Normal and Abnormal Immune Responses
I Cascade of Events in Typical Immune Responses (Box 4-1)
A Localized antigen-nonspecific responses at site of antigen exposure
1. Fast: activation of alternate or lectin complement pathway leading to inflammatory response, opsonization, and bacterial killing
2. Fast: interferon-mediated protection against viral infection and natural killer (NK) cell killing of virus-infected cells
3. Soon after: migration of phagocytes (neutrophils, macrophages, dendritic cells [DCs]) to site of antigen and phagocytosis
4. Early: pathogen-associated molecular patterns (PAMPs) on microbial structures (e.g., lipopolysaccharide and peptidoglycan) stimulate toll-like receptors (TLRs) and other receptors on DCs and macrophages that make cytokines
5. Early: acute phase response induced by interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF) secreted from macrophages and DCs
B Primary antigen-specific responses
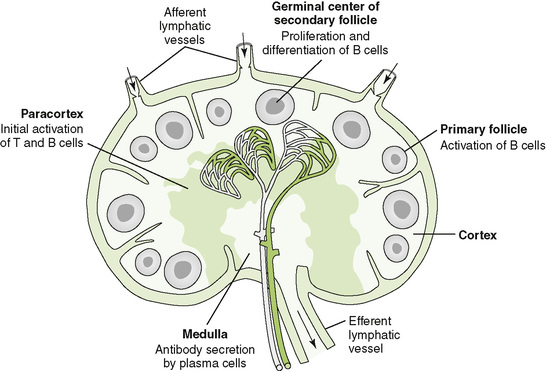
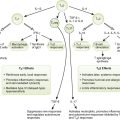
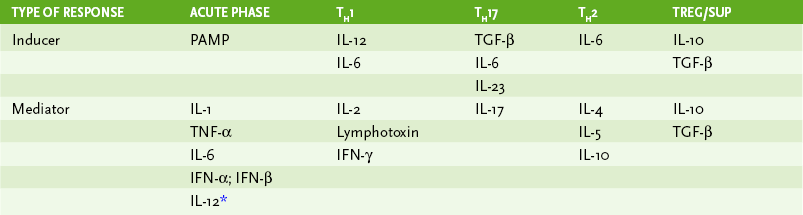
• Lymphocytes interact with antigen-presenting cells (APCs) in lymph nodes (Fig. 4-1), the spleen, and mucosal-associated lymphoid tissue, which includes tonsils, adenoids, appendix, and Peyer patches; cytokines define the nature of the response (Table 4-1).
TABLE 4-1
1. Initial activation of naive CD4 TH cells triggered by binding to antigenic peptides associated with class II major histocompatibility complex (MHC) molecules on DCs, but not other APCs
2. Activation of naive B cells (T dependent) expressing membrane immunoglobulin M (IgM) triggered by binding of antigen and interaction with TH cells
3. Proliferation of activated CD4 TH cells and differentiation into cytokine-secreting TH1 and TH2 subsets
4. Initial activation and proliferation of naive CD8 cytotoxic T (TC) cells triggered by binding of antigenic peptides associated with class I MHC molecules on DCs for recognition of infected cells, tumor cells, and grafts
5. Cytokine-induced proliferation of activated B cells and differentiation into memory cells and antibody-secreting plasma cells
• Class switching (gene recombination) and affinity maturation (somatic mutation) occur.
• TH1 cytokines stimulate B cells to make IgG1 (human).
• TH2 cytokines stimulate production of other IgG subclasses, IgE, or IgA.
6. Swelling of lymph nodes because of lymphoid proliferation
7. Exit of activated lymphocytes from lymph node or other peripheral lymphoid tissue and mobilization to site of infection
8. Activation of macrophages and DCs by interferon-γ (IFN-γ; TH1 cytokine), leading to enhancement of their antigen-presenting, antibacterial, antiviral, and antitumor activities
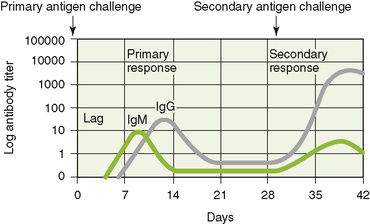
1. Rechallenge with an antigen produces a secondary specific response that is faster and stronger (anamnestic response) than primary response to the same antigen because DCs and any APCs can present antigen to T cells and because of the presence of memory B and T cells (Fig. 4-2).
2. Persistence of memory B cells accounts for the phenomenon called “original antigenic sin” (Box 4-2).
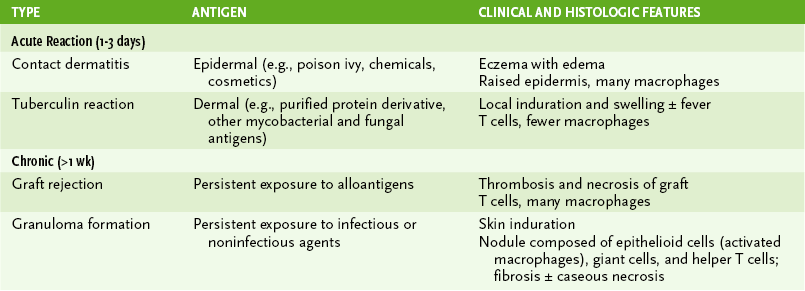
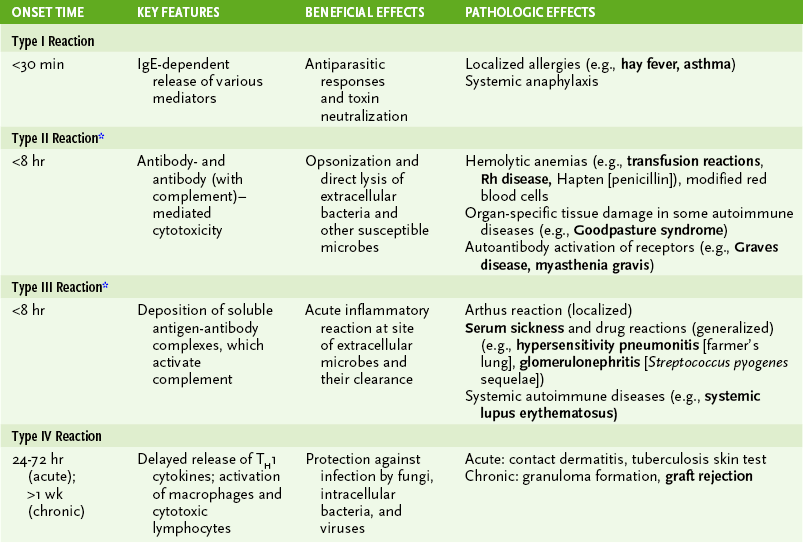
• Hypersensitivity reactions are important in the immune response to certain antigens, but they also cause pathologic changes associated with many autoimmune diseases and infections, especially viral infections (Table 4-2).
TABLE 4-2
*Antibody (with complement)–initiated autoimmune diseases like rheumatoid arthritis, systemic lupus erythematosus, and possibly multiple sclerosis develop into T cell–mediated chronic diseases.
A Type I (immediate) hypersensitivity
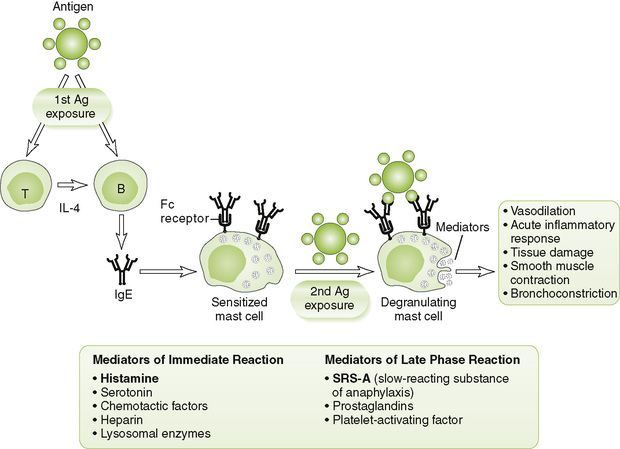
• IgE-mediated atopic (allergic) and anaphylactic reactions in previously exposed (sensitized) individuals (Fig. 4-3)
1. Initiation: cross-linkage by antigen (allergen) of IgE bound to Fc receptors on mast cells and basophils after reexposure of sensitized host to allergen
2. Effector mechanism: degranulation of mast cells and basophils releasing numerous vasoactive and other mediators, such as histamine and SRS-A (slow-reacting substance of anaphylaxis)
• Acute generalized anaphylaxis: shock, vascular collapse, respiratory collapse
• Chronic, recurrent localized reactions: asthma, allergic rhinitis (hay fever), and wheal and flare (hives)
4. Desensitization therapy: repeated injections of increasing doses of allergen induce production of IgG, which binds the allergen and prevents its binding to IgE on sensitized cells.
1. Initiation: binding of antibody to cell surface antigens
• Complement activated by cell surface antigen-antibody complex → cell lysis by membrane attack complex
• Antibody-dependent cellular cytotoxicity (ADCC) triggered by binding of antibody to Fc receptors on macrophages and NK cells → cell destruction
• Hemolytic transfusion reactions: antibodies to red blood cell (RBC) antigens
• Drug-induced thrombocytopenia and hemolytic anemia: antibodies to drugs absorbed on platelets and RBCs
• Hemolytic disease of the newborn (erythroblastosis fetalis): maternal antibody to antigens on fetal RBCs, especially Rh antigens (Box 4-3)
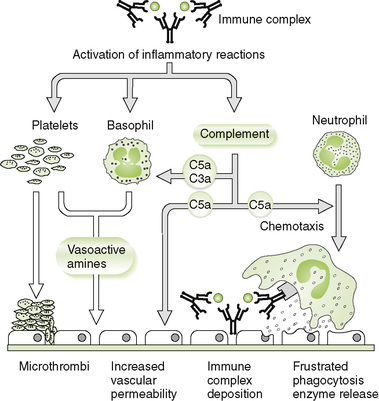
1. Initiation: formation of large amounts of circulating antigen-antibody (immune) complexes and their deposition in various tissues or on vessel walls
2. Effector mechanism: activation of the complement cascade by immune complexes leading to acute inflammatory reactions
• Arthus reaction: local skin reaction (redness and swelling) induced by intradermally injected antigen or insect bite
a. Intrapulmonary Arthus-type reaction can be induced by inhalation of bacterial spores or fungi (e.g., farmer’s lung).
• Serum sickness: generalized reaction developing 1 or 2 weeks after a second administration of immunoglobulin of another species (e.g., in passive immunization with horse serum)
a. Penicillin and other drugs can cause similar reaction marked by fever, urticaria, lymphadenopathy, and arthralgia.
• Vasculitis, nephritis, arthritis, and skin lesions associated with some infectious and autoimmune diseases
D Type IV (delayed-type) hypersensitivity
1. Initiation: antigen-stimulated release of cytokines (e.g., IL-2, IFN-γ, tumor necrosis factor-β [TNF-β]) from sensitized (activated) CD4 TH1 cells
• Primary inflammatory response: recruitment and activation of macrophages, which kill microbes and release various substances responsible for local inflammation and tissue damage
• Secondary cytolytic response: activation of CD8 TC cells and subsequent killing of target cells bearing antigen associated with class I MHC molecules
III Antimicrobial and Antitumor Host Defenses
• Table 4-4 summarizes the contribution of the various immune effector components in host responses to different types of pathogens.
TABLE 4-4
Role of Various Immune Effectors in Antimicrobial Responses
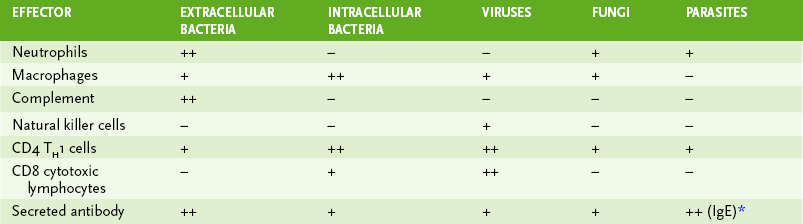
*IgE-mediated degranulation of mast cells is especially important in response to worm (helminthic) infections.
• Several anatomic and physiologic barriers inhibit entry of microbes into tissues (see Chapter 1, Fig. 1-1).
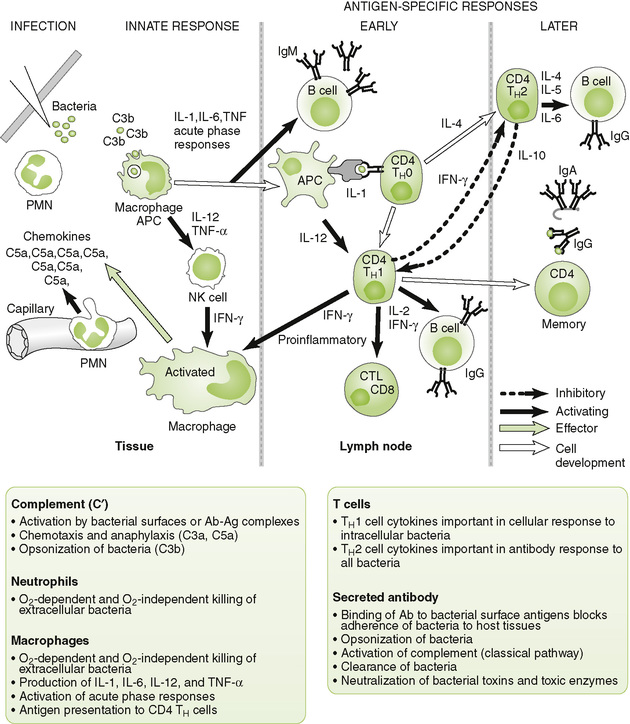
A Antibacterial responses (Fig. 4-5)
1. Initial innate (nonspecific) events
• Complement-mediated lysis, opsonization, and phagocytic destruction often can control infection by extracellular bacteria.
• PAMP stimulation of TLRs on DCs and macrophages stimulates cytokine production to stimulate acute, innate, and immune responses (Box 4-4).
• TH1 response (IFN-γ) is important in activating macrophages to control extracellular and intracellular bacteria (e.g., mycobacteria and Listeria monocytogenes) and wall-off infection (e.g., Mycobacterium tuberculosis).
• TH2 response stimulates and promotes class switch in B cells, thus promoting antibody production.
• Secreted antibody (B cell response) is most important against extracellular bacteria and toxins.
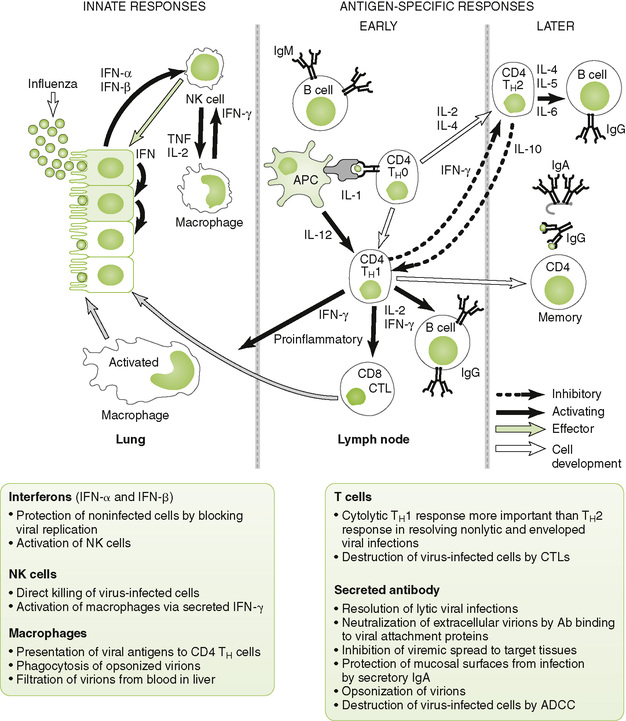
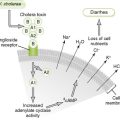
B Antiviral responses (Fig. 4-6)
1. Initial innate (nonspecific) events
• IFN-α and IFN-β secreted by infected cells protect surrounding noninfected cells from infection (local response) and trigger systemic immune responses (Box 4-5).
• Antibody neutralizes cell-free virus particles (virions; antibody), and cell-mediated immunity kills virus-infected cells, especially those not killed by virus replication.
• TH1 response is essential for control of enveloped and nonlytic viruses, which can replicate and spread within the host without killing infected cells.
a. TH1 cytokines promote antibody (IgG) production, which neutralizes virus and activation of cell-mediated responses, including CD8 cytotoxic T lymphocytes (CTLs), which kill infected cells.
• TH2 response stimulates antibody production.
a. Overactive TH2 response during early phase of viral infection can exacerbate disease by inhibiting development of protective inflammatory and cytolytic TH1 responses.
• Secreted antibody (B cell response) directed against viral surface antigens is essential in controlling infection by lytic viruses, which kill infected cells, and in preventing spread of virus by viremia.
C Antiparasite and antifungal responses
1. IgE-mediated degranulation of mast cells is critical to the control of parasitic worm infections.
2. Macrophages and delayed-type hypersensitivity (TH1 response) are essential for controlling most fungal infections and for intracellular parasites.
1. CTL-mediated destruction of tumor cells is a major antitumor effector response.
2. Antibody response plays a small or no role but may promote ADCC of tumor cells by NK cells and macrophages.
• Antibodies to tumor antigens, such as carcinoembryonic antigen, are monitors of tumor progression.
3. NK cells and activated macrophages exhibit nonspecific antitumor activity.
IV Vaccines and Immunization (see Chapters 8 and 20)
1. Administration of preformed antibodies, which provide rapid, temporary (2 or 3 months) protection against current infection or symptoms
2. Use of human gammaglobulin, rather than animal-derived serum, avoids possible induction of serum sickness.
1. Administration of agents that induce slowly developing but long-lasting immune protection against subsequent exposure to an infectious agent
2. Attenuated vaccines are live, weakened forms of an infectious agent; used primarily against viruses.
• Elicit both cell-mediated and antibody responses and long-term memory without need for multiple boosters
3. Inactivated vaccines include killed microbes and purified macromolecules.
• Elicit mainly antibody response and require periodic boosters to maintain immunity
a. Aggregation of antigens into particles increases their immunogenicity, such as hepatitis B and human papillomavirus–like particles.
• Bacterial polysaccharide capsular antigens elicit a relatively weak T-independent response, but when conjugated to a protein, they will elicit a strong T-dependent response with memory.
4. DNA vaccines are plasmids encoding a viral antigen (not yet licensed).
A Causes of autoimmune disorders
1. Inherited absence of tolerance to certain self antigens
2. Cross-reactivity of antimicrobial antibody with host tissue (e.g., antibody to group A streptococci)
3. Polyclonal activation induced by tumors or infection (e.g., multiple myeloma, Epstein-Barr virus infection)
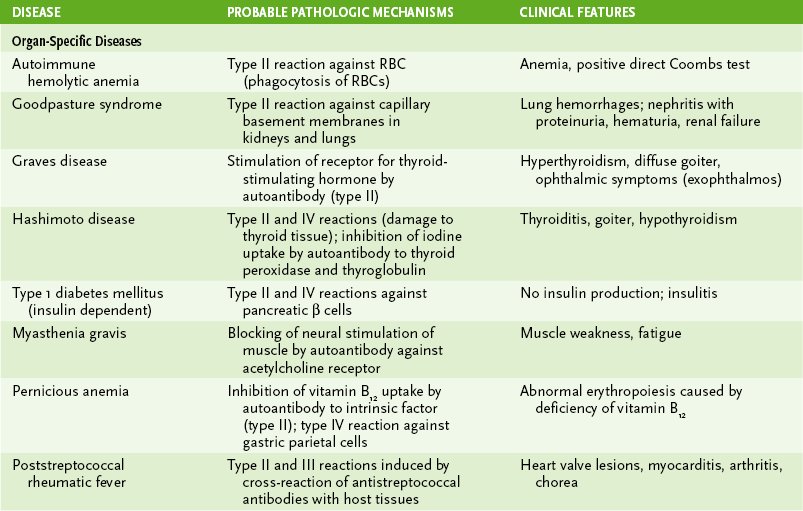
B Mechanisms of autoimmune pathology (Table 4-5)
1. Autoantibodies to cell surface proteins or circulating molecules blocking normal function or stimulating abnormal activity (also can be considered a type II hypersensitivity reaction)
2. Hypersensitivity-type reactions
a. Autoantibodies to cell surface antigens mediate antibody plus complement lysis and ADCC of host cells.
b. Examples: autoimmune hemolytic anemia, Goodpasture syndrome
1. The most common tissue graft is the fetus, and this requires that the mother have natural immunosuppression.
2. Trophoblasts express different HLA molecules (inhibit NK cells), decay antibody-accelerating factor (DAF; inhibits complement), inhibitors of T cells, and other immunosuppressive factors.
3. Cell-mediated immunity (CMI) autoimmunity wanes (rheumatoid arthritis), but antibody-mediated processes get worse (SLE)
1. Hyperacute rejection (minutes to hours): due to preexisting antidonor antibodies (e.g., ABO)
2. Acute (days to weeks): T cells reacting to HLA mismatch
3. Chronic (months): due to minor HLA mismatch
4. Graft versus host (e.g., bone marrow transplantation): T cells from graft react to host, release cytokines, kill cells, dysfunctional immune response
1. Neonates are naturally immunodeficient and susceptible to viral and intracellular microbial infections that cannot be controlled by maternal antibody due to inability to mount effective T cell responses.
2. Primary immunodeficiency results from congenital defects in some component of the immune system.
3. Secondary (acquired) immunodeficiency is associated with HIV infection, certain noninfectious diseases (e.g., nephrotic syndrome), cancer chemotherapy, and use of immunosuppressive drugs.
B Phagocyte disorders (see Chapter 1, Table 1-5)
C Complement abnormalities (see Chapter 1, section IV. E)
D Lymphocyte deficiencies (Table 4-6)
TABLE 4-6
Primary Lymphocyte Immunodeficiencies
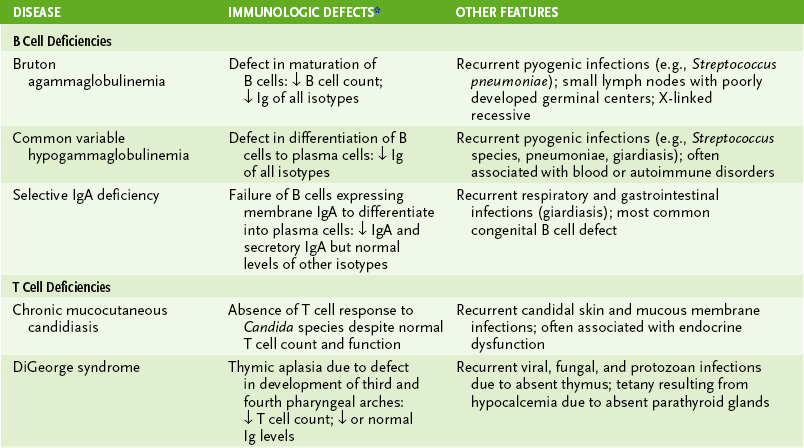
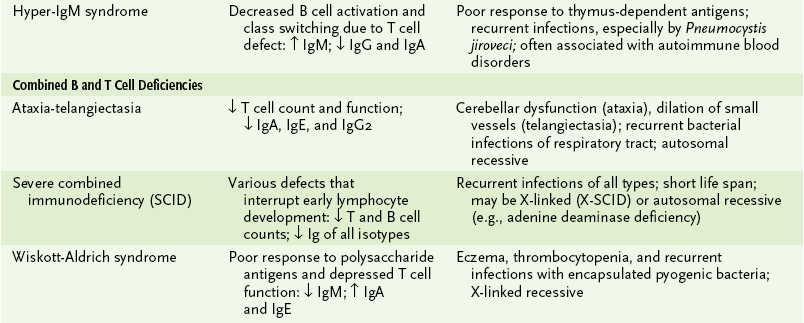
*↓, Below normal; ↑, above normal. Immunoglobulin (Ig) entries refer to serum antibody levels of indicated isotype.
1. Humoral deficiencies: decreased production of some or all antibody isotypes due to B cell defects or impaired TH cell function
2. Cell-mediated deficiencies: compromised delayed-type hypersensitivity response, TC-mediated cytotoxicity, or both
3. Combined immunodeficiencies: most severe with marked reduction in both cell-mediated and antibody responses