Nonsteroidal antiasthma agents
After reading this chapter, the reader will be able to:
1. Discuss the indications for nonsteroidal antiasthma agents.
2. List available nonsteroidal antiasthma agents used in respiratory therapy.
3. Differentiate between the specific nonsteroidal antiasthma agents.
4. Describe routes of administration available for various nonsteroidal antiasthma agents.
5. Describe the mode of action for various nonsteroidal antiasthma agents.
6. Discuss the use of nonsteroidal antiasthma agents in the treatment of asthma.
In Chapter 11, to present the antiinflammatory actions of glucocorticoids, the concept of airway inflammation was introduced, and some of the numerous cells and chemicals involved in an inflammatory response were described. Chapter 12 presents drug groups that also have an antiinflammatory effect through mechanisms different from those of the corticosteroids. Three subgroups of agents are included in the nonsteroidal antiasthma group: cromolyn-like drugs (mast cell stabilizers), antileukotrienes, and monoclonal antibodies. A brief summary of the immune mechanisms involved in allergic responses is given as an introduction to the specific mechanisms of action for the drug groups discussed in this chapter.
Clinical indications for nonsteroidal antiasthma agents
The general indication for clinical use of nonsteroidal antiasthma agents described in this chapter is prophylactic management (control) of mild persistent asthma (asthma requiring step 2 care, according to the classification presented in the 2007 National Asthma Education and Prevention Program [NAEPP] guidelines1).
The following are qualifications to the general indication for use of these agents:
• Cromolyn and antileukotrienes are typically recommended as alternatives to low-dose inhaled corticosteroids in asthma requiring step 2 care.1
• Cromolyn is often used with infants and young children as alternatives to inhaled corticosteroids in asthma requiring step 2 care because of the safety profiles of inhaled corticosteroids.1
• Antileukotrienes can be useful in combination with inhaled corticosteroids to reduce the dose of steroid.
All of the nonsteroidal antiasthma drugs described in this chapter are controllers, not relievers, and are used in asthma requiring antiinflammatory drug therapy. Box 12-1 summarizes reliever and controller agents used in drug therapy for asthma, listing cromolyn-like agents, antileukotrienes, and monoclonal antibodies as controllers. Use of rescue β2-agonist agents more than twice a week (i.e., asthma requiring step 2 care) is an indicator of the need to initiate controller drug therapy.
Identification of nonsteroidal antiasthma agents
Individual agents in the cromolyn-like agent group, antileukotriene group, and monoclonal antibody group are presented in Table 12-1 with generic and brand names, formulations and strengths, and usual recommended dosages.
TABLE 12-1
Nonsteroidal Antiasthma Medications: Generic and Brand Names, Formulations, and Usual Recommended Dosages*
| GENERIC DRUG | BRAND NAME | FORMULATION AND DOSAGE |
| Cromolyn-Like Agents (Mast Cell Stabilizers) | ||
| Cromolyn sodium | NasalCrom, Gastrocrom | SVN: 20 mg/ampoule or 20 mg/2 mL |
| Adults and children ≥2 yr: 20 mg inhaled 4 times daily | ||
| Spray: 40 mg/mL (5.2 mg per actuation) | ||
| Adults and children ≥2 yr: 1 spray each nostril, 3-6 times daily every 4-6 hr | ||
| Oral concentrate: 100 mg/5 mL | ||
| Adults and children ≥13 yr: 2 ampoules 4 times daily, 30 min before meals and at bedtime | ||
| Children 2-12 yr: 1 ampoule 4 times daily, 30 min before meals and at bedtime | ||
| Antileukotrienes | ||
| Zafirlukast | Accolate | Tablets: 10 mg and 20 mg |
| Adults and children ≥12 yr: 20 mg twice daily, without food | ||
| Children 5-11 yr: 10 mg twice daily | ||
| Montelukast | Singulair | Tablets: 10 mg and 4-mg and 5-mg cherry-flavored chewable; 4-mg packet of granules |
| Adults and children ≥15 yr: 1 10-mg tablet daily | ||
| Children 6-14 yr: 1 5-mg chewable tablet daily | ||
| Children 2-5 yr: 1 4-mg chewable tablet or 1 4-mg packet of granules daily | ||
| Children 6-23 mo: 1 4-mg packet of granules daily | ||
| Zileuton | Zyflo; Zyflo CR | Tablets: 600 mg |
| Adults and children ≥12 yr: 1 600-mg tablet 4 times per day; CR, 2 tablets twice daily, within 1 hr of morning and evening meals | ||
| Monoclonal Antibody | ||
| Omalizumab | Xolair | Adults and children ≥12 yr: Subcutaneous injection every 4 wk; dose dependent on weight and serum IgE level |
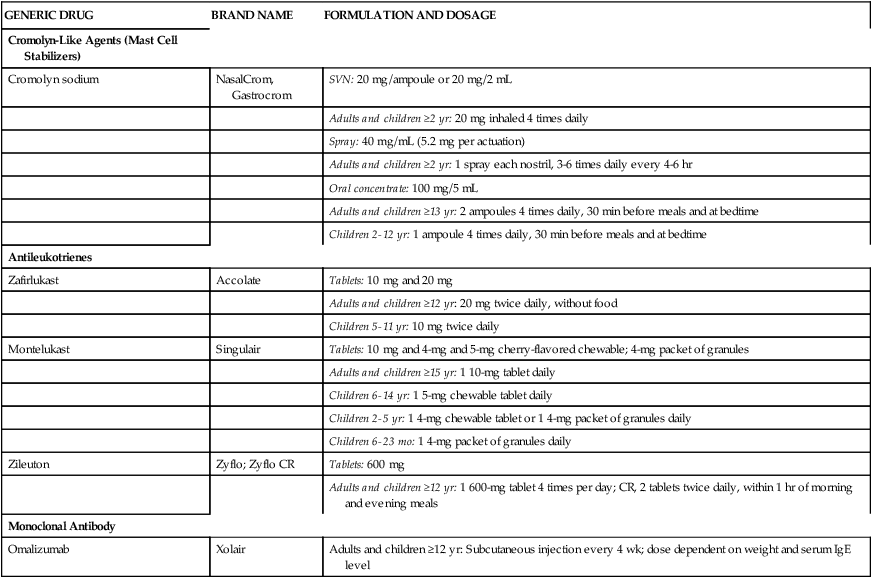
*Detailed prescribing information should be obtained from manufacturer’s package insert.
Mechanisms of inflammation in asthma
Asthma is a chronic inflammatory disorder of the airways.1 Asthma has been divided into extrinsic and intrinsic forms on the basis of the triggers for asthma. Extrinsic asthma is dependent on allergy, or atopy, whereas intrinsic asthma shows no evidence of sensitization to common inhaled allergens.2 The allergic form of asthma, which is immunoglobulin E (IgE)–mediated, is associated with younger subjects, and the intrinsic, or nonallergic, form is associated with later onset in adults in whom childhood asthma may not have been present. Holgate3 described asthma as an “evolving” disease in early childhood, when viruses are an important trigger, whereas in school and teen years, allergens stimulate an immune response. As asthma progresses and in adults, the disease becomes intrinsic and may be driven by T cells (lymphocytes) that release various cytokines, as described in Chapter 11. Asthma is chronic and persistent, with continuous inflammation and episodes of acute obstruction. Drazen and Turino4 described three components of asthma:
1. Acute asthma attack, which resolves spontaneously or with treatment
In both forms of asthma, allergic and nonallergic mediators and enzymes are released to act on target tissues in the airway, and cells involved in inflammation are recruited and activated in the airway. Airway inflammation is manifested in the responses of bronchoconstriction, airway swelling, mucus secretion and obstruction, and subsequent airway wall remodeling that furthers the responsiveness of the airway.5
Immunologic (allergic) response
Most instances of asthma are primarily an allergic response, which involves mast cells and IgE.1 The immunologic response is outlined in Box 12-2. An understanding of the immune response is fundamental to discussing asthma and the mediator antagonists presented in this chapter because allergy is essentially a mistaken immune response.
Generation of an immune response and specifically an allergic asthmatic response is considered to be initiated by the interaction of T lymphocytes with an antigen presented by other cells, such as macrophages or B lymphocytes.4 Activation of T lymphocytes results in production of IgE by B lymphocytes. Antigen-specific IgE binds to effector cells such as mast cells and is termed a cytophilic antibody because of this. When activated by subsequent exposure to an antigen or allergen, mast cells release physiologically active mediators of inflammation, such as prostaglandins, leukotrienes, proteases, histamine, platelet-activating factor (PAF), and certain cytokines.4 The cytokines released, which include tumor necrosis factor-α (TNF-α) and interleukin-4 (IL-4), can upregulate endothelial adhesion molecules.3
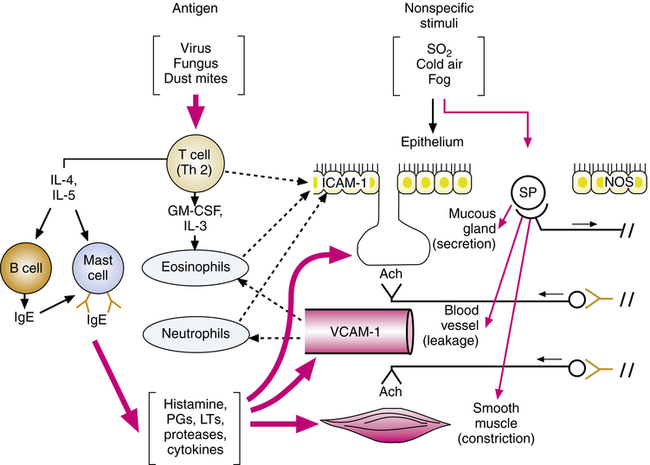
This cascade of mediators causes an inflammatory response manifested by vascular leakage, bronchoconstriction, mucus secretion, and mucosal swelling, all of which obstruct airflow in the bronchioles. T lymphocytes also release cytokines (e.g., interleukins), causing accumulation and activation of eosinophils, which also release chemicals to damage the airway. The process of initiating the inflammatory response and continuing it through amplification, as discussed next, is illustrated in Figure 12-1.
After being initiated by exposure to antigen, the inflammatory response in the airway is amplified by chemoattraction of more lymphocytes, eosinophils, basophils, and neutrophils and by an increase in mast cells. Adhesion molecules increase after stimulation of lymphocytes and mast cells by antigen or allergen. These molecules, found in epithelial cells (intercellular adhesion molecule-1 [ICAM-1]) and vascular endothelial cells (vascular cell adhesion molecule-1 [VCAM-1]) in the airway, are responsible for eosinophil, neutrophil, and lymphocyte recruitment from the microvascular circulation into the airways. The adhesion molecules enable leukocytes to marginate, cross the blood vessel wall, and migrate to the airway mucosa, continuing and further amplifying the inflammation begun.6 The increase and activation of eosinophils are associated with increased inflammation and severity in asthma.3
Nonspecific stimuli, such as fog, sulfur dioxide, dust, and cold air, can stimulate sensory receptors and cause reflex bronchoconstriction (see Chapter 7).5 Asthmatic patients are more sensitive to such stimuli, which reflects altered neural control or chronic inflammation sensitizing the airway, or both. Nerve fibers of the noncholinergic, nonadrenergic excitatory system, containing potent peptide mediators, contribute to local effects on smooth muscle and mucous glands and reflexively stimulate cholinergic activity. Some of these peptides include substance P (SP), neurokinin A (NKA), and neurokinin B (NKB); they are released from sensory C-fiber nerve endings. These neuropeptides can also contribute to inflammation and the features of asthma previously described, such as mucus hypersecretion, smooth muscle contraction, plasma leakage, inflammatory cell activation, and adhesion. Neutral endopeptidase (NEP) is an enzyme that inactivates neuropeptides to limit their activity; NEP is found on the surface of cells that contain receptors for neuropeptides (smooth muscle, airway epithelium, and vascular endothelium). An increased release of excitatory neuropeptides may be involved in asthma.5
Nitric oxide is formed in airway tissue through the action of the enzyme nitric oxide synthase (NOS). There is evidence that in asthma NOS is upregulated in airway epithelium.5 Nitric oxide, a potent vasodilator and bronchodilator, may be the neurotransmitter for the nonadrenergic, noncholinergic inhibitory nervous system (see Chapter 5). Nitric oxide, which can damage cells, possibly is induced by proinflammatory cytokines in asthma and contributes to the observed epithelial damage seen in Figure 12-1.6,7
Cromolyn (mast cell–stabilizing) agents
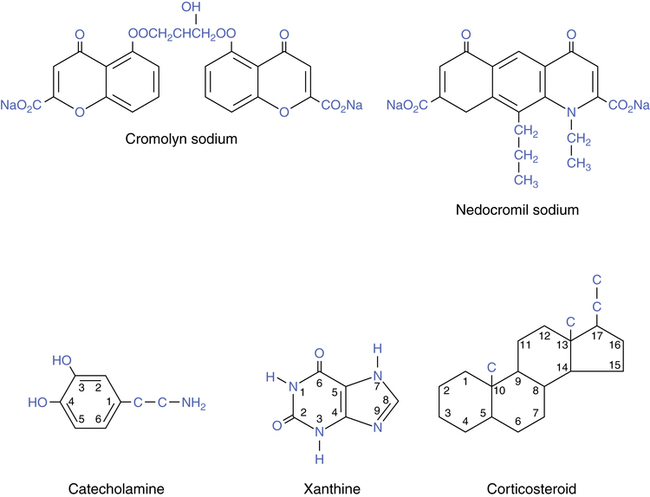
Cromolyn sodium, also termed disodium cromoglycate, is used as an inhaled prophylactic aerosol drug to prevent the inflammatory response in asthma. These drugs differ in structure and activity from the drug groups considered in previous chapters. Their chemical structures are illustrated in Figure 12-2. The basic catecholamine xanthine and steroid structures are given for comparison. Cromolyn is not related to the β agonists, xanthines, theophylline, or antiinflammatory glucocorticoids. Cromolyn has no intrinsic bronchodilating capability.
Cromolyn sodium (disodium cromoglycate)
Cromolyn sodium is used as a prophylactic agent in the treatment of asthma. Although it may not be used as often in clinical practice today as it was previously, this agent is an alternative in mild persistent asthma.1 The antiinflammatory, mast cell–stabilizing effect of cromolyn has led to uses other than asthma prophylaxis, including the following:
• Allergic rhinitis (nasal solution)
• Mastocytosis—to improve diarrhea, abdominal pain, headaches, nausea, and itching (oral)
Mode of action
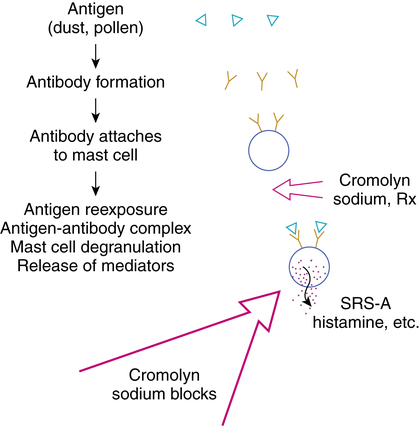
Cromolyn sodium is considered an antiasthmatic, an antiallergic, and a mast cell stabilizer. Pretreatment with inhaled cromolyn sodium results in inhibition of mast cell degranulation, blocking release of the chemical mediators of inflammation (Figure 12-3). By its action, cromolyn is effective in blocking the late phase reaction in asthma. (The late phase reaction in asthma is discussed in the review of corticosteroids in Chapter 11.)
• The mode of action of cromolyn sodium is prophylactic; pretreatment is necessary for inhibition of mast cell degranulation.
• Cromolyn sodium may inhibit mediator release by preventing calcium influx necessary for microfilament contraction and extrusion of mast cell granules.8,9
• Cromolyn sodium does not have an antagonist effect on any of the chemical mediators themselves.
• Cromolyn sodium does not operate through the cyclic adenosine 3’,5’-monophosphate (cAMP) system and does not affect α or β receptors.
• Antibody formation, attachment of antibody (IgE) to the mast cell, and antigen-antibody union are not prevented by cromolyn; cromolyn does prevent release of mediators.
• Cromolyn sodium can prevent or attenuate the late phase response in an asthmatic episode, which can otherwise cause more severe airway obstruction 6 to 8 hours after initial bronchoconstriction.10,11
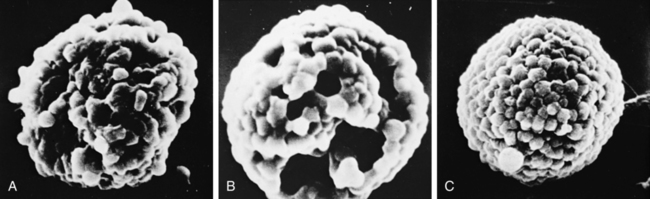
The protective effect of cromolyn in inhibiting mast cell degranulation has been captured by scanning electron microscopy and is shown in the sequence in Figure 12-4. Initial understanding of the activity of cromolyn focused on allergy-triggered mast cell release of mediators, and the drug came to be considered useful primarily in allergic asthma. There is evidence that the activity of cromolyn is not limited to preventing allergen-stimulated asthma. Cromolyn inhibits mast cell mediator release caused by nonallergic stimuli and may reduce reflex-induced asthma. The latter requires about twice the usual dose of cromolyn. Understanding of the broader protection given by cromolyn has supported its successful use in allergic and nonallergic asthma and specifically in exercise-induced asthma.12,13
Pharmacokinetics
Cromolyn sodium is a safe drug. It has an effectiveness similar to theophylline in controlling asthma, with a better therapeutic margin than theophylline.10 In studies comparing the two agents, subjects using theophylline reported more side effects, including nervousness, nausea, school behavioral problems, and more office visits. The overall incidence of adverse effects with cromolyn has been reported as 2%.14 Nasal congestion may be seen after beginning cromolyn sodium use. Dermatitis, myositis (muscle tissue inflammation), and gastroenteritis occurred in a very few patients.
Clinical efficacy of cromolyn sodium
The original trials of cromolyn sodium as a prophylactic agent in asthma were performed with the 20-mg Spinhaler formulation. Studies were conducted in adults and children and included intrinsic and extrinsic forms of asthma. The conclusion of these early studies was that inhaled cromolyn sodium is effective as a treatment for approximately 70% of patients.15 Edwards16 reviewed data supporting the use of cromolyn sodium in chronic asthma. In a long-term study by Konig and Shaffer,17 175 children were monitored for an average of 8.4 years (range 2.2 to 16.8 years). Their results showed that pulmonary function improved to normal values in both the group treated with corticosteroids and the group given cromolyn sodium. Cockcroft and Murdock18 found evidence of protection against antigen challenge, with attenuation of the early phase and the late phase asthmatic response with cromolyn sodium.
Use for cough associated with angiotensin-converting enzyme inhibitor.
Hargreaves and Benson19 reported that cromolyn sodium, administered as two actuations four times daily of the 5-mg MDI formulation, provided protection against the cough often seen as a side effect with use of angiotensin-converting enzyme (ACE) inhibitors.19 Cromolyn sodium significantly improved cough scores (frequency and severity) in 9 of the 10 patients in the study after 2 weeks. Cough was not completely suppressed in any of the 10 patients.
Anti–sickle cell effects.
Both the intranasal solution and the 20-mg inhaled powder capsule of cromolyn given as a single dose were observed to cause a striking decrease in sickle cell percentage in nine African children with severe sickle cell disease. Improvement was seen 24 hours after administration of the single dose. The reduction in sickling is hypothesized to be due to the blocking of calcium-activated potassium channels, which play a major part in water loss and erythrocyte dehydration.20
Clinical application of cromolyn sodium
1. The drug is prophylactic only and should not be used during acute bronchospasm. This is based on its mode of action because the drug must already be present to prevent mast cell degranulation. It has no bronchodilating action and may cause further bronchial irritation as an aerosol.
2. Abrupt withdrawal of oral corticosteroids and substitution of cromolyn sodium in asthmatic patients can result in inadequate adrenal function. Cromolyn has no effect on the adrenal system, and tapered withdrawal of corticosteroids is necessary while beginning cromolyn use with patients.
3. It may take 2 to 4 weeks for improvement in the patient’s symptoms, enabling a decrease in concomitant therapy such as bronchodilator or steroid use.
Guidelines for the management of asthma indicate that cromolyn sodium is used in subjects requiring regular use of β agonists for control of symptoms. It is considered an alternative to the use of inhaled corticosteroids, especially in children.1
Dosage regulation.
The protective effect of cromolyn in allergic, nonallergic, or reflex-induced asthma is dose-dependent. The usual dosage of 20 mg four times daily (80 mg/day) with the nebulized solution in some cases can be reduced to a maintenance dosage of 40 to 60 mg/day after the patient is stabilized for 1 or 2 months. Likewise, if stimuli for asthma increase in severity (e.g., heavy exercise in cold weather [skiing] as opposed to walking in warm weather), higher dosages or addition of a β agonist may be required. For seasonal allergy, cromolyn should be started at least 1 week before allergen exposure. The drug is protective if given 30 minutes before a specific allergen exposure (e.g., cat fur), and a single dose 15 minutes before exercise on an occasional, rather than a continuous, basis is effective. As stated previously, the degree of exercise and the conditions must be considered in estimating the protection required. Long-term continuous maintenance with cromolyn may be needed for patients with reflex-induced asthma or for patients with late-phase reactions or severe bronchial reactivity and lability.12,13
Antileukotriene agents
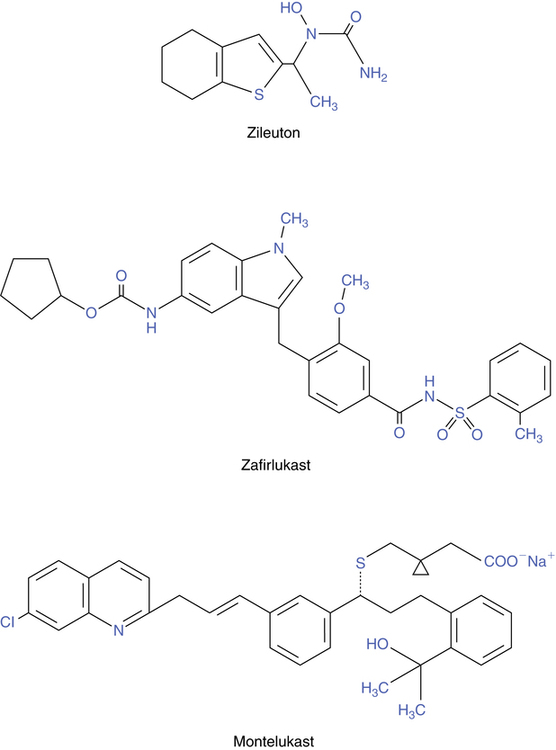
The name leukotriene is based on the fact that these molecules were originally isolated from leukocytes, and the carbon backbone has three double bonds in series, termed a triene. Chemical structures of the three antileukotriene drugs currently available in the United States are shown in Figure 12-5. Three of these agents (zafirlukast, montelukast, and pranlukast) attach to and block the receptor for leukotrienes; however, only zafirlukast and montelukast are approved for use in the United States. A fourth agent (zileuton) inhibits the synthesis of leukotrienes.
Leukotrienes and inflammation
The leukotrienes are members of a group of biologically active fatty acids, including prostaglandins, thromboxanes, and lipoxins, that are known as eicosanoids. These molecules are lipid mediators of inflammation that are synthesized from the fatty acid precursor arachidonic acid (5,8,11, 14-eicosatetraenoic acid). Arachidonic acid is found in cell nuclear membrane phospholipids. The leukotrienes mediate directly or indirectly at least some of the inflammatory process seen in asthma. They are potent bronchoconstrictors and stimulate other cells to cause airway edema, mucus secretion, ciliary beat inhibition, and recruitment of other inflammatory cells into the airway.21
Cell sources of leukotrienes
The leukotrienes and other lipid mediators are not preformed and stored in cells but rather are synthesized after a mechanical, chemical, or physical stimulus that activates phospholipase A2, an enzyme. These stimuli include antigen challenge of sensitized tissues and exposure to PAF or other cytokines. Certain cells, including eosinophils, mast cells, monocytes, macrophages, basophils, neutrophils, and B lymphocytes,22 have the necessary enzymes to synthesize leukotrienes and other mediators. Eosinophils, mast cells, and macrophages are present and recruited to the lung in asthma.23
Biochemical pathways
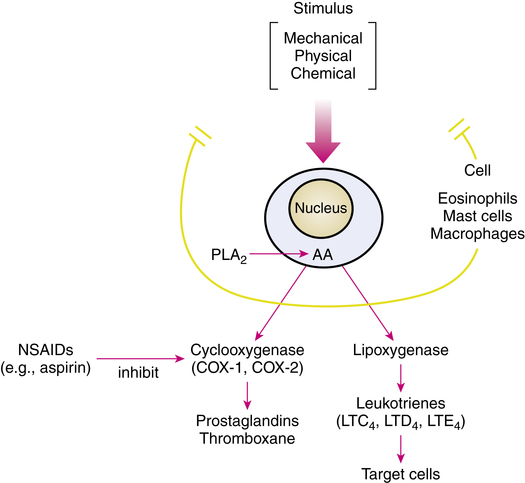
A simplified diagrammatic view of the arachidonic acid cascade, which results in the various lipid mediators, is given in Figure 12-6. Essentially, free arachidonic acid is converted to various lipid mediators by two routes: the cyclooxygenase (COX) and 5-lipoxygenase (5-LO) pathways. The COX pathway results in the prostaglandins and thromboxane, and the 5-LO pathway results in the leukotrienes. Aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen, inhibit the COX enzyme, blocking prostaglandin and thromboxane production. There are two forms of the COX enzyme: COX-1 and COX-2. Many NSAIDs are mainly COX-1-selective, such as aspirin, ketoprofen, and indomethacin; some are slightly COX-1-selective, such as ibuprofen and naproxen. Other agents, such as celecoxib and rofecoxib, used to treat arthritis, have primarily selective inhibition of COX-2. The 5-LO pathway results in the synthesis of leukotrienes; this pathway is the target for drugs in the antileukotriene group.
Leukotriene production
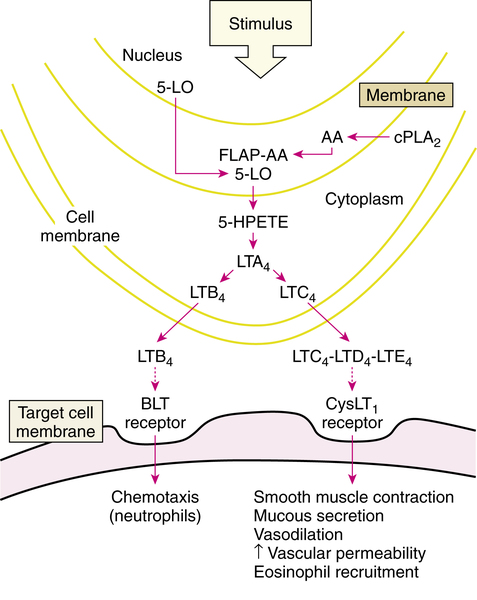
The lipoxygenase pathway resulting in leukotriene production is illustrated in Figure 12-7. After stimulation of an appropriate cell, the enzyme phospholipase A2 (PLA2), which is located in the cell cytoplasm, moves to the cell nuclear membrane. In the nuclear membrane, PLA2 hydrolyzes phospholipids to liberate free arachidonic acid (AA). AA binds to 5-LO-activating protein (FLAP) (AA-FLAP). Another enzyme, 5-LO, moves from both the nucleus and the cell cytoplasm to the nuclear membrane and interacts with the AA-FLAP complex to oxygenate the arachidonic acid. This results in 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which is converted to the unstable intermediate leukotriene A4 (LTA4). LTA4 is the source of all the other leukotrienes. LTA4 is converted either into leukotriene B4 (LTB4) or the cysteinyl leukotriene C4 (LTC4). LTB4 and LTC4 are exported from the cell to the extracellular space; LTC4 is converted to leukotrienes D4 and E4 (LTD4 and LTE4). These three leukotrienes are termed cysteinyl leukotrienes (CysLTs) because they each have the amino acid cysteine in their chemical structure. The three CysLTs—LTC4, LTD4, and LTE4—have been identified as the components of the previously termed SRS-A.
Cysteinyl leukotriene receptors and effects of leukotrienes
CysLTs attach to two subtypes of receptors: CysLT1 and CysLT2 receptors. The proasthmatic actions of CysLTs are mediated by the CysLT1 receptor, which is located on smooth muscle cells in the airway and other cell types. The human CysLT1 receptor has been cloned and characterized.24 Stimulation of the CysLT1 receptor causes bronchoconstriction, and CysLTs are more potent airway constrictors than histamine.25 In addition to direct bronchoconstriction, there is an increase in bronchial hyperresponsiveness to other irritants such as histamine. Other effects include mucus secretion in the airway, increased vascular permeability causing airway wall edema, and plasma exudation into the airway lumen. The resulting protein and cellular debris in the airway, together with the mucus secretion, increases secretion viscosity and may lead to airway occlusion such as that seen in asthma. CysLTs may also have an eosinophilic chemoattractant effect. Drugs that block the binding of leukotrienes to CysLT1 receptors are named with the generic suffix -lukast (e.g., zafirlukast, montelukast, and pranlukast). The CysLT2 receptor subtype mediates constriction of pulmonary vascular smooth muscle.25
CysLTs are produced largely by eosinophils, mast cells, and macrophages, all of which are cell types seen in the airways of asthmatics. Elevated levels of CysLTs may be markers of asthma. Leukocytes of asthmatics release more CysLTs than the leukocytes of nonasthmatics. Plasma levels of LTE4 correlate with asthma severity and are elevated in the urine of patients during an asthma attack, during exercise-induced asthma, and in the presence of nocturnal asthma symptoms.23 Urinary LTE4 is also elevated after challenge with allergen in atopic asthma or with aspirin in aspirin-sensitive asthmatics.21
Zileuton (zyflo)
Zileuton, available as Zyflo or Zyflo CR, is an orally active inhibitor of 5-LO. Its structure is shown in Figure 12-6. This drug is indicated for prophylaxis and long-term treatment of asthma and is approved for use in adults and children 12 years of age or older. It is considered a controller agent rather than a reliever and has no indication for use in an acute asthma episode.
Mode of action
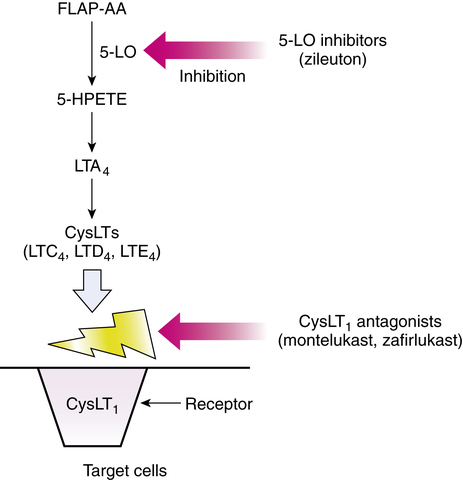
Taken orally, zileuton inhibits the 5-LO enzyme, which would otherwise catalyze the formation of leukotrienes from arachidonic acid. Specifically, 5-LO in the presence of FLAP catalyzes the conversion of arachidonic acid to the intermediate 5-HPETE, which is converted to LTA4 and ultimately the other leukotrienes. By interrupting the synthesis of these biologically active leukotrienes, their contribution to the inflammatory responses in asthma is effectively blocked. Both the (R)-enantiomers and the (S)-enantiomers are active as 5-LO inhibitors. The mode of action of zileuton is illustrated along with that of the other antileukotrienes in Figure 12-8.
Zafirlukast (accolate)
Zafirlukast (Accolate) is a synthetic asthma prophylactic agent (its structure is illustrated in Figure 12-6). It is indicated for prophylaxis and long-term treatment of asthma and has been approved for use in children 5 years of age or older. This drug inhibits asthma reactions induced by exercise, cold air, allergen, and aspirin.
Mode of action
Zafirlukast and montelukast are both leukotriene receptor antagonists and block the inflammatory effects of leukotrienes (Figure 12-9). Specifically, zafirlukast binds to the CysLT1 receptors, with no agonist effect. This activity causes competitive inhibition of LTC4, LTD4, and LTE4 and subsequent blockade of the inflammatory effects described previously (in the section on Leukotrienes and Inflammation).
Hazards and side effects
The most common side effects reported in healthy volunteers and patients were headache, infection, nausea, diarrhea, and generalized and abdominal pain. Infections were predominantly respiratory. Because zafirlukast is metabolized by liver enzymes, hepatic impairment (e.g., in cirrhosis) increases drug plasma levels. Although not noted in 6-month trials of zafirlukast, postmarketing surveillance indicated that doses greater than the 40-mg daily dose can cause elevations in serum aminotransferase concentrations.25 A case of hepatitis and hyperbilirubinemia with no other attributable cause has been reported in a patient receiving 40 mg a day for 100 days, indicating the possibility of liver enzyme dysfunction with the drug (this case was reported in the manufacturer’s drug literature).
Montelukast (singulair)
Montelukast (Singulair) is an orally active leukotriene receptor antagonist (its structure is illustrated in Figure 12-6). It is indicated for prophylaxis and long-term treatment of asthma (a controller) and has no bronchodilating effect for use in acute asthma treatment. Montelukast is also approved for allergic rhinitis. Montelukast is the only one of the three currently available antileukotriene agents that is approved for use in infants 6 month of age. Montelukast has been shown to have clinical efficacy in treating mild to moderate asthma and exercise-induced bronchoconstriction. Compared with placebo, montelukast significantly improved asthma control in children 12 to 23 months old, children 2 to 14 years old, adolescents older than 15 years of age, and adults.26–30 To date, no safety issues have appeared with pediatric use. The manufacturer states that the drug has not been proven safe and effective in infants younger than 6 months of age. However, Knorr and associates31 found that the 4-mg dose of granules was just as safe and effective in infants 3 to 6 months old as in children 6 to 24 months old.
Dosage and administration
Adults and adolescents 15 years and older: One 10-mg tablet daily, taken daily
Children 6 to 14 years: One 5-mg chewable tablet daily, taken daily
Children 2 to 5 years: One 4-mg chewable tablet daily or one 4-mg packet of oral granules daily
Children 12-23 months: One 4-mg packet of oral granules taken every evening
Children 6-23 months: One 4-mg packet of oral granules taken daily for allergic rhinitis
Mode of action
Similar to zafirlukast, montelukast is a competitive antagonist for the cysteinyl leukotrienes LTC4, LTD4, and LTE4. It binds with high affinity and selectivity to the CysLT1 receptor subtype (see Figure 12-9). Blockade of the CysLT1 receptor prevents leukotriene stimulation of the receptor on target cells such as airway smooth muscle and secretory glands. Montelukast has been shown to inhibit both early and late phase bronchoconstriction caused by antigen challenge.
Role of antileukotriene drugs in asthma management
Antileukotriene agents are recommended in the NAEPP guidelines for the treatment of mild to moderate asthma.1 Table 12-2 summarizes comparative features of the three currently available antileukotriene agents. Drazen and colleagues25 published an excellent review of asthma management with these agents.
TABLE 12-2
Summary of Comparative Features of the Three Currently Available Antileukotriene Agents
| ZILEUTON (ZYFLO) | ZAFIRLUKAST (ACCOLATE) | MONTELUKAST (SINGULAIR) | |
| Action | 5-LO inhibitor | CysLT1 receptor block | CysLT1 receptor block |
| Age range | ≥12 yr | ≥5 yr | ≥6 mo |
| Dose | 600-mg tablet qid or bid if extended release | 10-mg or 20-mg tablet bid | Adult: 10-mg tablet q evening |
| Children 6-14 yr: 5-mg tablet q evening | |||
| Children 2-5 yr: 4-mg tablet q evening | |||
| Administration | Can be taken with food | 1 hr before or 2 hr after meal | Taken with or without food |
| Drug interactions | Yes—theophylline, warfarin, propranolol | Yes—warfarin, theophylline, aspirin | No |
| Common side effects | Headache, dyspepsia, unspecified pain, liver enzyme elevations | Headache, infection, nausea, possible liver enzyme changes | Headache, influenza, abdominal pain |
| Contraindications | Active liver disease or elevated liver enzymes; hypersensitivity to components | Hypersensitivity to components | Hypersensitivity to components |
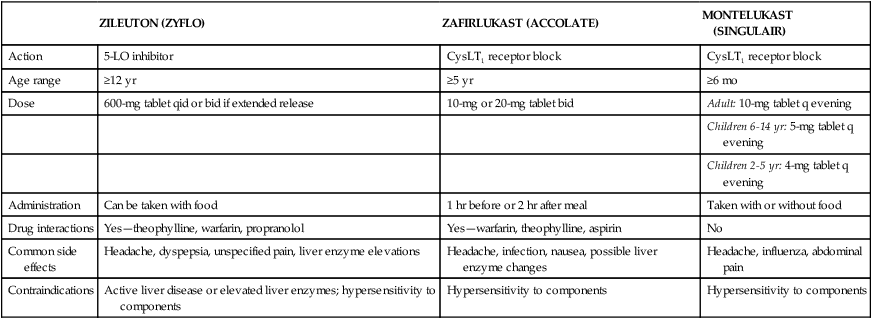
CysLT1, Cysteinyl leukotriene receptor subtype 1; 5-LO, lipoxygenase.
Protection against specific asthma triggers
Antileukotrienes are particularly useful in controlling asthma resulting from certain triggers, including exercise-induced asthma, aspirin-induced asthma, and, to a lesser extent, allergen-induced asthma.25,32
• Exercise-induced asthma: In exercise-induced asthma, cooling and drying of the airway promotes the generation of leukotrienes, resulting in bronchoconstriction. Although protection varies from complete to very little, the antileukotrienes develop no tolerance and may benefit patients who want to exercise or whose jobs require exercise under cold, dry conditions without the use of short-acting rescue β agonists.
• Aspirin-induced asthma: In 3% to 8% of asthma cases, aspirin or NSAIDs can cause bronchoconstriction as a result of an increase in LTC4 synthase activity. On the basis of such pathophysiology, which involves leukotriene production, leukotriene modifiers are the treatment of choice of patients with aspirin-induced asthma.
• Allergen-induced asthma: Antileukotrienes also block the early asthma response to allergen challenge and attenuate airway obstruction in the late-phase response. They are not completely effective in abolishing the late response that is also due to histamine release.
Chronic persistent asthma
The evidence to date supports the use of antileukotriene agents in the management of mild, moderate, or severe chronic asthma.25,33,34 In mild to moderate asthma, antileukotrienes improve lung function; reduce the need for rescue β-agonist use; and decrease asthma symptoms, including nocturnal symptoms. In moderate to severe asthma, the additive effect between antileukotrienes and inhaled corticosteroids is the basis for asthma control with lower steroid doses or without an increase in steroid dosing (inhaled or oral). The advantages and disadvantages of antileukotriene drug therapy in asthma are summarized in Box 12-3.
Antileukotriene drug therapy is effective in approximately 50% of patients (although this proportion is greater for aspirin-sensitive individuals), but there is no method to predict which patients will be responders.35,36 Considerable intersubject variability in response has been seen. In a study of exercise challenge, 20 mg of zafirlukast gave complete protection in three subjects, partial protection in four subjects, and no protection in one subject.44
Antileukotrienes in relation to corticosteroids
1. Choosing between an inhaled steroid and an antileukotriene in mild persistent asthma is based on offsetting advantages: the superior efficacy of inhaled steroids with possibly poor compliance versus the anticipated superior compliance of the orally administered antileukotrienes with their more limited antiinflammatory action.25
2. There is an additive effect between antileukotriene and inhaled corticosteroid therapy in mild to moderate asthma. A study by Laviolette and colleagues38 showed a greater response to inhaled beclomethasone alone compared with oral montelukast alone; however, the combination of the two treatments resulted in the greatest improvement in lung function, as seen in Figure 12-9. In another study, use of montelukast by adult asthmatics taking inhaled steroids long-term resulted in a 47% reduction in steroid dose compared with a 30% reduction in the placebo group.39
Churg-strauss syndrome
Churg-Strauss syndrome has been reported in a few patients treated with zafirlukast40 or montelukast (postmarketing letter).41 Churg-Strauss syndrome is a vasculitis of unknown etiology, usually occurring in adults 20 to 40 years old, marked by peripheral eosinophilia, eosinophilic infiltration of tissues, and necrotizing vasculitis that can result in major organ damage and death if left untreated. The syndrome is rare, with a prevalence of about 1 case per 15,000 to 20,000 patient-years of treatment. Patients to date who developed this syndrome have had difficult-to-control asthma and have been taking oral or high doses of inhaled corticosteroids.
It is unclear whether development of Churg-Strauss syndrome is an effect of antileukotriene treatment, or whether the syndrome is unmasked by a reduction in corticosteroid therapy allowed by the antileukotriene therapy.25 A review by Wechsler and associates41 of eight patients concluded that the occurrence of Churg-Strauss syndrome in asthmatic patients receiving antileukotriene treatment seemed to be due to unmasking of an underlying vasculitic syndrome diagnosed as moderate to severe asthma and treated with corticosteroids. Nevertheless, experience in humans with antileukotriene drugs, specifically CysLT1 receptor antagonists, is still new, and not all of the processes associated with 5-LO products are completely understood. For example, a type 2 leukotriene receptor (CysLT2), which is not blocked by the CysLT1 antagonists such as zafirlukast or montelukast, has been identified on human pulmonary vasculature.23 The effect of introducing a potential imbalance with CysLT1 antileukotriene therapy is not well understood. Although antileukotriene drugs seem to be safe and effective, additional clinical experience is needed.
Respiratory syncytial virus
There seems to be a link between respiratory syncytial virus (RSV) and asthma. It is believed that reactive airway disease is a common accompaniment to RSV.42 One idea to combat this phenomenon is to use a CysLT1 receptor antagonist, specifically montelukast. A published report by Bisgaard42 noted that 22% of patients receiving montelukast had a reduction of symptoms compared with only 4% receiving placebo. It is thought that the airway’s immune response in viral infections is very similar to the response after exposure to allergens. CysLTs are inflammatory mediators that are released in viral and allergic responses. The use of montelukast blocks these mediators in asthma and RSV infection.
Summary of clinical use of antileukotriene therapy
• Antileukotriene agents are prophylactic, controller drugs used in persistent asthma, including mild, moderate, and severe states; they are not indicated for acute relief or rescue therapy.
• Antileukotrienes can be tried as an alternative to inhaled corticosteroids or cromolyn-like agents in mild persistent asthma requiring more than as-needed β2 agonists.
• Antileukotrienes may not be optimal as monotherapy in persistent asthma.
• Antileukotrienes may allow reduction of high-dose inhaled corticosteroids or prevent an increase in the dose of inhaled corticosteroids, and they reduce or prevent the need for oral corticosteroids.
• Evidence to date shows these agents are safe and often effective choices in managing a wide range of asthma severity.
Monoclonal antibodies
Omalizumab (Xolair) is a subcutaneously injected monoclonal antibody. This drug is indicated for the treatment of moderate to severe asthma in adults and adolescents 12 years of age and older who have a positive skin test or in vitro reactivity to a perennial aeroallergen. It may be beneficial in treating seasonal allergic rhinitis.43
Mode of action
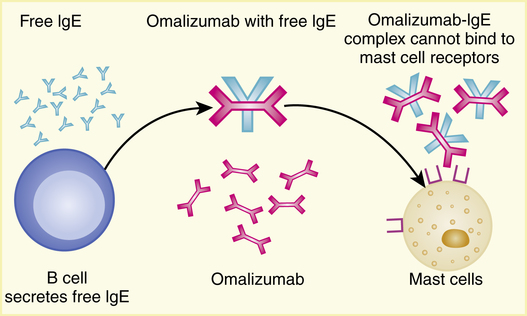
Omalizumab is a recombinant DNA-derived humanized IgG1(κ) murine monoclonal antibody that selectively binds to human IgE. The drug blocks the binding of IgE to the IgE receptor on the surface of mast cells and basophils (Figure 12-10). This blocking allows the reduction of mediators that can be released in an allergic response.
Pharmacokinetics
After parenteral administration, omalizumab is absorbed with an average bioavailability of 62%. Omalizumab is absorbed slowly, reaching peak concentrations after an average of 7 to 8 days. If doses greater than 0.5 mg/kg are given, a linear correlation exists, meaning the more given, the greater the drug availability. Omalizumab is eliminated primarily via the liver. The half-life of the drug averages 26 days and may be weight-related: increasing weight increases clearance.44
Role of omalizumab in asthma management
The literature at the time of this edition supports the use of omalizumab in uncontrolled moderate to severe asthma. Busse and colleagues45 found that omalizumab reduced asthma exacerbations and decreased corticosteroid and rescue medication use. Soler and colleagues46 found similar results in that asthma exacerbations per patient were reduced, and use of corticosteroids had decreased. Of patients taking omalizumab, 79% were able to decrease their steroid dose by 50% or more compared with only 55% in the placebo group. In an extension phase of a clinical trial for omalizumab, Buhl and associates47 found that patients receiving omalizumab had fewer exacerbations and that their corticosteroid use had decreased by approximately 180 μg/day. Lanier and colleagues48 discovered that patients being treated with omalizumab reduced their corticosteroid use by 108 μg/day compared with patients receiving placebo. It was noted that many patients in the placebo group were given more long-term β agonists and leukotriene inhibitors compared with the omalizumab group.
Summary of clinical use of omalizumab
The following points summarize the current understanding of the role of omalizumab in asthma:
• Omalizumab is a prophylactic agent used in uncontrolled moderate to severe persistent asthma; it is not indicated for acute relief or rescue therapy.
• Omalizumab is not a replacement for inhaled corticosteroids.
• Omalizumab is not optimal as monotherapy in persistent asthma.
• Omalizumab may allow reduction of high-dose inhaled corticosteroids or prevent an increase in the dose of inhaled corticosteroids.
• Omalizumab may allow reduction of asthmatic rescue agents.