Chapter 242 Nonpolio Enteroviruses
Etiology
Enteroviruses are non-enveloped, single-stranded, positive-sense viruses in the Picornaviridae (“small RNA virus”) family, which also includes the genera Rhinovirus, Hepatovirus (hepatitis A virus), and Parechovirus and genera containing related animal viruses. The original human enterovirus subgroups—polioviruses (Chapter 241), coxsackieviruses (named after Coxsackie, New York, where they were discovered), and echoviruses (named from the acronym enteric cytopathic human orphan viruses, applied before disease associations were identified)—were differentiated by their replication patterns in tissue culture and animals (Table 242-1). The human enteroviruses have been reclassified on the basis of nucleotide and amino acid sequences into 5 species, polioviruses and human enteroviruses A-D. Enterovirus types are distinguished by antigenic and genetic sequence differences; newer enteroviruses are classified by numbering. Although 100 or more types have been described, 10-15 account for the majority of disease. No disease is uniquely associated with any specific serotype, although certain manifestations are preferentially associated with specific serotypes.
| Family | Picornaviridae |
| Genus | Enterovirus |
| Subgroups* | Poliovirus serotypes 1-3 |
| Coxsackie A virus serotypes 1-22, 24 (23 reclassified as echovirus 9) | |
| Coxsackie B virus serotypes 1-6 | |
| Echovirus serotypes 1-9, 11-27, 29-33 (echoviruses 10 and 28 reclassified as non-enteroviruses; echovirus 34 reclassified as coxsackie A virus 24; echoviruses 22 and 23 reclassified within the genus Parechovirus) | |
| Numbered enterovirus serotypes (enterovirus 72 reclassified as hepatitis A virus) |
* The human enteroviruses have been alternatively classified on the basis of nucleotide and amino acid sequences into 5 species (polioviruses and human enteroviruses A-D).
Clinical Manifestations
Hand-Foot-and-Mouth Disease
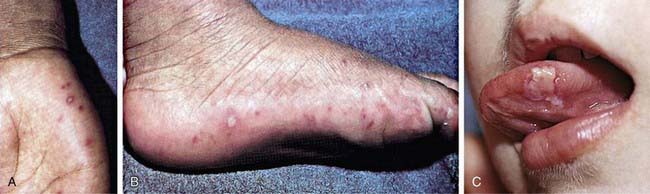
Hand-foot-and-mouth disease, one of the more distinctive rash syndromes, is most frequently caused by coxsackievirus A16, sometimes in large outbreaks, and can also be caused by enterovirus 71; coxsackie A viruses 5, 7, 9, and 10; coxsackie B viruses 2 and 5; and some echoviruses. It is usually a mild illness, with or without low-grade fever. The oropharynx is inflamed and contains scattered vesicles on the tongue, buccal mucosa, posterior pharynx, palate, gingiva, and/or lips (Fig. 242-1). These may ulcerate, leaving 4- to 8-mm shallow lesions with surrounding erythema. Maculopapular, vesicular, and/or pustular lesions may occur on the hands and fingers, feet, and buttocks and groin; the hands are more commonly involved than the feet (see Fig. 242-1). Lesions on the hands and feet are usually tender, 3- to 7-mm vesicles that occur more commonly on dorsal surfaces but frequently also on palms and soles. Vesicles resolve in about 1 wk. Buttock lesions do not usually progress to vesiculation. Disseminated vesicular rashes may complicate preexisting eczema. Hand-foot-and-mouth disease caused by enterovirus 71 is frequently more severe than coxsackievirus A16 disease, with high rates of neurologic and cardiopulmonary involvement, including brainstem encephalomyelitis, neurogenic pulmonary edema, pulmonary hemorrhage, shock, and rapid death, especially in young children. Coxsackievirus A16 also can occasionally be associated with complications such as myocarditis, pericarditis, and shock.
Myocarditis and Pericarditis
Enteroviruses account for approximately 25-35% of cases of myocarditis and pericarditis with proven cause (Chapters 433 and 434). Coxsackie B viruses are most commonly implicated, although coxsackie A viruses and echoviruses also may be causative. Adolescents and young adults, especially males, are disproportionately affected. Myopericarditis may be the dominant feature or it may be part of disseminated disease, as in neonates. Disease ranges from relatively mild to severe. Upper respiratory symptoms frequently precede fatigue, dyspnea, chest pain, congestive heart failure, and dysrhythmias. Presentations may mimic myocardial infarction; sudden death may also occur (including apparent sudden infant death syndrome). A pericardial friction rub indicates pericardial involvement. Chest radiography often demonstrates cardiac enlargement. Electrocardiography frequently reveals ST segment, T wave, and/or rhythm abnormalities, and echocardiography may confirm cardiac dilatation, reduced contractility, and/or pericardial effusion. Myocardial enzyme serum concentrations may be elevated. The acute mortality of enterovirus myocarditis is 0-4%. Recovery is complete without residual disability in the majority. Occasionally, chronic cardiomyopathy, inflammatory ventricular microaneurysms, or constrictive pericarditis may result. The role of persistent infection in chronic dilated cardiomyopathy is controversial. Enteroviruses have also been implicated in late adverse cardiac events following heart transplantation and acute coronary events, and in peripartum cardiomyopathy. Myocardial dysfunction observed in enterovirus 71 epidemics most commonly has occurred without evidence of myocarditis and may be of neurogenic origin; however, true myocarditis has also been described.
Differential Diagnosis
The differential diagnosis of enterovirus infections varies with the clinical presentation (Table 242-2).
Table 242-2 DIFFERENTIAL DIAGNOSIS OF ENTEROVIRUS INFECTIONS
| CLINICAL MANIFESTATION | BACTERIAL PATHOGENS | VIRAL PATHOGENS |
|---|---|---|
| Nonspecific febrile illness | Streptococcus pneumoniae, Haemophilus influenzae type b, Neisseria meningitidis | Influenza viruses, human herpesviruses 6 and 7 |
| Exanthems/enanthems | Group A streptococcus, Staphylococcus aureus, N. meningitidis | Herpes simplex virus, adenoviruses, varicella-zoster virus, Epstein-Barr virus, measles virus, rubella virus, human herpesviruses 6 and 7 |
| Respiratory illness/conjunctivitis | S. pneumoniae, H. influenzae (nontypable and type b), N. meningitidis, Mycoplasma pneumoniae, Chlamydia pneumoniae | Adenoviruses, influenza viruses, respiratory syncytial virus, parainfluenza viruses, rhinovirus, human metapneumovirus |
| Myocarditis/pericarditis | S. aureus, H. influenzae type b, M. pneumoniae | Adenoviruses, influenza virus, parvovirus, cytomegalovirus |
| Meningitis/encephalitis | S. pneumoniae, H. influenzae type b, N. meningitidis, Mycobacterium tuberculosis, Borrelia burgdorferi, M. pneumoniae, Bartonella henselae, Listeria monocytogenes | Herpes simplex virus, West Nile virus, influenza viruses, adenovirus, Epstein-Barr virus, mumps virus, lymphocytic choriomeningitis virus, arboviruses |
| Neonatal infections | Group B streptococcus, gram-negative enteric bacilli, L. monocytogenes, Enterococcus | Herpes simplex virus, adenoviruses, cytomegalovirus, rubella virus |
Abzug MJ. The enteroviruses: an emerging infectious disease? The real, the speculative, and the really speculative. Adv Exp Med Biol. 2008;609:1-15.
Centers for Disease Control and Prevention. Increased detections and severe neonatal disease associated with coxsackievirus B1 infection—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:553-556.
Chang LY, Huang LM, Gau SSF, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356:1226-1234.
Chen KT, Chang HL, Wang ST, et al. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics. 2007;120:e244-e252.
Chen TC, Weng KF, Chang SC, et al. Development of antiviral agents for enteroviruses. J Antimicrob Chemother. 2008;62:1169-1173.
Fowlkes AL, Honarmand S, Glaser C, et al. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J Infect Dis. 2008;198:1685-1691.
Gao SS, Chang LY, Huang LM, et al. Attention-deficit/hyperactivity-related symptoms among children with enterovirus 71 infection of the central nervous system. Pediatrics. 2008;122:e452-e458.
King RL, Lorch SA, Cohen DM, et al. Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger. Pediatrics. 2007;120:489-496.
Lee TC, Guo HR, Su HJJ, et al. Disease caused by enterovirus 71 infection. Pediatr Infect Dis J. 2009;28:904-910.
Perez-Velez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis. 2007;45:950-957.
Verboon-Maciolek MA, Groenendaal F, Cowan F, et al. White matter damage in neonatal enterovirus meningoencephalitis. Neurology. 2006;66:1267-1269.