. 1999;167:233-244.
82 Strakova Z, Srisuparp S, Fazleabas AT. IL-1β during in vitro decidualization in primate. J Reprod Immunol. 2002;55:35-47.
83 Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool. 1993;266:578-587.
84 Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat. 1991;192:329-346.
85 Bischof P, Meisser A, Campana A. Biochemistry and molecular biology of trophoblast invasion. Ann NY Acad Sci. 2001;943:157-162.
86 Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumour invasion. FASEB J. 1999;13:781-792.
87 Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:51-160.
88 Seppala M, Taylor RN, Koistinen H, et al. Glycodelin: A major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23:401-430.
89 Coffin JD, Poole TJ. Embryonic vascular development. Development. 1988;102:735-744.
90 Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest FLK-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835-846.
91 Vuorela P, Hatva E, Lymboussaki A, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56:489-494.
92 Hamai Y, Fujii T, Yamashita T, et al. Evidence for basic fibroblast growth factor as a crucial angiogenic growth factor, released from human trophoblasts during early gestation. Placenta. 1998;19:149-155.
93 Yamaguchi M, Imai T, Maeda T, et al. Cyclic adenosine 3′, 5′-monophosphate stimulation of placental proliferin and proliferin-related protein secretion. Endocrinology. 1995;136:2040-2046.
94 Lepercq J, Guerre-Millo M, Andre J, et al. Leptin: A potential marker of placental insufficiency. Gynecol Obstet Invest. 2003;55:151-155.
95 Pantos K, Nikas G, Makrakis E, et al. Clinical value of endometrial pinopodes detection in artificial donation cycles. Reprod Biomed Online. 2004;9:86-90.
96 Sterzik K, Dallenbach C, Schneider V, et al. In vitro fertilization: The degree of endometrial insufficiency varies with the type of ovarian stimulation. Fertil Steril. 1988;50:457-462.
97 Valbuena D, Jasper M, Remohi J, et al. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107-111.
98 Smith EM, Anthony FW, Gadd SC, Masson G. Trial of support treatment with human chorionic gonadotrophin in the luteal phase after treatment with buserelin and human menopausal gonadotrophin in women taking part in an in vitro fertilisation programme. BMJ. 1989;298:1483-1486.
99 Van Der Gaast MH, Beckers NG, Beier-Hellwig K, et al. Ovarian stimulation for IVF and endometrial receptivity—the missing link. Reprod Biomed Online. 2002;5:36-43.
100 Levi Setti PE, Albani E, Cavagna M, et al. The impact of embryo transfer on implantation—a review. Placenta. 2003;24(Suppl B):S20-S26.
101 Ubaldi F, Rienzi L, Baroni E, et al. Implantation in patients over 40 and raising FSH levels—a review. Placenta. 2003;24(Suppl B):S34-S38.