Chapter 201 Nonunion
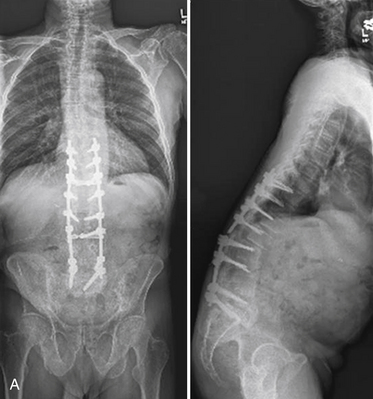
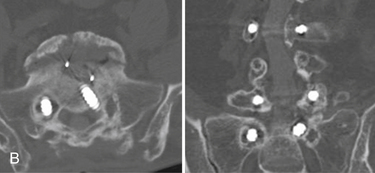
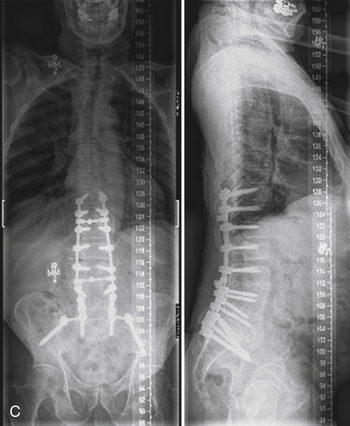
Recent advances and refinements in instrumentation have greatly expanded the capacity of surgeons to successfully treat complicated problems over the entire spine (Fig. 201-1). These systems aid the reestablishment of normal or near-normal alignment, may apply complex multidirectional force vectors, and may allow immediate immobilization over multiple spinal segments. As important as the meticulous and thoughtful use of this instrumentation is, careful consideration must be given to the material that will, for a lifetime, bear the stresses that instrumentation supports only temporarily. Solid bony union alone provides enduring spinal stability, and failure to achieve this goal can have consequences ranging from a benign radiographic finding to persistent pain or catastrophic construct failure. The nonunion rate varies with the type of operation performed, being higher when multiple-level fusions are attempted. A pseudarthrosis is defined as a documented failure of continuous bone formation over time that leads to a definitive absence of bone healing through a fracture or new bone formation at an intended arthrodesis site. From a practical standpoint, a nonunion in spine surgery has been defined as the absence of solid fusion 1 year after the operation with concomitant symptoms and signs.1 A large, population-based, prospective review of lumbar spine revisions showed that 23.6% of the indications were for pseudarthrosis.2 Overall, the true incidence of post–spine surgery nonunion is probably underestimated, because many cases are asymptomatic and require no treatment.
Biology of Bone Healing
Bone is a dynamic living tissue that undergoes constant remodeling.3 It is unique in its capacity to repair and regenerate after disruption.4 The amazing qualities of bone are derived from its unique composite structure of organic and inorganic materials. The organic component, chiefly a strongly cross-linked (type I) collagen, gives bone plasticity that allows substantial deformation without fracture and tolerates stress in tension.5 The inorganic component, chiefly in the form of hydroxyapatite, precipitates around the collagen fibers in a process of nucleation and maturation of mineral crystals.6 The inorganic-mineral component of bone gives the tissue tremendous strength in compression and bending.7
The cellular components of bone include osteoblasts, osteocytes, and osteoclasts, connected through an intricate and well-organized system of canals.8
Osteoblasts are derived from mesenchymal stem cells from the bone marrow and periosteum.9–12 They are responsible for bone formation. Osteoblasts fabricate bone in response to many stimuli and under different conditions such as growth, physiologic remodeling, fracture healing, and heterotopic ossification.13–15 Researchers have shown that new bone is formed in response to tumors and infections.16 An investigation has shown that osteoblasts have the ability to form bone during distraction osteogenesis,17 when substituting the void initially filled by autologous or allogeneic bone graft, demineralized bone matrix, or synthetic bone substitutes. When performing anterior cervical discectomy and fusion (ACDF) and plating, 97.5% of fusion with new bone formation has been achieved with either autograft or allograft.18 In a recent study, Jensen showed an 86% union rate after single- and multiple-level ACDF using patella allograft and plating.19 In a study from Japan, Momma has reported complete bone remodeling after 6 to 12 months on CT scan, after the use of B-tricalcium phosphate to fill a partial ventral vertebrectomy defect done for cervical decompression surgery.20
Osteoclasts are derived from hematopoietic stem cells. They exit the circulation close to the site to be remodeled.6,21 They are responsible for the bone resorption.
The mature osteoblast produces proteins such as type I collagen, osteocalcin, and alkaline phosphatase, a key enzyme in bone mineralization. Osteoblasts become entrapped in their own osteoid matrix and develop long cytoplasmic processes to remain in contact with surrounding cells.22 They then begin expressing a whole new set of genes to continue bone turnover and mineral homeostasis. These cells are now considered osteocytes (mature bone cells).6
The process of bone healing after injury is an indistinct continuous sequence of inflammation, repair, and remodeling.10,12 The inflammatory response to injury includes vascular dilatation with exudate and edema, as well as inflammatory cell (polymorphonuclear lymphocytes, macrophages, and lymphocytes) infiltration. A variety of hormones, cytokines, growth factors, and matrix proteins (e.g., bone morphogenetic protein [BMP]) is involved throughout the healing process. Nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, or chemotherapeutic agents given during the first week of healing may blunt the inflammatory response and impair bone healing.10,12,23 As the debris of the inflammatory phase is removed, fibroblasts begin laying down new matrix in the early phases of the repair. Initially, a fracture callus that is composed of fibrous tissue, cartilage, and woven bone may form to bridge the bony defect. This is then replaced by woven bone and, ultimately, by mature cortical or cancellous bone. This process may take 3 to 6 months or longer, depending on age and other factors.10,23 Although the general sequence of inflammation, repair, and remodeling that occurs in long bone fracture healing also occurs with bone graft repair, there are some distinct differences. Autograft bone used in spinal fusion is initially deprived of blood supply, although a robust nonspecific inflammatory response occurs as a result of preparation of the graft recipient bed. The collection of coagulated blood around the graft is somewhat analogous to the hematoma of an acute fracture, with the complex processes of inflammation ongoing within this milieu. Although some of the periosteum, endosteum, mesenchymal cells, and osteocytes within 0.2 to 0.3 mm of the borders survive transplant, most of the transplanted bone cells, separated from their blood supply, die.24 The cancellous portion of the bone graft may be revascularized within 2 weeks, and cortical bone is revascularized within 1 to 2 months. Cancellous bone is more rapidly remodeled and is initially strengthened during the remodeling phase, because osteoblasts are first laid down over the trabeculae.5
Cortical bone is weakened during initial remodeling, and the process is slower than in cancellous bone. Bone graft is gradually replaced with new bone in a process called creeping substitution24 Osteoclasts that act as cutting cones bore into the graft from the margins of host bone, followed by osteoblasts that lay down new bone. This process of healing and remodeling may leave as much as 50% to 90% of the original matrix, even after many years.25,26 The strength of cortical autograft is halved during the first 6 months after fusion but is gradually restored over 1 to 2 years. Autograft bone provides some living bone cells with the ability to make bone (i.e., osteogenic properties). It contains BMP and other substances capable of inducing cellular differentiation (i.e., it has osteoinductive properties), and it provides a scaffolding for bone growth (i.e., osteoconductive properties).27
Harvesting and Handling of the Bone Graft
Surgical exposure of the donor site should be performed to maximize the viability of the graft. Heating of bone with the electric cautery, although frequently unavoidable, has the potential of destroying those surface osteocytes most capable of surviving via diffusion of nutrients. A sharp periosteum elevator can be used to open the subperiosteal plane in a remarkably atraumatic fashion, with minimal blood loss. For tricortical bone grafts placed in compression, harvesting with an oscillating saw provides a graft that is better at resisting compressive loads than a graft harvested with an osteotome.28,29 The clinical significance of this is unknown. For bone used purely for onlay, one should bear in mind that 5 mm is the maximal thickness that can be nourished by diffusion of nutrients.30,31 If possible, the bone graft should be harvested within 30 minutes of planned use. The graft should be kept moist in a saline- or blood-soaked sponge before use. The graft should not be allowed to dry or come into contact with toxic chemicals (e.g., antibiotic solutions).32
Selection of Graft Material and Instrumentation
Ideally, graft material should have the capacity to form bone (i.e., osteogenic properties), induce undifferentiated mesenchymal cells to mature into osteoblasts (i.e., osteoinductive properties), serve as scaffolding for bone healing (i.e., osteoconductive properties), harbor no risk of infection, and be genetically identical to the patient. It should also be mechanically strong, durable, potentially viable, nonreactive to the host tissue, sterile, anatomic, and cost effective.33 Currently, the material that comes closest to these requirements is the patient’s own (autograft) bone. The most common choices for autograft in spine surgery include iliac crest, local bone, or rib. The disadvantages of an autograft include the potential for inadequate bone graft volume or quality, risk of wound hernia, pelvic fracture for iliac crest grafts, blood loss, infection, nerve injury, and, most commonly and bothersome at the iliac crest, chronic graft harvest site pain. The incidence of major complications with autograft harvesting can be as high as 10%33 or even 17.9% when using the same skin incision for iliac crest harvest and the primary spine procedure.34 Chronic persistent pain at the donor site ranges from 2.8% to 70% of patients,35–39 with most series reporting it to be about 20% to 30%.35–37
Allograft bone has the advantage of being readily available in multiple structural forms and without donor site morbidity. Allograft has some osteoinductive and osteoconductive, but no osteogenic, properties. Vascular ingrowth and new bone formation are delayed with allografts.40–42 The mode of preparation of allograft may have an impact on its success as a graft material. Allograft bone may be treated with freezing, freeze drying, or ethylene oxide to reduce its immunogenicity; however, because it is genetically dissimilar to the patient, an inflammatory response similar to graft rejection noted in other tissue transplants may occur.43,44 Fresh-frozen allograft appears to have a superior fusion rate to freeze-dried graft, with ethylene oxide–sterilized grafts demonstrating uniformly poor results.45
Cervical Spine
The reported outcomes of noninstrumented single-level ACDF procedures with the use of autologous ventral iliac crest bone graft (ICBG) include fusion rates between 83% and 100%.46–52 The use of allograft bone for single-level ACDF appears to yield results that are approximately equivalent to use of autograft bone.47,53–60 For multilevel ventral procedures, autograft classically appears to be superior.58,61–63 A recent study, however, shows equal fusion rates of 97.5% using either autograft or allograft and ventral plating for multilevel ACDF.18 For bone struts used over multiple segments, pseudarthrosis rates with allograft are higher (41%) than with autograft (27%).64 When supplemented with dorsal fusion, the pseudarthrosis rate falls to 26%.65
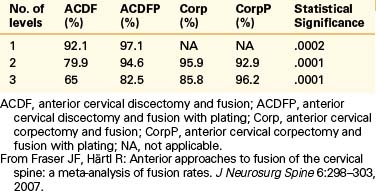
A separate study using notched fibular struts and a halo orthosis demonstrated delayed fusion, but seven of eight patients had good or excellent results.53 In fusions unsupplemented with dorsal instrumentation or a halo orthosis, autograft bone appears to be the favored graft material. For dorsal cervical fusions, autograft bone appears to be superior in some studies.4,66 Ventral plating to add stability to the ACDF construct, especially if multilevel, has clearly increased the union rate of the procedure.48,51,52,67–69 In a recent meta-analysis, the authors concluded that plating significantly increases the fusion rate of ACDF regardless of the number of levels. They also noted that corpectomies had higher fusion rates than multilevel ACDFs and that the use of plates improved the fusion rate of three-level but not two-level corpectomy surgery70 (Table 201-1).
The use of a cylindrical titanium mesh cage packed with bone salvaged from the corpectomy may avoid the need for harvesting a separate graft. Fusion rates using this method of reconstruction have been reported to be greater than 90%.71,72
Lumbar Spine
It is well established that instrumentation in the lumbar spine increases fusion rates. In 1997, Fischgrund et al.73 published a prospective, randomized trial comparing decompression and dorsolateral fusion with and without instrumentation in patients with degenerative spondylolisthesis and spinal stenosis. The average follow-up was 2 years. The fusion rate was significantly better with instrumentation than without (82% vs. 45%, respectively; P = .0015); however, no significant difference was found in clinical outcome. In 2004, Kornblum et al.74 analyzed the patients from the 1997 Fischgrund study (now with a follow-up of 5–14 years) and noted that the patients with a solid fusion did significantly better than patients with a nonunion. Other authors have also conducted prospective, randomized studies looking at the same issue. Whereas Zdeblick’s results also support rigid instrumentation,75 Thomsen et al.76 reported fusion in 68% of instrumented cases and in 85% of noninstrumented cases.
In the only randomized trial comparing circumferential fusion with dorsolateral fusion alone (to our knowledge), a significantly higher fusion rate was found with circumferential fusion (92% vs. 80%; P < .04).77 In a meta-analysis looking at different lumbar fusion procedures, Bono et al. concluded that the highest rate of fusion was obtained with a circumferential technique (91%; P = .06), followed by posterior interbody (89%; P = .05), anterior interbody (86%), and finally posterolateral (85%) techniques.78 The clinical relevance and cost of the circumferential procedure for fusion in the lumbar spine is still open to debate.
In a study of long-segment fusion to the sacrum (mean of 11.9 vertebrae) for adult spinal deformity, the pseudarthrosis rate was 24% (much higher than for short constructs). Half of these pseudarthroses occurred through the thoracolumbar junction and one fourth through the lumbosacral junction. Risk factors were kyphosis of 20 degrees or higher, positive sagittal balance of 5 cm, hip osteoarthritis, a thoracoabdominal approach, age older than 55 years, and incomplete lumbopelvic fixation (complete lumbopelvic fixation defined as L5-S1 interbody fusion and iliac screw fixation). Augmenting the number of fused levels into the upper thoracic spine did not improve the fusion rates.79
In the lumbar spine, allograft bone plays a limited role with dorsolateral fusion. However, its use as a ventral interbody strut (particularly femoral shaft allograft packed with cancellous autograft) when used with dorsal segmental instrumentation has been substantiated.80–82
Pediatric Spine Surgery
The use of allograft versus autograft bone has been well studied for scoliosis.83–86 In uninstrumented cases, autograft performed superiorly. Instrumented dorsal fusions supplemented with allograft bone performed comparably in a pediatric population (although it took a long time to achieve fusion). Ventral allograft struts supplemented with autologous bone (packed into the hollowed marrow space of the allograft), in conjunction with dorsal fusion and segmental instrumentation, yielded better results than allograft fusion without ventral graft supplementation.86 When treating high-grade pediatric isthmic spondylolisthesis, Molinari et al. performed a very interesting study. He divided patients in three groups with the following results:
Bone Graft Substitutes
Demineralized Bone Matrix
Demineralized bone matrix (DBM) facilitates bone fusion through osteoinduction and, to a lesser degree, through osteoconductive properties. During the fabrication of DBM, the demineralization of allograft reduces its antigenicity and may uncover osteoinductive factors, including BMP. However, BMP-2 and BMP-7 exist in nanogram concentrations in DBM, which is one million times less than the concentration of BMP required to produce a lumbar fusion clinically.88–90 DBM may vary in its osteoinductive capabilities, based on the cadaveric bone from which it is derived, by vendor, and even among batches of the same brand. Several animal studies favor DBM when compared with autogenous bone in achieving spinal fusion.91–93 Clinically, the data are more limited. A prospective trial comparing allograft and DBM with autograft in ventral cervical fusion demonstrated only a higher rate of graft collapse and pseudarthrosis in the allograft group.94 In the lumbar spine, the use of DBM plus local bone achieved the same fusion rates as ICBG for a single-level posterolateral fusion.95
Synthetic Bone Substitutes (Ceramics)
Numerous calcium-based synthetic products have emerged as bone graft substitutes and/or extenders in spine fusion. These products serve as scaffolds that support new bone ingrowth. During manufacturing, the porosity of these materials can be optimized for bony ingrowth.96 Calcium sulfate is not sufficient for use as an osteoconductive material, because it absorbs in only a few weeks, much before new bone has formed in a fusion.97 Hydroxyapatite takes several years to be reabsorbed, and its radiopacity makes the radiographic diagnosis of fusion difficult. Beta tricalcium phosphate absorbs in months, thus lasting an adequate period to conduct bone growth during fusion.97 For this reason, most ceramic products used in spine fusion these days are made of beta tricalcium phosphate and/or hydroxyapatite in combination with bovine collagen in varying ratios. Collagen affects the workability and reabsorption rate of the ceramic and may also serve as a carrier for osteoinductive agents, such as BMP.98
Animal studies show excellent fusion rates (superior to the control group with autologous bone) when ceramics are used in combination with bone marrow aspirate (osteoinductive and osteogenic) and very poor results when ceramics are used alone. The authors concluded that the association of bone marrow aspirate is paramount to the success of the procedure.99,100
In prospective case-controlled clinical series, the fusion rates for lumbar posterolateral fusion and transforaminal lumbar interbody fusion using ceramics in conjunction with bone marrow aspirate and/or local autograft yield similar good results as with ICBG (fusion rate from 92% to 100% for a single-level instrumented fusion).101–104 In the cervical spine, Momma has reported complete bone remodeling, after 6 to 12 months, on CT scan, after the use of beta tricalcium phosphate to fill a partial anterior vertebrectomy defect done for cervical decompression.20
Bone Morphogenetic Protein
BMPs are a group of growth factors originally discovered because of their ability to induce the formation of bone and cartilage. Originally, seven proteins from this group were discovered. Of these, six (BMP-2 through BMP-7) belong to the transforming growth factor-beta superfamily of proteins, whereas BMP-1 is a metalloprotease, involved in cartilage development. Since then more BMPs have been discovered, making a total of approximately 20 today.105 BMP bone induction is a sequential cascade. The key steps in this process are chemotaxis, mitosis, and differentiation as shown on early studies by Reddi.106 They are known to stimulate osteoblasts and inhibit osteoclasts.107,108 Currently, the Food and Drug Administration (FDA) has approved two BMPs for use in humans as bone growth inducers—BMP-2 and BMP-7. BMP-2 is the BMP used in almost the totality of current spinal fusion cases because of FDA regulation issues with BMP-7. Multiple preclinical animal studies have shown that the use of BMP results in similar, if not superior, fusion rates with biomechanically stronger fusion masses when compared with autogenous bone graft.109–111
Prospective, randomized clinical studies have shown that BMPs have at least comparable fusion rates and clinical outcomes when compared with ICBG in both interbody and posterolateral lumbar fusions.112–114
One prospective nonrandomized study in the ventral cervical spine reports that fusion rates of allograft and recombinant human bone morphogenetic protein (rhBMP-2; 0.9 mg per level) were slightly better than those of ICBG. However, 50% of the patients receiving the BMP had significant neck swelling.115 Another study reported 27.5% clinically significant neck swelling after ACDF with BMP.116 The safe dose of BMP and the best method for delivery are yet to be determined in the cervical spine. In 2008, the FDA issued a warning concerning its use in cervical surgery.117
Recently discovered bone morphogenetic protein-binding peptide (BBP) is a 19–amino acid peptide that has been shown to bind BMP and potentiate its effect of bone healing in animal studies. BBP may provide for improved fusion rates with a smaller dose of BMP required, potentially reducing cost, as well as potential side effects of BMP such as inflammation and ectopic bone formation.118
Influence of Electromagnetic Stimulation on Bone Healing
Direct current stimulation was first proposed in 1972 as a modality for improving fusion.119,120 Application of pulsed electromagnetic fields for nonunion in long bone fractures appears to have no hazardous side effects. Areas of tension are associated with a net positive charge, and compressive stresses are associated with a net negative charge (10–100 mV) and osteogenesis.120 Electromagnetic stimulation is believed to promote osteogenesis as a result of more rapid angiogenesis and decreased osteoclastic activity.121 The effect of improved osteogenesis may be mediated by growth factors.122 More recent evidence also suggests the activation of a second messenger system involved in bone remodeling. Three broad types of electromagnetic fields are used: implantable direct current, pulsed electromagnetic fields, and capacitively coupled electrical energy. Pulsed electromagnetic fields and capacitively coupled electrical energy are examples of external electromagnetic fields. These are delivered via external electrodes attached to a corset. Implantable direct current requires surgical placement of the electrodes and has been shown to be the most effective.123 Direct current may be more effective than external electrodes secondary to its increased precision in the distribution of current.124 The cost of these devices is not insignificant. Although some investigators have noted no significant benefit of electrical stimulation for canine spinal fusions,125,126 one randomized blinded study in 195 patients demonstrated a significant difference in fusion rates between stimulated (92%) and unstimulated (65%) groups.127 In a study of 59 patients who underwent reoperation for failed lumbar fusions, there was an 81% fusion rate in stimulated patients versus a 54% fusion rate in unstimulated patients.128 Another contemporary series found a 96% fusion rate with implanted stimulators versus 85% with no stimulation.129 Clinical trials have been faulted for having a high degree of variation in electrical stimulation protocols, surgical intervention, and disease-treated patient populations.123 Strong, consistent, and clear evidence supporting the effects of electrical stimulation on long-term fusion rates is yet to be published.
Factors Affecting Bone Healing
Smoking
Studies have shown a threefold to fourfold increase in the occurrence of nonunion of spinal fusions in smokers over that in nonsmokers.56,130,131 Smoking interferes with osteoblastic function,132 leads to increased bone resorption at fracture sites,133 and interferes with normal bone metabolism.134 Smoking has also been associated with bone mineral loss in several studies.135,136 Nicotine, the chemical most responsible for physical dependency, also has deleterious effects on spine fusion rates and revascularization of bone graft, as shown in animal studies.137 One particular article demonstrated decreased intertransverse fusion rates in rabbits receiving systemic nicotine and also suggested that the bone formed during nicotine use has inferior biomechanical properties.60 These studies strongly indicate that the use of nicotine patches or gums, in an effort to curb patient smoking perioperatively and during bone healing, may be ill advised. It is likely, however, that other components of cigarette smoke also have a deleterious effect on fusion, although this is less documented. Even the pulmonary compromise associated with smoking, as reflected in a decreased arterial partial oxygen pressure, has been suggested as a potential explanation for increased nonunion rates in smokers.61
A case-control study of two-level lumbar laminectomy and fusion demonstrated a nonunion rate of 40% in smokers and 8% in nonsmokers.131 Similarly, in a study of anterior cervical fusion with allograft, the authors found a higher rate of fusion in nonsmokers than in smokers (81% vs. 62%).138
Radiation Therapy
Radiation impairs bone healing,139–141 inhibiting cell proliferation and producing a vasculitic reaction that limits vessel ingrowth. Radiation delivered before long bone fracture in animals results in delayed fracture healing.142 In a canine model, bone healing was superior when radiation was given either preoperatively or only after 21 days postoperatively. The worst bone healing results were seen when radiation was administered on postoperative day 3.143 The total radiation dose, delivered preoperatively or postoperatively, has been shown to correlate well with reduction in strength of healing bone in an animal model.144 A total radiation dose exceeding 4000 cGy has been proposed as a risk factor for nonfusion in patients undergoing perioperative radiation for neoplasm.
Nutrition
Malnutrition has a negative impact on fracture healing,144 blunts the immune response, and impairs wound healing. A nutritional support team can provide vital preoperative evaluation and education to optimize nutritional status preoperatively and postoperatively.
Rheumatoid Arthritis and Ankylosing Spondylitis
Rheumatoid arthritis affects 1% of the world’s population, with 60% to 70% of patients with the disease eventually suffering cervical spine symptoms.145 In patients requiring fusion, bone healing is often compromised by osteoporosis,146 and the direct immunosuppressive effects of the disease itself (coupled with the effects of steroid medications)147 can lead to osteomyelitis.148 Despite these factors, the use of contemporary instrumentation and arthrodesis techniques in patients with rheumatoid arthritis has resulted in spine surgery fusion rates of 90% or higher in multiple centers.149–152
Nonunion does not seem to be a problem when operating on patients with ankylosing spondylitis. Bony union is usually the rule in patients with ankylosing spondylitis after surgery, and fusion rates exceeding 95% have been reported after the correction of spinal deformity.153–159
Age
Generally, skeletally immature patients have the greatest healing potential and heal more quickly.160 It is hypothesized that children may have a greater number of undifferentiated mesenchymal cells, and that these cells may be capable of more rapid differentiation when necessary.161,162 In one study looking at long thoracolumbar constructs, age greater than 55 years was a risk factor for nonunion.79
Nonsteroidal Anti-Inflammatory Drugs
There is clearly a negative association between spine fusion and use of NSAIDs in animal models.111,163–165 In the clinical setting, a recent meta-analysis looking at the use of NSAIDs and spine fusion rates found that the odds ratio of a nonunion was higher (3.0) in the anti-inflammatory use group only in lower-quality studies. The better-quality articles failed to show an association of NSAID use and nonunion. The authors conclude that higher-quality, prospective, controlled, randomized studies are needed to further elucidate this issue.166
Diagnosis of Nonunion
The diagnosis of pseudarthrosis remains a challenging endeavor that in most instances requires a high index of suspicion. It has classically been based on the triad of persistent pain, radiographic evidence of instability, and loss of correction/fixation. Diagnosis by imaging is often difficult and may require multiple modalities. Results may be misrepresented, not only by the limitations of technique but by the prejudgment of the surgeon. A study comparing radiographic analysis of ACDF fusion at 6 months between the surgeon and an independent panel had a correlation of k = 0.308. The correlation was even poorer when the surgeon noticed favorable clinical results.167
Flexion-Extension Radiographic Studies
There is controversy concerning the angular amount of motion accepted in a segment to correlate with a solid fusion. Most studies range from 0 to 5 degrees.168
One study developed a finite element model to simulate different types of lumbar fusion and concluded that, overall, a solid fusion should have less than 4.1 degrees of motion between the segments of interest. This study, however, did not account for instrumentation.169 Simmons considers a nonunion when more than 2 degrees of difference is seen between the flexion and extension films.170 One should keep in mind that with the advent of rigid instrumentation, pseudarthrosis may be present even without motion of the segment on dynamic radiographs.168
Computed Tomography
CT has become widely used for the diagnosis of nonunion after spine surgery. A recent prospective study of intraoperative evaluation (gold standard) versus imaging evaluation of pseudarthrosis after anterior cervical fusion found that CT most closely agrees with intraoperative findings (P < .05) compared with plain radiography and MRI.171 Another study comparing thin-slice CT and dynamic radiography for the diagnosis of nonunion found pour correlation between the two methods.172
Magnetic Resonance Imaging
Due to its cost and metal artifact on imaging after instrumentation, MRI is not a good choice for assessing bone union after fusion.168
Nuclear Medicine and Ultrasound
Bone scans have a low sensitivity and positive predictive value for identifying pseudarthrosis and are considered to be ineffective for the reliable diagnosis of nonunion after spinal fusion.168 Single-photon emission CT scans have a sensitivity and a specificity of about 50% and therefore cannot be used reliably for the diagnosis of pseudarthrosis.173 The use of ultrasound in detecting pseudarthroses has been explored in the past. In a series by Jacobson et al., it was used to evaluate pseudarthrosis after posterolateral spinal fusion. There was a reported sensitivity of 100%, but the specificity was only 60%.174 In conclusion, ultrasound and nuclear medicine modalities do not seem to play a major role in modern intervertebral pseudarthrosis investigation.
Technical Aspects and Results
Cervical Spine
Ventral cervical interbody fusion, using the Smith-Robinson technique, is one of the most common procedures performed by spine surgeons. Although this technique provides a high rate of solid arthrodesis, attention to some details can optimize the likelihood of solid success. Meticulous preparation of the recipient site, including the complete removal of the cartilaginous end plates, is required. Widening the exposure to the width of the uncovertebral joints helps provide a broad surface for fusion and helps localize the midline. Very often it is helpful to remove the overhanging ventral caudal lip of the rostral vertebral body. Performed early in the decompression, this phase allows for a better view of the dorsal interspace while also allowing for a smooth fit of the interbody graft. The final preparation of the recipient surfaces involves smoothing any irregular contours that may leave gaps in the interface between the graft and the recipient site. After the recipient site is prepared, the graft may be precisely contoured to just exceed the height of the recipient site. The graft should be impacted into the interspace, with great care taken to avoid driving it into the spinal canal. Controlled cervical traction, applied by the anesthesiologist, or an interspace spreader can provide the additional interspace height that is required to allow gentle tamping of the bone graft into position with only moderate force. Excess force, poor fit of the graft, or use of a small impactor may result in fragmentation of the graft, necessitating replacement. The implant should seat securely in the interspace, and the surgeon should be able to feel it settling solidly into position. Grafts that freely spin or move within the space after the release of cervical traction, or grafts that splinter, crack, or fray are suboptimal and should be replaced. Long interbody grafts require special consideration. A variety of strategies have been used that are largely based on the preference of the surgeon.
Symptomatic nonunion after ventral procedures can be managed in many ways. One option, when there is no overt ventral instrumentation failure and no symptomatic ventral compressive pathology, is simply a dorsal fusion, usually using instrumentation. A dorsal fusion that incorporates all levels included by the ventral procedure avoids reoperating through distorted anatomy. If overt hardware failure or residual ventral pathology demands reoperation, one may proceed from the side opposite the initial procedure. The decision to operate through a previous exposure is largely one of personal preference, unless a recurrent laryngeal nerve injury was sustained with the initial surgery (as documented by laryngoscopy), in which case the same-side incision is clearly indicated. Although the procedure is generally straightforward, a few points deserve mention with regard to ventral cervical operations.175 First, on exposure of the ventral spine, it may be difficult to precisely localize the level of suspected pseudarthrosis on gross inspection. Identifying adjacent disc space levels provides some guidance, as does an aggressive mobilization of the longus colli musculature, which provides lateral bony exposure that may reveal some preserved interspace laterally at the operated level. Second, the actual removal of graft from a previously operated level may be challenging. Usually a high-speed pneumatic drill is used to remove it. When ventral instrumentation is planned, particular care must be taken to avoid overzealous vertebral body resection adjacent to the graft site. Excessive straying into an adjacent vertebral body can quickly result in the loss of a suitable anchor point for the implant, necessitating inclusion of another level in the construct. Fluoroscopy can provide a helpful gauge of depth during drilling. One may drill down the old graft just slightly beyond its rostral and caudal margins, staying near the midline. This provides exposure of the posterior longitudinal ligament remote from previous surgical exposure. In this fashion, the surgeon can easily broaden the dural exposure and extend it rostrally and caudally, beginning on tissue away from the initial surgery. The technique of beginning far laterally and drilling on either side of the graft should be used with caution, because as the exposure deepens, one may inadvertently encounter a nerve root with little warning from normal anatomic landmarks.
In a series of 120 patients with post-ACDF pseudarthrosis, the union rates for revision surgery were 56% if a ventral approach was chosen for the revision, as opposed to 98% if the fusion was done from a dorsal approach.176 Two other studies corroborate the same findings.177,178 Others, however, have successfully achieved 100% of fusion when revising ACDF nonunion from the front.175,179
Thoracic Spine
The rigidity of the thoracic spine, afforded by the articulation of the ribs, enhances the integrity of most midthoracic constructs.180 The tremendous loads concentrated at both ends of the thoracic spine, by virtue of the leverage of the craniocervical and lumbosacral lever arms, stress constructs at these locations.
Berven published on the revision of 10 cases of thoracic nonunion treated with extension osteotomy, rigid dorsal-only instrumentation, and autograft. His results show 100% of fusion and 70% of patient satisfaction.181
Lumbar Spine
In a study treating dorsal surgery lumbar pseudarthrosis with anterior lumbar interbody fusion, the authors achieved solid fusion in 52 of 53 cases. Despite the excellent fusion rates, the functional results were dismal, with only approximately 30% of patients being able to return to work.182 Gertzbein et al. retrospectively reviewed 25 patients treated with circumferential fusion after pseudarthrosis following attempted dorsolateral fusion surgery. Besides a 100% fusion rate, only 52% of patients had considerable pain relief.183
It is well determined in lumbar nonunion history that even if fusion is achieved in the revision surgery, the clinical outcomes are usually not as good.184–187 On the other hand, it is also known that patients with successful fusion after revision surgery are far more likely to have a satisfactory outcome than are those who remain unfused.188–190
Summary
As in every other surgical arena, an understanding of the basic principles, coupled with common sense, best equips the surgeon to deal with adversity and achieve optimal results. Finally, it is important to always keep in mind that even though patients who attain fusion generally do better than those who go into a nonunion, achieving a firm arthrodesis between segments is not a guarantee of clinical improvement.
Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455-463.
Burkus J.K., Gornet M.F., Dickman C.A., et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
Buttermann G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426-435.
Kim Y.J., Bridwell K.H., Lenke L.G., et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31:2329-2336.
Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
Wang J.C., McDough P.W., Endow K., et al. The effect of cervical plating on one-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
Zdeblick T.A., Hughes S.S., Riew K.D., et al. Failed anterior cervical discectomy and arthrodesis: analysis and treatment of thirty-five patients. J Bone Joint Surg [Am]. 1997;79:523-532.
1. Steinmann J.C., Herkowitz H.N. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
2. Martin B.I., Mirza S.K., Comstock B.A., et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32:382-387.
3. Frost H.M. Tetracycline-based histologic analysis of bone remodeling. Calcif Tissue Res. 1969;3:211-237.
4. Stabler C.L., Eismont F.J., Brown M.D., et al. Failure of posterior cervical fusion using cadaveric bone graft in children. J Bone Joint Surg [Am]. 1985;67:370-375.
5. Triffitt J.T. The organic matrix of bone tissue. In: Urist M.R., editor. Fundamental and clinical bone physiology. Philadelphia: Lippincott-Raven; 1980:45.
6. Neuman W.F. Bone materials and calcification mechanisms. In: Urist M.R., editor. Fundamental and clinical bone physiology. Philadelphia: Lippincott-Raven; 1980:83.
7. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;174:28-42.
8. Recker R.R. Embryology, anatomy, and microstructure of bone. In: Coe F.L., Favus M.J., editors. Disorders of bone and mineral metabolism. Philadelphia: Lippincott-Raven; 1992:219.
9. Robling A.G., Castillo A.B., Turner C.H. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455-498.
10. Miller J.D., McCreadie B.R., Alford A.I. Form and function of bone. In: Einhorn T.A., O’Keefe R.J., Buckwalter J.A., editors. Orthopaedic basic science. ed 3. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007:129-159.
11. Brinker R.M., O’Connor D.P. Basic sciences. Bone. In Miller M.D., editor: Review of orthopaedics, ed 5, Philadelphia: Saunders, 2008.
12. Buckwalter J.A., Glimcher M.J., Cooper R.R., et al. Bone biology. Part II: formation, form, modeling, remodeling, and regulation of cell function. J Bone Joint Surg [Am]. 1995;77:1276-1289.
13. Micheli A., Trapani S., Brizzi I., et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
14. Beiner J.M., Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res.. 2002;403:S110-S119.
15. Järvinen T.A., Järvinen T.L., Kääriäinen M., et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745-764.
16. Aoki J., Yamamoto I., Hino M., et al. Reactive endosteal bone formation. Skelet Radiol. 1987;16:545-551.
17. Ilizarov G.A. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249-281.
18. Shen F.H., Matthews D.K., Yoon S.T., et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J Nov-Dec. 2003:451-459.
19. Jensen W.K., Moore T.A., Tribus C.B., et al. Use of patella allograft for anterior cervical diskectomy and fusion. J Spinal Disord Tech. 2009;6:392-398.
20. Momma F., Nakazawa T., Masaharu A. Repair and regeneration of vertebral body after antero-lateral partial vertebrectomy using B-tricalcium Phosphate. Neurol Med Chir (Tokyo). 2008;48:337-342.
21. Robling A.G., Turner C.H. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:319-338.
22. Klein-Nulend J., Bacabac R.G., Mullender M.G. Mechanobiology of bone tissue. Pathologie Biologie. 2005;53:576-580.
23. Dickman C.A., Maric Z. The biology of bone healing and techniques of spinal fusion. BNI Quarterly. 1994;10:2-12.
24. Muschler G.F., Lane J.M., Dawson E.G. The biology of spinal fusion. In: Cotler J.M., Cotler H.P., editors. Spinal fusion science and techniques. Berlin: Springer-Verlag; 1990:9.
25. Enneking W.F., Mindell E.R. Observations on massive retrieved human allografts. J Bone Joint Surg [Am]. 1991;73:1123-1142.
26. Urist M.R. Bone transplants and implants. In: Urist M.R., editor. Fundamental and clinical bone physiology. Philadelphia: JB Lippincott; 1980:331.
27. Cohen D.B., Chotivichit A., Fujita T., et al. Pseudarthrosis repair: autogenous iliac crest versus femoral ring allograft. Clin Orthop Relat Res. 2000;371:46-55.
28. Dougherty P.J., Jones A.A.M., Sharkey N., Benson D.R. Iliac crest bone graft: osteotome versus saw (abstract). Rosemont, IL: Cervical Spine Research Society; December 1992. 148
29. Heggeness M.H., Esses S.I. Classification of pseudarthroses of the lumbar spine. Spine. 1991;16:449-454.
30. Ray R.D. Bone grafting: transplants and implants. Instr Course Lect. 1956;13:177-186.
31. Ray R.D., Sabet T. Cellular survival versus induction: an experimental study in mice. J Bone Joint Surg [Am]. 1963;45:337-344.
32. Prolo D.J. Morphology and metabolism of fusion of the lumbar spine. Youmans J.R., editor. Neurological surgery, ed 4, vol. 3. Philadelphia: WB Saunders, 1996;2449.
33. Cook S.D., Whitecloud T.S. Use of osteoinductive implants to facilitate spine fusions. In: Bridwell K.H., DeWald R.L., editors. The textbook of spinal surgery. ed 2. Philadelphia: Lippincott-Raven; 1997:2379.
34. Younger E.M., Chapman E.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195.
35. Goulet J.A., Senunas L.E., DeSilva G.L., et al. Autogenous iliac crest bone graft. Clin Orthop Relat Res. 1997;339:76-81.
36. Silber J.S., Anderson D.G., Daffner S.D., et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134-139.
37. Sasso R.S., LeHuec J.C., Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion. A prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18:S77-S81.
38. Schnee C.L., Freese A., Weil R.J., et al. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine. 1997;22:2222-2227.
39. Singh K., Phillips F.M., Kuo E., et al. A prospective, randomized, double-blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis. A minimum of 4-year follow-up. Spine. 2007;32:2790-2796.
40. Enneking W.F. Histologic investigations of bone transplants in immunologically prepared animals. J Bone Joint Surg [Am]. 1957;39:597-615.
41. Prolo D.J. Biology of bone fusion. Clin Neurosurg. 1988;36:135-146.
42. Urist M.R., DeLange R.J., Finerman G.A.M. Bone cell differentiation and growth factors. Science. 1983;220:680-686.
43. Bonfiglio M., Jeter W.S., Smith C.L. The immune concept: its relation to bone transplantation. Ann NY Acad Sci. 1955;59:417-433.
44. Emery S.E., Hughes S.S., Junglas W.A., et al. The fate of anterior vertebral bone grafts in patients irradiated for neoplasm. Clin Orthop Relat Res. 1994;300:207-212.
45. Buttermann G.R., Glazer P.A., Bradford D.S. The use of bone allografts in the spine. Clin Orthop Relat Res. 1996;324:75-85.
46. Cauthen J.C., Kinard R.E., Vogler J.B., et al. Outcome analysis of noninstrumented anterior discectomy and interbody fusion in 348 patients. Spine. 1998;23:188-192.
47. Cloward R.B. The anterior approach for ruptured cervical discs. J Neurosurg. 1958;15:502-514.
48. Connolly P.J., Essess S.I., Kostuik J.P. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
49. Robinson R.A., Walker A.E., Ferlick D.C., et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg [Am]. 1962;44:1569-1587.
50. Simmons E.H., Bhalla S.K. Anterior cervical discectomy and fusion, a clinical and biomechanical study with eight years follow-up. J Bone Joint Surg [Br]. 1969;51:225-237.
51. Wang J.C., McDough P.W., Endow K., et al. The effect of cervical plating on one-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
52. Zoega B., Karrholm J., Lind B. One-level cervical fusion: a randomized study with or without plate fixation, using radiostereometry in 27 patients. Acta Orthop Scand. 1998;69:363-368.
53. Brown M.D., Malinan T.I., Davis P.B. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop Relat Res. 1976;119:231-236.
54. Cloward R.B. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion: method of use of banked bone. Ann Surg. 1952;136:987-992.
55. Grossman W.C., Peppelman W.C., Baum J.A., Kraus D.R. The use of freeze-dried fibular allograft in anterior cervical fusion. Spine. 1992;17:565-569.
56. Hanley E.N.Jr., Harvell J.C.Jr., Shapiro D.E., et al. Use of allograft bone in cervical spine surgery. Semin Spine Surg. 1989;1:262.
57. Malinan T.I., Rosomoff H.L., Sutton C.H. Human cadaveric femoral head homografts for anterior cervical spine fusions. Surg Neurol. 1977;7:249-251.
58. Rish B.L., McFadden J.T., Penix J.O. Anterior cervical fusion using homologous bone grafts: a comparative study. Surg Neurol. 1976;5:119-121.
59. Schneider J.R., Bright R.W. Anterior cervical fusion using preserved bone allografts. Transplant Proc. 1976;8(2 Suppl 1):73-76.
60. Silcox D.H.3rd, Daftari T., Boden S.D., et al. The effect of nicotine on spinal fusion. Spine. 1995;20:1549-1553.
61. Cloward R.B. Gas-sterilized cadaver bone grafts for spinal fusion operations: a simplified bone bank. Spine. 1980;5:4-10.
62. Fernyhough J.C., White J.L., LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula and autograft in 126 patients. Spine. 1991;16:S561-S564.
63. Zhang Z.H., Yin H., Yang K., et al. Anterior intervertebral disc excision and bone grafting in cervical spondylotic myelopathy. Spine. 1983;8:16-19.
64. Zdeblick T.A., Bohlman H.H. Cervical kyphosis and myelopathy: treatment by anterior corpectomy and strut grafting. J Bone Joint Surg [Am]. 1989;71:170-182.
65. Young W.F., Rosenwasser R.H. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
66. Rao S., Yadav A., Galvan R. Posterior cervical spine stabilization under local anesthesia. J Spinal Disord. 1990;3:250-254.
67. Geisler F.H., Caspar W., Pitzen T., et al. Reoperation in patients after anterior cervical plate stabilization in degenerative disease. Spine. 1998;23:911-920.
68. Wang J.C., McDough P.W., Endow K., et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25:41-45.
69. Zdeblick T.A., Ducker T.B. The use of freeze-dried allograft bone for anterior cervical fusion. Spine. 1991;16:726-729.
70. Fraser J.F., Härtl R. Anterior approaches to fusion of the cervical spine: a metanalysis of fusion rates. J Neurosurg Spine. 2007;6:298-303.
71. Holte D.C., O’Brien J.P., Renton P. Anterior lumbar fusion using a hybrid interbody graft. Eur Spine J. 1994;3:32.
72. Majd M.E., Vadhva M., Holt R.T. Anterior cervical reconstruction using titanium cages with anterior plating. Spine. 1999;24:1604-1610.
73. Fischgrund J.S., Mackay M., Herkowitz H.N., et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
74. Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
75. Zdeblick T.A. A prospective, randomized study of lumbar fusion: preliminary results. Spine. 1993;18:983-991.
76. Thomsen K., Christensen F.B., Eiskjaer S.P., et al. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22:2813-2822.
77. Christensen F.B., Hansen E.S., Eiskjaer S.P., et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine. 2002;27:2674-2683.
78. Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455-463.
79. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31:2329-2336.
80. Dennis S., Watkins R., Landaker S., et al. Comparison of disc space heights after anterior lumbar interbody fusion. Spine. 1989;14:876-878.
81. Pettine K.A., Salib R.M. Femoral diaphyseal allograft for anterior lumbar interbody fusion: long-term follow-up. Orthop Trans. 1993;17:12.
82. Kozak J.A., Heilman A.E., O’Brien J.P. Anterior lumbar fusion options: technique and graft materials. Clin Orthop Relat Res. 1994;300:45-51.
83. Bridwell K.H., O’Brien M.F., Lenke L.G., et al. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work? Spine. 1994;19:2658-2666.
84. Dodd C.A., Fergusson C.M., Freedman L., et al. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg [Br]. 1988;70:431-434.
85. Fabry G. Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991;11:465-468.
86. Kaufman H.H., Jones E. The principles of bony spinal fusion. Neurosurgery. 1989;24:264-270.
87. Molinari R.W., Bridwell K.H., Lenke L.G., et al. Complication in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis: a comparison of three surgical approaches. Spine. 1999;24:1701-1711.
88. Bae H.W., Zhao L., Kanim L.E., et al. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine. 2006;31:1299-1306. discussion 1307–1308
89. Burkus J.K., Sandhu H.S., Gornet M.F. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775-781.
90. Boden S.D., Kang J., Sandhu H., et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662-2673.
91. Martin G.J.Jr., Boden S.D., Titus L., et al. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637-645.
92. Peterson B., Whang P.G., Iglesias R., et al. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg [Am]. 2004;86:2243-2250.
93. Wang J.C., Alanay A., Mark D., et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007;16:1233-1240.
94. An H.S., Simpson J.M., Glover J.M., et al. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine. 1995;20:2211-2216.
95. Kang JD, An H, Hilibrand AS, et al. Grafton and local bone has comparable outcomes to iliac crest bone in single level lumbar fusions. Paper presented at American Academy of Orthopaedic Surgeons 75th Annual Meeting; March 5–9, 2008; San Francisco, CA.
96. Rihn A.J., Kirkpatrick K., Albert T.J. Graft options in posterolateral and posterior interbody lumbar fusion. Spine. 2010;35:1629-1639.
97. Jamali A., Hilpert A., Debes J., et al. Hydroxyapatite/calcium carbonate (HA/CC) vs. plaster of Paris: a histomorphometric and radiographic study in a rabbit tibial defect model. Calcif Tissue Int. 2002;71:172-178.
98. Glassman S.D., Dimar J.R., Carreon L.Y., et al. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine. 2005;30:1694-1698.
99. Tay B.K., Le A.X., Heilman M., et al. Use of a collagen-hydroxyapatite matrix in spinal fusion. A rabbit model. Spine. 1998;23:2276-2281.
100. Orii H., Sotome S., Chen J., et al. Beta-tricalcium phosphate (beta-TCP) graft combined with bone marrow stromal cells (MSCs) for posterolateral spine fusion. J Med Dent Sci. 2005;52:51-57.
101. Dai L.Y., Jiang L.S. Single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine. 2008;33:1299-1304.
102. Neen D., Noyes D., Shaw M., et al. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing HEALOS with autograft. Spine. 2006;31:E636-E640.
103. Kitchel S.H. A preliminary comparative study of radiographic results using mineralized collagen and bone marrow aspirate versus autologous bone in the same patients undergoing posterior lumbar interbody fusion with instrumented posterolateral lumbar fusion. Spine J. 2006;6:405-411. discussion 411–412
104. Rahm M.D., Carter J.D., Chaput C.D., et al. Clinical and radiographic assessment of transforaminal lumbar interbody fusion using HEALOS collagen-hydroxyapatite sponge with autologous bone marrow aspirate. Spine J. 2008;9:434-438.
105. Chen D., Zhao M., Mundy G. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233-241.
106. Reddi A.H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rat. Proc Natl Acad Sci U S A. 1972;69:1601-1605.
107. Centrella M., McCarthy T.L., Canalis E. Current concepts review. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg [Am]. 1991;73:1418-1428.
108. Atfi A., Baron R. PTH battles TGF-beta in bone. Nat Cell Biol. 2010;12:205-207.
109. Cook S.D., Dalton J.E., Tan E.H., et al. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994;19:1655-1663.
110. Wang J.C., Kanim L.E., Yoo S., et al. Effect of regional gene therapy with bone morphogenetic protein-2–producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg [Am]. 2003;85:905-911.
111. Martin G.J.Jr., Boden S.D., Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a non-steroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188-2193.
112. Burkus J.K., Gornet M.F., Dickman C.A., et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
113. Vaccaro A.R., Patel T., Fischgrund J., et al. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2003;12:495-500.
114. Glassman S.D., Carreon L., Djurasovic M., et al. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007;7:44-49.
115. Buttermann G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426-435.
116. Smucker J.D., Rhee J.M., Singh K., et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813-2819.
117. Schultz D.G. US Food and Drug Administration public health notification: life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. Available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm Accessed June 5, 2009
118. McGovern S.C., Fong W., Wang J.C. Can bone morphogenetic protein binding peptide increase efficiency of bone formation? Spine. 2010;35:1655-1659.
119. Aaron R.K., Coimbor D.M. Electrical stimulation of bone induction and grafting. In: Habal M.B., Reddi A.H., editors. Bone grafts and bone substitutes. Philadelphia: WB Saunders; 1992:173.
120. Bassett C.A.L. The role of pulsed electromagnetic fields in bone grafting. In: Habal M.B., Reddi A.H., editors. Bone grafts and bone substitutes. Philadelphia: WB Saunders; 1992:173.
121. Kahanovitz N. The use of adjunctive electrical stimulation to enhance healing of spine fusions. Spine. 1996;21:2523-2525.
122. Fitzsimmons R.J., Strong D., Mohan S., et al. Low amplitude, low frequency electric field-stimulated bone cell proliferation may in part be mediated by IGF II release. J Cell Physiol. 1992;150:84-89.
123. Oishi M., Onesti S.T. Electrical bone graft stimulation for spinal fusion: a review. Neurosurgery. 2000;47:1041-1056.
124. Jenis L.G., An H.S., Stein R., et al. Prospective comparison of the effect of direct current electrical stimulation and pulsed electromagnetic fields on instrumented posterolateral lumbar arthrodesis. J Spinal Disord. 2000;12:290-296.
125. Kahanovitz N., Arnoczky S.P., Hulse D., et al. The effect of postoperative electromagnetic pulsing on canine posterior spinal fusions. Spine. 1984;9:273-279.
126. Lindsey R.W., Grobman J., Leggon R.E., et al. Effects of bone graft and electrical stimulation on the strength of healing bony defects in dogs. Clin Orthop Relat Res. 1987;222:275-280.
127. Mooney V. A randomized double blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. 1990;15:708-712.
128. Kane W.J. Direct current electrical bone growth stimulation for spinal fusion. Spine. 1988;13:363-365.
129. Rogozinski A., Rogozinski C. Efficacy of implanted bone growth stimulation in instrumented lumbar fusion. Spine. 1996;21:2479-2483.
130. Blumenthal S., Baker J., Dossett A., Selby D.K. The role of anterior lumbar fusion for internal disc disruption. Spine. 1986;13:566-569.
131. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudoarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1983;8:942-943.
132. DeVernejoul M.C., Bielakoff J., Herve M., et al. Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in middle age men. Clin Orthop Relat Res. 1983;179:107-115.
133. Lau G.C., Luck J.V.Jr., Marshall G.J., Griffith G. The effect of cigarette smoking on fracture healing: an animal model. Clin Res. 1989;37:A132.
134. Hollo I., Gergely I., Boross M. Smoking results in calcitonin resistance. JAMA. 1977;237:2470.
135. McDerrmott M.T., Witte M.C. Bone mineral content in smokers. South Med J. 1988;81:477-480.
136. Nicita-Mauro V. Smoking, calcium, calcium antagonists, and aging. Exp Gerontol. 1990;25:393-399.
137. Daftari T.K., Whitesides T.E.Jr., Heller J.G., et al. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19:904-911.
138. Hilibrand A.S., Fye M.A., Emery S.E., et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg [Am]. 2001;83:668-673.
139. Datta R., Saha S. Quantitative determination of tolerance doses for preoperative and postoperative radiotherapy of bones. Med Phys. 1983;10:243-245.
140. Markbreiter L.A., Pelker R.R., Friedlander G.E., et al. The effect of radiation on the fracture repair process: a biomechanical evaluation of a closed fracture in a rat model. J Orthop Res. 1989;7:178-183.
141. Widmann R.F., Pelker R.R., Friedlander G.E., et al. Effects of prefracture irradiation on the biomechanical parameters of fracture healing. J Orthop Res. 1993;11:422-428.
142. Emery S.E., Brazinski M., Benusan J., et al. Effects of irradiation on the biological and biomechanical properties of anterior spine fusion in a canine model (abstract). Washington DC: Orthopaedic Research Society, 38th Annual Meeting; February 1992.
143. Finston R.A., Woodard H.Q., Laughlin J.S. Effects of external radiation on mineral metabolism in the bones of adult dogs. Clin Orthop Relat Res. 1966;46:183-201.
144. Rhoades J.E., Kasinkas W. Influence of hypoproteinemia on the formation of callus in experimental fracture. Surgery. 1942;11:38.
145. Winfield J., Young A., William P., Corbett M. Prospective study of the radiologic changes in the hands, feet, and cervical spine in adult rheumatoid disease. Ann Rheum Dis. 1983;42:613-618.
146. Sandelin J., Santavirta S., Laasonen E., Slätis P. Spontaneous fracture of atlas of cervical spine affected by rheumatoid arthritis. Scand J Rheumatol. 1985;14:167-170.
147. Sawin P.D., Dickman C.A., Crawford N.R., et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg Spine. 2001;94:76-81.
148. McGrath H.Jr., McCormick C., Carey M.E. Pyogenic cervical osteomyelitis presenting as a massive prevertebral abscess in a patient with rheumatoid arthritis. Am J Med. 1988;84:363-365.
149. Chan D.P., Ngian K.S., Cohen L. Posterior upper cervical fusion in rheumatoid arthritis. Spine. 1992;17:268-272.
150. Clark C.R., Whitehill R. Two views of the use of methylmethacrylate for stabilization of the cervical spine. Orthopaedics. 1989;12:589-596.
151. Hey L.A. Rheumatoid arthritis of the cervical spine. Wilkins R.E., Rengachary S.S., editors. Neurosurgery, ed 2, vol 3. New York: McGraw Hill, 1996;3789.
152. Vanden Berhe A., Ackerman C., Veys E., et al. Occipito-cervical fusion in rheumatoid arthritis. Acta Orthop Belg. 1991;57(suppl 1):94-98.
153. Simmons E.H. Surgical treatment of ankylosing spondylitis: surgical considerations. Rothman R.H., Simeone F.A., editors. The spine, ed 3, vol 2. Philadelphia: WB Saunders, 1992;1447.
154. McMaster M.J. Osteotomy of the cervical spine in ankylosing spondylitis. J Bone Joint Surg [Br]. 1997;79:197-203.
155. Belanger T.A., Milam RAIV, Roh J.S., et al. Cervicothoracic extension osteoteomy for chin-on-chest deformity in ankylosing spondylitis. J Bone Joint Surg [Am]. 2005;87:1732-1738.
156. Simmons E.D., DiStefano R.J., Zheng Y., et al. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine. 2006;31:3006-3012.
157. Langeloo D.D., Journee H.L., Pavlov P.W., et al. Cervical osteotomy in ankylosing spondylitis: evaluation of new developments. Eur Spine J. 2006;15:493-500.
158. Tokala D.P., Lam K.S., Freeman B.J., et al. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J. 2007;16:1471-1478.
159. El Saghir H., Boehm H. Surgical options in the treatment of the spinal disorders in ankylosing spondylitis. Clin Exp Rheumatol. 2002;20:101-105.
160. Buckwalter J.A., Woo S.L.-Y., Goldberg V.M., et al. Soft tissue aging and musculoskeletal function. J Bone Joint Surg [Am]. 1993;75:1533-1548.
161. Tonna E.A., Cronkite E.P. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop Relat Res. 1963;30:218-233.
162. Boden S.D., Schimandle J.H., Hutton W.C. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20:412-420.
163. Dimar J.R., Ante W.A., Zhang Y.P., et al. The effects on nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine. 1996;21:1870-1876.
164. Long J., Lewis S., Kuklo T., et al. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg [Am]. 2002;84:1763-1768.
165. Riew K.D., Long J., Rhee J., et al. Time-dependent inhibitory effects of indomethacin on spinal fusion. J Bone Joint Surg [Am]. 2003;85:632-634.
166. Dodwell E.R., Latorre J.G., Parisini E., et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87:193-202.
167. Skolasky R.L., Maggard A.M., Hilibrand A.S., et al. Agreement between surgeons and an independent panel with respect to surgical site fusion after single-level anterior cervical spine surgery: a prospective, multicenter study. Spine. 2006;31:E503-E506.
168. Raizman N.M., O’Brien J.R., Poehling-Monoghan K.L., et al. Pseudoarthrosis of the spine. J Am Acad Orthop Surg. 2009;17:494-503.
169. Bono C.M., Khandha A., Vadapalli S., et al. Residual sagittal motion after lumbar fusion: a finite element analysis with implications on radiographic flexion-extension criteria. Spine. 2007;32:417-422.
170. Simmons J.W. Posterior lumbar interbody fusion with posterior elements as chip grafts. Clin Orthop Relat Res. 1985;193:85-89.
171. Buchowski J.M., Liu G., Bunmaprasert T., et al. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine. 2008;33:1185-1191.
172. Santos E.R., Goss D.G., Morcom R.K., et al. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997-1001.
173. Albert T.J., Pinto M., Smith M.D., et al. Accuracy of SPECT scanning in diagnosing pseudoarthrosis: a prospective study. J Spinal Disord. 1998;11:197-199.
174. Jacobson J.A., Starok M., Pathria M.N., et al. Pseudarthrosis: sonographic evaluation after posterolateral spinal fusion: work in progress. Radiology. 1997;1204:853-858.
175. Coric D., Branch C.L., Jenkins J.D. Revision of anterior cervical pseudarthrosis with anterior allograft fusion and plating. J Neurosurg. 1997;86:969-974.
176. Carreon L., Glassman S.D., Campbell M.J. Treatment of anterior cervical pseudoarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154-156.
177. Phillips F.M., Carlson G., Emery S.E., et al. Anterior cervical pseudarthrosis: natural history and treatment. Spine. 1997;22:1585-1589.
178. Lowery G.L., Swank M.L., McDonough R.F. Surgical revision for failed anterior cervical fusions: articular pillar plating or anterior revision? Spine. 1995;20:2436-2441.
179. Zdeblick T.A., Hughes S.S., Riew K.D., et al. Failed anterior cervical discectomy and arthrodesis: analysis and treatment of thirty-five patients. J Bone Joint Surg [Am]. 1997;79:523-532.
180. Andriacchi T.P., Schultz A.B., Belytschko T.B. A model for studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7:497-507.
181. Berven S., Kao H., Deviren V., Hu S., Bradford D. Treatment of thoracic pseudarthrosis in the adult: is combined surgery necessary? Clin Orthop Relat Res. 2003;411:25-31.
182. Cohen D.B., Chotivichit A., Fujita T., et al. Pseudarthrosis repair: autogenous iliac crest versus femoral ring allograft. Clin Orthop Relat Res. 2000;371:46-55.
183. Gertzbein S.D., Hollopeter M.R., Hall S. Pseudarthrosis of the lumbar spine: outcome after circumferential fusion. Spine. 1998;23:2352-2356.
184. Kozak J.A., O’Brien J.P. Simultaneous combined anterior and posterior fusion: an independent analysis of a treatment for the disabled low-back pain patient. Spine. 1990;15:322-328.
185. Albert T.J., Pinto M., Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion: a functional and radiographic outcome study. Spine. 2000;25:123-129.
186. Stewart G., Sachs B.L. Patient outcomes after reoperation on the lumbar spine. J Bone Joint Surg [Am]. 1996;78:706-711.
187. Waddell G., Kummel E.G., Lotto W.N., et al. Failed lumbar disc surgery and repeat surgery following industrial injuries. J Bone Joint Surg [Am]. 1979;61:201-207.
188. Lauerman W.C., Bradford D.S., Ogilvie J.W., et al. Results of lumbar pseudarthrosis repair. J Spinal Disord. 1992;5:149-157.
189. Kornblatt M.D., Casey M.P., Jacobs R.R. Internal fixation in lumbosacral spine fusion: a biomechanical and clinical study. Clin Orthop Relat Res. 1986;203:141-150.
190. Kim S.S., Michelsen C.B. Revision surgery for failed back surgery syndrome. Spine. 1992;17:957-960.