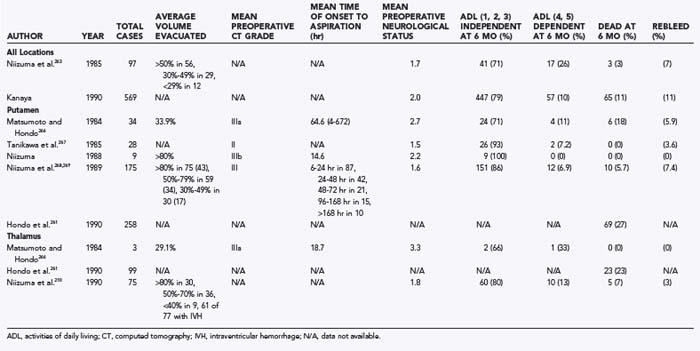
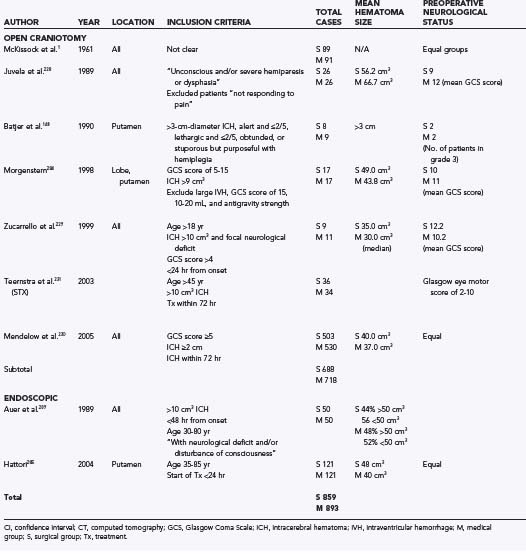
CHAPTER 358 Nonlesional Spontaneous Intracerebral Hemorrhage
Ever since McKissock, Richardson, and Taylor reported on their evaluation of the surgical and conservative treatment of 180 unselected patients with primary intracerebral hemorrhage (ICH) in 1961, operative approaches for ICH have been heavily debated1: “… With the possible exception of normotensive subjects, no group of patients fared better with operation than with conservative treatment. Indeed, in hypertensive women without angiographic midline displacement, the results of surgery were no less than disastrous.”
Historical Review
Magladery stated that the first recorded evidence of ICH and subarachnoid hemorrhage (SAH) dates back to Hippocrates (400 BC), who alluded to “sanguineous apoplexy.”2 According to Walton,3 Avicenna (AD 980-1037) described apoplexy as being due to “sanguineous humour effused suddenly about the ventricle” in the book Al Quanoun Fi’l Tibb (The Canon of Medicine).
As discussed by Donley,4 John James Wepfer in 1658 first described the relationship between circulating blood and cerebral function and the consequences of effusion of blood in the head in De Apoplexia. In their historical reviews, Clarke5 and Fazio and colleagues6 noted that Hoffman (1660-1742) first introduced the concept of ICH. They also indicated that Morgagni (1682-1771) described the difference between apoplexy associated with hemorrhage into the cerebral parenchyma and hemorrhage into the ventricular system in De Sedibus.
In 1888, MacEwen described the first successful operation for spontaneous ICH.7 In 1903, Cushing reported the first surgical evacuation of a cerebral hematoma and attributed increased intracranial pressure (ICP) to the mass effect caused by the hematoma.8 During the next 3 decades, the surgical treatment of ICH was occasionally reported.
Bagley first described surgical indications based on the location of the hematoma.9 He suggested that surgical treatment was ineffective for hemorrhages in the basal ganglia and was best reserved for subcortical hematomas associated with increased ICP. He also hypothesized that a ruptured aneurysm or rupture of an atherosclerotic or congenitally weak blood vessel wall without an aneurysm often caused spontaneous ICH. In 1932, Robinson suggested the possibility of spontaneous recovery in patients with small hemorrhages.10
In a review of nine cases, Craig and Adson suggested the possibility of ICH caused by Charcot-Bouchard aneurysms.11 Penfield suggested that ICH should be evacuated via a craniotomy and cortical incision rather than aspirated through a bur hole.12
The advent of cerebral angiography in 1929 provided an impetus for the surgical treatment of hematomas and resulted in multiple publications in the French literature in the 1940s and 1950s.13,14 In 1959, Lazorthes reported the results of his 52 cases,14 which sparked a resurgence of interest in the surgical management of ICH.
In 1961, McKissock and colleagues reported no difference in outcome between surgical and medical management and cast serious doubt on the benefit of surgical treatment.1 The advent of computed tomography (CT) in 1973 and magnetic resonance imaging (MRI) in 1982 has allowed much better recognition and understanding of the occurrence, evolution, and precise localization of ICH.
Epidemiology and Relevance
Age, sex, and race are the prime demographic factors in the prevalence of ICH.
Around 750,000 new strokes occur each year in the United States, which makes it the third most frequent cause of mortality and the number one cause of disability. Worldwide, annual incidence rates for stroke in individuals between 45 and 84 years of age range between 300 and 500 per 100,000.15–17
ICH causes 10% to 15% of first-ever strokes. In 2002, an estimated 67,000 patients suffered an ICH in the United States; of these patients, only 20% were expected to be functionally independent at 6 months.18 The worldwide incidence of ICH from all causes ranges from 10 to 20 cases per 100,000 population and increases with age. In the United States the incidence is 10 to 15 per 100,000 population per year, and up to 1993 ICH was more than twice as common as SAH.19 It is more common in men than women, particularly those older than 55 years, and in certain populations. There is a higher incidence of ICH in the Japanese and African American populations, 55 per 100,000,20,21 roughly about twice the incidence in white Western populations. If one takes these incidence figures and compares them with other data on the relative frequency of ICH, depending on the population chosen (variations with race and geography), ICH should account for 3% to 20% of all strokes.19,22 ICH is rare before the age of 45 years and becomes increasingly more frequent with advancing age. Among the group 80 years and older, it occurs 25 times more frequently than in the total population.23 The primary causes of spontaneous parenchymal bleeding in the young are vascular malformations, aneurysms, and drug abuse (cocaine, amphetamines, alcohol). Among the elderly, hypertension, tumors (primary and metastatic), vasculopathy, and coagulopathy (warfarin, heparin, aspirin, fibrinolytic agents) are the major contributing factors. In children, leukemia is a significant cause.
Because of the increasing age of the Western population, it is speculated that rates of ICH will rise steadily despite more accurate blood pressure control.24,25 Primary ICH is believed to account for 78% to 88% of cases.20,26
ICH carries an exceedingly high 30-day mortality rate of 35% to 52%; half of the deaths occur in the first 2 days.18,27
Causes
Hypertension
High blood pressure has consistently been reported as a major risk factor for ICH.27–31 A meta-analysis and review by Areisen and colleagues estimated a crude odds ratio (OR) of 3.68 for hypertension and ICH in comparison to normotensive individuals.24
Sturgeon and associates studied risk factors for ICH in a pooled cohort of the Atherosclerosis Risk in Communities study (ARIC) and the Cardiovascular Health Study (CHS).21 The ARIC cohort was recruited from 1987 to 1989 and included 15,792 men and women aged 45 to 64 years at baseline from four U.S. communities. The CHS cohort was recruited from 1989 to 1993 and included 5888 men and women 65 years or older at baseline from a sampling of four U.S. communities. Follow-up was in excess of 263,489 person-years. In this prospective study assessing baseline risk factors and subsequent occurrence of ICH, age, African American ethnicity (versus whites), and hypertension were positively associated with the development of ICH. Participants with systolic blood pressure of 160 mm Hg or greater or diastolic blood pressure of 110 mm Hg or greater had 5.55 (95% confidence interval [CI], 3.07 to 10.0) times the rate of ICH as nonhypertensive individuals.
Spontaneous ICH occurs predominantly in deep locations in the brain. The most common location is the putamen, followed by the subcortical white matter, cerebellum, and thalamus. In 100 unselected patients, Kase and associates found putaminal hemorrhage in 34, lobar hemorrhage in 24, thalamic hemorrhage in 20, cerebellar hemorrhage in 7, and pontine hemorrhage in 6.32
Hemorrhages in the caudate nucleus, putamen, thalamus, brainstem, and cerebellum occur in the distribution of small perforating arteries with a diameter of 50 to 200 µm. These deep penetrating arteries are small nonbranching end arteries that arise directly from much larger arteries (e.g., middle cerebral artery, anterior choroidal artery, anterior cerebral artery, posterior cerebral artery, posterior communicating artery, cerebellar arteries, basilar artery). Their small size and proximal position predispose them to the development of microatheroma and lipohyalinosis. Electron microscopic studies suggest that most bleeding occurs at or near the bifurcation of affected arteries, where prominent degeneration of the media and smooth muscles can be seen.33
The concept of ICH arising from rupture of miliary microaneurysms was first proposed by Charcot and Bouchard in 1868.34 Studies by Russell35 and by Cole and Yates36 confirmed the occurrence of microaneurysms in an anatomic distribution closely correlated to that of hypertensive hemorrhages and further defined the epidemiology of microaneurysms. Russell identified a strong association between miliary aneurysms 300 to 900 µm in diameter and hypertension. A few aneurysms were observed in his control group of normotensive individuals (diastolic blood pressure <110 mm Hg), but 84% of this group were 60 years or older. Cole and Yates observed that microaneurysms 50 to 2500 µm in diameter were uncommon in those younger than 50 years, even in hypertensive subjects. Therefore, both hypertension and age appear to be major factors in the formation of microaneurysms.
Fisher has further defined the pathologic process affecting small cerebral arteries in hypertension and coined the term lipohyalinosis to specify a destructive vascular process previously referred to by a variety of names, including “fibrinoid necrosis,” “angionecrosis,” and “hyaline arterionecrosis.”37–39 In Fisher’s view, raised arterial pressure alters the walls of small cerebral arterioles 80 to 300 µm in diameter and leads to focal subintimal fibrinoid deposition associated with the presence of fat-filled macrophages. As the process advances, the integrity of the elastica and media is lost, and the artery dilates locally to form a microaneurysm 500 to 1500 µm in diameter. Extravasation of red blood cells takes place through the damaged walls, and hemosiderin-filled macrophages are seen through and beyond the adventitia. Fisher found lipohyalinosis to be, by virtue of occlusion of the vascular lumen, the cause of many lacunar infarcts. He could not confirm that microaneurysms were the source of massive ICH.
Cerebral Amyloid Angiopathy
The characteristic feature of cerebral amyloid angiopathy (CAA) is the deposition of β-amyloid protein in the media and adventitia of small cortical and leptomeningeal arteries.40 The incidence of CAA rises steeply after the age of 70 and reportedly ranges from 23% to 48% in the 8th decade, from 37% to 46% in the 9th decade, and from 57% to 58% in the 10th decade.40 The most significant feature of CAA is the presence of multiple hemorrhages in unusual locations in the absence of hypertension.41 Vice versa, hypertension is present in only a minority of patients. Hematomas are mostly subcortical or lobar and are more frequently found in the occipital and parietal lobes. A distinct feature, in sharp contrast to nonamyloid ICH, is the multiplicity of hemorrhages over time and location. As for amyloid angiopathy in other locations in the body, the involved vessels exhibit Congo red birefringence in polarized light.41 Features of vessels with CAA include a severe degree of amyloid deposition and coexistent fibrinoid necrosis.42 Apolipoprotein E ε3/4 and ε4/4 seem to be associated with more severe forms of CAA, and severe degrees of CAA were associated with ICH. The annual risk for recurrent hemorrhage was found to be 10.5%.16
Anticoagulant Therapy
The incidence of ICH in patients taking warfarin after myocardial infarction is 1% per year.43 Sixty-one percent of ICHs occur in the first 6 months of anticoagulation therapy.44 Long-term anticoagulation therapy increases the risk for ICH 8- to 11-fold.45
The mortality rate of spontaneous ICH is as high as 67% in patients receiving oral anticoagulant therapy (OAT).45–47 The incidence of OAT-related ICH (OAT-ICH) is expected to increase in the coming years as a result of an anticipated rise in the incidence of atrial fibrillation attributable to an aging population.
A number of factors contribute to the increased risk for ICH in this group of patients, including advanced age, previous cerebrovascular disease, hypertension, and concomitant use of aspirin.32,43 In the Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial,47 which compared the effectiveness of aspirin (1300 mg/day) and warfarin (target international normalized ratio, 2.0 to 3.0) in preventing strokes in patients with transient ischemic attack or stroke caused by angiographically verified 50% to 99% stenosis of a major intracranial artery, major hemorrhage occurred in 3.2% of the aspirin group and 8.3% of the warfarin group. The study was terminated early because of a statistically significant higher rate of bleeding in the warfarin group and no statistically significant benefit in the primary end point of the study. In a review of eight placebo-controlled clinical trials for the prevention of stroke, Mayo and colleagues found that the risk for hemorrhagic stroke was 0.7% in 2981 patients treated with aspirin versus 0.37% in 2187 patients receiving placebo.48
ICH during treatment with heparin is rare and occurs mostly in patients being treated for acute embolic cerebral infarction and uncontrolled hypertension. In most patients, the activated partial thromboplastin time is excessively prolonged.49,50
The efficacy of fibrinolytic agents in the treatment of myocardial infarction is well known. ICH has been reported in 0.4% to 1.3% of patients with acute myocardial infarction treated with the single-chain tissue plasminogen activator (t-PA) alteplase.51 Thrombolysis in acute ischemic stroke increases the risk for severe, life-threatening hemorrhagic complications by up to 10-fold in comparison to controls. Intravenous t-PA was used in two studies, the European Cooperative Acute Stroke Study (ECASS)52 and the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study,53 with a therapeutic window of 6 and 3 hours, respectively. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group observed 2 patients (0.6%) with symptomatic and 1 patient (0.3%) with fatal hemorrhage in the placebo group (n = 312) and 20 patients (6.4%) with symptomatic and 9 patients (2.9%) with fatal hemorrhage in the recombinant tissue plasminogen activator (rt-PA) group (n = 312).53 In both ECASS 1 and 2, rt-PA increased the risk for parenchymal hematoma (OR, 3.0 and 4.2).52,54 However, the functional outcome at 3 months was better in the t-PA–treated group. Experimental focal cerebral ischemia causes a significant loss of the basal lamina components of cerebral microvessels.55 The mechanisms for this microvascular damage may include degradation of plasmin-generated laminin, activation of matrix metalloproteinases, transmigration of leukocytes through the vessel wall, and other processes. This loss in vessel wall integrity is associated with the development of petechial hemorrhage. Revascularization, coupled with hypertension, may lead to life-threatening hemorrhagic complications.
The pathophysiology of ICH in patients maintained on a regimen of anticoagulation therapy is not well known, but various factors have been hypothesized. Hart and colleagues theorized that the use of OAT merely unmasks intracerebral bleeding that would otherwise remain asymptomatic, especially in patients with underlying hypertension or cerebrovascular disease.56 This hypothesis is supported by the fact that gradient echo MRI indicates that microbleeding can be found even in neurologically normal individuals and is strongly associated with increased age and hypertension.57 The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) and the European Atrial Fibrillation Trial (EAFT) indicated that patients with primary underlying cerebrovascular disease had a remarkably higher risk for OAT-ICH.58–60 Furthermore, the presence of white matter lesions, so-called leukoaraiosis, is an independent predictor of spontaneous ICH.61 Therefore, the underlying mechanism of spontaneous ICH and OAT-ICH may be the same, with OAT acting as an exacerbating factor. This may also explain why the distribution of locations in the brain where OAT-ICH occurs is no different from that seen in patients with spontaneous ICH.46,47,62
OAT may also cause ICH directly. This is supported by the observation that higher intensities of anticoagulation clearly increase the risk for OAT-ICH.46,47,63–65 Oral anticoagulants interfere with the synthesis of vitamin K–dependent clotting factors, thereby resulting in low levels of factors VII, IX, and X and prothrombin. It is possible that adequate levels and functional forms of these clotting factors are essential to counteract the stress placed on blood vessels as part of normal daily activities and to prevent bleeding.66,67
The dynamics of hematoma expansion in OAT-ICH remain to be firmly elucidated. Because of persistent coagulopathy, hematoma expansion in OAT-ICH may be more common and occur over a longer time frame than seen with spontaneous ICH. In a retrospective study of 47 patients with OAT-ICH, hematoma expansion was found in 28% of those evaluated within 24 hours of onset.68 In another study, hematoma expansion up to the seventh day was found in 16% (9/57) of patients who were not receiving OAT versus 54% (7/13) of those who were.69
Although the evolution of hematomas in patients managed with anticoagulation therapy is protracted, their hematomas are about twice the size of those in patients not receiving anticoagulation therapy, and their mortality rate increases to 60% to 65%.43
Drug Abuse, Alcohol, and Smoking
Drugs
ICH has been associated with the abuse of multiple drugs such as amphetamines, pseudoephedrine, phenylpropanolamine, cocaine, the “crack” variant of cocaine, phencyclidine, and heroin.70–75 Typically, these hemorrhages are lobar and are attributed to a transient elevation in blood pressure, arteritis-like changes, or both. The arteritis-like changes in the vessel wall are thought to be the effects of either direct drug toxicity or hypersensitivity. However, many of the drug-associated ICHs in the young might not simply be due to induced hypertension and its sequelae but could also be due to a larger proportion with accompanying vascular malformations.76 Underlying lesions such as aneurysms, arteriovenous malformations (AVMs), or brain tumors can be found more frequently in this subgroup of patients.
In the young, cocaine is increasingly being reported as a cause of SAH and ICH. The hemorrhage usually takes place within hours of use and can be lobar or deep ganglionic. The use of cocaine is associated with a higher incidence of aneurysmal and AVM rupture than occurs with other sympathomimetics.73
Alcohol
Excellent data have been gathered from evaluation of a hospitalized Finnish population. Recent moderate use and heavy alcohol consumption appear to be independent risk factors.77 Patients in whom such a risk factor is suspected are usually younger.78 Pathophysiologic explanations put forward are a contribution to hypertension, impaired hemostasis, decreased level of circulating clotting factors, excessive fibrinolysis, and disseminated intravascular coagulation.79 It has been speculated that at the time of alcohol exposure, a transient increase in blood pressure in conjunction with the alcohol-induced cerebral arteriolar vasoconstriction can cause rupture of small cerebral arteries.80 This hypothesis is supported by the findings of Juvela and colleagues, who defined “recent drinking” as drinking within the last 24 hours and found that it was a more important risk factor than the amount of alcohol consumed within a week.77 The increase in blood pressure during consumption appears to be a more important factor than chronic hypertension with regard to specifically alcohol-induced ICH. Drinking (abuse) in general has been identified as a risk factor.81–85 In contrast, a recent evaluation of 242 ICH patients (age range, 34 to 97 years) in the city of Izumo, Japan, which apparently has the highest rate of ICH reported in Japan, cannot support the supposition that alcohol consumption is an associated risk factor. Nor was cigarette smoking or diabetes mellitus. Their results identified hypertension and, contrary to other studies, total serum cholesterol concentration as the main risk factors positively associated with ICH.86 However, there is also evidence that the pathophysiology of ICH varies by location within the brain, with frequent alcohol abuse apparently being more of a risk factor for lobar hemorrhage.27
Smoking
A very recent evaluation of 352 patients with ICH hospitalized in Tusla, Bosnia, revealed that smoking was the third most frequent risk factor (28%), after hypertension (84%) and heart disease (31%).87 However, smoking is most likely much more prevalent in the Bosnian population. In contrast, in a Korean population in Seoul that was also investigated for the role of lifestyle factors that may contribute to ICH, it was concluded that smoking was not a risk factor and hypertension was still the number one risk factor.88 A point was made that some of the pertinent risk factors for ICH might differ among the various ethnic groups (e.g., fat consumption).
An excellent prospective and unusual study is that of Kurth and coworkers from 2003. Male physicians previously reported to be healthy were monitored for 17.8 years. During follow-up, 108 ICHs and 31 SAHs occurred. Evaluation of their smoking habits suggested an increased risk for total hemorrhagic stroke, ICH, and SAH in current cigarette smokers. A graded increase in risk could be demonstrated that depended on how many cigarettes were smoked (less than 20 or 20 or more per day). The effect of smoking on ICH was about the same magnitude as the effect of smoking on ischemic stroke. Never smokers and past smokers had equal rates of ICH and SAH.89
Clinical Findings and Diagnosis
The symptoms and signs of ICH depend substantially on the location and size of the hematoma. The ictus oftentimes occurs during activity and is manifested as the sudden onset of a neurological deficit, which will then gradually progress. Important insights into the clinical features of patients with ICH can be taken from the prospective 1978 Harvard Cooperative Stroke Registry study.90
Impaired consciousness is the most relevant neurological sign. Such a finding was described in 60% of the patients reported by Mohr and coauthors.90 The degree of impaired consciousness depended on the location, size, and extension of the hematoma into deep structures or the ventricles.
A large hematoma results in increased ICP and direct compression or distortion of the thalamic and brainstem reticular activating system.91 Patients with hematomas in deep locations have a significant decrease in their level of consciousness and dense, lateralized neurological deficits. These locations in turn carry a worse prognosis (discussed later). Patients with more peripherally located hematomas are more alert with corresponding focal deficits.
The hallmark of brainstem involvement is a mixture of coma, long-tract signs, and cranial nerve deficits. The signs and symptoms of cerebellar hemorrhage are unique and so distinctly different from those of supratentorial ICH that a clinical diagnosis of location can be made after the physical examination, before performing a CT scan. The classic symptoms and typical findings were well summarized by Heros92 (Table 358-1).
| Symptoms |
Secondary Deterioration
A third to a fourth of the patients who are initially alert deteriorate in their level of consciousness within the first 24 hours.93,94 Rather than the initial clinical signs, the most accurate predictor is large hematoma volume and ventricular extension.94 Consequently, these patients need to be very closely monitored during the first 24 hours, especially if they are still awake and alert despite large hematoma volume. Expansion of the hematoma is the most common cause of neurological deterioration and death93,94 within the first 3 hours after the ictus. Progression of the mass effect secondary to edema can essentially occur within two distinct time intervals: early within the first 2 days and late within the second and third weeks.95
Hematoma Location and Clinical Profile
Spontaneous ICH occurs predominantly in deep locations in the brain. The most common location is the putamen, followed by the subcortical white matter, cerebellum, and thalamus. In 100 unselected patients, Kase and associates found putaminal hemorrhage in 34, lobar hemorrhage in 24, thalamic hemorrhage in 20, cerebellar hemorrhage in 7, and pontine hemorrhage in 6.32
Putaminal Hemorrhage
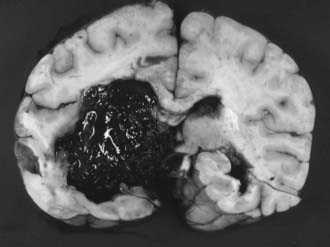
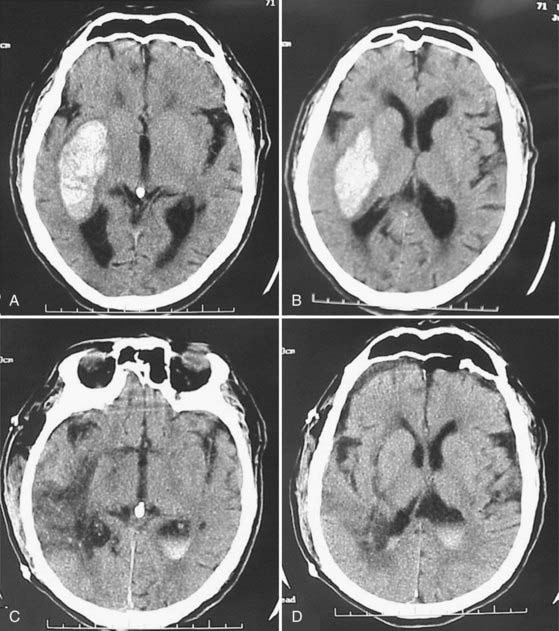
Putaminal hemorrhage is the most common form of ICH and can be manifested in different ways, depending on the size and extent of the hematoma. Initially, there is an acute onset of headaches. However, these headaches are different from the thunderclap type noted in patients with SAH. They are soon followed by a gradual progression of focal neurological signs and, depending on the overall size, by a worsening level of consciousness. A marked deficit from the beginning is unusual. Common neurological findings are hemiparesis, hemisensory syndrome, homonymous hemianopia, horizontal gaze palsy, and either aphasia (dominant hemisphere) or hemineglect (nondominant hemisphere).37,96 The pronounced neurological deficits associated with coma suggest large hematomas and carry a poor prognosis. Intraventricular extension of the hemorrhage is a sign of extensive parenchymal dissection or destruction and a bad prognosticator (Fig. 358-1).97,98 In contrast, patients who are alert and have only limited motor deficits, normal extraocular movements, full visual fields, and a hematoma that does not extend laterally or upward out of the putaminal region fare better. This has been ascribed to reversible compression of capsular fibers as opposed to destruction.97
Thalamic Hemorrhage
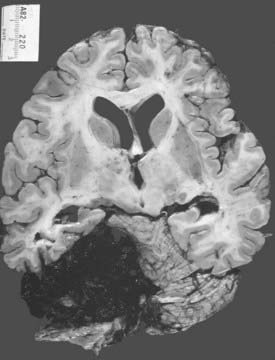
Thalamic hemorrhages account for 10% to 15% of all ICHs (Fig. 358-2).37,99 The bleeding originates from thalamic perforators of the posterior cerebral arteries and may extend laterally into the internal capsule, medially into the ventricles, superiorly into the corona radiata, and inferiorly into subthalamus and midbrain.100
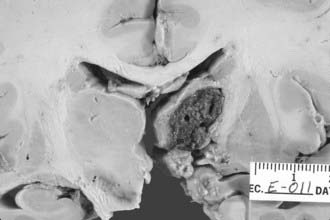
FIGURE 358-2 This medium-sized hypertensive thalamic hemorrhage, which spared the capsular fibers laterally, is well confined.
The signs and symptoms depend on the size and pattern of extension of the hematoma. In contrast to putaminal hemorrhages, thalamic bleeding will instantly result in gross neurological deficits with sensorimotor loss, a higher likelihood of vomiting, variable presence of headaches, and occasionally coma.6,99,101 The ocular findings in patients with thalamic lesions are pathognomonic: upward gaze palsy, convergence gaze, miotic unreactive pupils because of compression of the midbrain tectum, and less commonly, retraction nystagmus on upward gaze and skew deviation (vertical misalignment of the eyes because of abnormal prenuclear vestibular input to the ocular motor nuclei).6,37,99,101
Different characteristics of the so-called thalamic syndrome had already been described at the beginning of the 19th century by Dejerine and Roussy,102 followed by Lhermitte in 1925103 and Baudouin and associates in 1930.104 Fisher in 1959 emphasized language disorders and disturbances in ocular motility.105
Kumral and colleagues presented an excellent study from 1995 based on 100 patients and provided a very detailed correlation of different symptoms (sensorimotor, oculomotor, and neurobehavioral) to four defined topographic types of thalamic hemorrhage106:
Lobar Hemorrhage
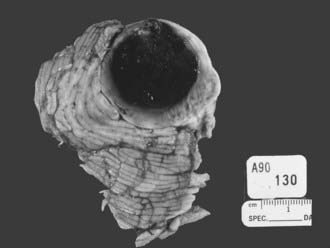
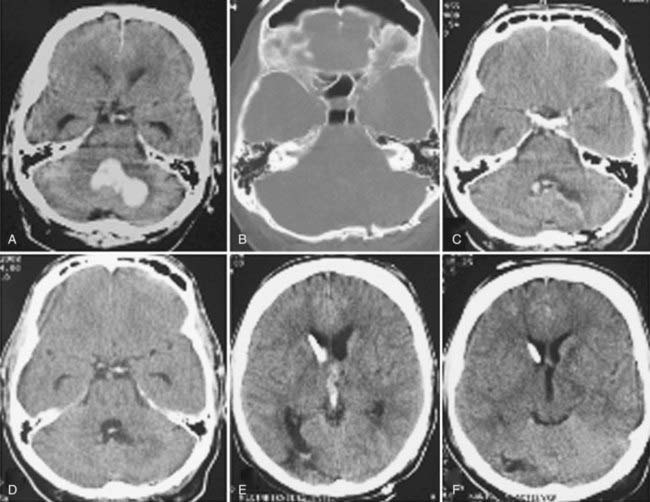
Lobar hemorrhages usually occur in the subcortical white matter and have a predilection for the parietal, temporal, and occipital lobes.107,108 In Ropper and Davis’ series of 26 patients with lobar hemorrhages, 11 (42%) were within the occipital lobe, 7 (27%) were in the temporal lobe, 4 (15%) were in the frontal lobe (Fig. 358-3), and 3 (12%) were in the parietal lobe.108 The frequent occurrence of lobar hemorrhages in the parieto-occipital lobes has been attributed to the higher concentration of intracerebral microaneurysms reported in anatomic studies.109
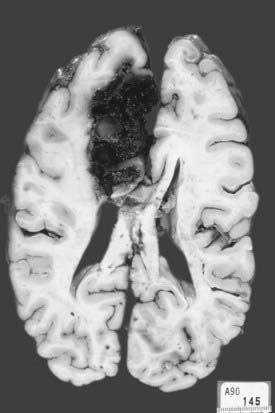
FIGURE 358-3 An extensive medial frontal intracerebral hematoma secondary to amyloid angiopathy caused this patient’s death.
Hypertension as a cause of lobar hemorrhage is unusual.107,108,110 Only 31% of the patients reported by Ropper and Davis had chronic hypertension.108 Kase and associates reported elevated blood pressure in only 50% of their patients on admission.107 In a series reported by Broderick and colleagues, hypertension contributed almost equally to lobar and deep hemispheric, cerebellar, and pontine hemorrhages.19 Other causes of lobar hemorrhages are AVMs, tumors, anticoagulation therapy, blood dyscrasias, and CAA.41,111,112 In a significant number of cases, no definite cause can be found.107 CAA is probably the most common cause in nonhypertensive patients 70 years and older.
The clinical manifestations of lobar ICH depend on the location and size of the hematoma.108 When compared with other forms of ICH, the frequency of associated hypertension and coma on admission is lower. The low incidence of coma is probably related to the peripheral location of the hematoma.108 Most patients complain of headache and vomiting. Seizures are also frequent.22,107,113 Hemiparesis is seldom pronounced.
The prognosis of patients with lobar ICH is relatively better than that of patients with other forms of ICH. Mortality rates range from 11% to 29%.107,108,114 Functional outcome in survivors also tends to be better.114 In their series of 22 patients, Kase and coauthors reported good outcomes in those with hematoma volumes of less than 20 cm3.107 Seventy percent survived after the surgical removal of hematomas that were 20 to 60 cm3. No patient with a hematoma volume greater than 60 cm3 survived.
Caudate Hemorrhage
Caudate hematomas represent approximately 5% to 7% of cases of ICH.98 The most common cause of caudate hemorrhage has been arterial hypertension.98,115–119
The head of the caudate nucleus receives its blood supply from Heubner’s artery and the anterior lenticulostriate and lateral lenticulostriate arteries, which also supply the anterior internal capsule and putamen.119 A rupture in these arteries causes parenchymal hemorrhage.
Patients have an abrupt headache and vomiting, followed by a decreased level of consciousness. They are usually disoriented, with evidence of neck stiffness.98 Occasional patients suffer seizures and exhibit horizontal gaze paresis. On CT, ventricular extension of the hematoma into the frontal horn with secondary hydrocephalus is common. Occasionally, the hemorrhage extends into the anterior portion of the thalamus. Such patients have transient, but significant short-term memory deficits.97 In Stein and colleagues’ series of 8 patients, most recovered fully with no significant neurological deficits.98 Weisberg also reported a small series of caudate hemorrhages, but the outcome in this series was poor, and stupor and a massive amount of intraventricular hemorrhage were associated with poor outcome.115 Liliang and coworkers looked at clinical data from 36 consecutive patients with hypertensive caudate hemorrhage.120 In this relatively large study, multivariate analysis and stepwise logistic regression revealed that hydrocephalus was the only independent prognostic factor for a poor outcome (P < .001).
Cerebellar Hemorrhage
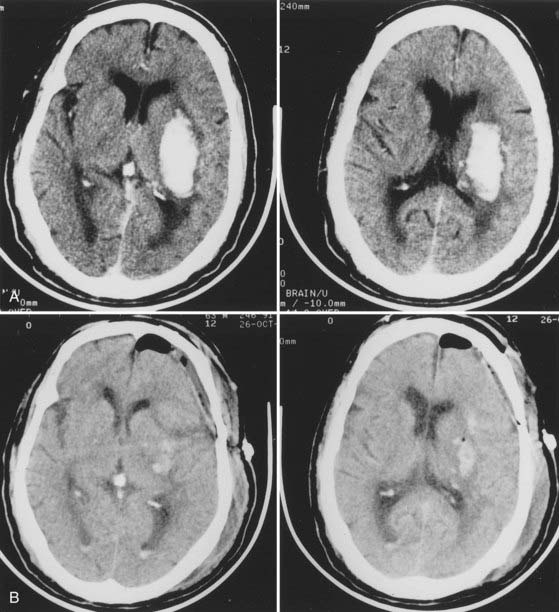
The frequency of cerebellar hemorrhage ranges from 5% to 10%.37,49,121,122 One of the major differences from supratentorial hemorrhages is the entirely different prognosis once coma has occurred. If the diagnosis is made early and surgical intervention is prompt, coma is reversible after a cerebellar hemorrhage.109,123–126 A very recent study by Smajlović and colleagues on the 30-day prognosis and risk factors in 352 patients treated for ICH confirmed once again that when compared with all other ICHs, cerebellar hematomas had the best outcome after the first month; brainstem and multilobar ICHs had the worst.87 The dentate nuclei are the most common substrate. The hematoma extends into the hemispheric white matter and often into the fourth ventricle, where it causes either brainstem compression or direct invasion (Fig. 358-4). Rarely, cerebellar hemorrhage involves only the vermis. Hypertension and anticoagulation are the two most important causative factors for cerebellar hemorrhage.123,127
Patients usually have headache and an inability to walk or stand. Vomiting is common and may or may not be associated with headache.123,127 Other symptoms include dizziness, neck stiffness, dysarthria, tinnitus, and singultus. Loss of consciousness at the onset is rare. On admission to the hospital, about a third of patients are obtunded.123 The early physical signs are appendicular or truncal ataxia, dysarthria, ipsilateral horizontal gaze palsy, peripheral facial palsy, nystagmus, and sixth nerve palsy. At least two of the three characteristic clinical signs—appendicular ataxia, ipsilateral gaze palsy, and peripheral facial palsy—were present in 73% of the patients reported by Ott and coworkers.123 In two patients with cerebellar hematomas and a peripheral facial nerve palsy on the hematoma side, Messert observed spontaneous unilateral eye closure on the contralateral side. In an effort to compensate for gaze dissociations and extraocular motor palsies, the eye on the noninvolved side of the face was closed. In other words, the open eye is on the hematoma side.128
The clinical course of cerebellar hemorrhage is unpredictable. These patients, whether alert or lethargic on admission, can deteriorate quickly to coma and die with no warning.123,129 Although the prognosis and final outcome are largely related to the patient’s initial preoperative condition, even comatose patients can make a good recovery.129 Little and colleagues reported on two groups of patients with cerebellar hemorrhage.130 The first group had an abrupt onset, progressive course, and low level of consciousness. CT in this group of patients, who required surgery, showed cerebellar hematomas 3 cm or greater in diameter, obstructive hydrocephalus, and extension of hemorrhage into the fourth ventricle.131,132 The second group of patients was awake and stable and had hematomas smaller than 3 cm in diameter. They were treated medically, with good outcomes.130
Brainstem Hemorrhage
The pons is the most common location for nonvascular causes of ICH in the brainstem. Spontaneous nontraumatic midbrain and medullary hematomas are comparably rare. The first to evaluate a large number of pontine hemorrhages was Attwater in 1911.133 After performing autopsies on 77 subjects with pontine hemorrhages, Attwater was able to differentiate between primary and secondary brainstem hemorrhages. He attributed some pontine hemorrhages to elevated ICP. Several years later in a monograph, Duret also described this phenomenon, which now bears his name as an eponym.134 Under the so-called Duret hemorrhage, we understand a characteristic slit-like bleeding in the upper brainstem (mesencephalon and pons).135 It needs to be mentioned, however, that this does not automatically imply spontaneous ICH as a cause. As Parizel and colleagues pointed out, they also occur in victims of craniocerebral trauma.135
In an autopsy review of 30 patients with pontine hemorrhages among 511 cases of ICH at Boston City Hospital, two thirds of the patients were comatose on initial evaluation and had massive hemorrhages that extended into the midbrain or fourth ventricle. Within 48 hours, 78% of the patients died. Fisher suggested that primary hemorrhage, by virtue of a pressure effect, causes the surrounding vessels to rupture and initiates a cascade of gradual enlargement of the hematoma.136 The bleeding in hypertensive patients was attributed to leakage from tiny penetrating vessels damaged by lipohyalinosis and containing small microaneurysms.49,109,136
Rupture of the paramedian perforating branches of the basilar artery is thought to be the cause of massive pontine hematomas (Fig. 358-5). The lesion usually begins in the midpons at the junction of the tegmentum and basis pontis and extends along the longitudinal axis of the brainstem into the midbrain, middle cerebellar peduncle, or the fourth ventricle.121
The clinical course of a hypertensive patient is typically one of rapid onset of coma. Awake patients may become symptomatic with headache, vomiting, and focal pontine signs such as facial or limb numbness, deafness, diplopia, quadriparesis, paraparesis, or hemiparesis. Occasionally, seizure (which can be a true convulsive episode), spasmodic decerebrate posturing, or violent shivering associated with autonomic dysfunction and rapidly developing hypothermia is reported. On examination, patients have an abnormal breathing pattern, apnea, cranial nerve and long-tract deficits, occasional decerebrate posturing, and multiple oculomotor findings.121,137,138 Weakness of the pontine and bulbar musculature is invariably associated with large median pontine hemorrhages, but it is seldom appreciated because of the depressed level of consciousness.
Pinpoint (miotic) reactive pupils are a hallmark of pontine lesions. Among the possible various ophthalmic findings in patients with pontine hemorrhage are absent horizontal gaze movement when the paramedian pontine reticular formation is bilaterally damaged, one-and-a-half syndrome after a unilateral pontine tegmental or abducens nucleus lesion that results in a horizontal conjugate gaze palsy in one direction and internuclear ophthalmoplegia in the other,139 and ocular bobbing. Fisher was the first to describe brisk, conjugate downward eye movements, followed by a slower “bobbing” back up to the primary position.140
Massive pontine hemorrhages are always fatal, but death may not be instantaneous.137 Some patients with medium-sized hematomas and most patients with small basal or lateral tegmental hematomas survive, but with various degrees of residual neurological deficits.
Brain Edema after Intracerebral Hemorrhage
One of the major challenges after primary ICH is the development of perihematomal edema, which forms rapidly after the ICH141 and contributes to a documented increase in perihematomal volume by approximately 75%. The causes of this perihematomal area of edema and cell death are not known decisively. Experimental data indicate that the presence of whole blood, but not intact red blood cells, induces the formation of edema. As red blood cells lyse, however, edema is observed, and the volume of the edema correlates with the volume of the lysed red blood cells. Hemoglobin and its degradation products induce edema formation and accumulation of reactive glial cells at the site of delivery.142 There are three distinct phases of edema formation after ICH. In the first hours after ICH, retraction of the clot begins. Intact red blood cells within the hematoma area have not been found to contribute to edema formation. As the coagulation cascade becomes activated over the following 24 to 48 hours, however, thrombin becomes activated and promotes edema formation and further disruption of the integrity of the blood-brain barrier.143 The third phase of edema formation starts when red blood cells in the hematoma begin to lyse and hemoglobin and its degradation products are deposited into the brain parenchyma, thus initiating a potent inflammatory reaction.144
In an observational study, Wu and colleagues studied 17 patients with spontaneous ICH treated medically.145 Hematoma size and absolute and relative brain edema volumes were measured. Hematoma enlargement occurred in 4 of the 17 ICH patients (24%) within the first 24 hours. Hematoma sizes were reduced significantly at day 10 (P < .05) because of clot lysis. However, both absolute and relative brain edema increased gradually with time (P < .01). These results suggest that delayed brain edema after ICH may result from hematoma lysis. The authors concluded that reducing early hematoma growth and limiting clot lysis–induced brain toxicity could be potential therapies for ICH.
Medical Management
The clinical course of ICH after medical management can be dismal. Mortality rates range from 27% to 77%.114,146–148 However, optimization of medical care with regard to managing blood pressure, controlling ICP, limiting hematoma expansion, and stabilizing the cardiorespiratory system can have important effects on outcome and help prevent deterioration.
Steroids
The use of steroids in patients with ICH is controversial. Batjer and associates used steroids (4 mg dexamethasone intravenously every 6 hours) in their protocol.148 The rationale for using steroids for the treatment of ICH is that they might lessen the damaging effects of cerebral edema, increased ICP, a disrupted blood-brain barrier, and stress. The first randomized study included 40 patients with ICH but showed no statistical difference in outcome associated with the use of steroids.149 This study, however, lacked case uniformity and appropriate, relevant stratification. Therefore, a well-designed, randomized, placebo-controlled study was performed in 1987.150 In their paper, Poungvarin and coworkers studied 93 patients 40 to 80 years old in a double-blind randomized design. Patients with documented primary supratentorial ICHs were randomly assigned to either dexamethasone (10 mg intravenously and then 5 mg every 6 hours) or placebo. The death rate at the 21st day was identical in both groups (dexamethasone versus placebo, 21 of 46 versus 21 of 47; P = .93). In contrast, the rate of complications (mostly infections and complications of diabetes) was 11 times higher in the dexamethasone group (P < .001). This led to early termination of the study.
Blood Pressure Management
The single most important factor in determining rapid expansion of an ICH is blood pressure. In studies of patients with hypertensive ICH, persistently elevated blood pressure increased the risk for hematoma progression.151–153 In a retrospective review of 320 patients with hypertensive ICH, 10 showed rapid expansion of the hematoma on serial CT.153 The consecutive scans were obtained an average of 1.7 and 48.9 hours after hemorrhage. Of the 10 patients with radiographic evidence of expansion, all had persistent hypertension, and half deteriorated neurologically. The average blood pressure in this group on admission was 179/110 mm Hg, and the average blood pressure recorded before deterioration was 190/121 mm Hg. The first 24 hours seems to be particularly critical. In a more recent retrospective study of 76 consecutive patients with hypertensive ICH, Ohwaki and colleagues observed that maximum systolic blood pressure was significantly associated with hematoma enlargement (P = .0074).154 A target systolic blood pressure of 160 mm Hg or greater was significantly associated with hematoma enlargement when compared with a pressure of 150 mm Hg or less (P = .025).
The degree to which blood pressure should be controlled is controversial. Patients with a history of chronic hypertension have impaired autoregulation, and overzealous lowering of blood pressure can lower cerebral perfusion pressure and produce secondary ischemic damage, especially in those with a decreased level of consciousness, who may have elevated ICP. Some authors recommend lowering systolic blood pressure to less than 160 mm Hg155; others recommend lowering it to normotensive levels but not below.156 In a prospective, randomized trial of putaminal ICH in which craniotomy was compared with medical therapy, patients were initially treated with sodium nitroprusside (Nipride) to decrease systolic blood pressure by 25% during the first 24 hours. During the next 48 to 72 hours, blood pressure decreased to normotensive levels.148 In this study, the mean admission systolic blood pressure was 234 mm Hg. Despite the tight control of blood pressure, the 6-month mortality rate was 77%. Because this study did not report the cause of death or the time of deterioration, it is difficult to determine whether the degree of blood pressure control was adequate.
In theory, there might be at least two conflicting trends in the immediate hours after ICH, namely, the development of perilesional microcirculatory insufficiency with a propensity to render the brain ischemic and, on the other hand, a propensity to rehemorrhage. The former would necessitate increased perfusion and the latter strict blood pressure control. Qureshi and colleagues looked at the rate of 24-hour blood pressure decline and mortality after spontaneous ICH.157 One hundred five patients with ICH were included in this retrospective study. Logistic regression analysis showed that the rate of decline in blood pressure in the first 24 hours was an independent predictor of mortality but did not affect the functional outcome of survivors. More recently, The Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial set out to answer the question about the optimal blood pressure range in the first 24 hours after ICH. This trial is a multicenter open-labeled pilot trial to determine the tolerability and safety of three escalating goals of antihypertensive therapy (110 to 140 mm Hg, 140 to 170 mm Hg, 170 to 210 mm Hg) for acute hypertension in 60 subjects with supratentorial ICH. The initial results of this trial were presented at the 2008 International Stroke Conference158: “Aggressive systolic blood pressure reduction to 110-140 mmHg in the first 24 hours using intravenous nicardipine was well tolerated with a low risk of hematoma expansion, neurological deterioration and in-hospital mortality. The results favor pharmacological reduction of systolic blood pressure in patients with acute ICH.”
Experimental rat studies suggest that although transient alterations in blood flow occur within minutes of hemorrhage, the severe alterations in perihematomal microcirculation that cause ischemia are not maximal until 4 hours thereafter.159,160 In addition, the formation of edema is not maximal until after 6 to 8 hours.161 However, the period associated with the maximal risk for progression of hemorrhage in the presence of persistent hypertension is 3 to 6 hours.23,153,162,163 Therefore, an argument can be made to reduce blood pressure dramatically during the first 4 hours after hemorrhage to decrease the risk for rehemorrhage and then to raise blood pressure slowly to perfuse ischemic areas.
The discussion whether an ischemic penumbra in ICH exists is ongoing. At present, a majority of researchers seem to favor that there is no ischemic penumbra, but rather a perilesional area with depressed metabolism (metabolic penumbra?) that can be detected with positron emission tomography,164 an area of reduced metabolic demand as detected by diffusion- and perfusion-weighted MRI,165 or only a perilesional area with no significant changes at all. This was suggested after sampling in eight dogs and serially measuring regional cerebral blood flow (CBF) with radiolabeled microspheres, cerebral oxygen extraction, the cerebral metabolic rate of oxygen consumption, glucose utilization, and lactate production.166
The last entry to date demonstrated vasogenic edema and only a mild perfusion deficit above the threshold for ischemia in an MRI rat ICH model, thus making a perihematomal penumbra unlikely.167 A prospective clinical study with perfusion-weighted MRI performed during the treatment of 18 ICH patients, however, confirmed the presence of a hypoperfused area around the ICH that disappeared completely after 1 week.168 Similar results were obtained from a single-photon emission CT study that compared early uptake within hours of hemorrhage and 6 to 9 months postictally.169 An interesting microdialysis study in 2006 revealed that the immediate zone around an evacuated ICH exhibits a similar biochemical pattern as the penumbra zone surrounding focal traumatic brain contusions.170
Intracranial Pressure Management
Elevated ICP is considered a major contributor to mortality after ICH and should be treated vigorously.171 ICP may be managed with osmotherapy, controlled hyperventilation, surgery, and other measures. A therapeutic goal for all treatment of elevated ICP is an ICP of less than 20 mm Hg and cerebral perfusion pressure greater than 70 mm Hg. Mannitol effectively and safely decreases ICP and can be used alone or with urea to potentiate its effect. In a study involving ICP-monitored patients, aggressive medical treatment with urea, mannitol, or both adequately controlled ICP and was associated with better outcomes and lower mortality rates.172 The beneficial effect of sustained hyperventilation on ICP is unresolved and has not been examined systematically in patients with this condition. In theory, reduction of ICP by hyperventilation ceases when the pH of cerebrospinal fluid reaches equilibrium. If hyperventilation is instituted for elevated ICP, PCO2 should be maintained between 30 and 35 mm Hg. Induced barbiturate coma and therapeutic hypothermia are considered last ditch options and not part of a standardized algorithm for the treatment of elevated ICP in patients with ICH. Short-acting barbiturates such as thiopental have been shown to effectively reduce elevated ICP.173,174 The effect is presumably mediated through reduction of CBF and cerebral blood volume. In addition to reducing the volume of the normal brain, barbiturates reduce brain swelling and may act as free radical scavengers.1,2 Moderate hypothermia (32°C to 35°C) may be neuroprotective and decreases ICP by lowering cerebral blood volume (by lowering metabolic demand).175
Seizures after Intracerebral Hemorrhage
Seizure activity can result in neuronal injury and worsening of an already critically ill patient and must be treated aggressively. The incidence of immediate seizures after supratentorial parenchymal ICH in published series ranges from 1.4% to 17%.176–183
In a prospective, observational study of 761 consecutive patients with nontraumatic, nonaneurysmal ICH, Passero and associates found the cumulative actuarial risk of experiencing seizures to be 7.2% in the first 5 days and 8.1% within 30 days.183 A lobar location of hemorrhage was also found to be an independent predictor of early seizures, as was previously recognized.176,178–180 Prophylactic antiepileptic therapy may be considered for 1 month. It should be tapered and discontinued if no seizure activity occurs during treatment, although data supporting this therapy are lacking.
Glycerol for Intracerebral Hemorrhage
A double-blind, randomized, placebo-controlled study of 216 patients examined the use of glycerol for the treatment of ICH.184 One hundred seven patients received active treatment and 109 were given placebo. Treatment consisted of 400 mL of 10% glycerol in saline administered over a period of 4 hours on 6 consecutive days. In experimental animals, glycerol reduces cerebral edema without a rebound effect and increases cerebral perfusion. In this study, however, glycerol had no effect on outcome and caused subclinical hemolysis in some patients.
Hemodilution for Intracerebral Hemorrhage
Hemodilution has also been studied in patients with ICH because it increases CBF and decreases blood viscosity in experimental animals. One prospective randomized study sought to answer this question.185 In this study, 164 patients with ICH within 12 hours of onset and a hematocrit higher than 35% were randomized to either hemodilution or control. Eighty-three patients were treated and 81 served as controls. Therapy consisted of removal of 350 mL of blood and infusion of 350 mL of dextran 40 in normal saline. There was no difference in outcome when the hematocrit level was decreased an average of 13%.185
Recombinant Activated Factor VII
As demonstrated by multiple studies, the volume of the hematoma is a critical determinant of mortality and functional outcome after ICH,163,186 and early hematoma growth is an important cause of neurological deterioration.23,162,187,188 Early hematoma growth occurs in the absence of coagulopathy and appears to result from continued bleeding or rebleeding at multiple sites within the first few hours after onset.189 Recombinant activated factor VII (rFVIIa) is approved to treat bleeding in patients with hemophilia, and it has been reported to reduce bleeding in patients without coagulopathy as well.190 Furthermore, a dose escalation safety study found that doses of rFVIIa ranging from 5 to 160 µg/kg of body weight were not associated with a high frequency of thromboembolic complications in patients with acute ICH.191 In 2005, Mayer and coauthors published the results of a double-blind, placebo-controlled trial in which 399 patients with ICH were randomly assigned to receive a single intravenous dose of 40, 80, or 160 µg/kg of rFVIIa or placebo.192 Treatment was instituted within 4 hours after the onset of symptoms. Mortality at 90 days was 29% in patients who received placebo versus 18% in the three rFVIIa groups combined (P = .02). Serious thromboembolic adverse events, mainly myocardial or cerebral infarction, occurred in 7% of rFVIIa-treated patients versus 2% of those given placebo (P = .12). From these data, Mayer and associates concluded that treatment with rFVIIa within 4 hours after the onset of ICH limits growth of the hematoma, reduces mortality, and improves functional outcomes at 90 days despite a small increase in the frequency of thromboembolic adverse events. To confirm the treatment advantage with respect to outcome, a phase III trial labeled with the acronym FAST (Factor Seven for Acute Hemorrhagic Stroke) was conducted by the same group.193 In this trial, 841 patients with ICH were randomly assigned to receive placebo (268 patients), 20 µg of rFVIIa per kilogram of body weight (276 patients), or 80 µg of rFVIIa per kilogram (297 patients) within 4 hours after onset of the ictus. Surprisingly, despite a reconfirmed significant reduction in volume of the hemorrhage, rFVIIa therapy did not improve survival or functional outcome. The proportion of patients with a poor clinical outcome at 90 days did not differ between groups (24% in the placebo group, 26% in the group receiving 20 µg/kg of rFVIIa, and 29% in the group receiving 80 µg/kg).
A well-defined subgroup of warfarin (Coumadin)-treated ICH patients might benefit from rFVIIa therapy. At the present time, there are no clear-cut recommendations, and the treatment modalities used are diverse.194 Successful administration of rFVIIa for treatment of spontaneous ICH in a patient taking oral anticoagulants for a prosthetic heart valve has previously been reported.195
Neuroprotective Agents
There has been some interest in the use of neuroprotective agents. Because ICH causes focal cerebral ischemia,160,196,197 neuroprotectants could interrupt the excitotoxic cascade and prevent neuronal death. In an experimental rat model of ICH, muscimol (γ-aminobutyric acid antagonist) and MK 801 (N-methyl-D-aspartate [NMDA] antagonist) increased the rats’ tolerance to larger hematomas. The area of white matter around the basal ganglia was also better preserved. In another study,160 pretreatment with nimodipine (Ca2+ channel blocker) and D-CPP-ene (NMDA receptor blocker) significantly reduced the ischemic volume and brain edema, respectively, at 24 hours in comparison to no treatment.
Summary
The four randomized medical trials for ICH, two testing steroids,149 one glycerol,184 and one hemodilution,185 did not show significant benefit for any therapy. General measures to control blood pressure, reduce ICP, prevent seizures, and maintain systemic health are important in preventing progression of the hemorrhage, edema, and brain ischemia. Despite disappointing results thus far, these parameters should be controlled aggressively. The optimal reduction in blood pressure needed to perfuse the brain adequately yet prevent rebleeding or progression is controversial. However, the patient’s state of consciousness is the best guide to prognosis1,198 and may help determine the degree of blood pressure and ICP control that should be instituted. If neurological deterioration occurs or ICP cannot be controlled, surgical evacuation should be considered.
Surgical Management
Experimental Rationale
The physiologic effect of ICH on the brain is multifactorial. In clinical practice, patients are particularly susceptible to neurological deterioration within the first 24 hours after hemorrhage,23,94 especially in the first 4 to 6 hours.23,162,163 Neurological status is, however, often affected disproportionate to the anatomic extent of the lesion. Furthermore, studies have failed to correlate elevated ICP with clinical findings.199 Animal models suggest that the hematoma’s effect on local rather than global CBF is responsible for progressive ischemia.159,160,196,200,201 The results of pathologic and experimental studies have indicated that a penumbra of progressive tissue damage and edema develops in regions immediately surrounding a hematoma.202,203 Mechanical injury203,204 as a result of elevated local tissue pressure, decreased CBF,205 reduced plasma levels,161 and inflammation related to accumulated clotting proteins206 and protease induction207 have all been implicated as mediators of this form of secondary injury. Clinical neurological deterioration, which occurs in approximately a third of patients with ICH,93 can develop as a direct consequence of this process.
Nath and coworkers studied the effect of various volumes of blood injected into the caudate nucleus of rats.196 Larger volumes produced larger areas of ischemia at 1 minute, and CBF decreased significantly in areas near the hematoma and ipsilateral frontal lobe. Cerebral perfusion pressure, however, remained unchanged, thus implying the presence of a local squeezing effect on the microcirculation rather than a generalized alteration in perfusion pressure.
Nehls and colleagues studied the amount of ipsilateral caudate blood flow that was below ischemic levels.159 At 5 minutes, 11.5% of the caudate volume was associated with blood flow of less than 25 mL/kg per minute. The ischemic area increased to a maximum of 38.9% at 4 hours. This time course suggested that interventions that reduce hematoma size might decrease ischemia. Therefore, in a subsequent study, the caudate balloon was deflated after 10 minutes in group 1 and after 24 hours in group 2. Group 1 had a higher mean CBF in the caudate nucleus and cortex and smaller areas of ischemia than did group 2. Group 1 also had a better neurological outcome. In further studies, the potential for limiting local ischemia was found to be less if the balloon was deflated after 2.5 hours.160 A pig model using a clot rather than a balloon showed that removal of the clot at 3 hours markedly reduced perihematomal edema and mass effect at 24 hours.161 Eliminating the hematoma prevented its serum proteins from diffusing into the adjacent white matter, thereby preventing subsequent edema. These experiments imply that early evacuation of ICH may decrease the ischemia caused by the hematoma by improving local blood flow, preventing the formation of edema, and preventing local mass effect.
Ropper and Zervas compared lesions made from whole blood, centrifuged blood, and inert plastic in the caudate nucleus.200 In all animals, regional CBF about the hematoma decreased. Relative hyperperfusion in one or both cortices was associated with whole blood on the second and third days, immediately with centrifuged blood, and never with inert plastic. These findings imply that sheer destruction of the caudate nucleus is not entirely responsible for changes in blood flow whereas certain elements within blood may be.
Jenkins and associates compared equal volumetric caudate injections of blood, oil (equal in viscosity to that of blood), and cerebrospinal fluid.201 They evaluated CBF and ischemic cell damage by light microscopy. CBF was immediately reduced adjacent to the lesion in all groups. With blood, however, CBF was reduced over a greater radius and throughout the ipsilateral cortex. In addition, at 4 hours, ischemic damage was present with both blood and oil but not with cerebrospinal fluid. This finding implies that both tissue pressure and vasoactive substances are components in the decreased regional CBF and that both play a role in ischemia at 4 hours.
In rats whose hematomas were “contained” within the caudate nucleus, global cerebral perfusion pressure was unaffected, whereas “unconfined” extension into the ventricles or subarachnoid space reduced cerebral perfusion pressure globally. This finding may explain the poorer outcome in this subgroup and may indicate a need to monitor ICP.160
Effect of Age
Age has a predictive role in the outcome and mortality rate of patients with ICH.162,208–211 It is an important factor that affects microglia and astrocyte reactions and plasticity. Brain swelling was more severe in old rats than in young rats at 3 days after ICH (P < .05).212 There were also more severe neurological deficits in the older rats at 1 day after ICH that persisted for the 4 weeks (P < .05).212 In a prospective outcome study of conservatively treated patients, old age was the most important predictor of a poor outcome.211 In a prospective, randomized trial that compared surgical and conservative treatment, surgically treated patients younger than 60 years had a significantly lower mortality rate than did those older than 60 years (25% versus 65%).209 In surgically treated patients older than 60 years, 67% had poor outcomes (activities of daily living [ADL] score >3), as compared with 50% of the patients younger than 60 years.208 The relationship between age and outcome is even more pronounced with thalamic hemorrhages. Patients who were younger than 59, 60 to 69 and older than 70 years had 59%, 33%, and 17% good or excellent outcomes, respectively. In a retrospective study of patients identified as having a “rapidly progressive” hematoma by serial CT, age older than 65 years was associated with 100% mortality in those whose deterioration prompted surgery. Age is thus an important element in any treatment decision, and age older than 60 years implies a poor prognosis, regardless of treatment.
Effect of Hematoma Volume
Expansion of the hematoma is the most common cause of underlying neurological deterioration within the first 3 hours after the onset of hemorrhage. In the era before CT, the volume of ICH could be inferred only from the angiographic distortion of vessels, thereby yielding imprecise estimates of the size and quality of the hematoma. CT made it possible to quantify the volume of hematomas and elucidate their characteristics, which helped delineate their natural history and clinical outcome. In experimental animal models of ICH, larger volumes were associated with larger areas of ischemia and poorer outcomes.214 In many retrospective* and prospective studies,209 the volume of hematomas based on CT measurements is a strong predictor of functional outcome and death in humans. The progression of hematomas has been examined by serial CT imaging, which helps predict further growth and potential clinical deterioration.23,94
Hematomas can be measured by direct computer imaging or estimated with simple calculations. The volume can be calculated by recording the largest diameter seen on CT, the diameter orthogonal to it, and the number of 1-cm slices on which the hemorrhage can be seen. The total volume can be estimated by using the formula for an ellipsoid, V =  × π × ABC ÷ 8, where A, B, and C represent the respective diameters of the three dimensions.163 Simplified, this equation yields an approximation of ABC/2, which has proved to be quite accurate.216
× π × ABC ÷ 8, where A, B, and C represent the respective diameters of the three dimensions.163 Simplified, this equation yields an approximation of ABC/2, which has proved to be quite accurate.216
Supratentorial Hematomas
In 1977, Hier and colleagues reviewed 5000 CT scans to correlate the volume of putaminal hematomas with clinical findings and prognosis.97 The authors defined three groups. Patients with small hematomas (<35 cm3) showed mild to moderate hemiparesis or hemisensory loss, preservation of higher cortical function, and a good prognosis, regardless of treatment. Patients with moderate hematomas (mean, 120 cm3) had classic flaccid hemiplegia, hemisensory defect, lateral gaze preference, homonymous hemianopia, and either aphasia or apractagnosia. Massive hematomas (>200 cm3) produced coma, fixed and dilated pupils, papilledema, absent eye movements, bilateral fixed plantar response, and rapid death. These correlations suggested that patients with moderate hematomas might be candidates for a controlled clinical comparison of surgical and conservative treatment.
In a retrospective review of 188 patients with supratentorial ICH, Broderick and coworkers showed that hematoma volume was the strongest predictor of the 30-day mortality rate and functional outcome for all locations (putaminal, thalamic, and subcortical).163 The 30-day mortality rates for deep hemorrhages less than 30 mL, between 30 and 60 mL, and greater than 60 mL were 23%, 64%, and 93%, respectively. Mortality rates for lobar hemorrhages at these volumes were 7%, 60%, and 71%, respectively. Half the patients who died did so within the first 2 days. Of 71 patients surviving with hematoma volumes larger than 30 cm3, only 1 (1.4%) was independent at 30 days. In contrast, of the 91 patients who survived with hematoma volumes of less than 30 cm3, 16 (18%) were independent. Combining hematoma volumes with admission Glasgow Coma Scale (GCS) scores proved to be a 97% sensitive and 97% specific test for 30-day mortality. For patients with GCS scores lower than 8 and hematoma volumes greater than 60 cm3, the probability of death at 30 days was 91%, whereas it was 19% for those with GCS scores higher than 9 and hematoma volumes of less than 30 cm3. Even though this study did not aim to evaluate the effectiveness of treatment, operative removal was associated with a decreased 30-day mortality rate, although overall surgical morbidity and mortality rates were not significantly different from those associated with conservative treatment.
Volpin and coworkers retrospectively reviewed the outcome of medical treatment and craniotomy in 132 supratentorial ICHs with respect to hematoma volume, regardless of location.217 When compared with conservative treatment, surgery decreased the mortality rate of comatose patients with hematoma volumes between 26 and 85 cm3, but the probability of discharge with a severe deficit was high. All patients with hematoma volumes greater than 85 cm3 died, irrespective of treatment, and all patients with hematoma volumes of less than 26 cm3 survived without surgery.
In contrast, a retrospective comparison of surgery and conservative therapy in 182 patients with putaminal hemorrhage showed that the size of the hematoma on CT was a statistically significant predictor of outcome regardless of the treatment modality.116 Localized hematomas or those extending into either limb of the internal capsule (groups I to III) were compared with those that extended into both limbs of the internal capsule, the thalamus, or both (groups IV and V). The 30-day mortality rate was significantly lower in groups I to III than in groups IV and V (12% versus 57%).
A randomized, prospective study comparing endoscopic removal of supratentorial ICH with medical management found surgery to be beneficial for hematomas of all volumes, especially subcortical hematomas.209 Interestingly, patients with hematoma volumes of less than 50 cm3 had better outcomes after surgery than after conservative treatment (25% versus 0% with an ADL score of 1), although the mortality rate was the same. For large hematomas (>50 cm3), there was no difference in functional outcome between the two groups, but the mortality rate was lower in the surgical group than in the conservative group (48% versus 90%). This study suggests that surgical evacuation may play a lifesaving role in patients with large hematomas by sparing viable local brain function by decreasing mass effect, progressive edema, or impaired cerebral perfusion. The overall lower surgical mortality rate (30%) in this study in comparison to others may reflect surgical technique and is discussed later in this chapter.
Large-volume thalamic hematomas are more devastating than similarly sized subcortical or putaminal hematomas. Of 29 patients with thalamic hemorrhages, those with volumes greater than 10 mL or with a maximal diameter greater than 3 cm had ADL scores of 4 or 5.216 Thalamic hematomas with a long axis greater than 3 cm are associated with poor outcomes.99,101,115 In a comparison of 75 patients with thalamic hemorrhages who underwent either stereotactic aspiration or conservative treatment, 31 of 40 (78%) surgical patients with hematoma diameters larger than 3.3 cm returned to useful activity in 6 months.210 This finding suggests that the less invasive nature of stereotactic aspiration may improve therapeutic outcomes irrespective of the size and location of the hematoma.
Hematoma volume also seems to be related to the risk for deterioration. In a retrospective study of 182 African Americans, the presence of ICH greater than 30 mL increased the risk for deterioration and death in the first 24 hours by 6.78- and 6.66-fold, respectively.94 Of 46 noncomatose patients, 15 deteriorated during the initial 24 hours. In this study, hematoma volume was a better early predictor of poor outcome than was the admission GCS score.
Understanding the natural time course of an acute ICH and its effect on clinical deterioration is critical to therapeutic decision making. Before CT was available, ICH was considered to be a monophasic event that stopped quickly as a result of clotting and tamponade by the surrounding regions.219 This impression is incorrect, as demonstrated by CT scans showing that hematomas expand over time.23,188 The radiographic progression of a hematoma and its correlation with the clinical course have been well studied. Rehemorrhage typically occurs within the first 6 hours of the primary ictus.162,163 Most of the extravasation of contrast material in the angiograms of patients with ICH is seen within 3 to 6 hours of onset.220,221 If deterioration occurs later than 6 hours after hemorrhage, other factors—edema, hydrocephalus, a new intraventricular hemorrhage, or a metabolic abnormality—must be contributing.
In a study of 419 patients, Fujii and coworkers obtained the first CT scan within 24 hours of onset and a follow-up scan 24 hours after admission.187 They found that the hematomas had enlarged in 14.3% of the patients. In another study, Kazui and colleagues performed sequential CT scans in 204 patients with acute ICH.188 Hematomas enlarged 40% in 20% of the patients. This was seen when the scans were obtained early; none of the patients showed an increase in hematoma size after 24 hours.
To define the progression over time more closely, Brott and associates prospectively studied 103 patients with ICH at all locations who underwent CT within 3 hours of onset.23 The patients were rescanned 1 and 20 hours after the first scan. On the 1-hour follow-up scan, 26% of patients exhibited hematoma growth (>33% enlargement). An additional 12% showed growth between 1 and 20 hours. Therefore, 38% of the patients exhibited hematoma progression within 24 hours of hemorrhage. Of these patients, 33% deteriorated within the first hour, and an additional 25% deteriorated within the next 20 hours. Thus, the clinical condition of more than 50% of all patients showing progression on serial CT deteriorated. This finding implies that early hematoma evacuation may not only reduce perihematomal ischemia160,196,197 and the toxic effect of blood products160,200,201 but also contain potential hemorrhagic progression.
Bae and colleagues reported similar results from their retrospective study of 320 patients who underwent serial CT for ICH.153 Three percent of patients showed rapid hematoma progression. The follow-up scan was performed at a mean of 48.9 hours (range, 10.5 to 149 hours), and 50% of the patients showing progression deteriorated before 24 hours had elapsed. In this study, the most important risk factor for progression was persistent hypertension.
When Qureshi and coworkers retrospectively reviewed 182 African American patients to identify independent predictors of early deterioration and death, 23% of the patients with GCS scores higher than 12 showed early deterioration (mean, 7.9 hours).94 An ICH volume greater than 30 mL and ventricular extension were independent predictors of early deterioration (OR, 6.78 and 4.67, respectively) and death (OR, 6.66 and 4.23, respectively).
The goals of surgical evacuation of a hematoma are to reduce the mass effect, block the release of neuropathic products from the hematoma, and prevent prolonged interaction between the hematoma and normal tissue, which can initiate pathologic processes.222 From the data available it is clear that within the spectrum of ICH there are some patients (with large or space-occupying ICHs) who require surgery for neurological deterioration and others with small hematomas who should be managed conservatively. There is equipoise about the management of patients between these two extremes. Some patients have a penumbra of functionally impaired but potentially viable tissue around the ICH. Surgical removal of the clot may improve the function and recovery in this penumbra.169 As suggested by the aforementioned studies, early surgery may play an important role in preventing secondary deterioration, and the timing of surgery becomes an important question that needs to be elucidated.
Timing of Surgery
Credible experimental evidence indicates that early evacuation of hematomas improves CBF,159,160,197,200,201 histologic changes,202 brain edema,161 ischemia,160 and outcome.197 The natural history of ICH reveals that 50% of related deaths occur within 48 hours of hemorrhage163 and that radiographic expansion or rebleeding occurs maximally within 3 to 4 hours but for as long as 24 hours thereafter.23 Therefore, early surgery may improve the outcome for many reasons. Extensive clinical evidence also supports early surgery.223–225 A single lenticulostriate branch rupture that bleeds for a brief time creates a significant hypertensive hematoma.226 Consequently, direct early vessel coagulation seems to be advantageous. Exacerbation occurs suddenly and most often within 4 to 6 hours of bleeding23; thus, early surgery may prevent clinical progression. Because secondary changes such as edema occur 7 to 8 hours after a hemorrhage, evacuation before that time may prevent these changes.
In an important retrospective study of ultra-early surgery, Kaneko and colleagues224 reviewed 100 putaminal hemorrhages, all of which were operated on within 7 hours. All patients were hemiplegic, with GCS scores between 6 and 12 and hematoma volumes greater than 20 to 30 cm3. The mortality rate was 7%, and the 6-month rate of “useful recovery” was 83%. Two patients died of rapid exacerbation before surgery, and two died of reaccumulation of hematoma. These results are favorable when compared with the series of Yukawa and Kanaya, which did not emphasize early surgery (28.6% mortality rate and 62.8% rate of useful recovery).227 The patients of Kaneko and colleagues had better immediate preoperative neurological grades,224 thus implying that earlier surgery limited the time available for further deterioration. Their study, however, did not address patients with GCS scores of 13 and hematoma volumes between 20 and 30 cm3.
These results are supported by a retrospective analysis in which it was shown that a subgroup of patients with moderate-sized putaminal hematomas had better outcomes when operated on within 6 hours of hemorrhage.225 In a prospective study by Juvela and associates, 52 patients with GCS scores between 7 and 10 did not benefit from surgery performed after 24 hours, but mortality rates improved when surgery took place within 13 hours.228
A randomized, controlled, prospective trial evaluated the feasibility of early surgery.229 The median onset from the time of hemorrhage to hospitalization was 3.3 hours, and the time to surgery was 8.5 hours (beyond the range of <6 hours). There was no difference in outcome between surgery and medical treatment, but there was a trend toward a lower 3-month morbidity rate with surgery. This study suggests that there are logistic barriers to ultra-early surgery, and this is where the bulk of the “brain attack” effort is directed (i.e., educating primary physicians, paramedics, and the public).
The need to gain robust evidence to support clinical decision making led to initiation of the Surgical Trial in Intracerebral Hemorrhage (STICH) funded by the Medical Research Council and the Stroke Association, which was activated in 1998.230 This prospective randomized trial compared early surgery with initial conservative treatment of patients with spontaneous supratentorial ICH. Early surgery combined hematoma evacuation (within 24 hours of randomization) with best medical treatment. Initial conservative treatment used best medical treatment, although delayed evacuation was allowed if it became necessary. Analysis was on an intention-to-treat basis. At 6 months, of 468 patients randomized to early surgery, 122 (26%) had a favorable outcome as compared with 118 (24%) of 496 randomized to initial conservative treatment (OR, 0.89 [95% CI, 0.66 to 1.19]; P = .414). These results suggested no overall benefit from early surgery when compared with initial conservative treatment. Detailed analysis of the CT images from STICH has shown that 42% of patients included in STICH had an associated intraventricular hemorrhage. The prognosis for patients with intraventricular hemorrhage with or without hydrocephalus is much worse than that for those with ICH alone. Removing these patients from the analysis plus focusing on superficial hematomas presents a better picture for surgery. When the prognosis-based Rankin score was used as the outcome variable, a significant benefit was observed for surgical patients in this subgroup (P = .013). Further analysis of the subgroup of patients with lobar hematomas from the trials of Auer and colleagues209 and Teernstra and associates231 supports the hypothesis that this subgroup might benefit from early surgery. This evidence supports the ongoing STICH-II trial, whose purpose is to evaluate the role of early surgery for superficial supratentorial lobar hematomas without intraventricular hemorrhage.
Infratentorial Hematomas
With cerebellar hemorrhages, identifying the appropriate clinical progression is paramount to guiding surgical evacuation. When hematomas are near the brainstem, however, irreversible deterioration can occur without warning. Many studies recommend surgery for all hematomas greater than 3 cm in diameter.96,123,232 Smaller lesions have a more benign course. However, the patient’s clinical profile must still be monitored carefully because spatial accommodation is, by necessity, more limited here than supratentorially.
Cerebellar hemorrhage tends to progress rapidly and to cause death because of its proximity to the brainstem. It has been recommended that patients with GCS scores of 13 or less or with hemorrhages of 4 mL or greater should undergo surgical evacuation.233 Other authors, however, contend that rapid progression of particular cerebellar and cranial nerve syndromes (see earlier) represents a surgical emergency despite these criteria.92,234
Timing of Surgery
With cerebellar hemorrhage, ultra-early surgery is vital in patients with signs of brainstem compression. At the earliest sign of clinical change, immediate evacuation without CT can save life and function. Without brainstem signs, however, the timing of surgery is controversial. Ott and associates studied the natural history of cerebellar hemorrhage.123 Their review of 56 patients with cerebellar hemorrhage showed that 9 of 28 patients treated conservatively died precipitously up to 2 weeks after hemorrhage. Fifty percent deteriorated within 2 days, and an additional 25% deteriorated within 1 week. Only after 8 days did awake or drowsy patients treated conservatively have a lower mortality rate than those undergoing surgery. Therefore, patients initially seen in the first week of hemorrhage may still benefit from surgery.
Yanaka and coworkers support the notion that even deeply comatose patients with cerebellar hemorrhage benefit from surgical evacuation, provided that evacuation is immediate, especially if the time between coma onset and surgery can be kept below 2 hours.125
Surgical Techniques
In 1903, Cushing first removed an intracerebral hematoma by craniotomy.8 In 1932, Bagley first described indications for removal based on location.9 In 1950, Fazio, a neurologist, suggested surgical removal based on pathologic studies.235
In 1961, McKissock and coworkers published the first pessimistic view of surgical treatment of ICH.1 They reported a 51% mortality rate in 244 operated patients and a 100% mortality rate in comatose patients. Since then, outcome studies have yielded controversial results. Operative mortality can range from 20% to 90% in comatose patients with deep ganglionic or thalamic bleeding.1,148,236 Most studies, however, have either failed or lacked the power to stratify patients appropriately or to identify the optimal timing of surgery in a well-designed, randomized, prospective manner (see earlier discussion). Because of this controversy, various less invasive methods of removal are practiced around the world: simple aspiration, stereotactic aspiration, fibrinolytic treatment, mechanically assisted aspiration, and endoscopy.237 In certain circumstances, some of these techniques may be more efficacious for deep putaminal or thalamic hemorrhages. Others are beneficial for subcortical hematomas. These techniques and the use of standard craniotomy for ICH are detailed in this section.
Table 358-2 summarizes the clinical retrospective studies that have compared surgery and medical management.
TABLE 358-2 Summary of Retrospective Trials: Surgery versus Medical Therapy for Intracerebral Hemorrhage
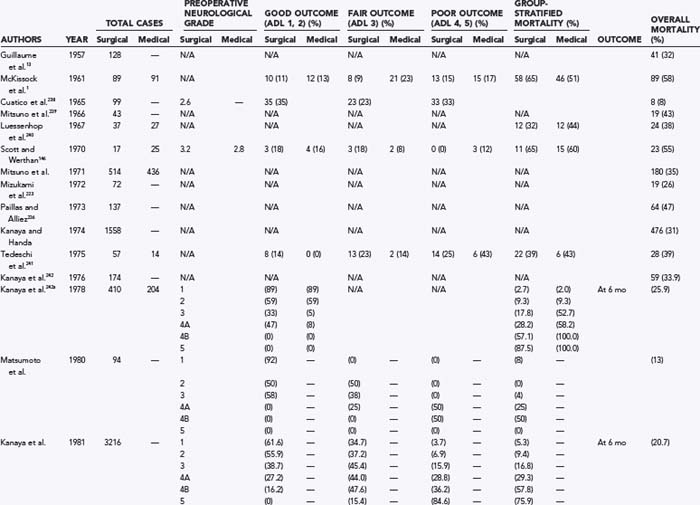
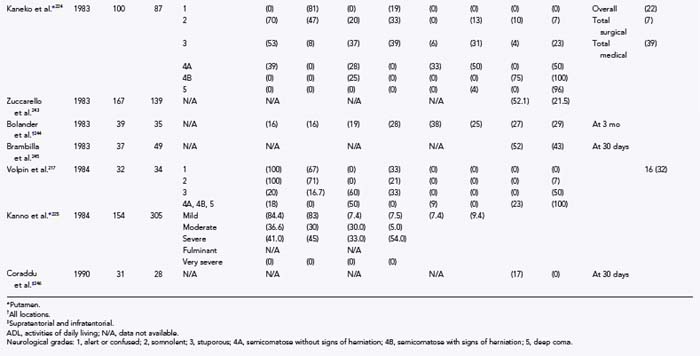
Craniotomy
We treat a putaminal hemorrhage as a microsurgical lesion requiring meticulous surgical technique to avoid adding insult to injury (Figs. 358-6 and 358-7). Accordingly, we favor a transcisternal-transsylvian-transinsular approach.247 The hematoma often extends to within millimeters of the insular cortex, and a small 2-cm insular corticotomy is often all that is needed to evacuate the largest hematoma. The operating microscope is used routinely, and its improved illumination and magnification make identification and bipolar coagulation of cavity wall bleeders straightforward. Alternatively, we rely on a malleable graduated suction device to suction and handle tissue.
Extreme caution is used to avoid traumatic manipulation of the capsular fibers at the depth of the surgical cavity. Hemostasis is ensured by elevating systolic pressure temporarily to identify potential rebleeding sites.247 Otherwise, blood pressure is maintained postoperatively in the normal range for the specific patient. If the hematoma extends significantly into the temporal lobe, the transtemporal approach can be used. The transfrontal approach is rarely used and is almost obsolete because the surgical track is, by necessity, deep. Suzuki and Sato once reported that the functional prognosis was better with the transsylvian than with the transcortical approach.248 Kanaya and Kuroda advocated transcortical approaches for large hematomas.249 The general surgical principles for evacuating hematomas at other locations also follow commonsense strategies: corticotomies are placed near the epicenter of the ICH, their length is minimized, and above all, eloquent tissue is avoided.
For infratentorial hematomas, a suboccipital craniotomy with the patient in the prone or lateral position is standard. Most commonly (i.e., for unilateral hemorrhage in the deep cerebellar nuclei), a paramedian straight incision is used. We strongly advocate a bone flap craniotomy rather than a craniectomy; the latter is slower, produces less pleasing cosmetic results, and may cause craniectomy headaches. A ventriculostomy is often necessary to relieve hydrocephalus.250 Figure 358-8 shows an example of evacuation of a cerebellar hematoma, coupled with postoperative intrathecal clot lysis with rt-PA, which resulted in prompt resolution of the intraventricular hematoma.
Burr Hole Aspiration
Theoretically, if evacuation were adequate, an expedient and simple procedure such as bur hole aspiration would be the optimal treatment approach. However, the unpredictable consistency of hematomas makes aspiration difficult. There is also a propensity to rebleed, which makes the lack of visualization riskier. Experimentally, within only hours of clot genesis, 80% of the clot becomes dense fibrous tissue.218 In 175 patients treated by stereotactic aspiration, 134 (76.6%) had more than 50% of the clot removed, and 13 (7.4%) exhibited postoperative rebleeding.251 Other series have reported poor localization of the hematoma and inadequate removal with this technique.252 These stereotactic results show the major limitations of simple aspiration: low effectiveness and high rates of recurrence.
Stereotactic Aspiration
Benes and coworkers first reported the use of stereotactic techniques in 1965 and achieved limited success in nontraumatic hematomas.253 In 1978, Backlund and von Holst performed the first successful stereotactic aspiration of an acute hemorrhage.254 Although no randomized, prospective, controlled studies have compared stereotactic aspiration with craniotomy and conservative therapy, studies claim favorable outcomes, especially with deep-seated lesions. Honda and associates retrospectively compared stereotactic aspiration and medical therapy for thalamic hemorrhages.255 Patients with hematomas smaller than 2.5 cm in diameter had significantly better ADL scores with aspiration. In addition, patients whose lesions were CT grade IIB or more favorable had better outcomes with aspiration. In a more recent study, a multiple-target aspiration technique was used in 64 consecutive patients with spontaneous hematomas within the basal ganglia.256 With use of this technique they were able to aspirate more than 80% of the hematoma volume in 73.4% of the patients. However, for fear of postoperative rehemorrhage, they did not treat these patients with this method before the third day after the onset of symptoms.
As interest in this technique increased, it was realized that certain shapes, consistencies, and locations make hematoma evacuation difficult. To make hematomas more amenable to aspiration, various devices and techniques have been developed and modified. Such developments include equipment that aims at physical fragmentation of the clots via the Archimedes screw principle,257,258 devices using high-pressure fluid irrigation,259,260 ultrasonic aspirators,261 or the Nucleotome probe.222 Chemical fragmentation by repeated injection of urokinase or (rt-PA) into the hematoma cavity was introduced to liquefy the clot and facilitate subsequent aspiration.210,251,258,262–265
Table 358-3 summarizes the clinical retrospective studies that have addressed stereotactic evacuation.
Fibrinolysis with Clot Aspiration
Fibrinolysis is used to facilitate clot dissolution by activating plasminogen, which in turn dissolves fibrin. In experimental animal models, adding fibrinolysis to stereotactic removal significantly improved CBF at 24 hours when compared with placebo.270,271 In this method, the hematoma is first evacuated by simple needle aspiration under CT guidance through a bur hole. The CT localization procedure can be done with a stereotactic device or by projecting the hematoma onto the scalp itself with a radiopaque marker derived from the images. Direct-image projection can be done on any CT scanner, and no special apparatus is needed. The disadvantage of the projection method is an approximately 5-mm error in comparison to stereotaxy.266
After localization, a bur hole is made with the patient under local anesthesia, and a 3- to 4-mm silicone tube is passed into the clot. The hematoma is aspirated with a syringe repeatedly until no more clot can be removed. A Dandy ventricular catheter is then placed into the hematoma bed, and urokinase (6000 units in 3 mL) is infused.262 Most studies use 6000 units of urokinase 2 to 4 times a day for 1 to 6 days until CT documents clot dissolution. In a canine model of intraventricular hemorrhage, urokinase caused clot lysis within 3 to 6 days, whereas in control groups, lysis took more than 7 days.272
Both urokinase and t-PA have been studied, but urokinase is used much more often. Urokinase is less expensive than t-PA, has a longer half-life, and has both fibrinolytic and fibrinogenolytic activity. Therefore, urokinase not only dissolves existing clot but also inhibits the formation of new ones.237 However, in an experimental animal study, the rate of hematoma dissolution after 6 hours of either t-PA or urokinase treatment was 89% for the former and 16.4% for the latter, thus suggesting that t-PA is more effective.273 Whether t-PA is more beneficial clinically has yet to be studied in a controlled setting.
An additional risk of fibrinolysis is rebleeding. Fibrinolytics exert their effect on the hematoma and protective fibrin clot. Therefore, in most studies, stereotactic aspiration is performed no earlier than 6 hours after hemorrhage. A study of serial CT scans suggested that the primary hemorrhage is completed within 6 hours,162 thus implying that hemostasis can be achieved by 6 hours and that the likelihood of inducing rebleeding can be reduced.
In Japan, where the incidence of spontaneous hematomas is high, there is increasing interest in stereotactic evacuation. Niizuma and coworkers published three consecutive retrospective reports on the effectiveness and potential pitfalls of stereotaxy and fibrinolysis.210,251,263 In 1985, 97 patients with ICH at all locations (cortex, thalamus, basal ganglia, cerebellum) and of all neurological grades were retrospectively reviewed within 24 hours of admission.263 Only 58% of the patients had 50% or more of the hematoma volume evacuated by initial aspiration before urokinase was administered. After urokinase infusion, the hematomas were 80% resolved in more than 70% of patients. The rebleeding rate was 7% (4% major, 3% minor), and the mortality rate was 3%.
In 1989, the same authors reported on 241 consecutive putaminal hematomas, 175 of which were evacuated stereotactically.251 Seventy-seven percent of the patients had 50% of the hematoma volume evacuated during the initial aspiration, and 82% of patients had 80% or better resolution after treatment with urokinase. Notably, 52 patients did not require fibrinolysis because their initial evacuation volumes were greater than 80%. The rebleeding rate of 7.4% (4% major, 3.4% minor) most likely underestimates the true rebleeding rate after fibrinolysis because just 30% of the patients did not require infusion. This improvement on initial aspiration of the hematoma may reflect improved technique and the particularity of the putaminal location.
In 1989, Niizuma and coworkers published a refined variation of stereotactic removal called “double track aspiration.”251 This technique enabled the evacuation of a larger proportion of the hematoma. The authors suggested that a hematoma is somewhat harder at its center, which is therefore more difficult to aspirate than its periphery. Consequently, the authors made two passes in which they aspirated anteriorly and then posteriorly. During the initial aspiration, a mean of 77% of the hematoma volume was evacuated, and 44% of the patients did not require treatment with urokinase. There were only 9 patients in the study, but none rebled. In the same study, stereotactic aspiration with craniotomy was compared retrospectively with conservative therapy in 241 patients with putaminal hematomas. Patients who were past 6 hours beyond ictus with a hemorrhage larger than 8 mL and lacked antigravity strength in the contralateral limb underwent stereotactic aspiration. Stuporous or semicomatose patients with a hematoma larger than 40 cm3 and clinical progression or those who were initially seen within 6 hours underwent craniotomy. The remainder received medical therapy. With stereotactic aspiration, more than 50% of the hematoma volume was aspirated in 77% of the patients. The rebleeding rate was 7.4% (3.4% major, 4% minor); 26% had a bleeding diathesis. There was no overall difference in outcome at 6 months between stereotaxis and craniotomy, and 80% of all stereotactic patients returned to useful lives. The findings suggested that stereotactic aspiration is an effective treatment of deep-seated lesions but should probably be avoided in coagulopathic patients.
The potential role of combining clot lysis with stereotactic aspiration was studied by Teernstra and colleagues in the Stereotactic Treatment of Intracerebral Hematoma by Means of a Plasminogen Activator (SICHPA) trial.231 In this prospective multicenter randomized trial, 36 of 71 patients were randomized to the surgical group within 72 hours of onset. Inclusion criteria were age older than 45 years, spontaneous supratentorial ICH greater than 10 mL, and Glasgow eye motor scores between 2 and 10. A statistically significant reduction in hematoma volume was noted in the surgically treated group. There was no significant reduction in 6-month mortality in the surgical group (56% and 59% in the surgical and medical treatment groups, respectively). With urokinase thrombolysis in the surgical group, a 22% rebleeding rate resulted and thus was deemed crucial in negating any benefit of reduced lesion mass.
Nonetheless, deep-seated lesions may respond to stereotactic aspiration better than to medical or surgical therapy. A review of 75 patients with thalamic hemorrhages treated by stereotactic evacuation showed that 44% were living independently at 6 months and 32% needed assistance.210 The mortality rate of 13% compared favorably with the 38% to 50% mortality rate associated with conservative treatment.99,101,274 This finding is consistent with a previous review of 99 cases of stereotactically aspirated thalamic hemorrhages, which were associated with a mortality rate of 23%.255 In a study of 175 putaminal hematomas by Niizuma and coworkers, 52% of the stereotactically aspirated group achieved independent outcomes (excellent or good grades) at 6 months, whereas only 7% of the craniotomy group achieved similar recoveries.251 The mortality rate was 6% and 7% in the stereotactic and craniotomy groups, respectively. Notably, patients undergoing immediate craniotomies had larger hematomas and worse neurological grades. Hondo and colleagues reported more than 400 stereotactic aspiration procedures in patients with hypertensive ICH.261,275 The 6-month outcome in patients with basal ganglionic ICH was better in those who underwent stereotactic aspiration than in those who underwent conventional surgery or best medical treatment alone.
A recent study by Vespa and associates using frameless stereotactic aspiration of deep intracerebral hematomas followed by local instillation of rt-PA reported promising safety results, including improved neurological outcomes that correlated well with the degree of hematoma removal.276 This study demonstrated not only an improvement in the level of consciousness but also an improvement in motor scores on the affected side.
Fragmentation Device–Assisted Aspiration
Devices that can physically fragment a hematoma are used to facilitate aspiration. In 1978, Backlund and von Holst first described aspiration of an acute hemorrhage with a “screw and suction” technique.254 This concept is based on the Archimedes water screw principle and involves inserting a long twist drill through a cannula and the application of suction. The rotating helical mandrel cuts portions of the clot, which are sucked into the cannula. This maneuver facilitates suctioning of hard clot. In 1982, Broseta and colleagues applied the technique to intracerebral hematomas in 16 patients and obtained a 13% rebleeding rate and a postoperative mortality rate of 81%.277 Using a thinner screw and a walled cannula and adding a motor, Kandel and Peresedov treated 32 patients in “grave condition.”257 Clot was removed completely in 88% of the patients, but the rebleeding rate was 16% and the mortality rate was 22%. These results suggest that fragmentation device–assisted removal of hematomas is associated with relatively high rates of rebleeding and excessive fibrinolysis. Moreover, it is notably time-consuming, and the degree of clot removal is variable and unpredictable.
One study of 28 putaminal and 18 thalamic hematomas evacuated with the Archimedes screw yielded good results.267 The average initial hematoma volume was 23 cm3 (range, 6 to 37 cm3), with an average aspiration volume of 81% (range, 54% to 100%). Only one patient hemorrhaged immediately after surgery. In general, the motor function of patients with thalamic hematomas improved, regardless of their preoperative functional condition or extension of blood into the internal capsule. Among the patients with putaminal hematomas, two thirds of those with severe preoperative motor weakness remained hemiplegic. This study contradicted previous data and suggested that use of the Archimedes screw could be associated with low rates of rebleeding and good motor recovery, especially with thalamic hemorrhages. This difference may reflect relatively smaller hematoma volumes and better preoperative neurological function.
Other less widely accepted devices include water irrigation systems, ultrasonic aspirators, and oscillating cutters. The stereotactic “aqua-stream” and aspirator is an automatic irrigator that effectively aspirates clot in vitro.259 In 1987, Matsumoto and associates used an ultrasonic aspiration device similar to the Cavitron on 375 patients and reported better outcomes than with either medical management or conventional craniotomy.275
The oscillating cutters contain a guillotine blade that is oriented parallel to the surface of the hematoma, and it is encased in a suctioning-irrigating cannula. The tip is closed and has an open side port for aspiration of the clot, which is amputated and removed with saline through a reservoir. The irrigation rate, vacuum pressure, and cutting speed were adjustable.237 An in vitro clot model with a vacuum pressure of 150 mm Hg dissolved 75% of a 4-hour-old clot in 15 minutes. However, the device was cumbersome and the procedure was time-consuming. The U.S. trial was terminated.
Neuroendoscopic Techniques
Endoscopically assisted keyhole approaches applied with the right indications potentially provide maximum efficiency for removal of lesions, maximum patient safety, and minimal invasiveness.209,278–280 Auer and associates randomized 100 patients with supratentorial ICH (subcortical, thalamic, or putaminal), aged 30 to 80 years, to receive either medical therapy or endoscopic evacuation of the hematoma.209 Patients were admitted to one hospital between 1983 and 1986. Inclusion criteria were as follows: hematoma greater than 10 cm3, neurological deficit or impaired consciousness, less than 48 hours elapsed from the onset of symptoms, medical clearance, and negative unilateral angiography. A 6-mm-diameter neuroendoscope (manufactured by Storz, Tuttlingen, West Germany) was placed through a bur hole and guided by intraoperative ultrasonography. Once the cavity was reached, it was rinsed intermittently with artificial body-temperature cerebrospinal fluid at 10 to 15 mm Hg through one channel while interval suctioning was performed through another. Hemostasis was achieved with a neodymium:yttrium-aluminum-garnet laser. An external drain was placed and left for days.
In 2006, Cho and coauthors published a series of 90 noncomatose patients with spontaneous basal ganglia hemorrhage randomized to treatment with either endoscopic surgery, stereotactic aspiration, or craniotomy.281 The endoscopic group had better Functional Independence Measure scores than the craniotomy group did (P = .001). The Barthel index was significantly better in the endoscopic group (50.45 ± 28.59) than in the craniotomy group (16.39 ± 20.93; P = .006). Patients in the endoscopic group showed greater improvement in the affected limbs than did patients in the craniotomy group (P = .004).
Similarly, Marquardt and colleagues reported that patients with deep-seated hematomas who were treated by stereotactic aspiration had markedly improved levels of consciousness and higher GCS scores.282
Thus, deep-seated lesions may lend themselves to minimally invasive operative procedures. This is being investigated and evaluated in the Minimally Invasive Surgery plus rt-PA for Intracerebral Hemorrhage Evacuation (MISTIE) trial.283 This ongoing prospective randomized controlled trial set out to investigate patients with deep-seated clots treated by bur hole aspiration and evacuation with a variety of devices, including hemolytic and thrombolytic drugs.
Table 358-4 summarizes the clinical prospective studies that have compared surgery and medical management.
Intracranial Pressure Monitoring
Papo and coworkers retrospectively reviewed a mixture of 66 conservatively and surgically treated patients who underwent either intermittent or continuous ICP monitoring.199 In all patients with a normal level of consciousness, ICP was less than 20 mm Hg; all comatose patients had very high ICP. In patients with intermediate levels of consciousness, however, there was no correlation between ICP and clinical course. Surgical treatment had mixed effects on ICP. If surgery was performed early, ICP decreased but did not return to preoperative levels. If surgery was performed late, either there was no change in ICP or it dropped initially and later returned to preoperative levels. These findings imply that ICP correlates poorly with outcome and that secondary destruction of brain tissue by the hematoma may be reversible if it is addressed early enough.
To further investigate the destructive effect of a hematoma and the optimal timing of its evacuation, Nakayama and colleagues studied ICP in surgically treated patients with putaminal ICH who had severe neurological deficits (semicomatose).286 Patients with smaller hematomas evacuated before 8 hours had lower ICP than did those with larger hematomas evacuated after 8 hours. In this setting, high ICP correlated with death and poor outcome. In this group, elevated ICP may reflect the secondary effects of hematoma-induced alterations in the microcirculation because experimentally, these effects are more pronounced when larger hematomas are evacuated late.159,160,196,197 ICP monitoring may help define a subgroup of comatose patients with elevated ICP who will benefit from early evacuation. Ropper and King also studied ICP in 10 comatose patients; none of the 3 patients with ICP less than 20 mm Hg died.287 Despite best medical treatment, there were four brain deaths among the 7 patients whose ICP was greater than 20 mm Hg. All conservatively treated patients died, whereas 3 patients with refractory ICP who underwent surgery survived. Therefore, ICP monitoring may guide therapy in deeply comatose patients whose ICP is elevated and thereby help prevent death.
In terms of response to medical treatment in a similar subgroup, Duff and associates showed that ICP monitoring resulted in improved outcome when treatment was guided by elevated pressures.172 Elevated ICP readings in patients with ICH also correlate with CT findings of lateral ventricular compression, intraventricular hemorrhage, and compression of the basal cisterns.288
Batjer HH, Reisch JS, Allen BC, et al. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103-1106.
Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987-993.
Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783-1787.
Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke. 1983;14:493-500.
McKissock W, Richardson A, Taylor J. Primary intracerebral haemorrhage; a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;2:221-226.
Mendelow A. Surgical trial for lobar intracerebral haemorrhage (STICH II). http://www.ncl.ac.uk/stich/, 2007. Available at
Mendelow AD. Mechanisms of ischemic brain damage with intracerebral hemorrhage. Stroke. 1993;24(12 Suppl):I115-I117.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397.
Mendelow AD, Unterberg A. Surgical treatment of intracerebral haemorrhage. Curr Opin Crit Care. 2007;13:169-174.
Qureshi AI, Safdar K, Weil J, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764-1767.
1 McKissock W, Richardson A, Taylor J. Primary intracerebral haemorrhage; a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;2:221-226.
2 Magladery J. The natural course of cerebrovascular hemorrhage. Clin Neurosurg. 1963;9:106-113.
3 Walton J. Subarachnoid Hemorrhage. Baltimore: Williams & Wilkins; 1956.
4 Donley J, Wepfer JJ. A renaissance student of apoplexy. Bull Johns Hopkins Hosp. 1909;20:1-2.
5 Clarke E. Cerebrovascular system—historical aspects. In: Newton T, Potts D, editors. Radiology of the Skull and Brain, Vol 2. Great Neck, NY: CV Mosby; 1974:875-889. book 1
6 Fazio C, Sacco G, Bugiani O. The thalamic hemorrhage. An anatomo-clinical study. Eur Neurol. 1973;9:30-43.
7 MacEwen W. An address on the surgery of the brain and spinal cord. BMJ. 1888;2:302-309.
8 Cushing H. The blood-pressure reaction of acute cerebral compression, illustrated by cases of intracranial hemorrhage. Am J Med Sci. 1903;125:1017-1044.
9 Bagley C. Spontaneous cerebral hemorrhage: discussion of four types, with surgical considerations. Arch Neurol Psychiatry. 1932;27:1113-1174.
10 Robinson G. Encapsulated brain hemorrhages. A study of their frequency and pathology. Arch Neurol Psychiatry. 1932;27:1441-1444.
11 Craig W, Adson A. Spontaneous intracerebral hemorrhage: etiology and surgical treatment with a report of nine cases. Arch Neurol Psychiatry. 1936;35:701-714.
12 Penfield W. The operative treatment of spontaneous intracerebral hemorrhage. Can Med Assoc J. 1933;28:369-379.
13 Guillaume J, Roger R, Maxars G, et al. Indications chirurgicales dans les hemorragies cerebrales. Presse Med. 1957;65:827-829.
14 Lazorthes G. Surgery of cerebral hemorrhage; report on the results of 52 surgically treated cases. J Neurosurg. 1959;16:355-364.
15 Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491-499.
16 O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240-245.
17 Feigin V, Anderson N, Gunn A, et al. The emerging role of therapeutic hypothermia in acute stroke. Lancet Neurol. 2003;2:529.
18 Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391-e413.
19 Broderick JP, Brott T, Tomsick T, et al. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188-191.
20 Qureshi AI, Giles WH, Croft JB. Racial differences in the incidence of intracerebral hemorrhage: effects of blood pressure and education. Neurology. 1999;52:1617-1621.
21 Sturgeon JD, Folsom AR, Longstreth WTJr, et al. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718-2725.
22 Massaro AR, Sacco RL, Mohr JP, et al. Clinical discriminators of lobar and deep hemorrhages: the Stroke Data Bank. Neurology. 1991;41:1881-1885.
23 Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
24 Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060-2065.
25 Hanggi D, Steiger HJ. Spontaneous intracerebral haemorrhage in adults: a literature overview. Acta Neurochir (Wien). 2008;150:371-379.
26 Foulkes MA, Wolf PA, Price TR, et al. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547-554.
27 Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190-1195.
28 Woodward M, Huxley H, Lam TH, et al. A comparison of the associations between risk factors and cardiovascular disease in Asia and Australasia. Eur J Cardiovasc Prev Rehabil. 2005;12:484-491.
29 Neaton JD, Wentworth DN, Cutler J, et al. Risk factors for death from different types of stroke. Multiple Risk Factor Intervention Trial Research Group. Ann Epidemiol. 1993;3:493-499.
30 Leppälä JM, Virtamo J, Fogelholm D, et al. Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke. 1999;30:2535-2540.
31 Thrift AG, McNeil JJ, Forbes A, et al. Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Melbourne Risk Factor Study (MERFS) Group. Stroke. 1996;27:2020-2025.
32 Kase CS, Mohr JP, Caplan LR, et al. Intracerebral hemorrhage. In: Barnett HH, Mohr JP, Steen BM, et al, editors. Stroke: Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone, 1999.
33 Cole F, Yates PO. Pseudo-aneurysms in relationship to massive cerebral hemorrhage. J Neurol Neurosurg Psychiatry. 1967;30:61-66.
34 Charcot J, Bouchard C. Nouvelles recherches sur la pathogenie de lhemorrhagie cerebrale. Arch Physiol Normale Pathol. 1868;1:110-127. 643-665, 725-734
35 Russell R. Observations on intracerebral aneurysms. Brain. 1963;86:425-442.
36 Cole FM, Yates PO. The occurrence and significance of intracerebral micro-aneurysms. J Pathol Bacteriol. 1967;93:393-411.
37 Fisher C. Clinical syndromes in cerebral hemorrhage. In: Fields E, editor. Pathogenesis and Treatment of Cerebrovascular Disease. Springfield, IL: Charles C Thomas, 1961.
38 Fisher C. Cerebral miliary aneurysms in hypertension. Am J Pathol. 1972;66:313-324.
39 Fisher C. The arterial lesions underlying lacunes. Acta Neuropathol. 1969;12:1-15.
40 Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14:915-923.
41 Gilles C, Brucher JM, Khoubessarian P, et al. Cerebral amyloid angiopathy as a cause of multiple intracerebral hemorrhages. Neurology. 1984;34:730-735.
42 Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637-649.
43 Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26:1471-1477.
44 Kase CS, Robinson RK, Stein RW, et al. Anticoagulant-related intracerebral hemorrhage. Neurology. 1985;35:943-948.
45 Franke CL, de Jonge J, van Swieten JC, et al. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726-730.
46 Rosand J, Eckman M, Knudsen KA, et al. Effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880-884.
47 Chimowitz M, Lynn M, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New Engl J Med. 2005;352:1305-1316.
48 Mayo NE, Levy AR, Goldberg MS. Aspirin and hemorrhagic stroke. Stroke. 1991;22:1213-1214.
49 Caplan L. Intracerebral hemorrhage. In: Tyler H, Dawson D, editors. Current Neurology, Vol 2. Boston: Houghton Mifflin; 1979.
50 Chamorro A, Vila N, Saiz A, et al. Early anticoagulation after large cerebral embolic infarction: a safety study. Neurology. 1995;45:861-865.
51 Camerlingo M, Casto L, Censori B, et al. Immediate anticoagulation with heparin for first-ever ischemic stroke in the carotid artery territories observed within 5 hours of onset. Arch Neurol. 1994;51:462-467.
52 Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
53 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
54 Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-1251.
55 Hamann GF, del Zoppo GJ, von Kummer R. Hemorrhagic transformation of cerebral infarction—possible mechanisms. Thromb Haemost. 1999;82(Suppl 1):92-94.
56 Hart R. What causes intracerebral hemorrhage during warfarin therapy. Neurology. 2000;55:907-908.
57 Roob G, Schmidt R, Kapeller P, et al. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991-994.
58 Gorter J. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Fibrillation Trial (EAFT) study groups. Neurology. 1999;53:1319-1327.
59 The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin. Ann Neurol. 1997;42:857-865.
60 Go A, Hylek E, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagultion and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
61 Smith E, Rosand J, Knudsen KA, et al. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193-197.
62 Nilsson OG, Lindgren A, Ståhl N, et al. Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden. J Neurol Neurosurg Psychiatry. 2000;69:601-607.
63 Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449-1457.
64 Hylek E, Singer D. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897-902.
65 Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med. 1996;156:409-416.
66 Perry DJ. Factor VII deficiency. Br J Haematol. 2002;118:689-700.
67 Mariani G, Herrmann F, Dolce A, et al. Clinical phenotypes and factor VII genotype in congenital factor VII deficiency (International Factor VII Deficiency Study Group). Thromb Haemost. 2005;93:481-487.
68 Yasaka M, Minematsu K, Naritomi H, et al. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89:278-283.
69 Flibotte JJ, Hagan N, O’Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059-1064.
70 Delaney P, Estes M. Intracranial hemorrhage with amphetamine abuse. Neurology. 1980;30:1125-1128.
71 Kase CS, Foster TE, Reed JE, et al. Intracerebral hemorrhage and phenylpropanolamine use. Neurology. 1987;37:399-404.
72 Toffol GJ, Biller J, Adams HPJr. Nontraumatic intracerebral hemorrhage in young adults. Arch Neurol. 1987;44:483-485.
73 Levine SR, Brust JC, Futrell N, et al. Cerebrovascular complications of the use of the “crack” form of alkaloidal cocaine. N Engl J Med. 1990;323:699-704.
74 Qureshi AI, Mohammad Y, Suri MF, et al. Cocaine use and hypertension are major risk factors for intracerebral hemorrhage in young African Americans. Ethn Dis. 2001;11:311-319.
75 Nanda A, Vannemreddy P, Willis B, et al. Stroke in the young: relationship of active cocaine use with stroke mechanism and outcome. Acta Neurochir Suppl. 2006;96:91-96.
76 McEvoy AW, Kitchen ND, Thomas DG, et al. Intracerebral haemorrhage and drug abuse in young adults. Br J Neurosurg. 2000;14:449-454.
77 Juvela S, Hillbom M, Palomäki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26:1558-1564.
78 Monforte R, Estruch R, Graus F, et al. High ethanol consumption as risk factor for intracerebral hemorrhage in young and middle-aged people. Stroke. 1990;21:1529-1532.
79 Gorelick PB. The status of alcohol as a risk factor for stroke. Stroke. 1989;20:1607-1610.
80 Altura BM, Altura BT, Gebrewold A. Alcohol-induced spasms of cerebral blood vessels: relation to cerebrovascular accidents and sudden death. Science. 1983;220:331-333.
81 Klatsky AL, Armstrong MA, Friedman GD, et al. Alcohol drinking and risk of hemorrhagic stroke. Neuroepidemiology. 2002;21:115-122.
82 Gill JS, Shipley MJ, Tsementzis SA, et al. Alcohol consumption—a risk factor for hemorrhagic and non-hemorrhagic stroke. Am J Med. 1991;90:489-497.
83 Thrift AG, Donnan GA, McNeil JJ. Heavy drinking, but not moderate or intermediate drinking, increases the risk of intracerebral hemorrhage. Epidemiology. 1999;10:307-312.
84 Jousilahti P, Rastenyte D, Tuomilehto J. Serum gamma-glutamyl transferase, self-reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851-1855.
85 Klatsky AL, Armstrong MA, Friedman GD. Alcohol use and subsequent cerebrovascular disease hospitalizations. Stroke. 1989;20:741-746.
86 Inagawa T. Risk factors for primary intracerebral hemorrhage in patients in Izumo City, Japan. Neurosurg Rev. 2007;30:225-234.
87 Smajlović D, Salihović D, Ibrahimagić O, et al. Analysis of risk factors, localization and 30-day prognosis of intracerebral hemorrhage. Bosn J Basic Med Sci. 2008;8:121-125.
88 Choi-Kwon S, Kim JS. Lifestyle factors and risk of stroke in Seoul, South Korea. J Stroke Cerebrovasc Dis. 1998;7:414-420.
89 Kurth T, Kase CS, Berger K, et al. Smoking and the risk of hemorrhagic stroke in men. Stroke. 2003;34:1151-1155.
90 Mohr JP, Caplan LR, Melski JW. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978;28:754-762.
91 Andrews BT, Chiles BW3rd, Olsen WL, et al. The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988;69:518-522.
92 Heros RC. Cerebellar hemorrhage and infarction. Stroke. 1982;13:106-109.
93 Mayer SA, Sacco RL, Shi T, et al. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379-1384.
94 Qureshi AI, Safdar K, Weil J, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764-1767.
95 Zazulia AR, Diringer MN, Derdeyn CP, et al. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167-1173.
96 Ojemann RG, Heros RC. Spontaneous brain hemorrhage. Stroke. 1983;14:468-475.
97 Hier DB, Davis KR, Richardson EPJr, et al. Hypertensive putaminal hemorrhage. Ann Neurol. 1977;1:152-159.
98 Stein RW, Kase CS, Hier DB, et al. Caudate hemorrhage. Neurology. 1984;34:1549-1554.
99 Walshe TM, Davis KR, Fisher CM. Thalamic hemorrhage: a computed tomographic–clinical correlation. Neurology. 1977;27:217-222.
100 Ojemann RG, Mohr JP. Hypertensive brain hemorrhage. Clin Neurosurg. 1976;23:220-244.
101 Barraquer-Bordas L, Illa I, Escartin A, et al. Thalamic hemorrhage. A study of 23 patients with diagnosis by computed tomography. Stroke. 1981;12:524-527.
102 Dejerine J, Roussy G. Le syndrome thalamique. Rev Neurol. 1906;14:521-532.
103 Lhermitte J. Les syndromes thalamiques dissocié: les formes analgique et hémialgique. Ann Med. 1925;17:488-501.
104 Baudouin A, Lhermitte J, Lerebouillet J. Une observation anatomo-clinique d’hémorragie du thalamus. Rev. Neurol. 1930;2:102-109.
105 Fisher C. The pathologic and clinical aspects of thalamic hemorrhage. Trans Am Neurol Assoc. 1959;84:56-59.
106 Kumral E, Kocaer T, Ertübey NO, et al. Thalamic hemorrhage: a prospective study of 100 patients. Stroke. 1995;26:964-970.
107 Kase CS, Williams JP, Wyatt DA, et al. Lobar intracerebral hematomas: clinical and CT analysis of 22 cases. Neurology. 1982;32:1146-1150.
108 Ropper AH, Davis KR. Lobar cerebral hemorrhages: acute clinical syndromes in 26 cases. Ann Neurol. 1980;8:141-147.
109 Cole FM, Yates P. Intracerebral microaneurysms and small cerebrovascular lesions. Brain. 1967;90:759-768.
110 McCormick WF, Rosenfield DB. Massive brain hemorrhage: a review of 144 cases and an examination of their causes. Stroke. 1973;4:946-954.
111 Tyler KL, Poletti CE, Heros RC. Cerebral amyloid angiopathy with multiple intracerebral hemorrhages. Case report. J Neurosurg. 1982;57:286-289.
112 Finelli PF, Kessimian N, Bernstein PW. Cerebral amyloid angiopathy manifesting as recurrent intracerebral hemorrhage. Arch Neurol. 1984;41:330-333.
113 Weisberg LA. Subcortical lobar intracerebral haemorrhage: clinical–computed tomographic correlations. J Neurol Neurosurg Psychiatry. 1985;48:1078-1084.
114 Helweg-Larsen S, Sommer W, Strange P, et al. Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke. 1984;15:1045-1048.
115 Weisberg LA. Caudate hemorrhage. Arch Neurol. 1984;41:971-974.
116 Waga S, Miyazaki M, Okada M, et al. Hypertensive putaminal hemorrhage: analysis of 182 patients. Surg Neurol. 1986;26:159-166.
117 Asakura K, Mizuno M, Yasui N. [Clinical analysis of 24 cases of caudate hemorrhage.]. Neurol Med Chir (Tokyo). 1989;29:1107-1112.
118 Fuh J. Caudate hemorrhage: clinical features, neuropsychological assessments and radiological findings. Clin Neurol Neurosurg. 1995;18:445-450.
119 Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30:100-108.
120 Liliang P, Liang C, Lu CH, et al. Hypertensive caudate hemorrhage prognostic predictor, outcome, and role of external ventricular drainage. Stroke. 2001;32:1195-1200.
121 Dinsdale HB. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol. 1964;10:200-217.
122 Freeman RE, Onofrio BM, Okazaki H, et al. Spontaneous intracerebellar hemorrhage. Diagnosis and surgical treatment. Neurology. 1973;23:84-90.
123 Ott KH, Kase CS, Ojemann RG, et al. Cerebellar hemorrhage: diagnosis and treatment. A review of 56 cases. Arch Neurol. 1974;31:160-167.
124 Brennan RW, Bergland RM. Acute cerebellar hemorrhage. Analysis of clinical findings and outcome in 12 cases. Neurology. 1977;27:527-532.
125 Yanaka K, Meguro K, Fujita K, et al. Immediate surgery reduces mortality in deeply comatose patients with spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2000;40:295-299.
126 Morioka J, Fujii M, Kato S, et al. Surgery for spontaneous intracerebral hemorrhage has greater remedial value than conservative therapy. Surg Neurol. 2006;65:67-72.
127 Fisher CM, Picard EH, Polak A, et al. Acute hypertensive cerebellar hemorrhage: diagnosis and surgical treatment. J Nerv Ment Dis. 1965;140:38-57.
128 Messert B, Leppik IE, Sato S. Diplopia and involuntary eye closure in spontaneous cerebellar hemorrhage. Stroke. 1976;7:305-307.
129 Yoshida S, Sasaki M, Oka H, et al. Acute hypertensive cerebellar hemorrhage with signs of lower brainstem compression. Surg Neurol. 1978;10:79-83.
130 Little JR, Tubman DE, Ethier R. Cerebellar hemorrhage in adults. Diagnosis by computerized tomography. J Neurosurg. 1978;48:575-579.
131 Elkind MS, Mohr JP. Cerebellar hemorrhage. New Horiz. 1997;5:352-358.
132 Pollak L, Rabey JM, Gur R, et al. Indication to surgical management of cerebellar hemorrhage. Clin Neurol Neurosurg. 1998;100:99-103.
133 Attwater H. Pontine hemorrhage. Guys Hosp Rep. 1911;65:339-389.
134 Duret H. Traumatismes Cranio-Cerebraux. Paris: Librairie Felix Alcan; 1919.
135 Parizel PM, Makkat S, Jorens PG, et al. Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage). Intensive Care Med. 2002;28:85-88.
136 Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536-550.
137 Steegmann AT. Primary pontile hemorrhage; with particular reference to respiratory failure. J Nerv Ment Dis. 1951;114:35-65.
138 Silverstein A. Primary pontile hemorrhage. A review of 50 cases. Confin Neurol. 1967;29:33-46.
139 Wall M, Wray SH. The one-and-a-half syndrome—a unilateral disorder of the pontine tegmentum: a study of 20 cases and review of the literature. Neurology. 1983;33:971-980.
140 Fisher C. Ocular bobbing. Arch Neurol. 1964;11:543-546.
141 Gebel JMJr, Jauch EC, Brott TG, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631-2635.
142 Xi G, Hua Y, Bhasin RR, et al. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32:2932-2938.
143 Gebel J, Brott T, Sila CA, et al. Decreased perihematomal edema in thrombolysis related intracerebral hemorrhage compared with spontaneous intracerebral hemorrhage. Stroke. 2000;31:596-600.
144 Huang F, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin in degradation products. J Neurosurg. 2002;96:287-293.
145 Wu G, Xi G, Huang F. Spontaneous intracerebral hemorrhage in humans: hematoma enlargement, clot lysis, and brain edema. Acta Neurochir Suppl. 2006;96:78-80.
146 Scott M, Werthan M. The fate of hypertensive patients with clinically proven spontaneous intracerebral hematomas treated without intracranial surgery. Stroke. 1970;1:286-300.
147 Kutsuzawa T, Ito K, Kawakami H. [Conservative treatment of hypertensive cerebral hemorrhage: results of 104 patients of acute stage (author’s transl).]. Neurol Med Chir (Tokyo). 1976;16:29-36.
148 Batjer HH, Reisch JS, Allen BC, et al. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103-1106.
149 Tellez H, Bauer RB. Dexamethasone as treatment in cerebrovascular disease. 1. A controlled study in intracerebral hemorrhage. Stroke. 1973;4:541-546.
150 Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229-1233.
151 Kelley RE, Berger JR, Scheinberg P, et al. Active bleeding in hypertensive intracerebral hemorrhage: computed tomography. Neurology. 1982;32:852-856.
152 Kase C. Pathophysiologie, diagnosis and management. In: Kase C, editor. Stroke, Vol 1. New York: Churchill Livingstone; 1986.
153 Bae HG, Lee KS, Yan IG, et al. Rapid expansion of hypertensive intracerebral hemorrhage. Neurosurgery. 1992;31:35-41.
154 Ohwaki K, Yano E, Nagashima H, et al. Blood pressure management in acute intracerebral hemorrhage. Relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364-1367.
155 Zabramski J, Hamilton M. Intracerebral hematomas. In: Spetzler RF, Carter LP, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:477-496.
156 Crowell R, Ojemann R. Cerebellar hemorrhage. In: Buchheit WA, Truex RCJ, editors. Surgery of the Posterior Fossa. New York: Raven Press; 1979:135-142.
157 Qureshi AI, Bliwise DL, Bliwise NG, et al. Rate of 24-hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: a retrospective analysis with a random effects regression model. Crit Care Med. 1999;27:480-485.
158 Qureshi A, for the ATACH Investigators. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH). Presented at the International Stroke Conference, New Orleans, LA, 2008.
159 Nehls DG, Mendelow AD, Graham DI, et al. Experimental intracerebral hemorrhage: progression of hemodynamic changes after production of a spontaneous mass lesion. Neurosurgery. 1988;23:439-444.
160 Mendelow AD. Mechanisms of ischemic brain damage with intracerebral hemorrhage. Stroke. 1993;24(12 Suppl):I115-I117.
161 Wagner KR, Xi G, Hua Y, et al. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27:490-497.
162 Fujitsu K, Muramoto M, Ikeda Y, et al. Indications for surgical treatment of putaminal hemorrhage. Comparative study based on serial CT and time-course analysis. J Neurosurg. 1990;73:518-525.
163 Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987-993.
164 Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin N Am. 2003;13:741-758.
165 Schellinger PD, Fiebach JB, Hoffmann K, et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke. 2003;34:1674-1679.
166 Qureshi AI, Wilson DA, Hanley DF, et al. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266-272.
167 Orakcioglu B, Becker K, Sakowitz DW, et al. MRI of the perihemorrhagic zone in a rat ICH model: effect of hematoma evacuation. Neurocrit Care. 2008;8:448-455.
168 Pascual AM, Lopez-Mut JV, Benlloch V, et al. Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: a longitudinal study. Cerebrovasc Dis. 2007;23:6-13.
169 Siddique MS, Fernandes HM, Wooldridge TD, et al. Reversible ischemia around intracerebral hemorrhage: a single-photon emission computerized tomography study. J Neurosurg. 2002;96:736-741.
170 Nilsson OG, Polito A, Säveland H, et al. Are primary supratentorial intracerebral hemorrhages surrounded by a biochemical penumbra? A microdialysis study. Neurosurgery. 2006;59:521-528.
171 Janny P, Papo I, Chazal J, et al. Intracranial hypertension and prognosis of spontaneous intracerebral haematomas. A correlative study of 60 patients. Acta Neurochir (Wien). 1982;61:181-186.
172 Duff TA, Ayeni S, Levin AB, et al. Nonsurgical management of spontaneous intracerebral hematoma. Neurosurgery. 1981;9:387-393.
173 Smith DS, Rehncrona S, Siesjö BK. Barbiturates as protective agents in brain ischemia and as free radical scavengers in vitro. Acta Physiol Scand Suppl. 1980;492:129-134.
174 Murphy PG, Davies MJ, Columb MO, et al. Effect of propofol and thiopentone on free radical mediated oxidative stress of the erythrocyte. Br J Anaesth. 1996;76:536-543.
175 Shiozaki T, Sugimoto H, Taneda M, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363-368.
176 Berger AR, Lipton RB, Lesser ML, et al. Early seizures following intracerebral hemorrhage: implications for therapy. Neurology. 1988;38:1363-1365.
177 Shinton RA, Gill JS, Melnick SC, et al. The frequency, characteristics and prognosis of epileptic seizures at the onset of stroke. J Neurol Neurosurg Psychiatry. 1988;51:273-276.
178 Faught E, Peters D, Bartolucci A, et al. Seizures after primary intracerebral hemorrhage. Neurology. 1989;39:1089-1093.
179 Sung CY, Chu NS. Epileptic seizures in intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989;52:1273-1276.
180 Weisberg LA, Shamsnia M, Elliott D. Seizures caused by nontraumatic parenchymal brain hemorrhages. Neurology. 1991;41:1197-1199.
181 Burn J, Dennis M, Bamford J, et al. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. Br J Med. 1997;315:1582-1587.
182 Labovitz DL, Hauser WA. Preventing stroke-related seizures: when should anticonvulsant drugs be started? Neurology. 2003;60:365-366.
183 Passero S, Rocchi R, Rossi S, et al. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175-1180.
184 Yu YL, Kumana CR, Lauder IJ, et al. Treatment of acute cerebral hemorrhage with intravenous glycerol. A double-blind, placebo-controlled, randomized trial. Stroke. 1992;23:967-971.
185 Haemodilution in acute stroke: results of the Italian haemodilution trial. Italian Acute Stroke Study Group. Lancet. 1988;1:318-321.
186 Hemphill JC3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-897.
187 Fujii Y, Tanaka R, Takeuchi S, et al. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51-57.
188 Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783-1787.
189 Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34:224-229.
190 Friederich PW, Henny CP, Messelink EJ, et al. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:201-205.
191 Mayer SA, Brun NC, Broderick J, et al. Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke. 2005;36:74-79.
192 Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
193 Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137.
194 Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82-92.
195 Conti S, La Torre D, Gambelunghe G, et al. Successful treatment with rFVIIa of spontaneous intracerebral hemorrhage in a patient with mechanical prosthetic heart valves. Clin Lab Haematol. 2005;27:283-285.
196 Nath FP, Jenkins A, Mendelow AD, et al. Early hemodynamic changes in experimental intracerebral hemorrhage. J Neurosurg. 1986;65:697-703.
197 Nehls DG, Mendelow DA, Graham DI, et al. Experimental intracerebral hemorrhage: early removal of a spontaneous mass lesion improves late outcome. Neurosurgery. 1990;27:674-682.
198 Benes V, Koukolik F, Obrovska D. Two types of spontaneous intracerebral hemorrhage due to hypertension. J Neurosurg. 1972;37:509-513.
199 Papo I, Janny P, Caruselli G, et al. Intracranial pressure time course in primary intracerebral hemorrhage. Neurosurgery. 1979;4:504-511.
200 Ropper AH, Zervas NT. Cerebral blood flow after experimental basal ganglia hemorrhage. Ann Neurol. 1982;11:266-271.
201 Jenkins A, Mendelow AD, Graham DI, et al. Experimental intracerebral haematoma: the role of blood constituents in early ischaemia. Br J Neurosurg. 1990;4:45-51.
202 Takasugi S, Ueda S, Matsumoto K. Chronological changes in spontaneous intracerebral hematoma—an experimental and clinical study. Stroke. 1985;16:651-658.
203 Yang GY, Betz AL, Chenevert TL, et al. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93-102.
204 Kingman TA, Mendelow AD, Graham DI, et al. Experimental intracerebral mass: time-related effects on local cerebral blood flow. J Neurosurg. 1987;67:732-738.
205 Bullock R, Brock-Utne J, van Dellen J, et al. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surg Neurol. 1988;29:101-107.
206 Lee KR, Betz AL, Kim S, et al. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neurochir (Wien). 1996;138:396-400.
207 Rosenberg GA, Navratil M. Inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921-926.
208 Hosaka Y, Kaneko M, Muraki M, et al. [Reevaluation of the effect of the operation in the per-acute stage. Review of 166 cases of hypertensive intracerebral hemorrhage (the lateral type) (author’s transl).]. Neurol Med Chir (Tokyo). 1980;20:907-913.
209 Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530-535.
210 Niizuma H, Yonemitsu T, Jokura H, et al. Stereotactic aspiration of thalamic hematoma. Overall results of 75 aspirated and 70 nonaspirated cases. Stereotact Funct Neurosurg. 1990;54-55:438-444.
211 Daverat P, Castel JP, Dartigues JF, et al. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991;22:1-6.
212 Gong Y, Hua Y, Keep RF, et al. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571-2575.
213 Auer J, Berent R, Weber T, et al. Subarachnoid haemorrhage with “Ecstasy” abuse in a young adult. Neurol Sci. 2002;23:199-201.
214 Nath F, Galbraith S. The effect of mannitol on cerebral white matter water content. J Neurosurg. 1986;65:41-43.
215 Durward QJ, Barnett HJ, Barr HW. Presentation and management of mesencephalic hematoma. Report of two cases. J Neurosurg. 1982;56:123-127.
216 Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke. 1983;14:493-500.
217 Volpin L, Cervellini P, Colombo F, et al. Spontaneous intracerebral hematomas: a new proposal about the usefulness and limits of surgical treatment. Neurosurgery. 1984;15:663-666.
218 Kaufman HH, Schochet S. Pathology, pathophysiology, and modeling. In: Kaufman HH, editor. Intracerebral Hematomas. New York: Raven Press; 1992:240.
219 Herbstein DJ, Schaumberg HH. Hypertensive intracerebral hematoma. An investigation of the initial hemorrhage and rebleeding using chromium Cr 51-labeled erythrocytes. Arch Neurol. 1974;30:412-414.
220 Takada I. [On the phenomena of extravasation of contrast media in cerebral angiogram of the case of hypertensive intracerebral hematoma and their clinical significance-analysis of 14 cases (author’s transl).]. No Shinkei Geka. 1976;4:471-478.
221 Ebina K, Okabe S, Manabe H, et al. [Experimental study on liquefaction of intracranial hematoma: usefulness of tissue-plasminogen activator (t-PA), a hematolytic agent, and its combination.]. No Shinkei Geka. 1990;18:927-934.
222 Kaufman HH. Treatment of deep spontaneous intracerebral hematomas. A review. Stroke. 1993;24(12 Suppl):I101-I106.
223 Mizukami M, Araki G, Mihara H, et al. [Surgical treatment of hypertensive cerebral hemorrhage. 4. The prognosis based on cerebral angiography.]. No To Shinkei. 1972;24:579-583.
224 Kaneko M, Tanaka K, Shimada T, et al. Long-term evaluation of ultra-early operation for hypertensive intracerebral hemorrhage in 100 cases. J Neurosurg. 1983;58:838-842.
225 Kanno T, Sano H, Shinomiya Y, et al. Role of surgery in hypertensive intracerebral hematoma. A comparative study of 305 nonsurgical and 154 surgical cases. J Neurosurg. 1984;61:1091-1099.
226 Takebayashi S, Kaneko M. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke. 1983;14:28-36.
227 Yukawa H, Kanaya H. [Indication for surgery in hypertensive intracerebral hemorrhage—a statistical study (author’s transl).]. Neurol Med Chir (Tokyo). 1978;18:361-365.
228 Juvela S, Heiskanen O, Poranen A, et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755-758.
229 Zuccarello M, Brott T, Derex L, et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30:1833-1839.
230 Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397.
231 Teernstra OP, Evers SM, Lodder J, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968-974.
232 Almaani WS, Awidi AS. Spontaneous intracranial bleeding in hemorrhagic diathesis. Surg Neurol. 1982;17:137-140.
233 Kobayashi S, Sato A, Kageyama Y, et al. Treatment of hypertensive cerebellar hemorrhage—surgical or conservative management? Neurosurgery. 1994;34:246-250.
234 Heros RC. Surgical treatment of cerebellar infarction. Stroke. 1992;23:937-938.
235 Fazio C. A neurologist’s study of problems concerning surgical treatment of spontaneous cerebral hemorrhage. Sc Med Ital. 1950;1:101-110.
236 Paillas JE, Alliez B. Surgical treatment of spontaneous intracerebral hemorrhage. Immediate and long-term results in 250 cases. J Neurosurg. 1973;39:145-151.
237 Kopitnik TJ. Spontaneous hemorrhage: innovative new techniques for decompression. In: Batjer H, editor. Cerebrovascula Disease. Philadelphia: Lippincott-Raven; 1997:641-647.
238 Cuatico W, Adib S, Gaston P. Spontaneous intracerebral hematomas: a surgical appraisal. J Neurosurg. 1965;22:569-575.
239 Mitsuno T, Kanaya H, Shirakata S, et al. Surgical treatment of hypertensive intracerebral hemorrhage. J Neurosurg. 1966;24:70-76.
240 Luessenhop AJ, Shevlin WA, Ferrero AA, et al. Surgical management of primary intracerebral hemorrhage. J Neurosurg. 1967;27:419-427.
241 Tedeschi G, Bernini FP, Cerillo A. Indications for surgical treatment of intracerebral hemorrhage. J Neurosurg. 1975;43:590-595.
242 Kanaya H, Nishimura K, Saiki I, et al. [Surgical treatment of hypertensive intracerebral hemorrhage. Experience with 174 cases (author’s transl).]. Neurol Med Chir (Tokyo). 1976;16:19-27.
242a Kanaya H, Yukawa H, Itoh Z, et al. A neurological grading for patients with hypertensive intracerebral hemorrhage and a classification for hematoma location on computed tomography. Proceedings of the Seventh Conference of Surgical Treatment of Stroke. Tokyo: Neuron; 1978. 265-270 (in Japanese)
243 Zuccarello M, Andrioli GG, Trincia G, et al. Spontaneous intracerebral haematomas. Aspects of treatment. Zentralbl Neurochir. 1983;44:209-213.
244 Bolander HG, Kourtopoulos H, Liliequist B, et al. Treatment of spontaneous intracerebral haemorrhage. A retrospective analysis of 74 consecutive cases with special reference to computertomographic data. Acta Neurochir (Wien). 1983;67:19-28.
245 Brambilla GL, Rodriguez y Baena R, Sangiovanni G, et al. Spontaneous intracerebral hemorrhage: medical or surgical treatment. J Neurosurg Sci. 1983;27:95-101.
246 Coraddu M, Nurchi GC, Floris F, et al. Considerations about the surgical indication of the spontaneous cerebral haematomas. J Neurosurg Sci. 1990;34:35-39.
247 Mizukami M. Hypertensive Intracerebral Hemorrhage. New York: Raven Press; 1983.
248 Suzuki J, Sato S. The new transinsular approach to the hypertensive intracerebral hematoma. Jpn J Surg. 1972;2:47-52.
249 Kanaya H, Kuroda K. Development in neurosurgical approaches to hypertensive intracerebral hemorrhage in Japan. In: Kaufman HH, editor. Intracerebral Hematomas. New York: Raven Press; 1992:197-209.
250 Crowell R, Ojemann R. Cerebellar hemorrhage. In: Buchheit W, Truex R, editors. Surgery of the Posterior Fossa. New York: Raven Press; 1979:135-142.
251 Niizuma H, Shimizu Y, Yonemitsu T, et al. Results of stereotactic aspiration in 175 cases of putaminal hemorrhage. Neurosurgery. 1989;24:814-819.
252 Donauer E, Faubert C. Management of spontaneous intracerebral and cerebellar hemorrhage. In: Kaufman HH, editor. Intracerebral Hematomas. New York: Raven Press; 1992:211-227.
253 Benes V, Vladyka V, Zverina E. Sterotaxic evacuation of typical brain haemorrhage. Acta Neurochir (Wien). 1965;13:419-426.
254 Backlund EO, von Holst H. Controlled subtotal evacuation of intracerebral haematomas by stereotactic technique. Surg Neurol. 1978;9:99-101.
255 Honda E, Hayashi T, Shimamoto H, et al. [A comparison between stereotaxic operation and conservative therapy for thalamic hemorrhage.]. No Shinkei Geka. 1988;16(5 Suppl):665-670.
256 Marquardt G, Wolff R, Seifert V. Multiple target aspiration technique for subacute stereotactic aspiration of hematomas within the basal ganglia. Surg Neurol. 2003;60:8-13.
257 Kandel EI, Peresedov VV. Stereotaxic evacuation of spontaneous intracerebral hematomas. J Neurosurg. 1985;62:206-213.
258 Nguyen JP, Decq P, Brugieres P, et al. A technique for stereotactic aspiration of deep intracerebral hematomas under computed tomographic control using a new device. Neurosurgery. 1992;31:330-334.
259 Ito H, Mukai H, Higashi S, et al. [Removal of hypertensive intracerebral hematoma with stereotactic aqua-stream and aspirator (SAS & A).]. No Shinkei Geka. 1989;17:939-943.
260 Mukai H, Yamashita J, Kitamura A, et al. Stereotactic Aqua-Stream and Aspirator in the treatment of intracerebral hematoma. An experimental study. Stereotact Funct Neurosurg. 1991;57:221-227.
261 Hondo H, Uno M, Sasaki K, et al. Computed tomography controlled aspiration surgery for hypertensive intracerebral hemorrhage. Experience of more than 400 cases. Stereotact Funct Neurosurg. 1990;54-55:432-437.
262 Doi E, Moriwaki H, Komai N, et al. [Stereotactic evacuation of intracerebral hematomas.]. Neurol Med Chir (Tokyo). 1982;22:461-467.
263 Niizuma H, Otsuki T, Johkura H, et al. CT-guided stereotactic aspiration of intracerebral hematoma—result of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985;48:427-430.
264 Lippitz BE, Mayfrank L, Spetzger U, et al. Lysis of basal ganglia haematoma with recombinant tissue plasminogen activator (rtPA) after stereotactic aspiration: initial results. Acta Neurochir (Wien). 1994;127:157-160.
265 Montes JM, Wong JH, Fayad PB, et al. Stereotactic computed tomographic–guided aspiration and thrombolysis of intracerebral hematoma: protocol and preliminary experience. Stroke. 2000;31:834-840.
266 Matsumoto K, Hondo H. CT-guided stereotaxic evacuation of hypertensive intracerebral hematomas. J Neurosurg. 1984;61:440-448.
267 Tanikawa T, Amano K, Kawamura H, et al. CT-guided stereotactic surgery for evacuation of hypertensive intracerebral hematoma. Appl Neurophysiol. 1985;48:431-439.
268 Niizuma H, Suzuki J. Stereotactic aspiration of putaminal hemorrhage using a double track aspiration technique. Neurosurgery. 1988;22:432-436.
269 Niizuma H, Otsuki T, Yonemitsu T, et al. Experiences with CT-guided stereotaxic biopsies in 121 cases. Acta Neurochir Suppl. 1988;42:157-160.
270 Deinsberger W, Hartmann M, Vogel J, et al. Local fibrinolysis and aspiration of intracerebral hematomas in rats. An experimental study using MR monitoring. Neurol Res. 1998;20:349-352.
271 Deinsberger W, Vogel J, Fuchs C, et al. Fibrinolysis and aspiration of experimental intracerebral hematoma reduces the volume of ischemic brain in rats. Neurol Res. 1999;21:517-523.
272 Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553-572.
273 Ebina K, Okabe S, Manabe H, et al. [Experimental study on liquefaction of intracranial hematoma: usefulness of tissue-plasminogen activator (t-PA), a hematolytic agent, and its combination.]. No Shinkei Geka. 1990;18:927-934.
274 Weisberg LA. Primary pontine haemorrhage: clinical and computed tomographic correlations. J Neurol Neurosurg Psychiatry. 1986;49:346-352.
275 Matsumoto K, Hondo H, Tomido K. Aspiration surgery for hypertensive brain hemorrhage in the acute stage. In: Suzuki J, editor. Advances in Surgery for Cerebral Stroke: Proceedings of the International Symposium on Surgery for Cerebral Stroke. Tokyo: Springer-Verlag, 1987.
276 Vespa P, McArthur D, Miller C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2:274-281.
277 Broseta J, Gonzalez-Darder J, Barcia-Saloria JL. Stereotactic evacuation of intracerebral hematomas. Appl Neurophysiol. 1982;45:443-448.
278 Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
279 Ausman JI. An important study comparing an endoscopic and a medical approach in treating acute intracerebral hematomas [editorial]. Surg Neurol. 2008;69:437-438.
280 Miller CM, Vespa P, Saver JL, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:441-446.
281 Cho DY, Chen CC, Chang CS, et al. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006;65:547-555.
282 Marquardt G, Wolff R, Sager A, et al. Subacute stereotactic aspiration of haematomas within the basal ganglia reduces occurrence of complications in the course of haemorrhagic stroke in non-comatose patients. Cerebrovasc Dis. 2003;15:252-257.
283 Hanley D. MISTIE: Minimally Invasive Surgery plus rtPA for Intracerebral Hemorrhage Evacuation. Available at http://www.strokecenter.org/trials/TrialDetail.aspx?tid=690 Accessed June 15, 2008
284 Morgenstern LB, Frankowski RF, Shedden P, et al. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359-1363.
285 Hattori N, Katayama Y, Maya Y, et al. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101:417-420.
286 Nakayama K, Miyasaka Y, Sato K, et al. [Postoperative intracranial pressure in severe cases with hypertensive intracerebral hematoma.]. No To Shinkei. 1989;41:1149-1154.
287 Ropper AH, King RB. Intracranial pressure monitoring in comatose patients with cerebral hemorrhage. Arch Neurol. 1984;41:725-728.
288 Hara M, Kadowaki C, Shiogai T, et al. Correlation between intracranial pressure (ICP) and changes in CT images of cerebral hemorrhage. Neurol Res. 1998;20:225-230.