Nonthyroidal Illness Syndrome
A Form of Hypothyroidism
Nonthyroidal Illness Syndrome With Low Serum T4
Physiologic Interpretations of NTIS
Serum Hormone Levels and Tissue Hormone Supplies in NTIS
Is There Evidence for Substances in Serum Which Can Affect T4 Binding to Proteins?
Mechanism of Thyroid Hormone Suppression in NTIS
Is Thyroid Hormone Treatment of NTIS Advantageous or Disadvantageous?
Serum thyroid hormone levels drop during starvation and illness. In mild illness, this involves only a decrease in serum triiodothyronine (T3) levels. However, as the severity of the illness increases, there is a drop in both serum T3 and thyroxine (T4). This decrease of serum thyroid hormone levels is seen in starvation, sepsis, surgery, myocardial infarction, bypass, bone marrow transplantation, and in fact probably any severe illness.1–9 The condition has been called the euthyroid sick syndrome (ESS). An alternative designation, which does not presume the metabolic status of the patient, is nonthyroidal illness syndrome, or NTIS.
Low T3 States
Starvation, and more precisely carbohydrate deprivation, appears to rapidly inhibit deiodination of T4 to T3 by type 1 iodothyronine deiodinase in the liver, thus inhibiting generation of T3 and preventing metabolism of reverse T3 (rT3).10 Consequently there is a drop in serum T3 and elevation of reverse T3. Since starvation induces a decrease in basal metabolic rate,11 it has been argued, teleologically, that this decrease in thyroid hormone represents an adaptive response by the body to spare calories and protein by inducing some degree of hypothyroidism. Patients who have only a drop in serum T3, representing the mildest form of the NTIS, do not show clinical signs of hypothyroidism. Nor has it been shown that this decrease in serum T3 (in the absence of a drop in T4) has an adverse physiologic effect on the body or that it is associated with increased mortality.
Nonthyroidal Illness Syndrome With Low Serum T4
As the severity of illness, and often associated starvation, progresses, there is the gradual development of a more complex syndrome associated with low T3 and usually low T4 levels. Generally thyroid-stimulating hormone (TSH) levels are low or normal despite the low serum hormone levels, and rT3 levels are normal or elevated. A large proportion of patients in an intensive care unit setting have various degrees of severity of NTIS with low T3 and T4. Plikat et al. found that 23% of patients admitted to an ICU during a 2-year period had low free T3, low free T4, and low or normal TSH, and that these findings gave a greatly increased risk of death.12 Girvent et al. note that NTIS is highly prevalent in elderly patients with acute surgical problems and is associated with poor nutrition, higher sympathetic response, and worse postoperative outcome.13 Surprisingly, during the past 3 decades, many endocrinologists have assumed that NTIS is a beneficial physiologic response,14–17 but actual evidence for this view is unavailable.
A marked decrease in serum T3 and T4 in NTIS is associated with a high probability of death. NTIS was found in a group of 20 patients with severe trauma, among whom 5 died, and the drop in T3 correlated with the Apache II score.18 NTIS found in patients undergoing bone marrow transplantation was associated with a high probability of fatal outcome.19 NTIS was typical in elderly patients undergoing acute surgery and was associated with a worse prognosis.20 All of 45 non-dopamine-treated children with meningococcal septicemia had low T3, T4, and thyroxine-binding globulin (TBG), without elevated TSH. When serum T4 levels drop below 4 g/dL, the probability of death is about 50%, and with serum T4 levels below 2 g/dL, the probability of death reaches 80%.21–23 Obviously such associations do not prove that hypothyroidism is the cause of these complications or deaths, but the fact of hypothyroidism must at least raise the consideration of treatment.
Physiologic Interpretations of NTIS
Several conceptual explanations of NTIS can be followed through the literature:
1. The abnormalities represent test artifacts, and assays would indicate euthyroidism if proper tests were employed.
2. The serum thyroid hormone abnormalities are due to inhibitors of T4 binding to proteins, and the tests do not appropriately reflect free hormone levels. Proponents of this concept may or may not take the position that a binding inhibitor is present throughout body tissues, rather than simply in serum, and that the binding inhibitor may also inhibit uptake of hormone by cells or prevent binding to nuclear T3 receptors and thus inhibit action of hormone.
3. In NTIS, T3 levels in the pituitary are normal because of enhanced local deiodination. Thus the pituitary is actually euthyroid, while the rest of the body is hypothyroid. This presupposes enhanced intrapituitary T4 > T3 deiodination as the cause.
4. Serum hormone levels are artifactually low, the patients are biochemically euthyroid, and this is (teleologically) a beneficial physiologic response which should not be altered by treatment.
5. Serum hormone levels are in fact low, and the patients are biochemically hypothyroid, but this is (teleologically) a beneficial physiologic response and should not be altered by treatment.
6. Lastly, NTIS is in part a form of secondary hypothyroidism, the patient’s serum and tissue hormone levels are truly low, tissue hypothyroidism is present, this is probably disadvantageous to the patient, and therapy should be initiated if serum thyroxine levels are depressed below the danger level of 4 µg/dL.
Serum Hormone Levels and Tissue Hormone Supplies in NTIS
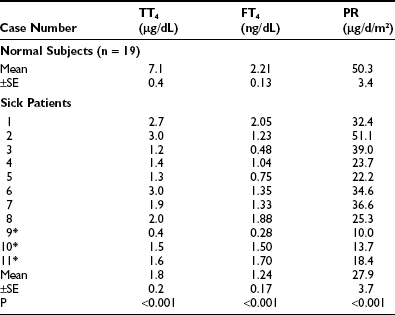
With few exceptions, reports on NTIS indicate that serum T3 and free T3 levels are low.24–30 Chopra and co-workers reported that free T3 levels were low (Fig. 15-1),31 or in a second report, often normal.32 However, it is important to note that in the second report, the patients with “NTIS” actually had average serum T4 levels that were above the normal mean and did not have significant NTIS. Sapin et al. compared free T3 levels found in patients with NTIS by direct dialysis, microchromatography, analogue, two-step immune extraction, and a labeled antibody RIA method.30 Results were significantly below normal by five of the methods and low in the most severe cases by one method. Faber et al. evaluated thyroid hormone levels in 34 seriously ill patients, most of whom had low T4 and free T4 index values, and found generally normal free T3 and free T4 using an ultrafiltration technique.33 A point to consider is that some ultrafiltration techniques fail to exclude thyroid hormone–binding proteins from the filtrate and give spuriously high free hormone values.34
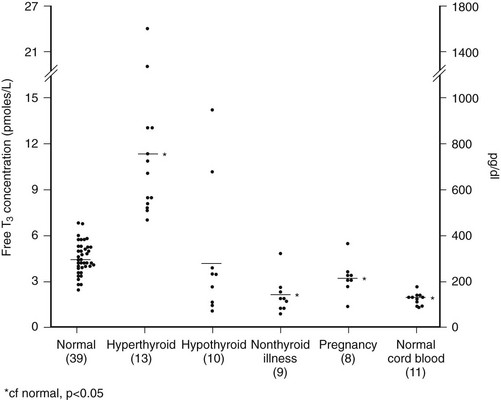
FIGURE 15-1 Free T3 concentrations in different groups of patients, as reported by Chopra et al.31 In this report, patients with NTIS have significantly lowered free T3 levels than normal subjects. NTIS, Nonthyroidal illness syndrome; T3, triiodothyronine.
Serum rT3 may be reduced, normal, or elevated and is not a reliable indicator of abnormal thyroid hormone supply. While it may be expected that rT3 should always be elevated, this is not true, and often it is within the normal range. Peeters et al.35 found in patients with NTIS, serum TSH, T4, T3, and the T3/rT3 ratio were lower, whereas serum rT3 was higher than in normal subjects (P < 0.0001). Liver D1 is down-regulated, and D3 (which is not evident in liver and skeletal muscle of healthy individuals) is induced, particularly in disease states associated with poor tissue perfusion. The level of rT3 reflects the action of several enzymes and presumably, as well, tissue metabolic function. Induction of D3 would tend to increase rT3. Degradation of rT3 is reduced by decreased function of the same D1 enzyme that generates T3. Moreover, formation of rT3 is limited by the low level of substrate (T4) in serum and in tissues and perhaps by inhibition of T4 entry into cells. Personal experience treating patients with NTIS (unpublished) shows that when T4 is given and repletes serum hormone levels, generation of rT3 rapidly increases, and levels often become significantly elevated.
Serum T4
Serum T4 levels are reduced in NTIS in proportion to the severity and, probably, length of the illness.24–35 In acute, short-term trauma such as cardiac bypass36 or in short-term starvation,37 there is no drop in serum T4. However, with increasing severity of trauma, illness, or infection, there is a drop in T4, which may become extreme. As indicated, serum T4 levels below 4 µg/dL are associated with a marked increased risk of death (up to 50%), and once T4 is below 2, prognosis becomes extremely guarded. In neonates, low total T4 and TSH are associated with a greater risk of death and severe intraventricular hemorrhage. It is suggested that thyroid hormone supplementation might be a potential benefit in infants with the lowest T4 values.27
Total serum T4 is reduced in part because of a reduction in TBG. One reason for this reduction appears to be because of cleavage of TBG. Schussler’s group recognized a rapid drop in TBG to 60% of baseline within 12 hours after bypass surgery, and their data suggest that this is due to cleavage of TBG by protease, which causes TBG to lose its T4-binding activity.38 Further studies by this group demonstrated the presence of a cleaved form of TBG present in serum of patients with sepsis.39
The impact of meningococcal sepsis on peripheral thyroid hormone metabolism and binding proteins was studied in 69 children with meningococcal sepsis. All children had decreased total T3 and total T3/rT3 ratios without elevated TSH. Lower total T4 levels were related to increased turnover of TBG by elastase. Lowered TBG is a partial explanation for lower total T4 and T3 in NTIS.40
Serum Free Thyroxine
A major problem in understanding NTIS is in analyzing data on the level of free T4. Free T4 is believed by most workers to represent hormone availability to tissues, although it is in fact intracellular T3 that binds to the receptors. The results of free T4 assays in NTIS are definitely method dependent. They may be influenced by a variety of variables, including (alleged) inhibitors present in serum or the effect of agents such as drugs, metabolites, or free fatty acids in the serum or assay. Assays which include an estimate of TBG capacity to estimate free hormone typically return low values for calculated free thyroxine in NTIS. Methods using T3 analogs in the assay also give levels that are depressed. The free T4 level determined by dialysis varies widely, as does T4 measured by ultrafiltration25–29; the majority of reports are of normal or low values but in some samples, elevated values.25,26,41–43
In theory, methods utilizing equilibrium dialysis may allow dilution of dialyzable inhibitors. Compounds such as 3-carboxy-4-methyl-5-propyl-2-furan-propanoic acid, indoxyl sulfate, and hippuric acid, can accumulate in severe renal failure.44 However, these compounds probably do not interfere with serum hormone assays. Free fatty acids, if elevated to 2 to 5 mmol/L, can displace T4 binding to TBG and elevate free T4. Free fatty acids almost never reach such levels in vivo.45,46 However, even small quantities of heparin (0.08 units/kg given IV, or 5000 units given SC), commonly given to patients in an ICU, can lead to in vitro generation of free fatty acids during extended serum dialysis for “free T4” assay and falsely augment apparent free hormone levels.47 This is probably a widespread and serious problem, which explains many instances of apparently elevated free T4 levels in patients with acute illness.
Results obtained using ultrafiltration also are variable. Wang et al.48 found that in patients with NTIS, free T4 measured by ultrafiltration was uniformly low (average of 11.7 ng/L), but when measured by equilibrium dialysis, free T4 was near normal, at 18 ng/L. By ultrafiltration, free T3 was also (not surprisingly) found to be low and similar to free T3 by radioimmune assay. Chopra32 found levels below the normal mean, ±2 SD, when measured by dialysis; 6 of 9 were low when measured by ultrafiltration, and 7 of 9 were low when measured by standard resin-uptake-corrected free T4. The means of the NTIS patients in this study were clearly below the mean of normals.
Is There Evidence for Substances in Serum Which Can Affect T4 Binding to Proteins?
Mendel et al.49 carefully review the studies that have claimed the presence of dialyzable inhibitors of binding and point out that many of these studies must be viewed with caution.44,45,50–53 Numerous artifacts are present in both dialysis assays and ultrafiltration assays. They also point out that while the low free T4 by resin uptake assays found in NTIS generally do not agree with the clinical status of the patient, it is equally true that clinical assessment generally does not fit with the high free T4 results found by some equilibrium dialysis assays in NTIS.
An argument that completely refutes the importance of factors in serum inhibiting binding of thyroid hormone is provided in the clinical study of Brent and Hershman (Fig. 15-2).54 These researchers gave 1.5 µg of T4 per kg body weight daily to 12 of 24 patients with severe NTIS and followed serum hormone levels over 14 days. T4 levels returned to the normal range within 3 days of therapy. Thus the thyroxine pool was easily replenished, and T4 levels reached normal values. Not surprisingly, because of reduced T4>T3 deiodination, T3 levels did not return to the normal range until the end of the study period in the few patients who survived. However, the ability of intravenous thyroxine in replacement doses to promptly restore the plasma pool to normal clearly shows that neither a loss of serum TBG nor an inhibitor of binding could be the main cause of low serum T4 in this group of severely ill patients.
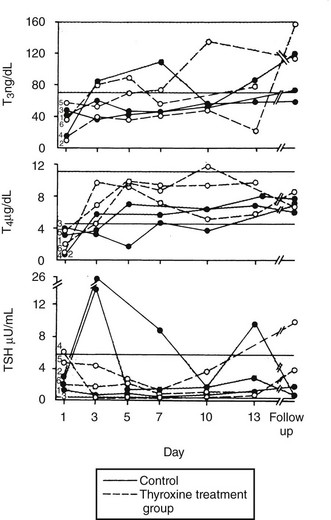
FIGURE 15-2 Patients with severe NTIS were randomized and left untreated (control, solid lines) or given IV T4 (thyroxine-treated group, dashed lines) over 2 weeks.54 Serum T3, T4, and TSH concentrations are shown for the survivors of the control (filled circles) and T4-treated groups (open circles) during the study period and at the time of follow-up. NTIS, Nonthyroidal illness syndrome; IV, intravenous; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
TSH Levels
Serum TSH in NTIS is typically normal or reduced and may be markedly low, although usually not less than 0.05 µU/mL.16,24,25,28,29,31,55 However, to use usual endocrinology logic, these TSH levels are almost always inappropriately low for the observed serum T4 and T3. Third-generation assays with sensitivity down to 0.001 U/mL may allow differentiation of patients with hyperthyroidism from those with NTIS, although there can be overlap in these very disparate conditions.56 Serum TSH in patients with NTIS may have reduced biological activity, perhaps because of reduced thyrotropin-releasing hormone (TRH) secretion and reduced glycosylation. Some patients are found with a TSH level above normal, and elevation of TSH above normal commonly occurs transiently if patients recover from NTIS (Fig. 15-3).16,29,54 This elevation of TSH strongly suggests that the patients are recovering from a hypothyroid state, during which the ability of the pituitary to respond had been temporarily inhibited.
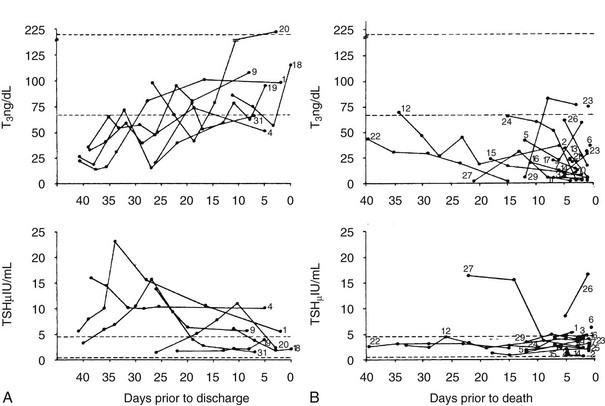
FIGURE 15-3 T3 and TSH concentrations are shown in patients with nonthyroidal illness who were eventually discharged from hospital (left panels).29 The broken line indicates ±2 SD of the mean value in the normal subjects. The right panel displays T3 and TSH concentrations in patients with NTIS who died. Subjects are indicated by numbers. Note the elevated TSH in some patients who recovered and the generally dropping T3 and low TSH levels in patients who died.29 NTIS, Nonthyroidal illness syndrome; SD, standard deviation; T3, triiodothyronine; TSH, thyroid-stimulating hormone.
Responsiveness of the pituitary to TRH during NTIS is variable: many patients respond less than normal,57 and others respond normally.58 “Normal” responsiveness in the presence of low TSH may suggest that there is a hypothalamic abnormality as a cause of the low TSH and low T4. There is also a diminution or loss of the diurnal rhythm of TSH,59 and in some studies, there is evidence for reduction of TSH glycosylation, with lower TSH bioactivity.60 A logical alternative explanation is that the low TSH is in fact the proximate cause of the low thyroid hormone levels. Hypothalamic function is impaired in patients with NTIS and, because of low TRH, results in low TSH and thus low output of thyroid hormones by the thyroid.
There is other evidence of diminished hypothalamic function in patients with serious illness. Serum testosterone drops rapidly, as do follicle-stimulating hormone (FSH) and luteinizing hormone (LH).61,62 Typically serum cortisol is elevated as part of a stress response, but this is not always the case. Some patients develop hypotension in association with apparent transient central hypoadrenalism, have low or normal serum ACTH, and cortisol levels under 20 µg/dL. The patients respond dramatically to cortisol replacement and may manifest normal adrenal function at a later time if they recover.
Centrally mediated hyposomatotropism, hypothyroidism, and pronounced hypoandrogenism were observed in a study of patients in the catabolic state of critical illness. In these patients, pulsatile LH secretion and mean LH secretions are very low, even in the presence of extremely low circulating total testosterone and low estradiol. Pulsatile growth hormone (GH) and TSH secretion are also, as is known, suppressed. Interleukin 1 β (IL-1β) levels are normal, whereas IL-6 and tumor necrosis factor α (TNF-α) are elevated. Exogenous IV gonadotropin-releasing hormone (GnRH) partially return serum testosterone levels toward normal but do not completely overcome hypoandrogenism, suggesting that combined deficiency of GH, GnRH, and TSH secretagogues may be important in this low androgen syndrome.63
Thyroid Hormone Turnover
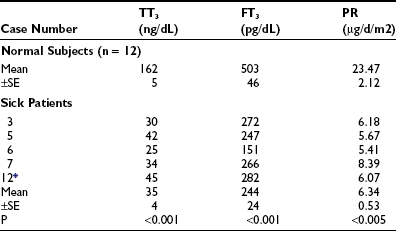
Kaptein et al.64,65 studied a group of patients who were critically ill, all of whom had total T4 below 4 µg/dL, low fT4 index, low normal free T4 by dialysis, and TSH which was normal or slightly elevated. In these patients, the mean T4 by dialysis was significantly below the normal mean. There was on average a 35% decrease in thyroxine disposal per day (Table 15-1). The T4 production rate in NTIS was significantly below the mean of 17 normal subjects (p < 0.005). In a similar study of T3 kinetics,65 free T3 was found to be 50% of normal serum values. The production rate of T3 was reduced by 83% (Table 15-2). These two studies document a dramatic reduction in provision of T4 and T3 to peripheral tissues, which would logically indicate that the effects of hormone lack (hypothyroidism) should be present. A third study reported dramatically reduced total T4 and T3 turnover, with normal thyroidal secretion of T3 in patients with NTIS due to uremia.66 However, this was a calculated rather than directly measured value, was highly variable, and does not negate the extreme reduction in T3 supply due to diminished T4>T3 conversion in peripheral organs.
T4 Entry into Cells and Generation of T3
Using deiodination of T4 as an index of cellular transport of T4 into rat hepatocytes, Lim et al.67 and Vos et al.68 found that serum from patients with NTIS inhibited T4 uptake. Sera from critically ill NTIS patients caused reduced T4 uptake compared to control sera in one study, and the authors considered elevated nonesterified fatty acids (NEFA) and bilirubin and reduced albumin to play a role. Serum from patients with mild NTIS did not cause impaired deiodination of T4 and T3.69 Inhibition of uptake of T4 into hepatocytes caused by sera of patients with NTIS also was observed by Sarne and Refetoff.70 There is a diminution in the “reducing equivalents” available for the deiodination of T4 to T3 in liver, and presumably elsewhere, thus lowering transport and the function of the type 1 iodothyronine deiodinase.71 In animals, and probably in man, there is also a drop in the level of type 1 iodothyronine deiodinase enzyme, apparently due to hypothyroidism, since it can be reversed by giving T3. Recently a study was performed on blood, liver, and skeletal-muscle biopsies of patients immediately after death in intensive care unit settings. Liver T4 deiodinase 1 was found to be down-regulated, and deiodinase 3 was induced in liver and muscle, especially in situations associated with poor tissue perfusion. These changes contribute to the low generation of T3 and its increased metabolism in NTIS, thus lowering the intracellular T3 levels.35
Thyroid Hormone in Tissues
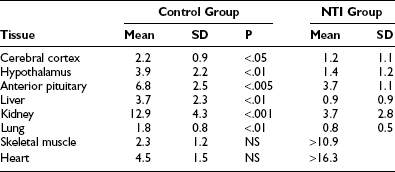
There are few significant data on thyroid hormone in tissues of patients with NTIS.72 In one study, there was of a dramatically reduced level of T3 in tissues (Table 15-3). While most samples had very low levels of T3 compared to normal tissues, some patients with NTIS showed sporadically and inexplicably high levels of T3 in certain tissues, especially skeletal muscle and heart.
Table 15-3
Tissue T3 Concentrations in Nonthyroidal Illness Syndrome (nmol of T3/kg of Wet Weight)72
Peeters et al.73 investigated 79 patients who died after intensive care, some of whom received thyroid hormone treatment. Tissue iodothyronine levels were positively correlated with serum levels, indicating that the decrease in serum T3 during illness is associated with decreased levels of tissue T3. Higher serum T3 levels in patients who received thyroid hormone treatment were accompanied by higher levels of liver and muscle T3, with evidence for tissue-specific regulation. Tissue rT3 and the T3/rT3 ratio were correlated with tissue deiodinase activities. Monocarboxylate transporter 8 expression was not related to the ratio of the serum over tissue concentration of the different iodothyronines.73
Information on expression of TRs in human tissues during illness is limited. Increased expression of the messenger ribonucleic acid (mRNA) for thyroid hormone receptors α1, α2, and β1 has been reported in cardiac tissue of patients with dilated cardiomyopathy; α1 and α2 isoforms also had increased expression in ischemic heart disease.74 Rodriguez-Perez et al. studied subcutaneous fat and skeletal muscle in patients with septic shock.75 In muscle, mRNA for TRβ1 and RXR gamma was reduced, and mRNA for RXR alpha was increased, compared to normals. In adipose tissue, MCT8, TRβ1, TRα1, and RXR gamma mRNAs were lower. The authors conclude that in these patients, tissue responses were oriented toward decreased hormone levels and decreased hormone action. In animals, starvation and illness are associated with a reduction in thyroid hormone receptor levels. In experimental studies in mice, LPS induces NTIS, and this is associated with an early decrease in binding of the RXR/TR dimer to DNA due to limiting amounts of RXR, and later an up to 50% decrease in levels of RXR and TR protein.76–77
Are Patients with NTIS Clinically Hypothyroid?
It is straightforward that the clinical parameters of severe hypothyroidism are absent in patients with NTIS. However, these patients usually present with a serious illness and are diagnostically challenging in view of their complicated state. Many are febrile, have extensive edema, have sepsis or pneumonia, may have hypermetabolism associated with burns, have severe cardiac or pulmonary disease, and in general have features that could easily mask evidence of hypothyroidism. Further, the common clinical picture of hypothyroidism does not develop within 2 to 3 weeks of complete thyroid hormone deprivation, but rather requires a much longer period for expression. General laboratory tests are also suspect. Thus starvation or disease-induced alterations in cholesterol, liver enzymes, TBG, creatine kinase, and even basal metabolic rate generally rule out the use of these associated markers for evidence of hypothyroidism. Angiotensin-converting enzyme levels are low,78 as seen in hypothyroidism, while high-affinity testosterone-binding globulin (TeBG) and osteocalcin levels are not altered.79 Antithrombin III levels are reduced in a septic rat model of NTIS. T3 supplementation returned the sepsis-induced decrease in antithrombin III levels toward normal.80
Mechanism of Thyroid Hormone Suppression in NTIS
It is probable that the cause of NTIS is multifactorial and may differ in different groups of patients. Specifically, the changes in liver disease and renal disease are probably somewhat different from those occurring in other forms of illness. Certainly one important cause of the drop in serum T3 is a decreased generation of T3 by type 1 iodothyronine deiodinase.81 If reduced entry of T4 into cells was a primary event and the major problem, then serum T4 levels would become elevated rather than suppressed. Some studies have suggested that individuals with NTIS may have selenium deficiency, and this may contribute to a malfunction of the selenium-dependent iodothyronine deiodinase.82 However, supplements of 500 μg of selenium given to patients in a surgical ICU during the first 5 days after serious injury caused only modest changes in thyroid hormones. The data did not suggest a major role for selenium deficiency in this condition.83
The overall degradation of thyroid hormone, both thyroxine and T3, is radically diminished in the NTIS syndrome in the presence of low hormone serum levels. The reduced degradation cannot produce the lowering of serum hormone levels; a primary reduction in degradation would increase serum hormone. The change in degradation must be secondary to the low hormone supply. Schussler and co-workers have observed a sharp drop in TBG levels during cardiac bypass surgery, which their studies indicate is due to some selective consumption of TBG. It is possible that this occurs because of activation of serine protease inhibitors (serpins) at sites of inflammation, which cleave the TBG into an inactive form.38
Considerable evidence suggests that an alteration in hypothalamic and pituitary function causes the low production of T4, which in turn causes the low production of T3. In rats, starvation reduces hypothalamic mRNA for TRH, reduces portal serum TRH, and lowers pituitary TSH content.84 A recent study documents low TRH mRNA in hypothalamic paraventricular nuclei85 in NTIS patients (Fig. 15-4). Responses to administered TRH vary in different reports, being suppressed or even augmented.57,58 Administration of TRH has been suggested as an effective means of restoring serum hormone levels to normal in individuals with NTIS. A recent report of great significance by Van den Berghe and co-workers proves that administration of TRH to patients with severe NTIS leads directly to increased TSH levels, increased T4 levels, and increased T3 levels.86 This data is strong support (albeit not proof) for the role of diminished hypothalamic function as an important factor causing NTIS.
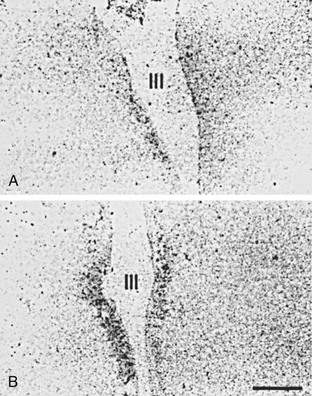
FIGURE 15-4 In situ hybridization study demonstrating mRNA for TRH in the periventricular nuclei of A, a subject who died with NTIS and B, a subject who died accidentally. In patients with NTIS, mRNA for TRH is significantly reduced.85 mRNA, Messenger ribonucleic acid; NTIS, nonthyroidal illness syndrome; TRH, thyrotropin-releasing hormone.
Quite possibly the production of TRH, and responses to TRH, are reduced by cytokines (to be discussed later) or by glucocorticoids.87 The diurnal variation in glucocorticoid levels at least in part controls the normal diurnal variation in TSH levels, perhaps by affecting pituitary responsiveness to TRH.88 High levels of glucocorticoids in Cushing’s disease suppress TSH and cause a modest reduction in serum hormone levels.89 High levels of glucocorticoids are known to suppress pituitary response to TRH in man.87 Stress-induced elevation of glucocorticoids in animals causes suppression of TSH and serum T4 and T3 hormone levels.90 Thus stress-induced glucocorticoid elevation may be one factor affecting TRH and TSH production.
Why should pituitary production of TSH be diminished in the presence of low serum thyroid hormone levels? A possibility is that there is augmented intrapituitary conversion of T4 to T3, thus allowing the pituitary to remain “euthyroid” while the rest of the body is actually hypothyroid. There is experimental support for this idea in a uremic rat model of NTIS.91 Another suggestion is that some other metabolite of thyroxine may be involved in control of pituitary responsiveness. For example, possibly triiodoacetic acid (triac) or tetraiodoacetic acid (tetrac) generated by metabolism of thyroxine could control pituitary responsiveness,92 but there is no experimental proof of this idea, and even if true, it would mean that the pituitary was normal but the rest of the body hypothyroid. As suggested earlier, elevated serum cortisol levels could play a role. The most obvious possibility is that low TSH stems from diminished TRH production, as previously described. It must also be remembered that the defect in pituitary function is not restricted to TSH, but that LH and FSH are also suppressed in seriously ill patients, and testosterone is reduced, in contrast to the generally augmented glucocorticoid response. Quite possibly these changes are the effect on the hypothalamus of neural integration of multiple factors including stress, starvation, glucocorticoids, and cytokines.
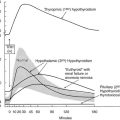
Van den Berghe has proposed that the changes in endocrine function seen during severe illness have a biphasic course. Possibly the initial suppression of T3 levels represents a genetically engineered adaptive response of the organism, allowing reduced metabolic rate and conservation of energy and protein stores for a longer period of time, while the animal or man goes through a period of starvation. However, the circumstances surrounding severe illness, and the resuscitative efforts applied in an intensive care unit over 1 or more weeks, presumably have not resulted in some genetically induced metabolic response, since survival under such extreme organ failure is a very recent phenomenon. This second phase of the syndrome, with associated suppression of thyroid hormone and other pituitary hormones and a variety of other changes, represents in this construction a maladaptive response. Patients in this situation tend to have elevated insulin levels, nitrogen wasting, retention of fats if calories are made available, and a variety of other metabolic abnormalities that include neuropathy and cardiomyopathy. These authors consider that provision of multiple hormonal support, including thyroid hormone, growth hormone, and androgens, may be beneficial.93–96
Cytokines in NTIS
In a series of septic patients studied shortly after admission to an ICU, total T4, free T4, total T3, and TSH were depressed, and IL-1β, soluble interleukin-2 receptor (sIL-2R), IL-6, and TNF-α were elevated.97 The hypothalamo-pituitary-adrenal axis was activated as expected. The data suggest central suppression of TSH as the cause of the problem, but the relation to cytokines is unclear, as seen in the following reports. Hermus et al.98 showed that continuous infusion of IL-1 in rats causes suppression of TSH, T3, and free T4. Higher doses of IL-1 were accompanied by a febrile reaction and suppression of food intake, which presumably played some role in the altered thyroid hormone economy. IL-1 did not reproduce the diminution in hepatic 5′-deiodinase activity believed to be so characteristic of NTIS. IL-1 is also known to impair thyroid hormone synthesis by human thyrocytes and is enhanced in many diseases associated with NTIS.99 van der Poll et al.100 studied the effect of IL-1 receptor blockade in human volunteers to determine if it could alter the NTIS induced by endotoxin. Blockade of IL-1 activity was achieved by infusing recombinant human IL-1 receptor antagonist, but this did not prevent the drop in T4, free T4, T3, and TSH or the rise in rT3 caused by endotoxin. This is evidence against an important role for IL-1.
Interferon γ
Interferon-γ (IFN-γ) 100 μg/m2 administered subcutaneously to normal volunteers did not alter TNF-α levels, caused a small elevation of IL-6 levels, and thus did not support a role for IFN-γ in the pathogenesis of the euthyroid sick syndrome in humans.101
Tumor Necrosis Factor
TNF is another proinflammatory cytokine that is thought to be involved in many of the illnesses associated with NTIS.102,103 Infusion of recombinant TNF in man by van der Poll et al.104 produced a decrease in serum T3 and TSH and an increase in rT3. Free T4 was transiently elevated in association with a significant rise in FFA levels. These studies suggest that TNF could be involved when recombinant IL-6 given to humans activates the hypothalamic pituitary axis; as noted previously, this could secondarily suppress TSH production. However, Chopra et al.102 did not find TNF to be closely correlated with hormone changes in NTIS. van der Poll et al.103,104 gave human subjects endotoxin, which caused lowering of T4, free T4, T3, and TSH. TNF blockade by a recombinant TNF receptor-IgG fusion protein did not alter the response, indicating that TNF did not cause the changes in hormone economy induced by administration of endotoxin. Nagaya et al.105 have proposed a mechanism through which TNF could reduce serum T3. TNF-α was found during in vitro studies to activate nuclear factor κB (NF-κB), which in turn inhibits the T3-induced expression of 5′-DI, which would lower T3 generation in liver.
Interleukin 6
Serum IL-6 is often elevated in NTIS,106 and its level is inversely related to T3 levels.107 Stouthard et al.108 gave recombinant human IL-6 chronically to human volunteers. Short-term infusion of IL-6 caused a suppression of TSH, but daily injections over 42 days caused only a modest decrease in T3 and a transient increase in rT3 and free T4 concentrations. IL-6 could be involved in the NTIS syndrome, although the mechanism was not defined. In an animal model of NTIS studied by Wiersinga and collaborators,109 antibody blockade of IL-6 failed to prevent the induced changes in thyroid hormone economy typical of NTIS. Boelen et al. studied the levels of IFN, IL-8, and IL-10 in patients with NTIS and found no evidence that they had a pathogenic role.110 Short-term administration of recombinant IFN-γ to normal subjects caused a minimal elevation of IL-6, no alteration in TNF, and did not significantly alter thyroid hormone levels.111 Michalaki et al. observed that serum T3 drops early after abdominal surgery as an early manifestation of the NTIS syndrome, prior to an increase in serum IL-6 or TNF-α, suggesting that these changes in cytokines do not induce the drop in T3.112
The potential interaction between cytokines and the hypothalamic-pituitary-thyroid axis is certainly complicated, and cytokines themselves operate in a network. For example, IL-1 and TNF can stimulate secretion of IL-6. Activation of TNF and IL-1 production is associated with the occurrence of cytokine inhibitors in serum, which are actually fragments of the cytokine receptor or actual receptor antagonists. Soluble TNF receptor and IL-1 RA are receptor antagonists, which can inhibit the function of the free cytokines. These molecules are increased in many infectious, inflammatory, and neoplastic conditions. Boelen et al.113 found evidence that the NTIS is “an acute phase response” generated by activation of a cytokine network. Soluble TNF, soluble TNF receptor, soluble IL-2 receptor antagonist, and IL-6 all inversely correlated with serum T3 levels.
Other Factors Altering Serum T4 Supply
In healthy men going through two 4.5-hour-long sessions of induced hypoglycemia, TSH, fT3 and fT4 are significantly reduced.114 Perinatal asphyxia, recognized by low Apgar scores, is associated with a depression of TSH, T4, and T3, and the reductions are greatest in infants with hypoxic/ischemic encephalopathy. In this study, 6 of 11 infants with fT4 < 2ng/dL died. These data suggest, not surprisingly, that reduced substrate or O2 supply to the CNS could induce hypothalamic/pituitary dysfunction.114,115
Glucagon
Administration of glucagon to dogs caused a significant fall in serum T3, suggesting that stress-induced hyperglucagonemia may be a contributor to the NTIS syndrome by altering intracellular metabolism of T4.116
Dopamine
Dopamine given in support of renal function and cardiac function must play a role in many patients who develop low hormone levels while in an intensive care unit setting. Dopamine inhibits TSH secretion directly, depresses further the already abnormal thyroid hormone production, and induces significant worsening of the low hormone levels. Withdrawal of dopamine infusion is followed by a prompt dramatic elevation of TSH, a rise in T4 and T3, and an increase of the T3/rT3 ratio. All of these changes suggested to Van den Berghe et al.117 that dopamine makes some patients with NTIS hypothyroid, inducing a condition of iatrogenic hypothyroidism, and that treatment (presumably by administering thyroid hormone), “should be evaluated.”
Leptin
Leptin plays a key role in control of thyroid hormone levels during starvation in animals. During starvation, leptin levels drop. With this there is diminished stimulation of TRH, thus diminished secretion of TSH, and lowered thyroid hormone levels. Administration of leptin appears to work via the arcuate nucleus of the hypothalamus to induce production of pro-opiomelanocortin (POMC), and thus α-melanocyte-stimulating hormone (αMSH), and reduce Agouti-related protein (AgRP). Normally αMSH stimulates the melanocortin 4 receptor (MC4R), whereas AgRP suppresses it. Presumably through these actions, a lack of leptin during starvation leads to diminished stimulation of the MC4R receptor on the TRH neurons in ventricular nuclear centers and thus diminished TRH secretion. Administration of leptin partially reverses this sequence.118 These actions appear to be part of an energy-conserving scheme related to thyroid changes during starvation and are associated with leptin-induced increase in appetite, decreased energy expenditure, and modified neuroendocrine function. Naturally the relevance of this to human physiology is as yet unclear, but the data strongly suggest that leptin is involved in the down-regulation of thyroid function during acute starvation.119–120 In clinical trials, stimulation of growth hormone secretion by GH secretagogues leads to increased insulin and leptin levels in severely ill ICU patients. To date, studies of leptin levels in patients with NTIS have indicated they are normal or elevated, not low.86,121
Atrial Natriuretic Peptides
Atrial natriuretic peptides, including amino acids 1 to 30, amino acids 31 to 67 (known as vessel dilator), 79 to 98 (kaliuretic hormone), and 99 to 126 (atrial natriuretic hormone), derived from the ANH prohormone, significantly decreased circulating concentrations of total T4, free T4, and free T3, when given to healthy humans for 60 minutes. A reciprocal increase in TSH lasted for 2 or 3 hours after cessation of the administration of these hormones, suggesting that the effect was a direct inhibition of thyroid hormone release from the thyroid gland rather than an action of the hormones upon the hypothalamus or pituitary. No data are available on these factors in NTIS122 (Table 15-4).
Table 15-4
Summary of Observations in Nonthyroidal Illness Syndrome
1. Hypothalamic mRNA for TRH is reduced, and cytokines may be responsible.
2. TSH levels are inappropriately low for serum hormone levels, presumably because of reduced secretion.
3. TRH injection causes an elevation of TSH, T4, and T3, reversing many aspects of the syndrome and suggesting low TRH secretion may be a primary problem.
4. Measured serum levels of apparent free T4 and T3 may be low or normal, but no assay can be certified to be free of artifact.
5. Inhibitors of T4, and T3, binding to serum proteins and possibly receptors, have been postulated but remain of unproven significance.
6. T4 and T3 production rates have been clearly demonstrated to be markedly reduced.
7. Based on scant data, levels of hormone in most tissues are greatly reduced in proportion to decreased blood hormone levels.
8. Serum hormone was (in the one study available) restored by administration of physiologic doses of hormone.
9. Replacement hormone therapy has not been shown to be disadvantageous, and in some studies it appears to be beneficial.
10. Major abnormalities are also present in GH, LH, T, and insulin physiology.
Diagnosis
Serum cortisol should be measured. Transient, apparently central, hypoadrenalism may occur in severe illness.123–125 Cortisol should be above 20 µg/dL, and commonly is above 30. If below 20, ACTH should be drawn, and the patient should be given supportive cortisol therapy. Serum cortisol should certainly be determined if thyroid hormone is to be given. Since CBG may be reduced, it is advisable to measure serum free cortisol if possible. It is useful to determine FSH in postmenopausal women as a sign of pituitary function, but this is less clearly valuable in men. If there is a reason to consider hypopituitarism, a CAT scan of the pituitary is appropriate, or at least a skull film.
Is Thyroid Hormone Treatment of NTIS Advantageous or Disadvantageous?
Two valuable studies are available on replacement therapy using thyroid hormone in patients with NTIS. In the study by Brent and Hershman,54 replacement with 1.5 µg T4 IV per kilogram body weight daily, in 12 patients, promptly returned serum T4 levels to normal (thereby proving that a binding defect was not the cause of the low T4) but did not normalize T3 levels over a period of 2 to 3 weeks. However, in both the treated and control group, mortality was 80%.54 Clearly this excellent small study, which used for primary therapy what would now be considered the wrong hormone, failed to show either an advantageous or disadvantageous effect. It is possible that the failure to show a positive effect was due to the failure of T3 levels to be restored to normal. In a study of severely burned patients given 200 µg T3 daily, again there was no evidence of a beneficial or disadvantageous effect.126 Mortality was not so great, as in the Brent and Hershman study, but it is entirely possible that the high levels of T3 provided worsened the hypermetabolism known to be present in burn patients and could have, at these levels, been disadvantageous.
An important study by Acker et al. certainly advises caution regarding T4 therapy in patients with acute renal failure. Numerous studies in animals have documented a beneficial effect of T4 therapy in experimental acute renal failure.127 In a randomized, controlled prospective study of patients with acute renal failure (ARF), treated patients received 150 μg of thyroxine a total of four times intravenously over 2 days.128 The single difference recognized in the subsequent laboratory data was a suppression of TSH. T4 treatment had no effect on any measure of ARF severity. Among other questions, it is not clear that serum T3 levels were ever altered. However, mortality was higher in the thyroxine group (43% versus 13%) than in the control group. It is of interest that, as the authors state, “the observed mortality in the controls in this study was less than that typically seen in our institution in ARF and ICU patients, whereas the 43% mortality noted in the thyroid group better approximates both our experience and that reported in the literature for ICU patients.” It will be difficult to replicate this study (although this reader believes it should be replicated). But it is uncertain whether the small dose of thyroxine administered over 2 days actually is related to the mortality, considering that the mortality in the treated group was that usually observed, whereas the control happened to have a much lower mortality.128 The same group has also studied the effect of thyroid hormone treatment on posttransplant acute tubular necrosis. T3 treatment during the posttransplant period did not alter outcome in a beneficial or derogatory manner.129
Studies from animals are often quoted in the literature as an argument against treatment of NTIS or for the therapy. A study of sepsis induced in animals showed no difference in mortality, but some animals treated with thyroid hormone died earlier than did those that were untreated.130 Chopra et al. induced NTIS in rats by injection of turpentine oil. The reduction in T4, T3, free T4 index, and TSH were associated with no clear evidence of tissue hypothyroidism, and urinary nitrogen excretion was normal. Thyroid hormone replacement with T4 or T3 did not significantly alter enzyme activities or urinary nitrogen excretion.131 Healthy pigs were subjected to 20 minutes of regional myocardial ischemia by Hsu and collaborators,132 and this was associated with a drop in T3, free T3, and elevated rT3. Some animals were treated with 0.2 µg T3 per kilogram for five doses over 2 hours. While myocardial infarction size was not altered, the pigs treated with T3 showed a more rapid improvement in cardiac index. Oxygen consumption did not alter. It should be noted that the T3 levels fell back to normal levels within 4 hours of the last T3 dose, suggesting that more prolonged therapy might have been beneficial. Katzeff et al.133 studied a model of NTIS induced by caloric restriction in young rats. In these animals, T3 was reduced, and there was a decrease in LV relaxation time, SERCA2 mRNA, and αMHC mRNA. All changes were reversed to normal values by supplementation with T3, suggesting that the low-T3 syndrome was related to the pathologic cardiac changes. Sepsis and multisystem organ failure are often associated with disseminated intravascular coagulation and consumption of coag inhibitors such as antithrombin III. Chapital studied a model of sepsis in rats and showed that T3 supplementation reduced the decrease in antithrombin III levels, which presumably would reflect a beneficial effect.134 Dogs subjected to hemorrhagic shock recovered more cardiovascular function when given T3 intravenously than did untreated animals.135 Neurologic outcome after anoxia is improved in dogs by T3 treatment.136
Short-term studies on T3 replacement of patients in shock, in patients with respiratory disease, in subjects who are brain dead and potential organ donors, and in patients undergoing coronary artery bypass grafts all suggest modest cardiovascular benefits from the administration of T3. One study reports benefit by replacing T3 to elevate the depressed T3 levels in premature infants.137 Other studies found no apparent effects. Children treated with T3 postoperatively when they have undergone cardiac surgery also require less cardiac support. T3 administration (one dose of approximately 6 µg IV) did not alter cardiac performance in brain-dead transplant donors.138–139 Coronary artery bypass, as studied by Klemperer and collaborators,36 was associated with a drop in serum T3; IV administration of T3 elevated T3 above normal, augmented cardiac output, and reduced the need for pressor support but had no other effect. In this study, however, the patients had a very favorable prognosis and minimal NTIS, so the study primarily shows that administration of T3 had no adverse effect under these circumstances. In a study reported several years ago, T3 administration to critically ill neonates with severe respiratory distress appeared to improve survival. Infants of less than 37 weeks gestational age or weighing less than 220 grams were given prophylactic doses of thyroxine and T3 daily and had a lower mortality rate than untreated infants.137 Use of thyroid hormone replacement in children after cardiac surgery has been extensively reviewed by Haas et al., with the conclusion that it is a desirable treatment option, especially in high-risk patients.140 Goarin et al. studied the effect of T3 administration in brain-dead organ donors and found that although it returned T3 levels to normal, it did not improve hemodynamic status or myocardial function.141 Pingitore et al. gave T3 by IV infusion for 3 days to patients with chronic heart failure. Heart rate, plasma norepinephrine (down 52%), natriuretic peptide, and aldosterone (down 23%) were all significantly diminished, and ventricular performance improved, without side effects.142 In a randomized study of patients for 24 hours after coronary bypass, correction of the usual drop in serum T3 by IV T3 infusion had no beneficial or deleterious effect on cardiac parameters.143 Of interest, it also did not affect leucine flux or urinary nitrogen excretion, contrary to the usual assumption that a drop in serum T3 should spare body protein. The general outcome of these studies is that they weakly support the use of T3, and none of the studies found evidence of damage caused by treatment.144–150
If Thyroid Hormone Replacement is Given, What Should it be?
Clearly the high mortality rate in patients with T4 under 4 µg/dL suggests that this is a target group in whom thyroid hormone administration should be considered. In this group of patients, there appears to be no obvious contraindication to replacement therapy, with the possible exception of people who have cardiac decompensation or arrhythmias. Even here, the evidence is uncertain. There is no clear evidence that administration of replacement doses of T3 to patients with low cardiac output is disadvantageous, and in fact current studies using intravenous T3 in these patients indicate it is well tolerated and may be beneficial.151 Arrhythmias obviously also raise a question, but again, there is no evidence that replacement of thyroid hormone to a normal level would cause trouble in control of arrhythmias. Low free T3 levels are reported to be associated with an increased incidence of fibrillation after cardiac surgery in elderly patients.152 Thus even in this group of patients, it is reasonable to suggest therapy. It should also be noted that among patients with NTIS, there will certainly be patients who are clearly hypothyroid—based on known disease, treatment with dopamine, or elevated TSH—who need replacement therapy by any standard.
If therapy is to be given, it cannot be thyroxine alone, since this would fail to promptly elevate T3 levels.54 Treatment should include oral, or if this is impractical, intravenous T3, and probably should be at the replacement level of approximately 50 µg/day given in divided doses. It may be appropriate to give slightly higher doses, such as 75 µg/day for 3 to 4 days to increase the body pool more rapidly, followed by replacement doses as described. Coincidentally, it is appropriate to start replacement with T4. Serum levels of T4 and T3 should be followed at frequent intervals (every 48 hours) and dosages adjusted to achieve a serum T3 level at least low normal (70 to 100 ng/dL) prior to the next scheduled dose. If treatment is successful, T3 administration can gradually be reduced, and thyroxine administration can be increased to replacement levels as deiodination increases. Because of the marked diminution in T4 to T3 deiodination, and shunting of T4 toward rT3, replacement with T4 may initially only lead to elevation of rT3 and have very little effect upon T3 levels, or physiologic action. In this situation, continued administration of T3 would be preferred.
Additional Supportive Hormonal Therapy to Consider
Although this discussion concentrates on the potential value of treating patients with NTIS with replacement thyroid hormone, several important recent studies expand the concept to other areas, including treatment of the associated hyperglycemia, relative adrenal insufficiency, use of beta blockers in burn patients, and possible use of GHRH and testosterone. Van den Berghe and co-workers have suggested that the acute and prolonged critical illness responses are entirely different neuroendocrine conditions. In protracted severe illness, patients are kept alive with conditions that previously caused death. However, this process has unmasked a variety of nonspecific wasting syndromes that include protein loss, accumulation of fat stores, hyperglycemia and insulin resistance, hypoproteinemia, hypercalcemia, potassium depletion, and hypertriglyceridemia. In prolonged illness, cortisol values are elevated, although ACTH levels are low, indicating that other mechanisms are driving the steroid response. Growth hormone secretory pulses are reduced, and the mean concentration is low in prolonged critical illness. FSH and LH are reduced, and testosterone levels are reduced. These authors maintain that the reduced neuroendocrine drive, present in the chronic phase of illness in an intensive care setting, is unlikely to be an evolutionary preserved beneficial process. They suggest that the administration of hypothalamic physiotropic releasing peptides may be a safer strategy than the administration of peripherally active hormones.93
References
1. McIver, B, Gorman, CA. Euthyroid sick syndrome: An overview. Thyroid. 1997;7:125–132.
2. DeGroot, LJ. “Non-thyroidal illness syndrome” is functional central hypothyroidism, and if severe, hormone replacement is appropriate in light of present knowledge. J Endocrinol Invest. 2003;26:1163–1170.
3. Stathatos, N, Wartofsky, L. The euthyroid sick syndrome: is there a physiologic rationale for thyroid hormone treatment? J Endocrinol Invest. 2003;26:1174–1179.
4. Hennemann, G, Docter, R, Krenning, EP. Causes and effects of the low T3 syndrome during caloric deprivation and non-thyroidal illness: an overview. Acta Med Kaust. 1988;15:42–45.
5. Phillips, RH, Valente, WA, Caplan, ES, et al. Circulating thyroid hormone changes in acute trauma: prognostic implications for clinical outcome. J Trauma. 1984;24:116–119.
6. Vardarli, I, Schmidt, R, Wdowinski, JM, et al. The hypothalamo-hypophyseal-thyroid axis, plasma protein concentrations and the hypophyseogonadal axis in low T3 syndrome following acute myocardial infarct. Klin Wochenschrift. 1987;65:129–133.
7. Eber, B, Schumacher, M, Langsteger, W, et al. Changes in thyroid hormone parameters after acute myocardial infarction. Cardiology. 1995;86:152–156.
8. Holland, FW, Brown, PS, Weintraub, BD, et al. Cardiopulmonary bypass and thyroid function: a “euthyroid sick syndrome.”. Ann Thorac Surg. 1991;52:46–50.
9. Vexiau, P, Perez-Castiglioni, P, Socie, G, et al. The “euthyroid sick syndrome”: Incidence, risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Hematol. 1993;85:778–782.
10. Harris, ARC, Fang, SL, Vagenakis, AG, et al. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978;27:1680–1690.
11. Welle, SL, Campbell, RG. Decrease in resting metabolic rate during rapid weight loss is reversed by low-dose thyroid hormone treatment. Metabolism. 1986;35:289–291.
12. Plikat, K, Langgartner, J, Buettner, R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007 Feb;56(2):239–244.
13. Girvent, M, Maestro, S, Hernandez, R, et al. Euthyroid sick syndrome, associated endocrine abnormalities, and outcome in elderly patients undergoing emergency operation. Surgery. 1998;123:560–567.
14. Wartofsky, L, Burman, KD. Alterations in thyroid function in patients with systemic illnesses: the “Euthyroid Sick Syndrome”. Endocrine Rev. 1982;3:164–217.
15. Kaptein, EM. Clinical relevance of thyroid hormone alterations in nonthyroidal illness. Thyroid International. 1997;4:22–25.
16. Docter, R, Krenning, EP, de Jong, M, et al. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol. 1993;39:499–518.
17. Chopra, IJ, Huang, TS, Boado, R, et al. Evidence against benefit from replacement doses of thyroid hormones in nonthyroidal illness: studies using turpentine oil–injected rat. J Endocrinol Invest. 1987;10:559–564.
18. Schilling, JU, Zimmermann, T, Albrecht, S, et al. Low T3 syndrome in multiple trauma patients – a phenomenon or important pathogenetic factor? Medizinische Klinik. 1999;3:66–69.
19. Schulte, C, Reinhardt, W, Beelen, D, et al. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplantation. 1998;22:1171–1178.
20. Girvent, M, Maestro, S, Hernandez, R, et al. Euthyroid sick syndrome, associated endocrine abnormalities, and outcome in elderly patients undergoing emergency operation. Surgery. 1998;123:560–567.
21. Maldonado, LS, Murata, GH, Hershman, JM, et al. Do thyroid function tests independently predict survival in the critically ill? Thyroid. 1992;2:119.
22. Vaughan, GM, Mason, AD, McManus, WF, et al. Alterations of mental status and thyroid hormones after thermal injury. J Clin Endocrinol Metab. 1985;60:1221.
23. De Marinis, L, Mancini, A, Masala, R, et al. Evaluation of pituitary-thyroid axis response to acute myocardial infarction. J Endocrinol Invest. 1985;8:507.
24. Surks, MI, Hupart, KH, Pan, C, et al. Normal free thyroxine in critical nonthyroidal illnesses measured by ultrafiltration of undiluted serum and equilibrium dialysis. J Clin Endocrinol Metab. 1988;67:1031–1039.
25. Melmed, S, Geola, FL, Reed, AW, et al. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab. 1982;54:300–306.
26. Kaptein, EM, MacIntyre, SS, Weiner, JM, et al. Free thyroxine estimates in nonthyroidal illness: comparison of eight methods. J Clin Endocrinol Metab. 1981;52:1073–1077.
27. Kantor, M, et al. Admission thyroid evaluation in very-low-birth-weight infants: association with death and severe intraventricular hemorrhage. Thyroid. 2003;13:965.
28. Chopra, IJ, Solomon, DH, Hepner, GW, et al. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Int Med. 1979;90:905–912.
29. Bacci, V, Schussler, GC, Kaplan, TB. The relationship between serum triiodothyronine and thyrotropin during systemic illness. J Clin Endocrinol Metab. 1982;54:1229–1235.
30. Sapin, R, Schlienger, JL, Kaltenbach, G, et al. Determination of free triiodothyronine by six different methods in patients with nonthyroidal illness and in patients treated with amiodarone. Ann Clin Biochem. 1995;32:314–324.
31. Chopra, IJ, Taing, P, Mikus, L. Direct determination of free triiodothyronine (T3) in undiluted serum by equilibrium dialysis/radioimmunoassay. Thyroid. 1996;6:255–259.
32. Chopra IJ: Simultaneous measurement of free thyroxine and free 3,5,3′-triiodothyronine in undiluted serum by direct equilibrium dialysis/radioimmunoassay: evidence that free triiodothyronine and free thyroxine are normal in many patients with the low triiodothyronine syndrome, 1998.
33. Faber, J, Kirkegaard, C, Rasmussen, B, et al. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab. 1987 Aug;65(2):315–320.
34. Fritz, KS, Wilcox, RB, Nelson, JC. Quantifying spurious free T4 results attributable to thyroxine-binding proteins in serum dialysates and ultrafiltrates. Clin Chem. 2007 May;53(5):985–988.
35. Peeters, RP, Wouters, PJ, Kaptein, E, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003 Jul;88(7):3202–3211.
36. Klemperer, JD, Klein, I, Gomez, M, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522–1527.
37. Osburne, RC, Myers, EA, Rodbard, D, et al. Adaptation to hypocaloric feeding: physiologic significance of the fall in T3. Metabolism. 1983;32:9–13.
38. Afandi, B, Schussler, GC, Arafeh, A-H, et al. Selective consumption of thyroxine-binding globulin during cardiac bypass surgery. Metabolism. 2000;49:270–274.
39. Jirasakuldech, B, Schussler, GC, Yap, MG, et al. A characteristic serpin cleavage product of thyroxine-binding globulin appears in sepsis sera. J Clin Endocrinol Metab. 2000;85:3996–3999.
40. den Brinker, M, Joosten, KF, Visser, TJ, et al. J Clin Endocrinol Metab. 2005;90(10):5613–5620.
41. Uchimura, H, Nagataki, S, Tabuchi, T, et al. Measurements of free thyroxine: comparison of percent of free thyroxine in diluted and undiluted sera. J Clin Endocrinol Metab. 1976;42:561–566.
42. Nelson, JC, Weiss, RM. The effect of serum dilution on free thyroxine concentration in the low T4 syndrome of nonthyroidal illness. J Clin Endocrinol Metab. 1985;61:239–246.
43. Wang, R, Nelson, JC, Weiss, RM, et al. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10:31–39.
44. Kaptein, EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocrine Rev. 1996;17:45–63.
45. Liewendahl, K, Helenius, T, Naveri, H, et al. Fatty acid–induced increase in serum dialyzable free thyroxine after physical exercise: implication for nonthyroidal illness. J Clin Endocrinol Metab. 1992;74:1361–1365.
46. Mendel, CM, Frost, PH, Cavalieri, RR. Effect of free fatty acids on the concentration of free thyroxine in human serum: the role of albumin. J Clin Endocrinol Metab. 1986;63:1394–1399.
47. Jaume, JC, Mendel, CM, Frost, PH, et al. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentration as measured by equilibrium dialysis. Thyroid. 1996;6:79–83.
48. Wang, Y-S, Hershman, JM, Pekary, AE. Improved ultrafiltration method for simultaneous measurement of free thyroxine and free triiodothyronine in serum. Clin Chem. 1985;31:517–522.
49. Mendel, CM, Laufhton, CW, McMahon, FA, et al. Inability to detect an inhibitor of thyroxine-serum protein binding in sera from patients with nonthyroidal illness. Metabolism. 1991;40:491–502.
50. Chopra, IJ, Huang, T-S, Beredo, A, et al. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3,5,3’-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab. 1985;60:666.
51. Wang, R, Nelson, JC, Wilcox, RB. Salsalate administration—a potential pharmacological model of the sick euthyroid syndrome. J Clin Endocrinol Metab. 1998;83:3095–3099.
52. Csako, G, Zweig, MH, Benson, C, et al. On the albumin-dependence of the measurement of free thyroxine. II. Patients with nonthyroidal illness. Clin Chem. 1987;33:87–92.
53. Chopra, IJ, Chua Teco, GN, Mead, JF, et al. Relationship between serum free fatty acids and thyroid hormone binding inhibitor in nonthyroid illnesses. J Clin Endocrinol Metab. 1985;60:980–984.
54. Brent, GA, Hershman, JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and lower serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8.
55. Chopra, IJ. Nonthyroidal illness syndrome or euthyroid sick syndrome? Endocrine Pract. 1996;2:45–52.
56. Franklyn, JA, Black, EG, Betteridge, J, et al. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab. 1994;78:1368–1371.
57. Vierhapper, H, Laggner, A, Waldhausl, W, et al. Impaired secretion of TSH in critically ill patients with “low T4-syndrome. Acta Endocrinol. 1982;101:542–549.
58. Faber, J, Kirkegaard, C, Rasmussen, B, et al. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab. 1987;65:315–320.
59. Arem, R, Deppe, S. Fatal nonthyroidal illness may impair nocturnal thyrotropin levels. Am J Med. 1990;88:258–262.
60. Lee, H-Y, Suhl, J, Pekary, AE, et al. Secretion of thyrotropin with reduced concanavalin-A-binding activity in patients with severe nonthyroid illness. J Clin Endocrinol Metab. 1987;65:942.
61. Spratt, DI, Bigos, ST, Beitins, I, et al. Both hyper- and hypogonadotropic hypogonadism occur transiently in acute illness: bio- and immunoactive gonadotropins. J Clin Endocrinol Metab. 1992;75:1562–1570.
62. Spratt, DI, Cox, P, Orav, J, et al. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab. 1993;76:1548–1554.
63. Van den Berghe, G, Weekers, F, Baxter, RC, et al. Five-day pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic-pituitary-gonadal defects underlying profound hypoandrogenism in men with prolonged critical illness. J Clin Endocrinol Metab. 2001;86:3217–3226.
64. Kaptein, EM, Grieb, DA, Spencer, CA, et al. Thyroxine metabolism in the low thyroxine state of critical nonthyroidal illnesses. J Clin Endocrinol Metab. 1981;53:764–771.
65. Kaptein, EM, Robinson, WJ, Grieb, DA, et al. Peripheral serum thyroxine, triiodothyronine and reverse triiodothyronine kinetics in the low thyroxine state of acute nonthyroidal illnesses. A noncompartmental analysis. J Clin Invest. 1982;69:526–535.
66. Lim, VS, Fang, VS, Katz, AI, et al. Thyroid dysfunction in chronic renal failure. A study of the pituitary-thyroid axis and peripheral turnover, Kinetics of thyroxine and triiodothyronine. J Clin Invest. 1997;60(3):522–534.
67. Lim, C-F, Docter, R, Visser, TJ, et al. Inhibition of thyroxine transport into cultured rat hepatocytes by serum of nonuremic critically ill patients: effects of bilirubin and nonesterified fatty acids. J Clin Endocrinol Metab. 1993;76:1165–1172.
68. Vos, RA, de Jong, M, Bernard, BF, et al. Impaired thyroxine and 3,5,3′-triiodothyronine handling by rat hepatocytes in the presence of serum of patients with nonthyroidal illness. J Clin Endocrinol Metab. 1995;80:2364–2370.
69. Lim, C-F, Docter, R, Krenning, EP, et al. Transport of thyroxine into cultured hepatocytes: effects of mild nonthyroidal illness and calorie restriction in obese subjects. Clinical Endocrinol. 1994;40:79–85.
70. Sarne, DH, Refetoff, S. Measurement of thyroxine uptake from serum by cultured human hepatocytes as an index of thyroid status: reduced thyroxine uptake from serum of patients with nonthyroidal illness. J Clin Endocrinol Metab. 1985;61:1046–1052.
71. Krenning, D, et al. Decreased transport of thyroxine (T4), 3, 3′, 5-triiodothyronine (T3) and 3,3′,5′-triiodothyronine (rT3) into rat hepatocytes in primary culture due to a decrease of cellular ATP content and various drugs. FEBS Lett. 1982;140:229–233.
72. Arem, R, Wiener, GJ, Kaplan, SG, et al. Reduced tissue thyroid hormone levels in fatal illness. Metabolism. 1993;42:1102–1108.
73. Peeters, RP, van der Geyten, S, Wouters, PJ, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab. 2005;90(12):6498–6507.
74. d’Amati, G, di Gioia, CRT, Mentuccia, D, et al. Increased expression of thyroid hormone receptor isoforms in end-stage human congestive heart failure. J Clin Endocrinol Metab. 2001;86:2080–2084.
75. Rodriguez-Perez, A, Palos-Paz, F, Kaptein, E, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf). 2008;68(5):821–827.
76. Sanchez, B, Jolin, T. Triiodothyronine-receptor complex in rat brain: effects of thyroidectomy, fasting, food restriction, and diabetes. Endocrinology. 1991;129:361–367.
77. Beigneux, A, et al. Sick euthyroid syndrome is associated with decreased TR expression and DNA binding in mouse liver. Am J Physiol Endocrinol Metab. 2003;284:E228–E236.
78. Brent, GA, Hershman, JM, Reed, AW, et al. Serum angiotensin converting enzyme in severe nonthyroidal illness associated with low serum thyroxine concentration. Ann Intern Med. 1986;100:680–683.
79. Seppel, T, Becker, A, Lippert, F, et al. Serum sex hormone-binding globulin and osteocalcin in systemic nonthyroidal illness associated with low thyroid hormone concentrations. J Clin Endocrinol Metab. 1996;81:1663–1665.
80. Chapital, AD, Hendrick, SR, Lloyd, L, et al. The effects of triiodothyronine augmentation on antithrombin III levels in sepsis. Am Surg. 2001 Mar;67(3):253–255.
81. Kaplan, MM. Subcellular alterations causing reduced hepatic thyroxine-5′-monodeiodinase activity in fasted rats. Endocrinology. 1979;104:58–64.
82. Berger, MM, Lemarchand-Beraud, T, Cavadini, C, et al. Relations between the selenium status and the low T3 syndrome after major trauma. Intensive Care Med. 1996;22:575–581.
83. Berger, MM, Reymond, MJ, Shenkin, A, et al. Influence of selenium supplements on the post-traumatic alterations of the thyroid axis: a placebo-controlled trial. Intensive Care Med. 2001;27:91–100.
84. Blake, NG, Eckland, JA, Foster, OJF, et al. Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology. 1991;129:2714–2718.
85. Fliers, E, Guldenaar, SEF, Wiersinga, WM, et al. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab. 1997;82:4032–4036.
86. Van den Berghe, G, De Zegher, F, Baxter, RC, et al. Neuroendocrinology of prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab. 1998;83:309–319.
87. Nicoloff, JT, Fisher, DA, Appleman, MD, Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest. 1970;49:1922.
88. Brabant, G, Brabant, A, Ranft, U. Circadian and pulsatile thyrotropin secretion in euthyroid man under the influence of thyroid hormone and glucocorticoid administration. J Clin Endocrinol Metab. 1987;65:83.
89. Benker, G, Raida, M, Olbricht, T, et al. TSH secretion in Cushing’s syndrome: relation to glucocorticoid excess, diabetes, goiter, and the “sick euthyroid syndrome”. Clin Endocrinol. 1990;33:777–786.
90. Bianco, AC, Nunes, MT, Hell, NS, et al. The role of glucocorticoids in the stress-induced reduction of extrathyroidal 3,5,3′-triiodothyronine generation in rats. Endocrinology. 1987;120:1033–1038.
91. Lim, VS, Passo, C, Murata, Y, et al. Reduced triiodothyronine content in liver but not pituitary of the uremic rat model: demonstration of changes compatible with thyroid hormone deficiency in liver only. Endocrinology. 1984;114:280–286.
92. Beale E, Srivastava P, Liang H, et al: Triiodothyroacetic acid (T3AC): is it an intracellular autocrine acting form of thyroid hormone? Abstract No. OR1–1 presented at the 79th Annual Meeting of the Endocrine Society, Minneapolis, MN, 1997.
93. Van den Berghe, G, de Zegher, F, Bouillon, R. Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83:1827–1834.
94. Van den Berghe, G, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367.
95. Van den Berghe, G, Schoonheydt, K, Becx, P, et al. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005 Apr 26;64(8):1348–1353.
96. Weekers, F, Giulietti, AP, Michalaki, M, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003 Dec;144(12):5329–5338.
97. Mönig, H, Arendt, T, Meyer, M, et al. Activation of the hypothalamo-pituitary-adrenal axis in response to septic or non-septic diseases—implications for the euthyroid sick syndrome. Intensive Care Med. 1999;25:1402–1406.
98. Hermus, RM, Sweep, CG, van der Meer, MJ, et al. Continuous infusion of interleukin-1 beta induces a nonthyroidal illness syndrome in the rat. Endocrinology. 1992;131:2139–2146.
99. Cannon, JG, Tompkins, RG, Gelfand, JA, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84.
100. van der Poll, T, Van Zee, KJ, Endert, E, et al. Interleukin-1 receptor blockade does not affect endotoxin-induced changes in plasma thyroid hormone and thyrotropin concentrations in man. J Clin Endocrinol Metab. 1995;80:1341–1346.
101. de Metz, J, Romijn, JA, Endert, E, et al. Administration of interferon-γ in healthy subjects does not modulate thyroid hormone metabolism. Thyroid. 2000;10:87–91.
102. Chopra, IJ, Sakane, S, Chua Teco, GN. A study of the serum concentration of tumor necrosis factor—in thyroidal and nonthyroidal illnesses. J Clin Endocrinol Metab. 1991;72:1113–1116.
103. van der Poll, T, Endert, E, Coyle, SM, et al. Neutralization of TNF does not influence endotoxin-induced changes in thyroid hormone metabolism in humans. Am J Physiol. 1999;276:R357–R362.
104. van der Poll, T, Romijn, JA, Wiersinga, WM, et al. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. 1990;71:1567–1572.
105. Nagaya, T, Fujieda, M, Otsuka, G, et al. A potential role of activated NF-kappa B in the pathogenesis of euthyroid sick syndrome. J Clin Invest. 2000;106(3):393–402.
106. Bartalena, L, Brogioni, S, Grasso, L, et al. Relationship of the increased serum interleukin-6 concentration to changes of thyroid function in nonthyroidal illness. J Endocrinol Invest. 1994;17:269–274.
107. Boelen, A, Platvoet-ter Schiphorst, MC, Wiersinga, WM. Association between serum interleukin-6 and serum 3,5,3′-triiodothyronine in nonthyroidal illness. J Clin Endocrinol Metab. 1993;77:1695–1699.
108. Stouthard, JML, van der Poll, T, Endert, E, et al. Effects of acute and chronic interleukin 6 administration on thyroid hormone metabolism in humans. J Clin Endocrinol Metab. 1994;79:1342–1346.
109. Boelen, A, Platvoet-ter Schiphorst, MC, Wiersinga, WM. Immunoneutralization of interleukin 1, tumor necrosis factor, interleukin-6 or interferon does not prevent the LPS-induced sick euthyroid syndrome in mice. J Endocrinol. 1997;153:115–122.
110. Boelen, A, Platvoet-ter Schiphorst, MC, Wiersinga, WM. Relationship between serum 3,5,3′-triiodothyronine and serum interleukin 8, interleukin 10 or interferon gamma in patients with nonthyroidal illness. J Endocrinol Invest. 1996;19:480–483.
111. De Metz, J, Romijn, JA, Endert, E, et al. Administration of Interferon-γ in healthy subjects does not modulate thyroid hormone metabolism. Thyroid. 2000;10:87–91.
112. Michalaki, M, Vagenakis, AG, Makri, M, et al. Dissociation of the early decline in serum T3 concentration and serum IL-6 rise and TNFα in nonthyroidal illnss syndrome induced by abdominal surgery. J Clin Endocrinol Metab. 2001;86:4198–4205.
113. Boelen, A, Platvoet-ter Schiphorst, MC, Wiersinga, WM. Soluble cytokine receptors and the low 3,5,3′-triiodothyronine syndrome in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1995;80:971–976.
114. Schultes, B, et al. Acute and prolonged effects of insulin-induced hypoglycemia on the pituitary-thyroid axis in humans. Metabolism. 2002;51:1370–1374.
115. Pereira, D, Procianoy, R. Effect of perinatal asphyxia on thyroid-stimulating hormone and thyroid hormone levels. Acta Pediatr. 2003;92:339–345.
116. Custro, N, Scafidi, V, Costanzo, G, et al. Role of high blood glucagon in the reduction of serum levels of triiodothyronine in severe nonthyroid diseases. Minerva Endocrinol. 1989;14:221–226.
117. Van den Berghe, G, de Zegher, F, Lauwers, P. Dopamine and the sick euthyroid syndrome in critical illness. Clin Endocrinol. 1994;41:731–737.
118. Legradi, G, Emerson, CH, Ahima, RS, et al. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576.
119. Legradi, G, Emerson, CH, Ahima, RS, et al. Arcuate nucleus ablation prevents fasting-induced suppression of proTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1998;68:89–97.
120. Flier, JS, Harris, M, Hollenberg, AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest. 2000;105:859–861.
121. Bornstein, SR, et al. Leptin levels are elevated despite low thyroid hormone levels in the “euthyroid sick” syndrome. J Clin Endocrinol Metab. 1997;82(12):4278–4279.
122. Vesely, DL, San Miguel, GI, Hassan, I, et al. Atrial natriuretic hormone, vessel dilator, long-acting natriuretic hormone, and kaliuretic hormone decrease the circulating concentrations of total and free T4 and free T3 with reciprocal increase in TSH. J Clin Endocrinol Metab. 2001;86:5438–5442.
123. Kidess, AI, Caplan, RH, Reynertson, RH, et al. Transient corticotropin deficiency in critical illness. Mayo Clin Proc. 1993;68:435–441.
124. Merry, WH, Caplan, RH, Wickus, GG, et al. Acute adrenal failure due to transient corticotropin deficiency in postoperative patients. Surgery. 1994;116:1095–1100.
125. Lambert, SWJ, Bruining, HA, DeLong, FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337:1285–1292.
126. Becker, RA, Vaughan, GM, Ziegler, MG, et al. Hypermetabolic low triiodothyronine syndrome of burn injury. Critical Care Med. 1982;10:870–875.
127. Siegel, N, et al. Beneficial effect of thyroxine on recovery from toxic acute renal failure. Kidney Int. 1984;25:906–911.
128. Acker, CG, Singh, AR, Flick, RP, et al. A trial of thyroxine in acute renal failure. Kidney Int. 2000;57:293–298.
129. Acker, CG, Flick, R, Shapiro, R, et al. Thyroid hormone in the treatment of post-transplant acute tubular necrosis (ATN). Am J Transplant. 2002;2:57–61.
130. Little, JS. Effect of thyroid hormone on survival after bacterial infection. Endocrinology. 1985;117:1431–1435.
131. Chopra, IJ, Huang, TS, Boado, R, et al. Evidence against benefit from replacement doses of thyroid hormones in nonthyroidal illness: studies using turpentine oil-injected rat. J Endocrinol Invest. 1987;10:559.
132. Hsu, R-B, Huang, T-S, Chen, Y-S, et al. Effect of triiodothyronine administration in experimental myocardial injury. J Endocrinol Invest. 1995;18:702–709.
133. Katzeff, HL, Powell, SR, Ojamaa, K. Alterations in cardiac contractility and gene expression during low T3 syndrome: prevention with T3. Amer J Physiol. 1997;273:E951–E956.
134. Chapital, AD, Hendrick, SR, Lloyd, L, et al. The effects of triiodothyronine augmentation on antithrombin III levels in sepsis. Am Surg. 2001;67:253–255.
135. Shigematsu, H, Shatney, CH. The effect of triiodothyronine and reverse triiodothyronine on canine hemorrhagic shock. Nippon Geka Gakkai Zasshi. 1988;89:1587–1593.
136. Facktor, MA, Mayor, GH, Nachreiner, RF, et al. Thyroid hormone loss and replacement during resuscitation from cardiac arrest in dogs. Resuscitation. 1993;26:141–162.
137. Schoenberger, W, Grimm, W, Emmrich, P, et al. Thyroid administration lowers mortality in premature infants. Lancet. 1979;2:1181.
138. Novitzky, D, Cooper, D, Reichart, B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43:852–855.
139. Novitzky, D, et al. Improved cardiac allograft function following triiodothyronine therapy to both donor and recipient. Transplantation. 1990;49:311–316.
140. Haas, NA, Camphausen, CK, Kececioglu, D. Clinical review: thyroid hormone replacement in children after cardiac surgery: is it worth a try? Crit Care. 2006;10(3):213.
141. Goarin, JP, Cohen, S, Riou, B, et al. The effects of triiodothyronine on hemodynamic status and cardiac function in potential heart donors. Anesth Analg. 1996;83:41–47.
142. Pingitore, A, Galli, E, Barison, A, et al. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93(4):1351–1358.
143. Spratt, DI, Frohnauer, M, Cyr-Alves, H, et al. Physiological effects of nonthyroidal illness syndrome in patients after cardiac surgery. Am J Physiol Endocrinol Metab. 2007;293(1):E310–E315.
144. Hesch, R, et al. Treatment of dopamine-dependent shock with triiodothyronine. Endocr Res Commun. 1981;8:299–301.
145. Meyer, T, et al. Treatment of dopamine-dependent shock with triiodothyronine: preliminary results. Dtsch Med Wochenschr. 1979;104:1711–1714.
146. Dulchavsky, S, et al. Beneficial effects of thyroid hormone administration on metabolic and hemodynamic function in hemorrhagic shock. FASEB J. 1990;4:A952.
147. Dulchavsky, S, et al. T3 preserves respiratory function in sepsis. J Trauma. 1991;31:753–759.
148. Dulchavsky, S, Hendrick, S, Dutta, S. Pulmonary biophysical effects of triiodothyronine (T3) augmentation during sepsis-induced hypothyroidism. Trauma. 1993;35:104–109.
149. Klemperer, J, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522–1527.
150. Bennett-Guerro, E, et al. Duke T3 Study Group, Cardiovascular effects of intravenous triiodothyronine in patients undergoing coronary artery bypass graft surgery. A randomized, double-blind, placebo-controlled trial. J Am Med Assoc. 1996;275:687–692.
151. Hamilton, MA, Stevenson, LW. Thyroid hormone abnormalities in heart failure: possibilities for therapy. Thyroid. 1996;6:527–529.
152. Kokkonen, L, Majahalme, S, Kööbi, T, et al. Atrial fibrillation in elderly patients after cardiac surgery: postoperative hemodynamics and low postoperative serum triiodothyronine. J Cardiothorac Vasc Anesth. 2005 Apr;19(2):182–187.