Chapter 43 Nonsustained Ventricular Tachycardia
Nonsustained ventricular tachycardia (NSVT) has been recognized as a usually asymptomatic rhythm disorder detected in an extremely wide range of conditions, from asymptomatic, apparently healthy, young individuals to patients with significant heart disease. Because of its brevity, NSVT does not produce symptoms in most instances; it derives its clinical importance from the fact that its detection may have important prognostic implications, depending on the underlying pathology. In several clinical settings, NSVT is a marker of increased risk for subsequent sustained tachyarrhythmias and sudden cardiac death (SCD), whereas it may have no prognostic significance in a healthy individual, although evidence for occult pathology or an inherited channelopathy in apparently normal subjects who develop ventricular arrhythmia not related to physical exercise continues to accumulate.1–3 The main task of the physician is to detect the apparently healthy individuals in whom NSVT represents a sign of occult disease and to risk stratify patients with known disease who present with this arrhythmia. This, however, is not an easy task. The patient with NSVT still represents a clinical challenge with regard to proper management, and several crucial questions remain unanswered.
Definition
Traditionally, the term tachycardia (from the Greek words tachy, meaning fast, and cardia, meaning heart) is used to describe conditions in which the heart rate exceeds the conventional number of 100 beats/min for more than three consecutive beats, either in response to metabolic demand or other stimuli or because of disease.4 Sustained tachycardia is defined as tachycardia that lasts for more than 30 seconds (unless requiring termination because of hemodynamic collapse), whereas nonsustained tachycardia terminates spontaneously within 30 seconds.4 Thus, NSVT has been defined as three (sometimes five) or more consecutive beats arising below the atrioventricular (AV) node with an R-R interval of less than 600 ms (>100 beats/min) and lasting less than 30 seconds.5
This definition is by no means a universally accepted one. In the recent Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study,6 NSVT was detected by registering runs of 16 beats or more, with a rate of 125 beats/min, whereas a rate of 120 beats/min or more has been used by the Marburg trial and other studies.7,8 The period of a tachycardia run to qualify for NSVT has also varied. In the Electrophysiologic Study Versus Electrocardiographic Monitoring (ESVEM) study, the time cut-off was 15 seconds as opposed to the conventional number of 30 seconds, which has been used both by the Multicenter Automatic Defibrillator Implantation Trial (MADIT) and the Multicenter Unsustained Tachycardia Trial (MUSTT) .9–11 This implies that NSVT cases according to the MADIT and MUSTT criteria might well have been dealt with as sustained ventricular tachycardia (VT) episodes in the ESVEM cohort. In other studies, the terms ventricular ectopy or NSVT are often used without strictly defined diagnostic criteria.12
Epidemiology
Reliable epidemiologic data on NSVT are difficult to obtain. Usually, although not invariably, most patients remain asymptomatic, and the reproducibility of NSVT recordings is documented in only half of the patients with this arrhythmia. In the MADIT, NSVT was defined as reproducible when found in at least two recordings of three consecutive Holter electrocardiograms (ECGs) performed in weekly intervals. Reproducible NSVT was identified in only 50% of the patients with NSVT and did not seem to be an independent risk factor for future arrhythmic events.13 The reported prevalence of NSVT in various clinical conditions is presented in Table 43-1. Previous studies were based on Holter monitoring, thus reporting NSVT occurring within a short period. The advent of implantable permanent pacemakers, defibrillators, and monitoring devices with extensive ECG monitoring capabilities has allowed for a more accurate estimation of the incidence of NSVT in patients with heart disease. Evidence is now available from implantable cardioverter-defibrillator (ICD) data that NSVT is a distinct tachyarrhythmia that may cause syncope without causing death in patients with heart disease and that the incidence of polymorphic NSVT relative to sustained arrhythmia is greater than previously believed.14
Table 43-1 Reported Prevalence of Nonsustained Ventricular Tachycardia in Different Cardiac Conditions
| CONDITION | PREVALENCE |
|---|---|
| Apparently healthy individuals | 0%-3% |
| Non-ST ACS (2 to 9 days after admission) | 18%-25% |
| Acute MI (early phase) | 45%-75% |
| Reperfused acute MI (later than 1 week) | 7%-13% |
| Heart failure (LVEF <30% to 40%) | 30%-80% |
| DCM | 40%-50% |
| HCM | 25%-80% |
| Significant valve disease | ≤25% |
| Hypertension | 8% |
| Hypertension and left ventricular hypertrophy | 12%-28% |
DCM, Dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVEF, left ventricular ejection fraction; MI, myocardial infarction; Non-ST ACS, non–ST-segment elevation acute coronary syndrome.
In asymptomatic, apparently healthy persons, Holter recordings in older studies revealed a frequency of NSVT ranging from 0% to 3%.5,15,16 In non–ST-segment elevation acute coronary syndromes, NSVT is detected in 18% to 25% of patients 2 to 9 days after admission.17 In acute myocardial infarction (MI), NSVT during the first 24 hours is frequent (43% in patients without thrombolysis and up to 75% in re-perfused patients).5,18 In the first 7 days after MI, NSVT is detected in approximately 6.8% to 13.4% of patients in the reperfusion era, which is not much different from that reported in the pre-thrombolytic era (12%; Multicenter Postinfarction Research Program).18–23 NSVT can be detected in approximately 2% of patients with ischemia and preserved left ventricular function.24 Recently, the CARISMA study group reported on long-term cardiac arrhythmias recorded by a cardiac monitor implanted 2 to 5 days after MI in patients with left ventricular ejection fraction (LVEF) of 40% or less. The detected incidence of NSVT over a 2-year period was 13%.6
In patients with heart failure and LVEF less than 30% to 40%, the reported prevalence of NSVT is 30% to 80%.25,26 It seems that ischemic heart failure carries a higher risk for NSVT. In the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) trial, in which only one third of patients had ischemic heart disease, the prevalence of NSVT was 33%. Whereas in the Congestive Heart Failure: Survival Trial of Antiarrhythmic Therapy (CHF-STAT) trial, in which the ischemic patients comprised two thirds of the whole cohort, the prevalence was 80%.25,26 In dilated cardiomyopathy (DCM), NSVT has been detected in 40% to 50% of patients.27–29 In hypertrophic cardiomyopathy (HCM), 20% to 30% of patients may have NSVT, whereas in patients with a history of cardiac arrest, this proportion approaches 80%.30–32 These estimations may not reflect the true incidence of NSVT in HCM, since they are based on highly selected referral populations. In patients with valvular disease, the incidence of NSVT is considerable (up to 25% in aortic stenosis and in significant mitral regurgitation) and appears to be a marker of underlying left ventricular pathology.33,34 In patients with arterial hypertension, NSVT is correlated to the degree of cardiac hypertrophy and subendocardial fibrosis.35,36 Approximately 12% to 28% of patients with hypertension and left ventricular hypertrophy present with NSVT as opposed to 8% of patients with hypertension alone.35,36 Ventricular extrasystolic activity can be detected with Holter monitoring in up to 50% of patients with repaired tetralogy of Fallot, and recent studies have detected a 4% to 14% prevalence of sustained VT.37–39
Clinical Electrocardiography
Electrocardiographic Patterns
Nonsustained Ventricular Tachycardia Associated with a “Normal” Heart
In the apparently normal population, NSVT episodes without consistent morphology patterns may be recorded either at rest or after exercise. 15,16,40–44 When NSVT is documented in the context of a history of established monomorphic VT, it may demonstrate the same morphology as clinical sustained arrhythmia. VTs with a left bundle branch block (LBBB) pattern and inferior axis originate in the right ventricular outflow tract (RVOT).45 Left ventricular outflow tract (LVOT) tachycardias may produce a right bundle branch block (RBBB) morphology with inferior axis or a variable QRS morphology, depending on the site of origin. Tachycardias originating in the left coronary cusp have a QRS morphology consistent with an M or W pattern in lead V1, tachycardias originating in the right coronary cusp have an LBBB pattern, and those from the aortomitral continuity usually result in a qR pattern.46 Idiopathic left VT may also be caused by re-entry within the Purkinje network and may originate within one of the fascicles of the left bundle branch (fascicular tachycardias), and usually the posterior fascicle is involved, resulting in a tachycardia with RBBB and left-axis deviation. However, cases with an inferior axis, that is, of anterior fascicular origin, have also been described.47,48
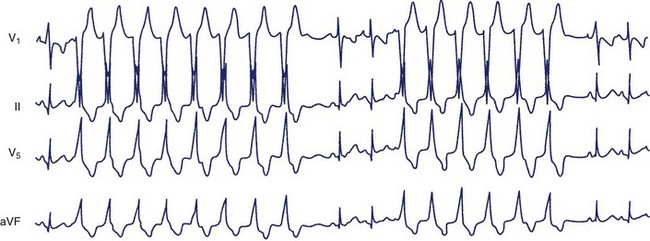
The most systematic attempts to characterize the electrocardiographic pattern of NSVT have been reported to be successful in patients with the so-called repetitive monomorphic VT, originally described by Gallavardin.49 This arrhythmia represents a form of the spectrum of idiopathic ventricular outflow tachycardias, which includes repetitive uniform premature ventricular contractions (PVCs) and exercise-triggered paroxysmal VT. They usually originate in the RVOT and less often in the LVOT and are most probably caused by triggered activity secondary to cyclic adenosine monophosphate–mediated delayed afterdepolarizations.45 The relatively consistent recording of frequent arrhythmia episodes in patients with repetitive monomorphic VT has made possible the accumulation of useful information on its morphology. Holter studies have revealed that the tachycardia begins with a fusion complex or with an ectopic beat that has the same morphology as the subsequent beats (Figure 43-1).50 The salvos of VT are generally short (3 to 15 beats) and the coupling interval of the first beat is usually long (>400 ms). The coupling intervals of successive beats may gradually become prolonged.51–53 Many patients mostly have ventricular premature beats (VPBs) with only occasional episodes of tachycardia, whereas others manifest mainly with short runs of VT, where the tachycardia beats far exceed the number of sinus beats. The rate during tachycardia is generally 110 to 150 beats/min. The arrhythmia is only present within a critical window of heart rates (upper and lower thresholds).50 Thus, the tachycardia often occurs during exercise but disappears as the heart rate increases and returns during the recovery period following exercise. Some patients develop sustained episodes (>30 seconds) during the recovery phase, and this behavior differentiates repetitive monomorphic VT from the exercise-triggered paroxysmal VT first described by Wilson and others in patients with apparently normal hearts.54–56 It should be noted, however, that repetitive behavior has been documented in various clinical settings, including cardiomyopathy and previous MI, as well as tachycardias originating in the aortic valve cusps.51,57
In catecholaminergic polymorphic VT, a familial condition mainly caused by mutations in the genes encoding the cardiac ryanodine receptor channel as well as calsequestrin involved in calcium kinetics, episodes of provoked tachycardia are typically nonsustained.58 They may originate from the LVOT and less frequently from the RVOT or the right ventricular apex. QRS morphology suggests an outflow tract origin of the initiating beat in more than 50% of patients, and subsequent beats portray a polymorphic or typically bi-directional VT morphology.59
Ischemic Heart Disease
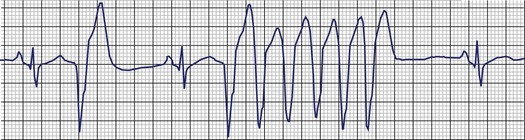
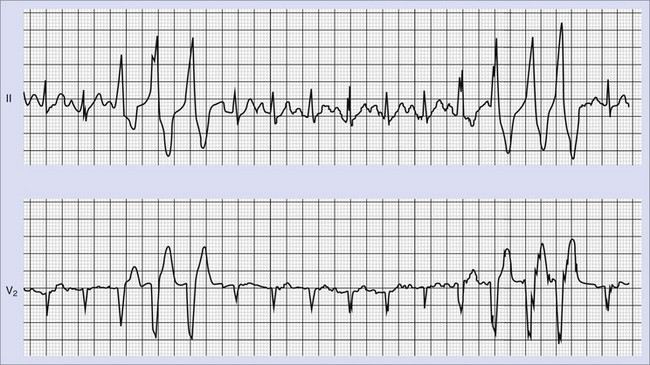
Frequent and complex ventricular ectopy as well as NSVT occur more commonly in patients with left ventricular dysfunction. Usually, monomorphic VT is seen in the context of re-entry at the borders of a ventricular scar caused by previous MI, whereas ischemia most of time induces polymorphic VT or ventricular fibrillation (VF).60,61 The QRS morphology during monomorphic tachycardia may show an RBBB or LBBB pattern or may even be nonspecific (Figure 43-2). Both RBBB and LBBB patterns can be seen in the same patient when the infarct scar involves the interventricular septum (Figure 43-3). Spontaneous reversions to sinus rhythm, thus documenting nonsustained VF, can occur (Figure 43-4), and data from patients with ICDs reveal that up to 40% of all VF episodes are nonsustained.14
Cardiomyopathies and Other Conditions
VTs (both sustained and nonsustained) in DCM may present with multiple morphologies or an LBBB or RBBB pattern. In approximately one third of the cases of idiopathic DCM, and probably in a small percentage of ischemic patients, sustained or nonsustained VT is caused by bundle branch re-entry.62 The necessary condition for bundle branch re-entry seems to be prolonged conduction in the His-Purkinje system, which is reflected in the H-V interval that is prolonged during sinus rhythm and prolonged or equal to the baseline sinus rhythm during VT.63 The circuit involves the right and left branch bundles, with antegrade conduction occurring most of the times through the right branch. As a rule, AV dissociation is present. These tachycardias are usually unstable; the 12-lead ECG, when obtainable, may show an LBBB or RBBB pattern, depending on the orientation of activation of the bundle branches.
No characteristic electrocardiographic morphology of NSVT exists in patients with HCM. Relatively slow, often asymptomatic nonsustained episodes of monomorphic VT may be documented on prolonged ambulatory recordings. When nonsustained or sustained VT is induced by programmed stimulation, it is more often polymorphic (60% of patients) than monomorphic (30%).32,64,65
In other conditions such as valvular disease and systemic hypertension, ventricular arrhythmias are common but less well characterized and usually represent polymorphic rhythms.34–36 Most of the reported patients with mitral valve prolapse demonstrate LBBB morphology during tachycardia, which raises the possibility that the mitral valve prolapse might be an incidental finding or that the arrhythmia is caused by other mechanisms not directly related to the mechanical stress imposed on the ventricle by the valvular apparatus.66,67
The tachycardias in arrhythmogenic right ventricular dysplasia (ARVD) arise from the right ventricle and typically present with LBBB morphology with a left- or even right-axis deviation.68
Long QT Syndrome
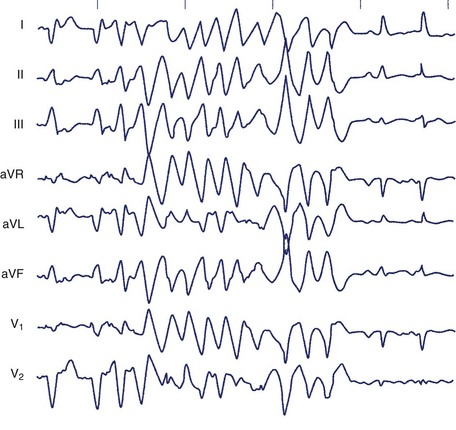
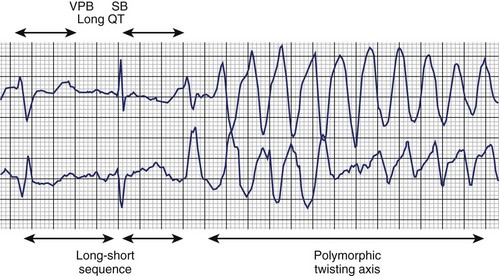
The typical morphology of recorded arrhythmias in long QT syndrome (LQTS; congenital or acquired) is that of torsades de pointes.69 The term refers to the electrocardiographic appearance of spike-like QRS complexes that rotate irregularly around the isoelectric line at rates of 200 to 250 beats/min (Figure 43-5). Typically, the coupling interval of the initial beat of the torsade is long (600 to 800 ms), whereas the last QRS complex of the episode is larger than the normal QRS during sinus rhythm. This is a VT that is frequently nonsustained and sometimes sustained. The tachycardia, which usually occurs in the setting of bradycardia or long postectopic pauses, is often repetitive and may trigger VF. It is seen primarily in association with prolongation of the Q-T interval that may be appreciable before the onset of arrhythmias or after a pause. Not all patients with the LQTS have polymorphic VT with a characteristic torsades de pointes configuration, and this pattern can be seen in some patients without the LQTS. A variety of torsade initiating with a short coupling interval in patients without any evidence of LQTS has also been described.70
Clinical Evaluation
Trans-thoracic Echocardiography
Trans-thoracic echocardiography may detect signs of cardiomyopathy, ARVD and other structural abnormalities, and impaired left ventricular function. Overwhelming evidence indicates that in patients with heart disease, in general, LVEF is the major determinant of cardiac and total mortality.1 The results of the MADIT, MUSTT, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), and the Marburg Cardiomyopathy Study (MACAS) trials have also established the importance of LVEF as the most critical prognostic factor in patients with ischemic heart disease and NSVT.8,10,11,71 Left ventricular function assessment with echocardiography or radionuclide ventriculography is therefore mandatory for the risk stratification and subsequent management of patients with NSVT. However, LVEF does not always predict ICD intervention in patients with ischemic or nonischemic cardiomyopathy.72 Analysis of arrhythmic death in 674 patients enrolled in MUSTT showed that patients whose only factor is ejection fraction (EF) of 30% or less have a predicted 2-year arrhythmic death risk of 5% or less.73 Multiple additional factors influence arrhythmic death in patients with LVEF less than 40% and NSVT such as inducible VT, history of heart failure, NSVT not discovered within 10 days after coronary artery bypass grafting (CABG), and intraventricular conduction delay or LBBB.73
Tests for Myocardial Ischemia
Functional tests such as treadmill exercise electrocardiography, myocardial perfusion, and stress echocardiography are required to demonstrate myocardial ischemia in patients with NSVT. Acute myocardial ischemia is an established cause of polymorphic ventricular rhythms.61 The association of monomorphic VT, a substrate-dependent arrhythmia, with acute ischemia is less well characterized, but ischemia may induce monomorphic VT in the presence of a myocardial scar.74–76
Apart from demonstration of ischemia, exercise tasting may also be helpful in patients with LQTS, exercise-triggered RVOT tachycardia, and cathecholaminergic polymorphic VT. NSVT induced by treadmill exercise testing aimed at evaluating presumed LQTS suggests catecholaminergic polymorphic VT rather than LQTS.77
Ambulatory Electrocardiographic Monitoring
Holter monitoring is a valuable diagnostic tool in detecting patients with NSVT, and 7-day Holter monitoring improves detection and allows better characterization of ventricular arrhythmic episodes.78 Detection of NSVT has been reported to predict SCD in patients with non–ST-segment elevation acute coronary syndromes, thrombolysed MI, DCM, HCM, and arrhythmogenic right ventricular dysplasia.17,18,25,31,79–81 However, whether NSVT provokes arrhythmia or is simply a surrogate marker of a more severe underlying cardiac condition is not known. In patients with revascularization of most infarct-related arteries (78%), NSVT early after MI (4 to 16 days) carried a significant but low RR for the composite endpoint of cardiac death, VT, or VF but not for arrhythmic events considered alone.20 In the Danish Multicenter Randomized Study on Thrombolytic Therapy Versus Acute Coronary Angioplasty in Acute Myocardial Infarction-2 (DANAMI-2) trial, the prognostic value of NSVT was limited regardless of reperfusion strategy, and in the study of Makikallio et al, no Holter variable predicted sudden death among infarct survivors with an LVEF of 35% or less.22,79 It seems that in the β-blocking era, all common arrhythmia risk variables, including NSVT, have diminished the predictive power in identifying patients with previous MI at risk of SCD.82
Both in MACAS and CHF-STAT, in patients with CM or ischemic cardiomyopathy, NSVT was a predictor of arrhythmia in univariate analysis but not in multivariate analysis, as opposed to LVEF.8,26 In patients with HCM, NSVT was associated with SCD, but a relation between the risk of arrhythmic death and the frequency, duration, and rate of NSVT episodes could not be demonstrated.31
The suppression of frequent ventricular ectopy or NSVT runs following β-blockade or amiodarone therapy does not imply a favorable diagnosis. Mortality was increased in the Cardiac Arrhythmia Suppression Trials (CAST and CAST II) despite reduced ectopic activity, whereas mortality was not reduced by amiodarone in the Veterans Administration CHF-STAT despite the elimination of ventricular ectopy.83–85 Thus, Holter monitoring may be useful for risk stratification purposes by means of detecting episodes of NSVT in certain clinical settings, but its use for the subsequent follow-up and evaluation of treatment is limited.
Assessment of Autonomic Tone
Heart rate variability (HRV) has been used as an independent risk factor for cardiac mortality in patients with previous MI and has been claimed to be a specific predictor of arrhythmic rather than total cardiac mortality.86–88 In these patients, a depressed baroreflex sensitivity has been associated with increased cardiac mortality and SCD and with a higher predictive power (in values below 3 ms/mm Hg) compared with LVEF, signal-averaged ECG (SAECG), or HRV.89,90 Analysis of patients in the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) trial has shown that NSVT, HRV, and depressed baroreflex sensitivity were all significantly and independently associated with increased mortality. Depressed baroreflex sensitivity, in particular, identified a subgroup with the same mortality risk as patients with NSVT and reduced LVEF.21
However, the Multiple Risk Factor Analysis Trial (MRFAT) show that in the β-blocking era, the common arrhythmia risk variables, particularly the autonomic and standard ECG markers, have limited predictive power in identifying patients at risk of SCD.82 In 43 ± 15 months of follow-up in 675 patients, SCD was weakly predicted only by reduced LVEF (<40%), NSVT, and abnormal SAECG but not by autonomic markers or ECG variables. The positive predictive accuracy of these markers (low LVEF, NSVT, and abnormal SAECG), however, was low at 8%, 12%, and 13%, respectively.82 The smaller Bucindolo Evaluation in Acute Myocardial Infarction Trial (BEAT) study also refuted the predictive value of HRV, the prognostic information of which was found to be contained completely in heart rate.91 In patients with nonischemic cardiomyopathy, results on the value of HRV have been conflicting.7,92,93 Heart rate turbulence and deceleration capacity are newer noninvasive measures of cardiac autonomic regulation that are currently under study.94 More data are clearly needed to establish the clinical usefulness of autonomic markers, particularly in the setting of NSVT.
Signal-Averaged Electrocardiography
The clinical value of SAECG in patients with NSVT by means of determining prognosis and identifying patients in need for an aggressive antiarrhythmic management has not been established yet. In patients presenting with unexplained syncope, the presence of late potentials is a good predictor of induction of sustained VT, and in survivors of MI an abnormal SAECG has been associated with increased risk of arrhythmic and total mortality.95–98 However, its positive predictive value in this setting is low (<30%) as opposed to its high negative predictive value (90%). Thus, a negative SAECG might obviate the need for further investigations when the suspicion of a ventricular arrhythmia is low, but in the case of a high suspicion of ventricular arrhythmia, a negative SAECG is not sufficient evidence for the exclusion of sustained VT or NSVT as the cause of syncope. Furthermore, in patients treated with thrombolysis or β-blockers, the predictive ability of SAECG is limited.82,99 In patients with DCM, SAECG does not predict SCD, but in arrhythmogenic right ventricular dysplasia, SAECG can identify those with more extensive disease and a propensity for inducible VT at programmed electrical stimulation, and is now considered a minor diagnostic criterion.8,81,100
T-Wave Alternans
T-wave alternans (TWA) is a test that is thought to reflect dispersion of repolarization and has been shown to predict VT inducibility and future arrhythmic events better than SAECG.101 In patients similar to those in MADIT II, a microvolt TWA (MTWA) test was found to be better than QRS duration at identifying high-risk patients as well as patients unlikely to benefit from ICD therapy.102 Recently, in patients with reduced LV function (LVEF <40%) and NSVT, MTWA also predicted unstable ventricular tachyarrhythmias better than electrophysiology testing and LVEF less than 30%.103 However, in a later study, although TWA predicted higher total mortality in a MADIT II–like population, the risk of tachyarrhythmic events did not differ according to TWA results.104 The Alternans Before Cardioverter Defibrillator (ABCD) study was the first trial to use MTWA to guide prophylactic ICD insertion in patients with LVEF less than 40% and NSVT. Risk stratification strategies using noninvasive MTWA versus invasive electrophysiological study (EPS) were comparable at 1 year, with very low positive predictive values and very high negative predictive values and complementary when applied in combination. Strategies that use MTWA, EPS, or both might identify subsets of patients least likely to benefit from ICD insertion.105
Electrophysiology Testing
Induction of sustained arrhythmia by programmed electrical stimulationstill retains a predictive power in patients with ischemia who have impaired left ventricular function. According to a meta-analysis, in patients with ischemia and NSVT, the induction of sustained VT is associated with a two- to three-fold increased risk of arrhythmia-related death in a previous meta-analysis.106 In NSVT, in the context of reduced LVEF (<40%), inducibility of sustained monomorphic VT at baseline programmed electrical stimulation was associated with a 2-year actuarial risk of SCD or cardiac arrest of 50% compared with a 6% risk in patients without inducible VT.107 Analysis of patients enrolled in the MUSTT as well as of those in the registry revealed that noninducible patients have a significantly lower risk of cardiac arrest or SCD compared with inducible patients at 2 and 5 years (12% vs. 24% and 18% vs. 32%, respectively).108 Still, however, as these results indicate, patients with noninducible sustained VT are not free of risk of SCD. The MUSTT investigators have further analyzed the relation of EF and inducible ventricular tachyarrhythmias to mode of death in 1791 patients who were enrolled in MUSTT and did not receive antiarrhythmic therapy. Total and arrhythmic mortality were higher in patients with an EF less than 30% than in those whose EFs were 30% to 40%. The relative contribution of arrhythmic events to total mortality was significantly higher in patients with inducible tachyarrhythmia (58% of deaths in inducible patients vs. 46% in noninducible patients; P = .004). The higher percentage of events that were arrhythmic among patients with inducible tachyarrhythmia appeared more distinct among patients with an EF of 30% or greater (61% of events were arrhythmic among inducible patients with an EF ≥30% and only 42% among noninducible patients; P = .002). This study therefore suggested that the major usefulness of EPS may be restricted to patients having an EF between 30% and 40%.109 The prognostic significance of VT inducibility appears to be similar to that of VT induced by one, two, or three extrastimuli.110 These results should be considered in the context of evidence from analysis of stored ICD data that has shown that little association exists between spontaneous and induced ventricular arrhythmias.111
The role of programmed electrical stimulation has not been established in the patient with ischemia and relatively preserved left ventricular function (LVEF >40%), although some evidence indicates that inducibility of sustained VT or VF at programmed electrical stimulation might retain discriminative ability by means of identifying a higher risk patient cohort.112,113 The prognostic usefulness of programmed stimulation in patients with nonischemic DCM, including those with NSVT, remains controversial.114–116 Retrospective cohort analysis of 54 patients from the Mayo Clinic showed that programmed electrical stimulation did not differentiate patients with and those without appropriate ICD shocks.114 However, inducibility of polymorphic VT or VF is a much stronger predictor of recurrences of fast VT as opposed to sustained monomorphic VT induction in DCM patients with ICD for secondary prevention.117 Daubert et al, in the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial, reported that inducibility tested via ICD was found in 29 of 204 patients (VT in 13, VF in 16) and that at follow-up, 34.5% of the inducible group (10 of 29) had ICD therapy for VT or VF or arrhythmic death versus 12% (21 of 175) of noninducible patients (hazard ratio [HR], 2.60; P = .014).118 Thus, inducibility of ventricular arrhythmias, especially polymorphic VT or VF, indicates increased likelihood of subsequent ICD therapies and might be considered a useful risk stratifier.
Programmed ventricular stimulation is of diagnostic as well as prognostic value in risk stratifying patients with repaired tetralogy of Fallot.119 In multivariate analysis of a study on 252 patients with repaired tetralogy, inducible sustained monomorphic or polymorphic VT was an independent risk factor for subsequent events (RR, 4.7; 95% confidence interval [CI], 1.2–18.5; P < .0268).119
Little evidence exists for benefit from EPS in patients with HCM and NSVT. In patients with a history of cardiac arrest, polymorphic VT that deteriorates into VF was induced in approximately half only.32,64,65
Cardiac Magnetic Resonance Imaging
In patients with previous MI, necrotic myocardium and viable myocardium coexisting within the same wall segments, as detected by delayed enhancement cardiac magnetic resonance imaging (CMRI), predicts the occurrence of NSVT in patients without left ventricular dilation, whereas left ventricular mass and end-systolic volume are predictors of NSVT in those with left ventricular dilation.120 In patients with HCM, the presence of delayed enhancement on contrast-enhanced CMRI is associated with increased likelihood of NSVT compared with those without this finding (28% vs. 4%), and the presence of two-dimensional strain, which is used to identify myocardial fibrosis, in more than three left ventricular segments is an independent predictor of NSVT.121,122
Clinical and Prognostic Significance
The physician who cares for a patient presenting with an episode of NSVT has two tasks. First, he or she needs to establish whether the underlying occult pathology is responsible for the arrhythmia; second, in a case of diagnosed heart disease, the patient should be risk stratified to determine the appropriate management and therapy. The clinical approach to the patient with NSVT should therefore always be considered within the particular clinical context in which the arrhythmia occurs. In several settings, the patient with NSVT represents a clinical challenge with regard to proper management, and several crucial questions remain unanswered.123 The occurrence of an abrupt ventricular arrhythmia is a multi-factorial process involving a continually changing complex substrate of myocardial scarring, ischemia, and adrenergic and genetic factors. We are therefore dealing with a probabilistic event in which each of the currently considered risk factors such as NSVT identifies only a small fraction of the risk process.
The independent prognostic significance of NSVT depends on the underlying condition (Table 43-2). It currently is not known whether NSVT bears a cause-and-effect relationship with sustained ventricular tachyarrhythmias or if it is merely a surrogate marker of left ventricular dysfunction and electric instability. Even when it does hold prognostic significance, NSVT does not necessarily imply the mechanism of death. Certain patient groups have a high mortality rate because of the progression of their disease. Death in these patients may be arrhythmic, but this does not imply that mere prevention of NSVT will unconditionally prolong life significantly. The cardiac mortality rate at 2 years in MADIT was still 11% despite the use of defibrillators.10 Furthermore, reduction of arrhythmic death does not necessarily imply a concomitant reduction in total mortality, and even a nonsignificant increase in mortality has been demonstrated despite the reduction of SCD by using amiodarone in patients with acute MI.124–126
Table 43-2 Clinical Significance of Nonsustained Ventricular Tachycardia
| CLINICAL SETTING | SIGNIFICANCE |
|---|---|
| APPARENTLY NORMAL HEART | |
| Random finding | No adverse prognostic significance in the absence of occult pathology |
| During or after exercise | May predict IHD and increased cardiac mortality |
| Valvular disease, hypertension | Prognostic significance unknown |
| ISCHEMIC HEART DISEASE | |
| Acute MI <13–24 h | No adverse prognostic significance |
| Acute MI >13–24 h | Adverse prognostic significance |
| Chronic IHD with LVEF >40% | Unknown. No adverse prognostic significance(?) |
| Chronic IHD with LVEF <40% | Adverse prognostic significance |
| DCM | Independent prognostic significance not established as opposed to LVEF |
| HCM | Adverse prognostic significance, especially in the young |
| Long QT syndrome, CPVT, repaired congenital abnormalities | Adverse prognostic significance |
| ARVC, Brugada syndrome | Probably adverse prognostic significance |
ARVD, Arrhythmogenic right ventricular cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; VF, ventricular fibrillation.
Apparently Healthy Individuals
Early reports from longitudinal community-based studies indicated that the presence of complex ventricular ectopy and NSVT increase the risk of heart disease subsequently observed.15 In other studies, NSVT was not found to adversely influence prognosis, with cardiac event rates not exceeding those detected in age-matched populations without the arrhythmia.16,40 Polymorphic NSVT, as opposed to monomorphic NSVT, may indicate an increased risk.127 Thus, recording of spontaneous NSVT in apparently healthy individuals does not imply an adverse prognosis, provided that occult cardiomyopathy and genetic arrhythmia disorders have been excluded. These conditions, however, may remain latent for several years, and although apparently healthy individuals presenting with NSVT can be reassured about their prognosis, long-term follow-up is advisable.
NSVT originating from the RVOT may occasionally cause syncope, although the risk of death is very low. Short cycle length during NSVT and a history of syncope are predictors of the coexistence of VF and polymorphic VT.128 Subjects with RVOT VT might have subtle structural and functional abnormalities of RVOT as detected by CMRI.129 Detection of NSVT in patients with ARVD indicates intermediate risk for future arrhythmic events.130 The diagnosis of RVOT VT needs to be differentiated from the diagnosis of ARVD or cardiomyopathy, which can be accomplished by a series of noninvasive ECG and imaging studies such as Holter monitoring, SAECG, CMRI and, if needed, endomyocardial biopsy.81
Exercise-Induced Nonsustained Ventricular Tachycardia
The occurrence of premature ventricular demoralizations during exercise in apparently healthy subjects has not been associated with an increase in cardiovascular mortality and was considered to be a normal response to exertion.41,42 However, the Paris Prospective Study, in agreement with earlier data, reported that runs of two or more consecutive ventricular depolarizations during exercise or at recovery may occur in up to 3% of healthy men and indicated an increase in cardiovascular mortality within the next 23 years by a factor of more than 2.5.43,131 This increased RR persisted even after adjusting for other characteristics and known factors predisposing to coronary artery disease (CAD). Interestingly, among subjects with a positive exercise test for ischemia, only 3% had ectopy, whereas among subjects with exercise-induced ectopy, only 6% had a positive exercise test for ischemia. Thus, although the precise mechanism of arrhythmia is unknown in this setting and various forms of occult cardiomyopathy cannot be excluded, it does not appear to be a direct consequence of ischemia. This study argues that exercise-induced nonsustained ventricular arrhythmia may predict CAD, even in the absence of evidence of ischemia in asymptomatic individuals. These results were also verified in a later study on patients who had been referred for symptom-limited exercise testing without a history of heart failure, valve disease, or arrhythmia. Frequent ventricular ectopy during recovery after exercise is a better predictor of an increased risk of death than ventricular ectopy occurring only during exercise.44 Exercise testing may also induce catecholaminergic polymorphic VT. When recognized, this condition requires aggressive management.59
Ventricular tachyarrhythmias and NSVT commonly occur in trained athletes during ambulatory Holter electrocardiography and are usually associated with a benign course, and resumption of training is safe.132
Ischemic Heart Disease
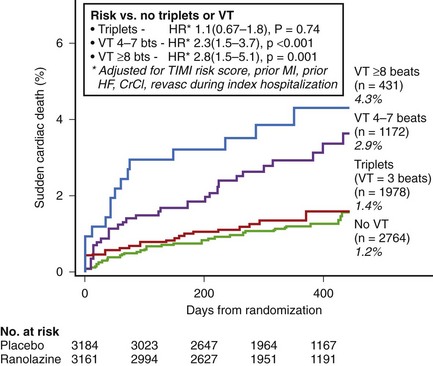
In non–ST-segment elevation acute coronary syndromes, NSVT is common after admission, and even short episodes of VT lasting 4 to 7 beats are independently associated with the risk of SCD over the subsequent year. In the Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-segment Elevation Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial on 6300 patients who had 7-day continuous ECG monitoring, a greater than twofold increase in the risk of SCD was seen in patients with both short (4 to 7 beats) and longer (>8 beats) episodes of VT occurring greater than 48 hours after admission compared with patients with no VT (Figure 43-6).17 This relationship was unchanged after adjustment for clinical characteristics, including LVEF and natriuretic peptides. No increased risk of SCD was observed in patients with ventricular triplets. In several subgroups (history of heart failure, depressed ventricular function, QTc >450 ms), the presence of VT was associated with a greater than 10% incidence of SCD at 1 year. Earlier episodes within 48 hours after admission did not carry the same risk. Thus, extended continuous ECG monitoring beyond 48 hours after admission for non–ST-segment elevation acute coronary syndrome to detect the presence of even short episodes of VT may potentially identify patients at higher risk for SCD.
In acute MI, NSVT during the first 13 to 24 hours does not carry a prognostic significance.18,133 Following this period, NSVT after MI predicted higher mortality in earlier studies. In the Multi-Center Postinfarction Research Program (MPIP) study in the pre-thrombolytic era, the presence of NSVT was associated with a twofold increase in total and arrhythmic deaths, independent of LVEF.134,135 A similar relationship between repetitive forms and mortality, and the joint predictive value of NSVT and low EF was noted in the Multicenter Investigation of Limitation of Infarct Size (MILIS).136 Evidence from the reperfusion era suggests that in the patient with ischemic heart disease, NSVT no longer appears to be an independent predictor of death if other factors such as the EF are taken into account. In the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico 2 (GISSI-2) trial, NSVT was a significant predictor of mortality in univariate analysis, but not independently in multivariate analysis involving other clinical variables.19 Similarly, in ESVEM, although univariate analysis suggested an association between the presenting arrhythmia and the outcome, multivariate analysis failed to establish the predictive value of the presenting arrhythmia. LVEF was the single most important predictor of arrhythmic death or cardiac arrest in patients with life-threatening arrhythmias who were treated with antiarrhythmic drugs.9 NSVT has not been found to be an independent predictor of arrhythmic or total mortality in the studies of Hohnloser et al (325 patients with MI) and DANAMI-2 (501 patients with fibrinolysis and 516 patients with primary angioplasty).20,22 In a study on 2130 infarct survivors, Makikallio et al reported that NSVT predicted SCD only in patients with LVEF greater than 35%. No Holter variable was predictive of outcome in the presence of an LVEF of 35% or more.79 In the modern era, only ATRAMI has found NSVT to be an independent predictor of adverse prognosis.21 It seems that it is important not only how often but under what circumstances NSVT occurs. MUSTT data have shown prognostic differences in patients with in-hospital, as opposed to out-of-hospital, identified NSVT.137 Overall mortality rates at 2-year and 5-year follow-up were 24% and 48%, respectively, for inpatients and 18% and 38%, respectively, for outpatients (adjusted P = .018). In patients subjected to surgical coronary revascularization, the occurrence of NSVT within the early (less than 10 days) post-revascularization period portends a far better outcome than when it occurs later after CABG (10 to 30 days) or in non-postoperative settings. Indeed, the approximate 2-year mortality rates for patients with early post-revascularization NSVT, late post-revascularization NSVT, and non-postoperative NSVT were 14%, 23% and 24%, respectively.138 When, however, sustained VT is inducible in patients with early postoperative NSVT, the outcome is worse compared with noninducible patients.139 In the MUSTT, racial differences were also shown to influence the outcome of patients with NSVT and reduced left ventricular function.140
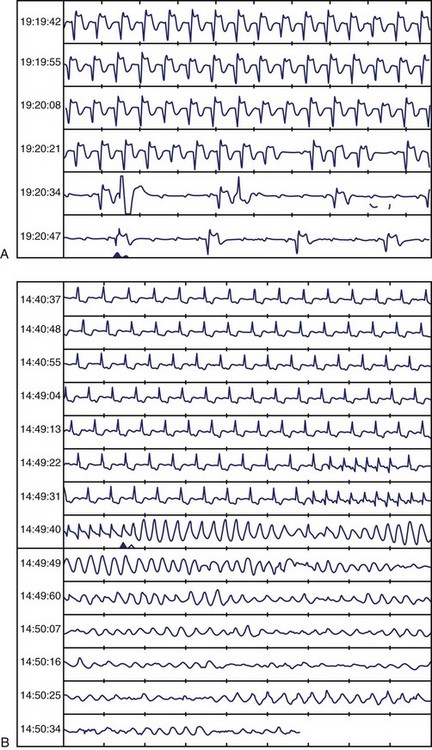
Recently, CARISMA reported on long-term cardiac arrhythmias recorded by a cardiac monitor implanted 2 to 5 days after MI in patients with an LVEF of 40% or less.6 Clinically significant bradyarrhythmias and tachyarrhythmias were documented in a substantial proportion of patients, most of them asymptomatic, within the next 2 years. The most significant arrhythmia was intermittent high-degree AV block (10% incidence), which was associated with a very high risk of cardiac death (>30% within the next 2 years), and the most frequent was atrial fibrillation (28%). Nonsustained VT (VT ≥125 beats/min for ≥16 beats lasting <30 seconds) was the most frequent ventricular arrhythmia recorded in the study (incidence of 13%) and was associated with cardiac death over the next 2 years only in univariate analysis (Figure 43-7). It seems, therefore, that with reperfusion and use of β-blockers, NSVT after MI may not be an independent predictor of mortality, especially after EF is taken into account, and its prognostic significance is ambiguous. In patients with non–ST-segment elevation acute coronary syndrome, NSVT occurring beyond 48 hours after admission may indicate an increased risk for SCD.
Not much data on patients with stable ischemic heart disease and an LVEF over 40% exist. In an older trial, NSVT was reported in approximately 2% of patients with ischemia and preserved left ventricular function, apparently excluding previous MI, and did not appear to carry a significant predictive ability.24
Heart Failure
No convincing data exist in patients with heart failure. Although the GESICA–Grupo de Estudios Multicentricos en Argentina (GEMA) investigators identified NSVT as an independent predictor of total mortality in patients with heart failure (35% to 40% ischemic heart disease) and an LVEF of 35% or less, in CHF-STAT (70% to 75% ischemic heart disease), after adjusting other variables, especially for LVEF, NSVT was not an independent predictor of SCD or total mortality in patients with heart failure and an LVEF less than 35% to 50%.26,85 Similar results were published by the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) investigators.12 Only during the recovery period after exercise, frequent ventricular ectopy has been found to carry an adverse prognostic significance in patients with heart failure.141 However, in the study by Verma et al, in 421 primary prevention patients with both ischemic and nonischemic cardiomyopathy undergoing ICD implantation, 79 (19%) had received appropriate ICD therapies.72 NSVT detected on Holter monitoring and no β-blocker use, but not LVEF, was predictive for ICD-derived arrhythmias in patients with either ischemic or nonischemic cardiomyopathies.
Cardiomyopathies
Reports investigating the association between NSVT and cardiac mortality in patients with nonischemic DCM are not consistent. MACAS has reported on noninvasive arrhythmia risk stratification in patients with DCM.8 On univariate analysis, nonsustained VT and frequent VPBs showed a significant association with a higher arrhythmia risk, but on multivariate analysis, only LVEF was found to be a significant predictor of major arrhythmic events with an RR of 2.3 per 10% decrease of EF. SAECG, baroreflex sensitivity, HRV, and TWA were not helpful in arrhythmia risk stratification. Patients with NSVT in the context of LVEF less than 30% had an eightfold arrhythmia risk compared with patients with LVEF greater than 30% without nonsustained VT.8 In a recent study on 179 patients with DCM, multivariate analysis detected LVEF, NSVT, and QT dynamicity (QTe slope assessed by dedicated software) as significant predictors of arrhythmic events. Among the patients with a low LVEF, NSVT, steeper QTe-slope, or both identified a subgroup at highest arrhythmic risk.142 In another study, after medical stabilization with an angiotensin-converting enzyme inhibitor (88%) and a β-blocker, NSVT did not increase the risk of major ventricular arrhythmias in patients with DCM and LVEF of 35% or greater. Conversely, the number and length of NSVT runs were significantly related to the occurrence of major ventricular arrhythmias in the patients with an LVEF greater than 35%.143 The frequency and complexity of ventricular arrhythmias increase with decreasing function of the left ventricle and with the presence of symptoms of congestive heart failure in patients with nonischemic DCM. However, cardiac death in patients with nonischemic DCM and functional class IV heart failure is more frequently caused by bradyarrhythmia or electromechanical dissociation rather than by ventricular tachyarrhythmias.4,29
Frequent and prolonged (>10 beats) episodes of NSVT on Holter monitoring most probably indicate an increased risk of SCD in HCM, especially in young patients.31,144,145 In a study of 531 patients with HCM, a relation between arrhythmic mortality and the frequency, duration, and rate of NSVT episodes could not be demonstrated.31 However, NSVT was associated with a substantial increase in the risk of SCD in young patients with hypertrophic obstructive cardiomyopathy.31 Adabag et al reported NSVT in Holter recordings in 31% of 178 patients with HCM, and NSVT was associated with greater left ventricular hypertrophy and more severe symptoms.146 Over a follow-up of 5.5 ± 3.4 years, 11 (6%) patients died suddenly (annual mortality rate, 1.1%), including 5 patients with NSVT. For SCD, NSVT on Holter ECG had negative and positive predictive values of 95% and 9%, respectively, and sensitivity and specificity of 45% and 69%, respectively. Therefore, NSVT had a low positive predictive value and relatively high negative predictive value for SCD in this HCM population. In another study from the same group, delayed enhancement on contrast-enhanced CMRI (believed to represent myocardial fibrosis) was found as an independent predictor of NSVT (RR, 7.3; 95% CI, 2.6–20.4; P < .0001).
Asymptomatic patients with ARVD and NSVT have a propensity for increased arrhythmic risk and a rate of appropriate ICD intervention of 3.7% per year.80 However, the predictive value of NSVT has not been prospectively assessed in this setting.147
Other Conditions
The presence of NSVT in patients with idiopathic ventricular outflow tract arrhythmias indicates a higher propensity for inducibility of VT during EPS compared with isolated PVCs (48% vs. 4%). At exercise, the incidence of VT in this group is 10%.45
In patients with tachycardiopathy, the presence of nonsustained VT, in addition to the PVC burden, longer PVC duration, multi-form PVCs, and right ventricular PVCs may be associated with the development of cardiomyopathy.148
The occurrence of NSVT in hypertensive patients requires evaluation of ischemic heart disease. However, the prognostic value of NSVT in patients with lone hypertension (without evidence for concomitant ischemic heart disease) remains unclear. Evidence that elimination of NSVT improves survival in patients with hypertension is not available.35,36 Also, no convincing evidence exists to prove that nonsustained VT is an independent predictor of SCD in patients with valve disease, including mitral leaflet prolapse. Initial studies suggested that patients with mitral valve prolapse had an increased incidence of NSVT and other arrhythmias.149,150 Kilgfield et al estimated the annual risk of SCD in patients with mitral valve prolapse at 1.9 per 10,000 patients, lower than the annual risk of SCD from all causes in the adult population in the United States.151 Thus, patients with mitral valve prolapse are not at increased risk of SCD. NSVT in patients with mitral valve prolapse is related primarily to coexisting mitral regurgitation and secondarily chronic hemodynamic and myocardial abnormalities, but they do not independently contribute to the risk of death.
Ventricular arrhythmias have an adverse prognosis in patients with repaired tetralogy of Fallot, and programmed stimulation carries a prognostic value.38,39,119
Patients with Chagas’ cardiomyopathy presenting with either sustained VT or NSVT have a major risk for mortality in the presence of moderate left ventricular systolic dysfunction (LVEF <40%).152
In cases of LQTS, detection of spontaneous episodes of torsades de pointes indicate an adverse prognosis.4
Management and Evidence-Based Therapy
Nonsustained Ventricular Tachycardia in Structurally Normal Heart
Sometimes, episodes of NSVT in patients without structural heart disease may cause symptoms and require treatment. Because RVOT VT does not increase the risk of SCD, asymptomatic patients do not require treatment. In symptomatic patients, treatment should be directed toward relief of tachycardia-related symptoms with β-blockers or calcium channel blockers and adenosine, if needed, in an acute setting. If medication is not effective, radiofrequency catheter ablation is recommended; it is successful in more than 80% of cases with a low risk of relapse during follow-up.153
Nonsustained Ventricular Tachycardia in Inherited Channelopathies
β-Blocker therapy is the treatment of choice in cathecholaminergic polymorphic VT, and some evidence exists that combination of β-blockers with calcium channel blockers is superior to β-blockers alone.154 Defibrillator implantation may be necessary in addition to β-blockers in some patients with catecholaminergic polymorphic VT.
Usually, cardiac events in patients with LQTS are caused by prolonged polymorphic NSVT or sustained VT (torsades de pointes) that might degenerate into VF.155 However, transient, asymptomatic runs of polymorphic NSVT are also common. β-Blocker treatment is the therapy of choice in patients with LQTS (especially LQT1 and LQT2); it is effective in 70% of patients, whereas the remaining 30% of patients continue to have cardiac events despite β-blocker treatment.156 Those patients with recurrent syncope despite β-blocker therapy and those with cardiac arrest should be considered for ICD therapy, which is successful in preventing death.157
Although no systematic studies evaluating prognostic significance of NSVT in Brugada syndrome have been conducted, it is generally agreed that symptomatic patients (i.e., those with syncope or cardiac arrest presumably caused by NSVT or sustained VT) should be treated with ICDs.158
Patients with ARVD are at high risk of sudden death.81 These patients may present with asymptomatic NSVT despite only subtle right ventricular abnormalities. The results of antiarrhythmic medications have been disappointing, and ICDs seem to be the therapy of choice in patients with documented arrhythmias or in those who are symptomatic.80 Catheter ablation of VT, when feasible, is successful in reducing further episodes but cannot offer absolute protection without ICD backup.159
Nonsustained Ventricular Tachycardia in Hypertrophic Cardiomyopathy
The effects of pharmacologic antiarrhythmic therapy in patients with HCM are disappointing.64,65 β-Blockers may decrease the risk of SCD; however, the risk of death on β-blocker remains significant. Amiodarone was shown to suppress VT induction in some patients while causing conduction abnormalities and facilitating induction of VT in others.64,65 Therefore, this drug cannot be considered as therapy of choice in high-risk patients with HCM. Defibrillator implantation is the therapy of choice in the primary and secondary prevention of CD in patients with HCM.160 Patients with a history of syncope, a family history of SCD, or history of NSVT who had received defibrillators as primary prevention had a 5% per year appropriate discharge rate. In patients resuscitated from cardiac arrest (secondary prevention), the rate of appropriate discharges was 11%. More than one third of the patients who had an appropriate discharge of the defibrillator were taking amiodarone, further emphasizing the limited role of antiarrhythmic therapy in these patients. 160 These observations indicate that presence of high-risk factors, including frequent NSVT accompanied by history of syncope or family history of SCD at a young age, may justify defibrillator implantation as primary prevention in patients with HCM.
Nonsustained Ventricular Tachycardia in Dilated Cardiomyopathy
Two large trials evaluated amiodarone therapy in patients with ischemic and nonischemic cardiomyopathy. In the GESICA trial, amiodarone therapy was associated with decreased mortality, whereas no such effect was observed in the CHF-STAT trial.25,85 The reasons for the different frequencies of NSVT in both studies (34% and 79%, respectively) and the differences in the effectiveness of amiodarone therapy are not clear and might be related to the varying characteristics of patient populations (with the GESICA trial enrolling patients with more advanced left ventricular dysfunction and a high number of patients with Chagas’ disease). Results of SCD-HeFT demonstrated that amiodarone is not effective in preventing mortality in patients with nonischemic DCM (HR 1.07, in 813 patients randomized to amiodarone or placebo).71 The SCD-HeFT study of 2521 patients with ischemic (52%) and nonischemic (48%) cardiomyopathies (EF <36% and New York Heart Association [NYHA] class II or III) randomized the subjects to amiodarone, ICD therapy, or conventional therapy. A history of NSVT was reported in 23% of patients. During a mean 45-month follow-up, a 7.2% annual mortality was observed in the placebo group. ICD therapy was associated with a 23% reduction in mortality (HR 0.77, P = .007) in all patients combined. Patients with nonischemic cardiomyopathy had an HR of 0.73, and those with ischemic cardiomyopathy had an HR of 0.79 (no significant difference existed between the two subgroups). The SCD-HeFT data seem to end the controversy regarding the usefulness of amiodarone for primary prevention of SCD in patients with nonischemic (and also in ischemic) cardiomyopathy. Dronedarone also did not fulfill hopes regarding its prevention of mortality in patients with heart failure; therefore, this drug needs to be taken off the list of medications used in such patients.161
The effect of defibrillator implantation as primary prevention of cardiac mortality in patients with nonischemic DCM and NSVT or ventricular ectopy was the subject of the DEFINITE trial.162 In this study, 458 patients with nonischemic DCM with EF less than 36%, symptomatic congestive heart failure, and NSVT or more than 10 PVCs per hour were randomized to defibrillator versus no defibrillator therapy (229 patients in each arm). The mean age of the patients was 58 years. They were predominantly males (71%; mean EF, 21%). NSVT was present in 23% of the patients. During a mean 29-month follow-up, 68 deaths occurred, 28 in the ICD arm and 40 in the non-ICD arm (P = .08 from the log-rank test). Two-year mortality rate was 14.1% in the non-ICD arm and 7.9% in the ICD arm (HR, 0.65; CI, 0.40–1.06). The HR for SCD was 0.20 (P = .006). Patients with NYHA class III demonstrated significant benefit from ICD therapy with an HR of 0.37 (P = .02). A trend for reduced total mortality was observed with ICD. Thus, results from DEFINITE and SCD-HeFT show that patients with nonischemic cardiomyopathy with EF less than 36% and NYHA class II or III have a high (7%) annual mortality despite optimal pharmacologic therapy and that the risk of mortality could be substantially reduced by ICD therapy.
The purpose of the Amiodarone Versus Implantable Cardioverter-Defibrillator: Randomized Trial in Patients With Nonischemic Dilated Cardiomyopathy and Asymptomatic Nonsustained Ventricular Tachycardia (AMIOVIRT) was to compare total mortality during therapy with amiodarone or ICD in 103 patients with nonischemic DCM and NSVT.163 The primary endpoint was total mortality. Secondary endpoints included arrhythmia-free survival, quality of life, and costs. The study was stopped when the prospective stopping rule for futility was reached. The percentage of patients surviving at 1 year (90% vs. 96%) and 3 years (88% vs. 87%) in the amiodarone and ICD groups, respectively, were not statistically different (P = .8). Quality of life was also similar with each therapy (P = not significant). A trend toward a more beneficial cost profile and improved arrhythmia-free survival was observed with amiodarone therapy. Mortality and quality of life in patients with nonischemic DCM and NSVT treated with amiodarone or ICD are not statistically different. ICD was also not found superior to amiodarone in the Cardiomyopathy Trial (CAT) study on patients with recent onset of DCM ≤9 months) and EF 30% or less.164
Current indications for ICD or cardiac resynchronization therapy (CRT) therapy include primary prevention indications beyond those based on syncope or arrhythmic events. In light of the results from the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial and subsequently from the Cardiac Resynchronization–Heart Failure (CARE-HF) trial, CRT has been approved as treatment in patients with an LVEF of 35% or less, NYHA class III or IV, and QRS greater than 120 ms.165,166 CRT provides benefit in patients with heart failure with wide QRS complex; this benefit is observed in patients with ischemic and nonischemic cardiomyopathies, but it might be higher in patients with nonischemic cardimyopathy.167,168
Nonsustained Ventricular Tachycardia in Coronary Artery Disease
The management of patients with previous MI and NSVT should first include modern therapy for ischemic heart disease, including revascularization procedures, the use of β-blockers, statins, and angiotensin-receptor blockers or inhibitors. This therapy is the most effective antiarrhythmic measure in patients with CAD as demonstrated by the Coronary Artery Bypass Graft Patch (CABG-Patch) trial.169 The CAST and CAST II trials demonstrated that class IC antiarrhythmic agents (encainide, flecainide, and moricizine) confer increased (not decreased) mortality in patients with previous MI.83,84,170 Subsequently, d-sotalol (class III drug) tested in the Survival With Oral d-Sotalol (SWORD) trial also was associated with increased mortality in comparison with placebo.171 The European Myocardial Infarct Amiodarone Trial (EMIAT) and the Canadian Amiodarone Myocardial Infarction Arrhythmia Trial (CAMIAT) failed to demonstrate beneficial effect of amiodarone on survival.125,126 Therefore, no antiarrhythmic drug is suitable for the primary prevention of cardiac death, with the exception of β-blockers, which were shown in several studies to reduce total and cardiac mortality in patients with previous MI. However, in low-risk to moderate-risk patients with previous MI and NSVT from the Beta-Blocker Heart Attack Trial, there was no reduction in mortality in patients with NSVT.172
In the MERLIN study, among patients assigned to ranolazine, an episode of VT lasting at least 8 beats was not associated with SCD compared with patients with no VT lasting at least 8 beats (2.5% vs. 1.6%; HR, 1.1; 95% CI, 0.3–3.5; P = .90), whereas among patients assigned to placebo, VT lasting at least 8 beats was significantly associated with SCD (5.4% vs. 1.5%; HR, 2.9; 95% CI; 1.6–5.3; P < .001) (P for interaction = .15).17 These findings indicate that ranolazine most likely contributed to decreased mortality in patients with NSVT. However, these findings require confirmation in prospective studies.
In the 1990s, the implantable defibrillator became a feasible alternative to pharmacologic antiarrhythmic therapy for primary prevention of SCD in patients with CAD and NSVT. In the MADIT study, 196 patients with previous MI, an EF of 35% or less, a documented episode of NSVT, and inducible and nonsuppressible sustained VT were randomized to receive an implanted defibrillator or conventional (primary amiodarone) non-ICD therapy. Fifteen deaths occurred in 95 patients randomized to ICD therapy and 39 deaths in 101 patients randomized to conventional therapy, yielding a 54% reduction in total mortality associated with ICD during a mean 27-month follow-up. This was the first study demonstrating that ICD therapy is beneficial as primary prevention in high-risk patients with previous MI. Antiarrhythmic therapy guided by invasive EPS has historically been used to treat patients with NSVT and inducible sustained VT. MUSTT used the concept of EPS-guided antiarrhythmics to determine whether this approach can reduce mortality in survivors of MI with EF 40% or less and NSVT.11 The main study enrolled 704 patients with CAD, asymptomatic NSVT, EF 40% or less, and inducible sustained VT. Patients with induced, sustained VT were randomized to no antiarrhythmic therapy or to EPS-guided therapy. The EPS-guided therapy included pharmacologic antiarrhythmic therapy and implantable defibrillators as indicated, based on EPS. The risk of cardiac arrest or death from arrhythmia among patients who received treatment with defibrillators in MUSTT was significantly lower than that among patients who did not receive ICDs (RR, 0.24; 95% CI, 0.13–0.45; P < .001). Patients assigned to EPS-guided therapy who were treated with only pharmacologic antiarrhythmics had a similar rate of death and cardiac arrest as those in the control group who had not been treated with antiarrhythmic therapy. These studies demonstrated that ICDs used in patients with previous MI and depressed EF and NSVT significantly reduce the risk of SCD and total mortality.173
Subsequently, MADIT II hypothesized that patients with previous MI and markedly depressed left ventricular function (EF ≤30%)—regardless of the presence or absence of other risk stratifiers, including frequent ventricular ectopy and inducibility of sustained VT—will benefit from ICD therapy.174 MADIT II enrolled 1232 patients with previous MI (742 randomized to ICD therapy and 490 to the non-ICD arm) and demonstrated that ICD therapy was associated with a significant 30% reduction in total mortality during a 20-month follow-up and a sustained 8-year survival benefit.175 SCD-HeFT (with 52% of the patients having ischemic cardiomyopathy) confirmed the MADIT II findings, indicating substantial benefit from ICD therapy in patients with a low EF (<36%) and NYHA classes II and III. MADIT II and SCD-HeFT significantly changed the clinical approach to patients with previous MI and NSVT. In light of MADIT II, patients with previous MI and EF of 30% or less should undergo ICD therapy regardless of the presence or absence of NSVT. Based on SCD-HeFT, patients with previous MI and EF of 31% to 35% should undergo ICD implantation if they are in NYHA class II or III. Therefore, on the basis of the MUSTT findings, patients with previous MI and NSVT and EF of 36% to 40% should undergo EPS and, if inducible, they should be treated with ICDs.
Two trials have investigated the optimal timing of ICD in patients with previous MI and reduced left ventricular function and other risk factors. The Defibrillator in Acute Myocardial Infarction Trial (DINAMIT), a randomized trial, compared ICD therapy (in 332 patients) with no ICD therapy (in 342 patients) 6 to 40 days after MI in patients with LVEF 35% or less and impaired cardiac autonomic function (manifested as depressed HRV or an elevated average 24-hour heart rate on Holter monitoring).176 Prophylactic ICD therapy did not reduce overall mortality during an approximately 3-year follow-up, although it was associated with a reduction in the rate of arrhythmic death. The Immediate Risk Stratification Improves Survival (IRIS) investigators randomized 898 patients with an LVEF of 40% or less and a heart rate of 90 or more beats/min on the first available ECG (602 patients), or NSVT (≥150 beats/min) during Holter monitoring (208 patients), or both criteria (88 patients), 5 to 31 days after MI, to treatment with an ICD (n = 445) and to medical therapy alone (n = 453).177 During a mean follow-up of 37 months, prophylactic ICD therapy did not reduce overall mortality. Fewer SCDs occurred in the ICD group than in the control group (27 vs. 60; HR, 0.55; 95% CI, 0.31–1.00; P = .049), but the number of non-SCDs was higher (68 vs. 39; HR, 1.92; 95% CI, 1.29–2.84; P = .001). Thus, ICD after MI, when indicated, should be considered at least 40 days later than the event.
Current guidelines for ICDs include MUSTT-based class I indication, formulated as follows: “ICD therapy is indicated in patients with NSVT due to prior MI, LVEF <40%, and inducible VF or sustained VT at electrophysiological study (Evidence level B).”178 However, this category of patients is very much limited to individuals with LVEF of 36% to 40%, since other (MADIT II and SCD-HeFT based) approved indications that do not require documented NSVT cover patients with an LVEF less than 35%.4
Summary
NSVT can be recorded in an extremely wide range of conditions, from apparently healthy individuals to patients with significant heart disease. The prognostic significance of NSVT is strongly influenced by the type and severity of underlying heart disease. The current evidence-based therapy in patients with NSVT is summarized in Table 43-3. ICD therapy remains the mainstay of current antiarrhythmic regimen in high-risk conditions complicated by NSVT.
Table 43-3 Management and Evidence-Based Therapy in Patients with NSVT Depending on Underlying Conditions
| NSVT SETTING | MANAGEMENT | EVIDENCE-BASED THERAPY |
|---|---|---|
| NONISCHEMIC CONDITIONS | ||
| Asymptomatic, no overt heart disease | Evaluate for ischemic heart disease and other disorders | No treatment necessary |
| Symptomatic with RVOT morphology of NSVT | EPS | β-Blockers, verapamil, adenosine Differentiate with ARVD Radiofrequency ablation, if needed |
| Hypertrophic cardiomyopathy | Evaluate additional risk factors: Previous cardiac arrest (although most are lethal) Unexplained syncope Massive left ventricular hypertrophy (≥30 mm) Hypotensive or attenuated blood pressure response to upright exercise |
β-Blockers, CCBs (in nonobstructive form), septal myectomy/ablation ICD when frequent and prolonged (>10 beats) episodes of NSVT and additional risk factor |
| Nonischemic dilated cardiomyopathy | Value of EPS not established | Optimal CCB therapy (medical and CRT, if indicated) Ablation for bundle branch re-entry ICD for syncope or LVEF ≤30% to 35% and NYHA class II/III |
| ARVC | Value of EPS not established | ICD with sustained VT or VF or left ventricular involvement and/or one or more affected family members |
| LQTS | Genotype analysis useful | β-Blockers ICD if cardiac arrest with β-blockers (especially in LQT2 and LQT3) |
| CPVT | Value of EPS not established | β-Blockers ICD, if cardiac arrest on β-blockers |
| Brugada syndrome | Value of EPS disputed | ICD with syncope |
| ISCHEMIC CONDITIONS | ||
| Acute MI, NSVT <24 hours | Routine for acute MI | Primary angioplasty, thrombolysis |
| NSVT >24 hours until predischarge | Routine for acute MI | Optimal CAD therapy* Revascularization |
| Asymptomatic CAD without MI; or MI with EF >40% | EPS if EF <40% | No need for specific management |
| Syncope in CAD without MI; or MI with EF >40% | EPS | Optimal CAD therapy* If EPS-inducible: ICD† optimal CAD therapy |
| MI with EF of 31% to 40% | EPS | Optimal CAD therapy If EPS-inducible: ICD |
| MI with LVEF ≤35% and NYHA II/III | No further testing needed | Optimal CAD therapy ICD therapy |
| MI with EF ≤30% | No further testing needed | Optimal CAD therapy ICD therapy |
ARVC, Arrhythmogenic right ventricular cardiomyopathy; ARVD, arrhythmogenic right ventricular dysplasia; CAD, coronary artery disease; CCB, calcium channel blocker; CPVT, catecholaminergic polymorphic ventricular tachycardia; EF, ejection fraction; EPS, electrophysiology study; ICD, implantable cardioverter-defibrillator; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; RVOT, right ventricular outflow tract; VF, ventricular fibrillation; VT, ventricular tachycardia.
* Optimal CAD therapy defined as administration of aspirin, β-blockers, statins, angiotensin receptor blocker/angiotensin-converting enzyme inhibitor (ARB/ACEI), and revascularization therapy.
† ICD is recommended if indications exist at least 40 days after MI.
Key References
ACC/AHA/ESC. 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2006;8:746-837.
Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. N Engl J Med. 2005;352:225-237.
Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: The Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study, Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group. Circulation. 2010;122:1258-1264.
Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
Camm AJ, Katritsis D. Risk stratification of patients with ventricular arrhythmias. In Zipes DP, Jalife J, editors: Clinical electrophysiology. From cell to bedside, ed 3, Philadelphia: Saunders, 2000.
Goldenberg I, Gillespie J, Moss AJ, et al. Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II: Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: An extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265-1271.
Grimm W, Christ M, Bach J, Müller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883-2891.
Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2157.
Katritsis D, Gill J, Camm AJ. Repetitive monomorphic ventricular tachycardia. In Zipes DP, Jalife J, editors: Clinical electrophysiology. From cell to bedside, ed 2, Philadelphia: Saunders, 1995.
Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2010;121:445-456.
Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events, MADIT-CRT Trial Investigators. N Engl J Med. 2009;361:1329-1338.
Scirica BM, Braunwald E, Belardinelli L, et al. Relationship between nonsustained ventricular tachycardia after non-ST-elevation acute coronary syndrome and sudden cardiac death: Observations from the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndrome-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2010;122:455-462.
Stein KM. Noninvasive risk stratification for sudden death: Signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog Cardiovasc Dis. 2008;51:106-117.
Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115:2750-2760.
1 Camm AJ, Katritsis D. Risk stratification of patients with ventricular arrhythmias. In Zipes DP, Jalife J, editors: Clinical electrophysiology. From cell to bedside, ed 3, Philadelphia: Saunders, 2000.
2 Chimenti C, Calabrese F, Thiene G, Pieroni M, Maseri A, Frustaci A. Inflammatory left ventricular microaneurysms as a cause of apparently idiopathic ventricular tachyarrhythmias. Circulation. 2001;104:168-173.
3 Lehnart SE, Ackerman MJ, Benson DWJr, et al. Inherited arrhythmias: A National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325-2345.
4 ACC/AHA/ESC. 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2006;8:746-837.
5 Buxton AE, Duc J, Berger EE, Torres V. Nonsustained ventricular tachycardia. Cardiol Clin. 2000;18:327-336.
6 Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: The Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study, Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group. Circulation. 2010;122:1258-1264.
7 Kinder C, Tamburro P, Kopp D, et al. The clinical significance of nonsustained ventricular tachycardia: Current perspectives. PACE. 1994;17:637-664.
8 Grimm W, Christ M, Bach J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883-2891.
9 Caruso AC, Marcus FI, Hahn EA, et al. Predictors of arrhythmic death and cardiac arrest in the ESVEM trial. Electrophysiologic study versus electromagnetic monitoring. Circulation. 1997;96:1888-1892.
10 Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
11 Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
12 Teerlink JR, Jalaluddin M, Anderson S, et al. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation. 2000;101:40-46.
13 Senges JC, Becker R, Schreiner KD, et al. Variability of Holter electrocardiographic findings in patients fulfilling the noninvasive MADIT criteria. Multicenter Automatic Defibrillator Implantation Trial. Pacing Clin Electrophysiol. 2002;25:183-190.
14 Farmer DM, Swygman CA, Wang PJ, et al. Evidence that nonsustained polymorphic ventricular tachycardia causes syncope (data from implantable cardioverter defibrillators). Am J Cardiol. 2003;91:606-609.
15 Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: The Framingham heart study. Ann Intern Med. 1992;117:990-996.
16 Engstrom G, Hedblad B, Janzon L, et al. Ventricular arrhythmias during 24-h ambulatory ECG recording: Incidence, risk factors and prognosis in men with and without a history of cardiovascular disease. J Intern Med. 1992;246:363-372.
17 Scirica BM, Braunwald E, Belardinelli L, et al. Relationship between nonsustained ventricular tachycardia after non-ST-elevation acute coronary syndrome and sudden cardiac death: Observations from the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndrome-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2010;122:455-462.
18 Heidbüchel H, Tack J, Vanneste L, et al. Significance of arrhythmias during the first 24 hours of acute myocardial infarction treated with alteplase and effect of early administration of a beta-blocker or a bradycardiac agent on their incidence. Circulation. 1994;89:1051-1059.
19 Maggioni AP, Zuanetti G, Franzosi MG, et al. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era: GISSI-2 results. Circulation. 1993;87:312-322.
20 Hohnloser SH, Klingenheben T, Zabel M, et al. Prevalence, characteristics and prognostic value during long-term follow-up of nonsustained ventricular tachycardia after myocardial infarction in the thrombolytic era. J Am Coll Cardiol. 1999;33:1895-1902.
21 La Rovere MT, Pinna GD, Hohnloser SH, et al. Autonomic tone and reflexes after myocardial infarction. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials, ATRAMI Investigators. Circulation. 2001;103:2072-2077.
22 Høfsten DE, Wachtell K, Lund B, et al. Prevalence and prognostic implications of non-sustained ventricular tachycardia in ST-segment elevation myocardial infarction after revascularization with either fibrinolysis or primary angioplasty. Eur Heart J. 2007;28:407-414.
23 Bigger JTJr, Fleiss JL, Rolnitzky LM. Prevalence, characteristics and significance of ventricular tachycardia detected by 24-hour continuous electrocardiographic recordings in the late hospital phase of acute myocardial infarction. Am J Cardiol. 1986;58(13):1151-1160.
24 Callif RM, McKinnis RA, Burks J, et al. Prognostic implications of ventricular arrhythmias during 24 hour ambulatory monitoring in patients undergoing cardiac catheterization for coronary artery disease. Am J Cardiol. 1982;50:23-31.
25 Doval HC, Nul DR, Grancelli HO, et al. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA-GEMA Investigators. Circulation. 1996;94:3198-3203.
26 Singh SN, Fisher SG, Carson PE, Fletcher RD. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. J Am Coll Cardiol. 1998;32:942-947.
27 Neri R, Mestroni L, Salvi A, Camerini F. Arrhythmias in dilated cardiomyopathy. Postgrad Med J. 1986;62:593-597.
28 Meinertz T, Hofmann T, Kasper W, et al. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1984;53:902-907.
29 Spirito P, Rapezzi C, Autore C, et al. Prognosis of asymptomatic patients with hypertrophic cardiomyopathy and nonsustained ventricular tachycardia. Circulation. 1994;90:2743-2747.
30 Olshausen KV, Stienen U, Schwartz F, et al. Long-term prognostic significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1988;61:146-151.
31 Monserrat L, Elliott PM, Gimeno JR, et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873-879.
32 Fananapazir L, Epstein SE. Hemodynamic and electrophysiologic evaluation of patients with hypertrophic cardiomyopathy surviving cardiac arrest. Am J Cardiol. 1991;67:280-287.
33 Klein RC. Ventricular arrhythmias in aortic valve disease: Analysis of 102 patients. Am J Cardiol. 1984;53:1079-1083.
34 Martinez-Rubio A, Schwammenthal Y, Schwammenthal E, et al. Patients with valvular heart disease presenting with sustained ventricular tachyarrhythmias and syncope. Results of programmed ventricular stimulation and long-term follow-up. Circulation. 1997;96:500-508.
35 McLenaghan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317:787-792.
36 Pringle SD, Dunn FG, Macfarlane PW, et al. Significance of ventricular arrhythmias in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1992;69:913-917.
37 Chandar JS, Wolff GS, Garson AJr, et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol. 1990;65:655-661.
38 Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet. 2000;356:975-981.
39 Khairy P, Aboulhosn J, Gurvitz MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: A multi-institutional study, for the Alliance for Adult Research in Congenital Cardiology (AARCC). Circulation. 2010;122:868-875.
40 Kennedy HL, Whitlock JA, Sprague MK, et al. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193-198.
41 Drory Y, Pines A, Fisman EZ, Kellermann JJ. Persistence of arrhythmia exercise response in healthy young men. Am J Cardiol. 1990;66(15):1092-1094.
42 Nair CK, Aronow WS, Sketch MH, et al. Diagnostic and prognostic significance of exercise-induced premature ventricular complexes in men and women: A four year follow-up. J Am Coll Cardiol. 1983;1:1201-1206.
43 Jouven X, Zureik M, Desnos M, et al. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med. 2000;343:826-833.
44 Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781-790.
45 Kim RJ, Iwai S, Markowitz SM, et al. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol. 2007;49:2035-2043.
46 Lin D, Ilkhanoff L, Gerstenfeld E, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5:663-669.
47 Katritsis D, Ahsan A, Anderson M, et al. Catheter ablation for successful management of fascicular tachycardia. An approach guided by the recording of fascicular potentials. Heart. 1996;75:384-388.
48 Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: Unidirectional block and macroreentry within the Purkinje network. Circulation. 2002;105:462-469.
49 Gallavardin L. Extrasystolie ventriculaire a paroxysms tachycardiques prolonges. Arch Mal Coeur. 1922;15:298-306.
50 Katritsis D, Gill J, Camm AJ. Repetitive monomorphic ventricular tachycardia. In Zipes DP, Jalife J, editors: Clinical electrophysiology. From cell to bedside, ed 2, Philadelphia: Saunders, 1995.
51 Buxton AE, Marschlinski FE, Doherty JU, et al. Repetitive monomorphic ventricular tachycardia: Clinical and electrophysiologic characteristics in patients with and patients without organic heart disease. Am J Cardiol. 1984;54:997-1002.
52 Rahilly GT, Prystowsky EN, Zipes DP, et al. Clinical and electrophysiologic findings in patients with repetitive monomorphic ventricular tachycardia and otherwise normal electrocardiogram. Am J Cardiol. 1982;50:459-468.
53 Zimmerman M, Maisonblanche P, Cauchemez B, et al. Determinants of the spontaneous ectopic activity in repetitive idiopathic monomorphic ventricular tachycardia. J Am Coll Cardiol. 1986;7:1219-1227.
54 Wilson FN, Wishart SW, Macleod AG, et al. A clinical type of paroxysmal tachycardia of ventricular origin in which paroxysms are induced by exertion. Am Heart J. 1932;8:155-162.
55 Wu D, Kou H-C, Hung J-S. Exercise-triggered paroxysmal ventricular tachycardia. A repetitive rhythmic activity possibly related to afterdepolarization. Ann Intern Med. 1981;95:410.
56 Palileo EV, Ashley WW, Swiryn S, et al. Exercise provocable right ventricular outflow tract tachycardia. Am Heart J. 1982;104:185-193.
57 Ouyang F, Fotuhi P, Ho SY, et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: Electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002;39:500-508.
58 Sy RW, Gollob MH, Klein GJ, et al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8(6):864-871.
59 Napolitano C, Priori SG. Diagnosis and treatment of cathecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675-678.
60 Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation. 1990;82:1561.
61 Wolfe CL, Nibley C, Bhandari A, et al. Polymorphous ventricular tachycardia associated with acute myocardial infarction. Circulation. 1991;84:1543-1551.
62 Caceres J, Jazayeri M, McKinnie J, et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989;79:256.
63 Blanck Z, Jazayeri M, Dhala A, et al. Bundle branch reentry: A mechanism of ventricular tachycardia in the absence of myocardial or valvular dysfunction. J Am Coll Cardiol. 1989;22:1718-1722.
64 Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2010;121:445-456.
65 McKenna WJ, Behr ER. Hypertrophic cardiomyopathy: Management, risk stratification, and prevention of sudden death. Heart. 2002;87:169-176.
66 Avierinos JF, Gersh BJ, Melton LJIII, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355-1361.
67 Grigioni F, Enriquez-Sarano M, Ling LH, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34:2078-2085.
68 Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289-1300.
69 Morita H, Wu J, Zipes DP. The QT syndromes: Long and short. Lancet. 2008;372:750-763.
70 Leenhardt A, Glaser E, Burguera M, et al. Short-coupled variant of torsade de pointes: A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1993;89:206-215.
71 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure, for the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. N Engl J Med. 2005;352:225-237.
72 Verma A, Sarak B, Kaplan AJ, et al. Predictors of appropriate implantable cardioverter defibrillator (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2010;33:320-329.
73 Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: Lessons from the MUSTT study, for the MUSTT Investigators. J Am Coll Cardiol. 2007;50:1150-1157.
74 Mehta D, Curwin J, Gomes A, Fuster V. Sudden death in coronary artery disease: Acute ischemia versus myocardial substrate. Circulation. 1997;96:3215-3223.
75 Furukawa T, Moroe K, Mayrovitz HN, et al. Arrhythmogenic effects of graded coronary blood flow reduction superimposed upon prior myocardial infarction in dogs. Circulation. 1991;84:368-377.
76 Garan H, McComb JM, Ruskin JN. Spontaneous and electrically induced ventricular arrhythmias during acute ischemia superimposed on 2 week old canine myocardial infarction. J Am Coll Cardiol. 1988;11:603-611.
77 Horner JM, Ackerman MJ. Ventricular ectopy during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm. 2008;5:1690-1694.
78 Pastor-Pérez FJ, Manzano-Fernández S, Goya-Esteban R, et al. Comparison of detection of arrhythmias in patients with chronic heart failure secondary to non-ischemic versus ischemic cardiomyopathy by 1 versus 7-day Holter monitoring. Am J Cardiol. 2010;106:677-681.
79 Makikallio TH, Barthel P, Schneider R, et al. Prediction of sudden cardiac death after acute myocardial infarction: Role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762-769.
80 Corrado D, Calkins H, Link MS, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144-1152.
81 Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806-814.
82 Huikuri HV, Tapanainen JM, Lindgren K, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652-658.
83 Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412.
84 The Cardiac Arrhythmia Suppression Trial-II Investigators. Effect of antiarrhythmic agent moricizine on survival after myocardial infarction. The Cardiac Arrhythmia Suppression Trial-II. N Engl J Med. 1992;327:227-233.
85 Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77-82.
86 Algra A, Tijssen JGP, Roelandt JRTC, et al. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation. 1993;88:180-185.
87 Hartikainen JEK, Malik M, Staunton A, et al. Distinction between arrhythmic and nonarrhythmic death after acute myocardial infarction based on heart rate variability, signal-averaged electrocardiogram, ventricular arrhythmias and left ventricular ejection fraction. J Am Coll Cardiol. 1996;28:296-304.
88 Camm AJ, Pratt CM, Schwartz PJ, et al. Mortality in patients after a recent myocardial infarction: A randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification, for the AzimiLide post Infarct surVival Evaluation (ALIVE) Investigators. Circulation. 2004;109:990-996.
89 La Rovere MT, Specchia G, Mortara A, et al. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. Circulation. 1988;78:816-824.
90 Farrell TG, Cripps TR, Malik M, et al. Baroreflex sensitivity and electrophysiological correlates in patients after acute myocardial infarction. Circulation. 1991;83:945-952.
91 Abildstrom SZ, Jensen BT, Agner E, et al. Heart rate versus heart rate variability in risk prediction after myocardial infarction, for the BEAT Study Group. J Cardiovasc Electrophysiol. 2003;14:168-173.
92 Fauchier L, Babuty D, Cosnay P, et al. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1999;33:1203-1207.
93 Rashba EJ, Estes NA, Wang P, et al. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: Results from the DEFINITE trial. Heart Rhythm. 2006;3:281-286.
94 Stein KM. Noninvasive risk stratification for sudden death: Signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog Cardiovasc Dis. 2008;51:106-117.
95 Kuchar DL, Thorburn CW, Sammel NL. Signal-averaged electrocardiogram for evaluation of recurrent syncope. Am J Cardiol. 1986;58:949-953.
96 Winters SL, Stewart D, Gomes JA. Signal averaging of the surface QRS complex predicts inducibility of ventricular tachycardia in patients with syncope of unknown origin: A prospective study. J Am Coll Cardiol. 1987;10:775-781.
97 McClements BM, Adgey AJ. Value of signal averaged electrocardiography, radionuclide ventriculography, Holter monitoring and clinical variables for prediction of arrhythmic events in survivors of acute myocardial infarction in the thrombolytic era. J Am Coll Cardiol. 1993;21:1419-1427.
98 Steinberg JS, Regan A, Sciacca RR, et al. Predicting arrhythmic events after acute myocardial infarction using the signal-averaged electrocardiogram. Am J Cardiol. 1992;69:13-21.
99 Bauer A, Guzik P, Barthel P, et al. Reduced prognostic power of ventricular late potentials in post-infarction patients of the reperfusion era. Eur Heart J. 2005;26:755-761.
100 Wichter T, Martinez-Rubio A. Value of the signal-averaged ECG in patients with arrhythmogenic right ventricular disease. Circulation. 1992;86:319.
101 Armoundas AA, Rosenbaum DS, Ruskin JN, et al. Prognostic significance of electrical alternans versus signal averaged electrocardiography in predicting the outcome of electrophysiological testing and arrhythmia-free survival. Heart. 1998;80:251-256.
102 Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: A solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885-1889.
103 Amit G, Rosenbaum DS, Super DM, Costantini O. Microvolt T-wave alternans and electrophysiologic testing predict distinct arrhythmia substrates: Implications for identifying patients at risk for sudden cardiac death. Heart Rhythm. 2010;7:763-768.
104 Chow T, Kereiakes DJ, Onufer J, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial, for the MASTER Trial Investigators. J Am Coll Cardiol. 2008;52:1607-1615.
105 Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: Strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention, for the ABCD Trial Investigators. J Am Coll Cardiol. 2009;53:471-479.
106 Kowey PR, Taylor JE, Marinchak RA, et al. Does programmed stimulation really help in the evaluation of patients with nonsustained ventricular tachycardia? Results of a meta-analysis. Am Heart J. 1992;123:481-485.
107 Wilber DJ, Olshansky B, Moran JF, Scanlon PJ. Electrophysiological testing and nonsustained ventricular tachycardia. Use and limitations in patients with coronary artery disease and impaired ventricular function. Circulation. 1990;82:350-358.
108 Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937-1945.
109 Buxton AE, Lee KL, Hafley GE, et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease: An analysis of patients enrolled in the multicenter unsustained tachycardia trial, for the MUSTT Investigators. Circulation. 2002;106:2466-2472.
110 Piccini JP, Hafley GE, Lee KL, et al. Mode of induction of ventricular tachycardia and prognosis in patients with coronary disease: The Multicenter UnSustained Tachycardia Trial (MUSTT), for the MUSTT Investigators. J Cardiovasc Electrophysiol. 2009;20:850-855.
111 Monahan KM, Hadjis T, Hallett N, et al. Relation of induced to spontaneous ventricular tachycardia from analysis of stored far-field implantable defibrillator electrograms. Am J Cardiol. 1999;83:349-353.
112 Wellens HJJ, Dovendans P, Smeets J, et al. Arrhythmia risk: Electrophysiological studies and monophasic action potentials. PACE. 1999;20(II):2560-2565.
113 Rodriguez LM, Sternick EB, Smeets JLRM, et al. Induction of ventricular fibrillation predicts sudden death in patients treated with amiodarone because of ventricular tachyarrhythmias after myocardial infarction. Heart. 1999;75:23-28.
114 Brilakis ES, Shen WK, Hammill SC, et al. Role of programmed ventricular stimulation and implantable cardioverter defibrillators in patients with idiopathic dilated cardiomyopathy and syncope. Pacing Clin Electrophysiol. 2001;24:1623-1630.
115 Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT. Circulation. 2002;105:1453-1458.
116 Grimm W, Hoffmann J, Menz V, et al. Programmed ventricular stimulation for arrhythmia risk prediction in patients with idiopathic dilated cardiomyopathy and nonsustained ventricular tachycardia. J Am Coll Cardiol. 1998;32:739-745.
117 Rolf S, Haverkamp W, Borggrefe M, et al. Induction of ventricular fibrillation rather than ventricular tachycardia predicts tachyarrhythmia recurrences in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillator for secondary prophylaxis. Europace. 2009;11:289-296.
118 Daubert JP, Winters SL, Subacius H, et al. Ventricular arrhythmia inducibility predicts subsequent ICD activation in nonischemic cardiomyopathy patients: A DEFINITE substudy, for the Defibrillators In Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Pacing Clin Electrophysiol. 2009;32:755-761.
119 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: A multicenter study. Circulation. 2004;109:1994-2000.
120 Di Bella G, Passino C, Aquaro GD, et al. Different substrates of non-sustained ventricular tachycardia in post-infarction patients with and without left ventricular dilatation. J Card Fail. 2010;16:61-68.
121 Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369-1374.
122 Di Salvo G, Pacileo G, Limongelli G, et al. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr. 2010;23:581-590.
123 Katritsis DG, Camm AJ. Nonsustained ventricular tachycardia: Where do we stand? Eur Heart J. 2004;25:1093-1099.
124 Elizari M, Martinez JM, Belzitic C, et al. Mortality following early administration of amiodarone in acute myocardial infarction: Results of the GEMICA trial. Circulation. 1996;94(Suppl 1):90.
125 Cairns JA, Connolly SJ, Gent M, et al. Post-myocardial infarction mortality in patients with ventricular premature depolarizations. Circulation. 1991;84:550-557.
126 Julian DG, Camm AJ, Frangin J, et al. Randomised trial of effect of amiodarone on mortality in patients with left ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet. 1997;349:662-663.
127 Viskin S, Belhassen B. Polymorphic ventricular tachyarrhythmias in the absence of organic heart disease: Classification, differential diagnosis, and implications for therapy. Prog Cardiovasc Dis. 1998;41:17-34.
128 Shimizu W. Arrhythmias originating from the right ventricular outflow tract: How to distinguish “malignant” from “benign”? Heart Rhythm. 2009;6:1507-1511.
129 Carlson MD, White RD, Trohman RG, et al. Right ventricular outflow tract ventricular tachycardia: Detection of previously unrecognized anatomic abnormalities using cine magnetic resonance imaging. J Am Coll Cardiol. 1994;24:720-727.
130 Corrado D, Basso C, Pilichou K, Thiene G. Molecular biology and the clinical management of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart. 2010;97(7):530-539.
131 Udall JA, Ellestad MH. Predictive implications of ventricular premature contractions associated with treadmill stress testing. Circulation. 1977;56(6):985-989.
132 Biffi A, Maron BJ, Culasso F, et al. Patterns of ventricular tachyarrhythmias associated with training, deconditioning and retraining in elite athletes without cardiovascular abnormalities. Am J Cardiolx. 2011;107:697-703.
133 Cheema AN, Sheu K, Parker M, et al. Nonsustained ventricular tachycardia in the setting of acute myocardial infarction: Tachycardia characteristics and their prognostic implications. Circulation. 1998;98:2030-2036.
134 The Multicenter Post-infarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309:331-336.
135 Bigger JT, Fleiss JL, Rolnitzky LM. Prevalence, characteristics and significance of ventricular tachycardia detected by 24-hour continuous electrocardiographic recordings in the late hospital phase of acute myocardial infarction. Am J Cardiol. 1986;58:1151-1160.
136 Mukharji J, Rude RE, Poole WK, et al. Risk factors for sudden death after acute myocardial infarction: Two-year follow-up. Am J Cardiol. 1984;54:31-36.
137 Pires LA, Lehmann MH, Buxton AE, et alfor the Multicenter Unsustained Tachycardia Trial Investigators. Differences in inducibility and prognosis of in-hospital versus out-of-hospital identified nonsustained ventricular tachycardia in patients with coronary artery disease: Clinical and trial design implications. J Am Coll Cardiol. 2001;38:1156-1162.
138 Pires LA, Hafley GE, Lee KL, et al. Prognostic significance of nonsustained ventricular tachycardia after coronary bypass surgery in patients with left ventricular dysfunction, for the Multicenter Unsustained Tachycardia Trial Investigators. J Cardiovasc Electrophysiol. 2002;13:757-763.
139 Mittal S, Lomnitz DJ, Mirchandani S, et al. Prognostic significance of nonsustained ventricular tachycardia after revascularization. J Cardiovasc Electrophysiol. 2002;13:342-346.
140 Russo AM, Hafley GE, Lee KL, et al. for the Multicenter UnSustained Tachycardia Trial Investigators: Racial differences in outcome in the Multicenter UnSustained Tachycardia Trial (MUSTT): A comparison of whites versus blacks. Circulation. 2003;108:67-72.
141 O’Neill JO, Young JB, Pothier CE, et al. Severe frequent ventricular ectopy after exercise as a predictor of death in patients with heart failure. J Am Coll Cardiol. 2004;44:820-826.
142 Iacoviello M, Forleo C, Guida P, et al. Ventricular repolarization dynamicity provides independent prognostic information toward major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2007;50:225-231.
143 Zecchin M, Di Lenarda A, Gregori D, et al. Are nonsustained ventricular tachycardias predictive of major arrhythmias in patients with dilated cardiomyopathy on optimal medical treatment? Pacing Clin Electrophysiol. 2008;31:290-299.
144 Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2008;121:445-456.
145 Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881-1891.
146 Adabag AS, Casey SA, Kuskowski MA, et al. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;45:697-704.
147 Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289-1300.
148 Munoz FD, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011 Feb 18. doi: 10.1111/j.1540-8167.2011.02021.x. [Epub ahead of print]
149 Winkle RA, Lopes MG, Fitzgerald JW, et al. Arrhythmias in patients with mitral valve prolapse. Circulation. 1975;52:73-81.
150 Duren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: A prospective study. J Am Coll Cardiol. 1988;11:42-47.
151 Kilgfield P, Levy D, Devreux RB, Savage DD. Arrhythmias and sudden death in mitral valve prolapse. Am Heart J. 1987;113:1298-1307.
152 Sarabanda AV, Marin-Neto JA. Predictors of mortality in patients with Chagas’ cardiomyopathy and ventricular tachycardia not treated with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 2011;34:54-62.
153 Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115:2750-2760.
154 Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149-1154.
155 Zareba W, Moss AJ, le Cessie S, et al. Risk of cardiac events in long QT syndrome family members. J Am Coll Cardiol. 1995;26:1685-1691.
156 Moss AJ, Zareba W, Hall WJ, et al. Effectiveness and limitations of beta-blocker therapy in congenital LQTS. Circulation. 2000;101:616-623.
157 Zareba W, Moss AJ, Daubert JP, et al. Implantable cardioverter-defibrillator in high-risk LQTS patients. J Cardiovasc Electrophysiol. 2003;14:337-341.
158 Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670.
159 Arbelo E, Josephson ME. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2010;21:473-486.
160 Maron BJ, Shen WK, Link MS, et al. Efficacy of implantable-cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365-373.
161 Kober L, Torp-Pedersen C, McMurray JJV, et al. Increased mortality after dronedarone therapy for severe heart failure, for the Dronedarone Study Group. N Engl J Med. 2008;358:2678-2687.
162 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2157.
163 Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT, for the AMIOVIRT Investigators. J Am Coll Cardiol. 2003;41:1707-1712.
164 Bänsch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation. 2002;105(12):1453-1458.
165 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
166 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure, for the Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. N Engl J Med. 2005;352:1539-1549.
167 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events, for the MADIT-CRT Trial Investigators. N Engl J Med. 2009;361:1329-1338.
168 McLeod CJ, Shen WK, Rea RF, et al. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm. 2011;8:377-382.
169 Bigger JTJr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569-1575.
170 Camm AJ, Yap YG. Clinical trials of antiarrhythmic drugs in postmyocardial infarction and congestive heart failure patients. J Cardiovasc Pharmacol Ther. 2001;6:99-106.
171 Waldo AL, Camm AJ, deRuyter H, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7-12.
172 Viscoli CM, Horwitz RI, Singer BH. Beta-blockers after myocardial infarction: Influence of first-year clinical course on long-term effectiveness. Ann Intern Med. 1993;118:99-105.
173 Prystowsky EN, Nisam S. Prophylactic implantable cardioverter defibrillator trials. Am J Cardiol. 2000;86:1214-1215.
174 Goldenberg I, Gillespie J, Moss AJ, et al. for the Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: An extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265-1271.
175 Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149-1154.
176 Hohnloser SH, Kuck KH, Dorian P, et al. for the DINAMIT Investigators: Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-2488.
177 Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction, for the IRIS Investigators. N Engl J Med. 2009;361:1427-1436.
178 Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive summary, for the American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. Heart Rhythm. 2008;5:934-955.